EGFR- and Integrin αVβ3-Targeting Peptides as Potential Radiometal-Labeled Radiopharmaceuticals for Cancer Theranostics
Abstract
1. Introduction
2. EGFR and Integrin αVβ3 as Cancer Targets
3. EGFR- and Integrin αvβ3-Targeting Peptides
3.1. EGFR-Targeting Peptides
3.2. Integrin αvβ3-Targeting Peptides
4. Radiolabeled Peptides as Valuable Tools for Imaging and the Treatment of Cancer
4.1. Strategies for Radiolabeling Peptides
4.1.1. Direct Labeling
4.1.2. Indirect Labeling
5. Preclinical Studies of Radiolabeled EGFR and Integrin αvβ3-Targeting Peptides
5.1. EGFR-Targeting Peptides
Use of EGFR-Targeting Peptides for Therapeutic Purposes
5.2. Integrin αVβ3-Targeting Peptides
Use of Integrin αVβ3-Targeting Peptides for Therapeutic Purposes
6. Clinical Studies
7. Dual-Targeting Peptides
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, H.; Pan, Y.; Zhang, Y.; Tang, H. Challenges for the application of EGFR-targeting peptide GE11 in tumor diagnosis and treatment. J. Control. Release 2022, 349, 592–605. [Google Scholar] [CrossRef]
- Li, Z.B.; Cai, W.; Cao, Q.; Chen, K.; Wu, Z.; He, L.; Chen, X. 64Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor αvβ3 integrin expression. J. Nucl. Med. 2007, 48, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Cong, L.; Yan, B.; Jiang, Y. The role of cell-penetrating peptides in potential anti-cancer therapy. Clin. Transl. Med. 2022, 12, e822. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.J.; King, A.T.; Katsifis, A.; Matesic, L.; Jamie, J.F. Methods to Enhance the Metabolic Stability of Peptide-Based PET Radiopharmaceuticals. Molecules 2020, 25, 2314. [Google Scholar] [CrossRef]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Bessman, N.J.; Freed, D.M.; Lemmon, M.A. Putting together structures of epidermal growth factor receptors. Curr. Opin. Struct. Biol. 2014, 29, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Guy, P.M.; Platko, J.V.; Cantley, L.C.; Cerione, R.A.; Carraway, K.L., 3rd. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 1994, 91, 8132–8136. [Google Scholar] [CrossRef]
- Hubbard, S.R. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997, 16, 5572–5581. [Google Scholar] [CrossRef]
- Mohammadi, M.; Schlessinger, J.; Hubbard, S.R. Structure of the FGF receptor tyrosine kinase domain reveals a novel autoinhibitory mechanism. Cell 1996, 86, 577–587. [Google Scholar] [CrossRef]
- Kamath, S.; Buolamwini, J.K. Targeting EGFR and HER-2 receptor tyrosine kinases for cancer drug discovery and development. Med. Res. Rev. 2006, 26, 569–594. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Stefani, C.; Miricescu, D.; Stanescu, S., II; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth Factors, PI3K/AKT/mTOR and MAPK Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Desai, P.P.; Tyagi, A.; Lampe, J.B.; Srivastava, Y.; Donkor, M.; Jones, H.P.; Dzyuba, S.V.; Crossley, E.; Williams, N.S.; et al. Short peptides based on the conserved regions of MIEN1 protein exhibit anticancer activity by targeting the MIEN1 signaling pathway. J. Biol. Chem. 2024, 300, 105680. [Google Scholar] [CrossRef] [PubMed]
- Shostak, K.; Chariot, A. EGFR and NF-κB: Partners in cancer. Trends Mol. Med. 2015, 21, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Genta, I.; Chiesa, E.; Colzani, B.; Modena, T.; Conti, B.; Dorati, R. GE11 Peptide as an Active Targeting Agent in Antitumor Therapy: A Minireview. Pharmaceutics 2017, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Pierschbacher, M.D. Arg-Gly-Asp: A versatile cell recognition signal. Cell 1986, 44, 517–518. [Google Scholar] [CrossRef]
- Cai, W.; Chen, X. Anti-angiogenic cancer therapy based on integrin αvβ3 antagonism. Anti-Cancer Agents Med. Chem. 2006, 6, 407–428. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Alghisi, G.C.; Rüegg, C. Vascular integrins in tumor angiogenesis: Mediators and therapeutic targets. Endothelium 2006, 13, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Cheresh, D.A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2002, 2, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Haier, J. Integrins in cancer treatment. Curr. Cancer Drug Targets 2006, 6, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Debordeaux, F.; Chansel-Debordeaux, L.; Pinaquy, J.B.; Fernandez, P.; Schulz, J. What about αvβ3 integrins in molecular imaging in oncology? Nucl. Med. Biol. 2018, 62–63, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C. Integrin αvβ3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr. Drug Targets 2003, 4, 123–131. [Google Scholar] [CrossRef]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, R.; Wu, X.; Sun, Y.; Yao, M.; Li, J.; Xu, Y.; Gu, J. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J. 2005, 19, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, K.; Liu, W.; Wei, Y.; Sha, R.; Long, Y.; Han, Y.; Sun, P.; Wu, H.; Li, G.; et al. Synthesis and evaluation of [18F]FP-Lys-GE11 as a new radiolabeled peptide probe for epidermal growth factor receptor (EGFR) imaging. Nucl. Med. Biol. 2020, 90–91, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Hosseinimehr, S.J.; Khalaj, A.; Noaparast, Z.; Abedi, S.M.; Sabzevari, O. Tc labeled HYNIC-EDDA/tricine-GE11 peptide as a successful tumor targeting agent. Med. Chem. Res. 2018, 27, 890–902. [Google Scholar] [CrossRef]
- Rahmanian, N.; Hosseinimehr, S.J.; Khalaj, A.; Noaparast, Z.; Abedi, S.M.; Sabzevari, O. 99mTc-radiolabeled GE11-modified peptide for ovarian tumor targeting. DARU J. Pharm. Sci. 2017, 25, 13. [Google Scholar] [CrossRef] [PubMed]
- Abourbeh, G.; Shir, A.; Mishani, E.; Ogris, M.; Rödl, W.; Wagner, E.; Levitzki, A. PolyIC GE11 polyplex inhibits EGFR-overexpressing tumors. IUBMB Life 2012, 64, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Striese, F.; Sihver, W.; Gao, F.; Bergmann, R.; Walther, M.; Pietzsch, J.; Steinbach, J.; Pietzsch, H.J. Exploring pitfalls of 64Cu-labeled EGFR-targeting peptide GE11 as a potential PET tracer. Amino Acids 2018, 50, 1415–1431. [Google Scholar] [CrossRef]
- Jiao, H.; Zhao, X.; Han, J.; Zhang, J.; Wang, J. Synthesis of a novel 99mTc labeled GE11 peptide for EGFR SPECT imaging. Int. J. Radiat. Biol. 2020, 96, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Judmann, B.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Towards Radiolabeled EGFR-Specific Peptides: Alternatives to GE11. Pharmaceuticals 2023, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, I.M.; Vakili, M.R.; Soleimani, A.H.; Tabatabaei Dakhili, S.A.; Munira, S.; Paladino, M.; Martin, G.; Jirik, F.R.; Hall, D.G.; Weinfeld, M.; et al. Biodistribution and Activity of EGFR Targeted Polymeric Micelles Delivering a New Inhibitor of DNA Repair to Orthotopic Colorectal Cancer Xenografts with Metastasis. Mol. Pharm. 2022, 19, 1825–1838. [Google Scholar] [CrossRef]
- Decker, S.; Taschauer, A.; Geppl, E.; Pirhofer, V.; Schauer, M.; Pöschl, S.; Kopp, F.; Richter, L.; Ecker, G.F.; Sami, H.; et al. Structure-based peptide ligand design for improved epidermal growth factor receptor targeted gene delivery. Eur. J. Pharm. Biopharm. 2022, 176, 211–221. [Google Scholar] [CrossRef]
- Furman, O.; Zaporozhets, A.; Tobi, D.; Bazylevich, A.; Firer, M.A.; Patsenker, L.; Gellerman, G.; Lubin, B.C.R. Novel Cyclic Peptides for Targeting EGFR and EGRvIII Mutation for Drug Delivery. Pharmaceutics 2022, 14, 1505. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Duan, J.; Liu, X.; Bock, S.; Tian, Y.; Huang, Z. Biological evaluation of a novel doxorubicin-peptide conjugate for targeted delivery to EGF receptor-overexpressing tumor cells. Mol. Pharm. 2011, 8, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Stroobant, P.; Rice, A.P.; Gullick, W.J.; Cheng, D.J.; Kerr, I.M.; Waterfield, M.D. Purification and characterization of vaccinia virus growth factor. Cell 1985, 42, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.W.; Yang, X.Z.; Du, X.; Fu, L.Y.; Zhang, T.Z.; Shan, H.W.; Zhao, J.; Wang, F.J. Structure optimisation to improve the delivery efficiency and cell selectivity of a tumour-targeting cell-penetrating peptide. J. Drug Target. 2018, 26, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh-Mivehroud, M.; Mahmoudpour, A.; Dastmalchi, S. Identification of new peptide ligands for epidermal growth factor receptor using phage display and computationally modeling their mode of binding. Chem. Biol. Drug Des. 2012, 79, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Joshi, B.P.; Duan, X.; Pant, A.; Qiu, Z.; Kuick, R.; Owens, S.R.; Wang, T.D. EGFR Overexpressed in Colonic Neoplasia Can be Detected on Wide-Field Endoscopic Imaging. Clin. Transl. Gastroenterol. 2015, 6, e101. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, D.; Peng, J.; Deng, H.; Guo, Y.; Xu, L.X.; Miller, A.D.; Xu, Y. Novel peptide ligand directs liposomes toward EGF-R high-expressing cancer cells in vitro and in vivo. FASEB J. 2009, 23, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Yang, X.; Xu, J.; Lai, W.; Wang, Z.; Hu, Z.; Tian, J.; Geng, L.; Fang, Q. Tumor detection using magnetosome nanoparticles functionalized with a newly screened EGFR/HER2 targeting peptide. Biomaterials 2017, 115, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Joo, H.; Tsai, J.; Jasti, B.; Li, X. A Rational Approach for Creating Peptides Mimicking Antibody Binding. Sci. Rep. 2019, 9, 997. [Google Scholar] [CrossRef]
- Sobral, D.V.; Fuscaldi, L.L.; Durante, A.C.R.; Rangel, M.G.; Oliveira, L.R.; Mendonça, F.F.; Miranda, A.C.C.; Cabeza, J.M.; Montor, W.R.; Cabral, F.R.; et al. Radiochemical and biological properties of peptides designed to interact with EGF receptor: Relevance for glioblastoma. Nucl. Med. Biol. 2020, 88–89, 14–23. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, B.; He, X.; Qiu, Z.; Zhang, J.; Zhang, M.; Liu, H.; Pang, X.; Cui, Y. The challenges and opportunities of αvβ3-based therapeutics in cancer: From bench to clinical trials. Pharmacol. Res. 2023, 189, 106694. [Google Scholar] [CrossRef]
- Hernandez, R.; Czerwinski, A.; Chakravarty, R.; Graves, S.A.; Yang, Y.; England, C.G.; Nickles, R.J.; Valenzuela, F.; Cai, W. Evaluation of two novel 64Cu-labeled RGD peptide radiotracers for enhanced PET imaging of tumor integrin αvβ3. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1859–1868. [Google Scholar] [CrossRef][Green Version]
- Zuo, H. iRGD: A Promising Peptide for Cancer Imaging and a Potential Therapeutic Agent for Various Cancers. J. Oncol. 2019, 2019, 9367845. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, W.; Zhang, Y.; Meza, L.; Tseng, H.; Takada, Y.; Ames, J.B.; Lam, K.S. Optimization of RGD-Containing Cyclic Peptides against αvβ3 Integrin. Mol. Cancer Ther. 2016, 15, 232–240. [Google Scholar] [CrossRef]
- Indrevoll, B.; Kindberg, G.M.; Solbakken, M.; Bjurgert, E.; Johansen, J.H.; Karlsen, H.; Mendizabal, M.; Cuthbertson, A. NC-100717: A versatile RGD peptide scaffold for angiogenesis imaging. Bioorg. Med. Chem. Lett. 2006, 16, 6190–6193. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, E.; Wang, B.; Ruoslahti, E. Phage Libraries Displaying Cyclic Peptides with Different Ring Sizes: Ligand Specificities of the RGD-Directed Integrins. Bio/Technology 1995, 13, 265–270. [Google Scholar] [CrossRef]
- Del Gatto, A.; Zaccaro, L.; Grieco, P.; Novellino, E.; Zannetti, A.; Del Vecchio, S.; Iommelli, F.; Salvatore, M.; Pedone, C.; Saviano, M. Novel and Selective αvβ3 Receptor Peptide Antagonist: Design, Synthesis, and Biological Behavior. J. Med. Chem. 2006, 49, 3416–3420. [Google Scholar] [CrossRef]
- Zhang, L.; Shan, X.; Meng, X.; Gu, T.; Guo, L.; An, X.; Jiang, Q.; Ge, H.; Ning, X. Novel Integrin αvβ3-Specific Ligand for the Sensitive Diagnosis of Glioblastoma. Mol. Pharm. 2019, 16, 3977–3984. [Google Scholar] [CrossRef]
- Ma, Y.; Ai, G.; Zhang, C.; Zhao, M.; Dong, X.; Han, Z.; Wang, Z.; Zhang, M.; Liu, Y.; Gao, W.; et al. Novel Linear Peptides with High Affinity to αvβ3 Integrin for Precise Tumor Identification. Theranostics 2017, 7, 1511–1523. [Google Scholar] [CrossRef]
- Kręcisz, P.; Czarnecka, K.; Królicki, L.; Mikiciuk-Olasik, E.; Szymański, P. Radiolabeled Peptides and Antibodies in Medicine. Bioconjug. Chem. 2021, 32, 25–42. [Google Scholar] [CrossRef]
- Durante, A.C.R.; Sobral, D.V.; Miranda, A.C.C.; Almeida, É.L.d.V.; Fuscaldi, L.; de Barboza, M.R.F.F.; Malavolta, L. Comparative Study of Two Oxidizing Agents, Chloramine T and Iodo-Gen®, for the Radiolabeling of β-CIT with Iodine-131: Relevance for Parkinson’s Disease. Pharmaceuticals 2019, 12, 25. [Google Scholar] [CrossRef]
- Sobral, D.V.; Fuscaldi, L.L.; Durante, A.C.R.; Mendonca, F.F.; de Oliveira, L.R.; Miranda, A.C.C.; Mejia, J.; Montor, W.R.; de Barboza, M.F.; Malavolta, L. Comparative Evaluation of Radiochemical and Biological Properties of 131I- and [99mTc]Tc(CO)3-Labeled RGD Analogues Planned to Interact with the αvβ3 Integrin Expressed in Glioblastoma. Pharmaceuticals 2022, 15, 116. [Google Scholar] [CrossRef]
- Baishya, R.; Nayak, D.K.; Chatterjee, N.; Halder, K.K.; Karmakar, S.; Debnath, M.C. Synthesis, Characterization, and Biological Evaluation of 99mTc(CO)3-Labeled Peptides for Potential Use as Tumor Targeted Radiopharmaceuticals. Chem. Biol. Drug Des. 2014, 83, 58–70. [Google Scholar] [CrossRef]
- Vats, K.; Satpati, D.; Sharma, R.; Sarma, H.D.; Banerjee, S. Synthesis and comparative in vivo evaluation of 99mTc(CO)3-labeled PEGylated and non-PEGylated cRGDfK peptide monomers. Chem. Biol. Drug Des. 2017, 89, 371–378. [Google Scholar] [CrossRef]
- Brechbiel, M.W. Bifunctional chelates for metal nuclides. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 166–173. [Google Scholar]
- Haubner, R.; Weber, W.A.; Beer, A.J.; Vabuliene, E.; Reim, D.; Sarbia, M.; Becker, K.F.; Goebel, M.; Hein, R.; Wester, H.J.; et al. Noninvasive visualization of the activated αvβ3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005, 2, e70. [Google Scholar] [CrossRef]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Tohme, M.; Park, R.; Khankaldyyan, V.; Gonzales-Gomez, I.; Bading, J.R.; Laug, W.E.; Conti, P.S. Pegylated Arg-Gly-Asp Peptide: 64Cu Labeling and PET Imaging of Brain Tumor αvβ3-Integrin Expression. J. Nucl. Med. 2004, 45, 1776. [Google Scholar]
- Liolios, C.; Sachpekidis, C.; Kolocouris, A.; Dimitrakopoulou-Strauss, A.; Bouziotis, P. PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors. Molecules 2021, 26, 1792. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Z.; Chen, K.; Yan, Y.; Watzlowik, P.; Wester, H.J.; Chin, F.T.; Chen, X. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol. Imaging Biol. 2010, 12, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yue, J.B. Current status and advances in arginine-glycine-aspartic acid peptide-based molecular imaging to evaluate the effects of anti-angiogenic therapies. Precis. Radiat. Oncol. 2019, 3, 29–34. [Google Scholar] [CrossRef]
- Imberti, C.; Terry, S.Y.; Cullinane, C.; Clarke, F.; Cornish, G.H.; Ramakrishnan, N.K.; Roselt, P.; Cope, A.P.; Hicks, R.J.; Blower, P.J.; et al. Enhancing PET Signal at Target Tissue in Vivo: Dendritic and Multimeric Tris(hydroxypyridinone) Conjugates for Molecular Imaging of αvβ3 Integrin Expression with Gallium-68. Bioconjug. Chem. 2017, 28, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.S.; Kang, K.W.; Lee, H.Y.; Han, S.W.; Kim, T.Y.; Lee, Y.S.; Jeong, J.M.; Lee, D.S. Whole-body distribution and radiation dosimetry of 68Ga-NOTA-RGD, a positron emission tomography agent for angiogenesis imaging. Cancer Biother. Radiopharm. 2012, 27, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Li, W.; Guo, N.; Ma, Y.; Zhu, L.; Kiesewetter, D.O.; Shen, B.; Niu, G.; Chen, X. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjug. Chem. 2011, 22, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ye, Y.; Wadas, T.J.; Lewis, J.S.; Welch, M.J.; Achilefu, S.; Anderson, C.J. 64Cu-labeled CB-TE2A and diamsar-conjugated RGD peptide analogs for targeting angiogenesis: Comparison of their biological activity. Nucl. Med. Biol. 2009, 36, 277–285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kazemi, Z.; Zahmatkesh, M.H.; Abedi, S.M.; Hosseinimehr, S.J. Biological Evaluation of 99mTc-HYNIC-EDDA/tricine-(Ser)-D4 Peptide for Tumor Targeting. Curr. Radiopharm. 2017, 10, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zahmatkesh, M.H.; Abedi, S.M.; Hosseinimehr, S.J. 99mTc-HYNIC-D4 Peptide: A New Small Radiolabeled Peptide for Non Small Cell Lung Tumor Targeting. Anticancer Agents Med. Chem. 2017, 17, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Haddad Zahmatkesh, M.; Abedi, S.M.; Hosseinimehr, S.J. Preparation and biological evaluation of 99mTc-HYNIC-(Ser)3-D4 peptide for targeting and imaging of non-small-cell lung cancer. Future Oncol. 2017, 13, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Dissoki, S.; Hagooly, A.; Elmachily, S.; Mishani, E. Labeling approaches for the GE11 peptide, an epidermal growth factor receptor biomarker. J. Label. Compd. Rad. 2011, 54, 693–701. [Google Scholar] [CrossRef]
- Ogawa, K.; Takeda, T.; Yokokawa, M.; Yu, J.; Makino, A.; Kiyono, Y.; Shiba, K.; Kinuya, S.; Odani, A. Comparison of Radioiodine- or Radiobromine-Labeled RGD Peptides between Direct and Indirect Labeling Methods. Chem. Pharm. Bull. 2018, 66, 651–659. [Google Scholar] [CrossRef]
- Dejesus, O.T. Synthesis of [64Cu]Cu-NOTA-Bn-GE11 for PET imaging of EGFR-rich tumors. Curr. Radiopharm. 2012, 5, 15–18. [Google Scholar] [CrossRef]
- Judmann, B.; Braun, D.; Schirrmacher, R.; Wangler, B.; Fricker, G.; Wangler, C. Toward the Development of GE11-Based Radioligands for Imaging of Epidermal Growth Factor Receptor-Positive Tumors. ACS Omega 2022, 7, 27690–27702. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, S.G.; Kim, D.W. A novel dual-labeled small peptide as a multimodal imaging agent for targeting wild-type EGFR in tumors. PLoS ONE 2022, 17, e0263474. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Xiong, Z.; Cheng, Z.; Fisher, D.R.; Liu, S.; Gambhir, S.S.; Chen, X. microPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J. Nucl. Med. 2005, 46, 1707–1718. [Google Scholar]
- Shi, J.; Kim, Y.S.; Zhai, S.; Liu, Z.; Chen, X.; Liu, S. Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly3 and PEG4 linkers. Bioconjug. Chem. 2009, 20, 750–759. [Google Scholar] [CrossRef]
- Liu, S.; Vorobyova, I.; Park, R.; Conti, P.S. Biodistribution and Radiation Dosimetry of the Integrin Marker 64Cu-BaBaSar-RGD2 Determined from Whole-Body PET/CT in a Non-human Primate. Front. Phys. 2017, 5, 54. [Google Scholar] [CrossRef]
- Oxboel, J.; Brandt-Larsen, M.; Schjoeth-Eskesen, C.; Myschetzky, R.; El-Ali, H.H.; Madsen, J.; Kjaer, A. Comparison of two new angiogenesis PET tracers 68Ga-NODAGA-E[c(RGDyK)]2 and 64Cu-NODAGA-E[c(RGDyK)]2; in vivo imaging studies in human xenograft tumors. Nucl. Med. Biol. 2014, 41, 259–267. [Google Scholar] [CrossRef]
- Cai, H.; Li, Z.; Huang, C.W.; Park, R.; Conti, P.S. 64Cu labeled AmBaSar-RGD2 for micro-PET imaging of integrin αvβ3 expression. Curr. Radiopharm. 2011, 4, 68–74. [Google Scholar] [CrossRef]
- Minamimoto, R.; Karam, A.; Jamali, M.; Barkhodari, A.; Gambhir, S.S.; Dorigo, O.; Iagaru, A. Pilot prospective evaluation of 18F-FPPRGD2 PET/CT in patients with cervical and ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Toriihara, A.; Duan, H.; Thompson, H.M.; Park, S.; Hatami, N.; Baratto, L.; Fan, A.C.; Iagaru, A. 18F-FPPRGD2 PET/CT in patients with metastatic renal cell cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Mittra, E.S.; Goris, M.L.; Iagaru, A.H.; Kardan, A.; Burton, L.; Berganos, R.; Chang, E.; Liu, S.; Shen, B.; Chin, F.T.; et al. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: A PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology 2011, 260, 182–191. [Google Scholar] [CrossRef]
- Glaser, M.; Morrison, M.; Solbakken, M.; Arukwe, J.; Karlsen, H.; Wiggen, U.; Champion, S.; Kindberg, G.M.; Cuthbertson, A. Radiosynthesis and Biodistribution of Cyclic RGD Peptides Conjugated with Novel [18F]Fluorinated Aldehyde-Containing Prosthetic Groups. Bioconjug. Chem. 2008, 19, 951–957. [Google Scholar] [CrossRef]
- Cai, H.; Conti, P.S. RGD-based PET tracers for imaging receptor integrin αvβ3 expression. J. Label. Comp. Radiopharm. 2013, 56, 264–279. [Google Scholar] [CrossRef]
- Doss, M.; Kolb, H.C.; Zhang, J.J.; Bélanger, M.J.; Stubbs, J.B.; Stabin, M.G.; Hostetler, E.D.; Alpaugh, R.K.; von Mehren, M.; Walsh, J.C.; et al. Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J. Nucl. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef]
- Kolb, H.; Walsh, J.; Walsh, J.; Liang, Q.; Zhao, T.; Gao, D.; Secrest, J.; Gomez, L.; Scott, P. 18F-RGD-K5: A cyclic triazole-bearing RGD peptide for imaging integrin αvβ3 expression in vivo. J. Nucl. Med. 2009, 50, 329. [Google Scholar]
- Gaertner, F.C.; Kessler, H.; Wester, H.J.; Schwaiger, M.; Beer, A.J. Radiolabelled RGD peptides for imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39 (Suppl. S1), S126–S138. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Jiang, H.; Xu, Y.; Zhang, H.; Cheng, Z. One-step radiosynthesis of 18F-AlF-NOTA-RGD2 for tumor angiogenesis PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1732–1741. [Google Scholar] [CrossRef]
- Guo, J.; Guo, N.; Lang, L.; Kiesewetter, D.O.; Xie, Q.; Li, Q.; Eden, H.S.; Niu, G.; Chen, X. 18F-alfatide II and 18F-FDG dual-tracer dynamic PET for parametric, early prediction of tumor response to therapy. J. Nucl. Med. 2014, 55, 154–160. [Google Scholar] [CrossRef]
- Provost, C.; Prignon, A.; Rozenblum-Beddok, L.; Bruyer, Q.; Dumont, S.; Merabtene, F.; Nataf, V.; Bouteiller, C.; Talbot, J.N. Comparison and evaluation of two RGD peptides labelled with 68Ga or 18F for PET imaging of angiogenesis in animal models of human glioblastoma or lung carcinoma. Oncotarget 2018, 9, 19307–19316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vatsa, R.; Shukla, J.; Kumar, S.; Chakraboarty, S.; Dash, A.; Singh, G.; Mittal, B.R. Effect of Macro-Cyclic Bifunctional Chelators DOTA and NODAGA on Radiolabeling and In Vivo Biodistribution of Ga-68 Cyclic RGD Dimer. Cancer Biother. Radiopharm. 2019, 34, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Chakravarty, R.; Vatsa, R.; Bhusari, P.; Sarma, H.D.; Shukla, J.; Mittal, B.R.; Dash, A. Toward realization of ‘mix-and-use’ approach in ⁶⁸Ga radiopharmacy: Preparation, evaluation and preliminary clinical utilization of ⁶⁸Ga-labeled NODAGA-coupled RGD peptide derivative. Nucl. Med. Biol. 2016, 43, 116–123. [Google Scholar] [CrossRef]
- Shi, J.; Jin, Z.; Liu, X.; Fan, D.; Sun, Y.; Zhao, H.; Zhu, Z.; Liu, Z.; Jia, B.; Wang, F. PET Imaging of Neovascularization with 68Ga-3PRGD2 for Assessing Tumor Early Response to Endostar Antiangiogenic Therapy. Mol. Pharm. 2014, 11, 3915–3922. [Google Scholar] [CrossRef]
- Kazmierczak, P.M.; Todica, A.; Gildehaus, F.J.; Hirner-Eppeneder, H.; Brendel, M.; Eschbach, R.S.; Hellmann, M.; Nikolaou, K.; Reiser, M.F.; Wester, H.J.; et al. 68Ga-TRAP-(RGD)3 Hybrid Imaging for the In Vivo Monitoring of αvβ3-Integrin Expression as Biomarker of Anti-Angiogenic Therapy Effects in Experimental Breast Cancer. PLoS ONE 2016, 11, e0168248. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Niu, G.; Shi, J.; Liu, S.; Wang, F.; Liu, S.; Chen, X. 68Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: Promising agents for tumor integrin αvβ3 PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 947–957. [Google Scholar] [CrossRef]
- Knetsch, P.A.; Zhai, C.; Rangger, C.; Blatzer, M.; Haas, H.; Kaeopookum, P.; Haubner, R.; Decristoforo, C. [68Ga]FSC-(RGD)3 a trimeric RGD peptide for imaging αvβ3 integrin expression based on a novel siderophore derived chelating scaffold-synthesis and evaluation. Nucl. Med. Biol. 2015, 42, 115–122. [Google Scholar] [CrossRef]
- Kondo, N.; Wakamori, K.; Hirata, M.; Temma, T. Radioiodinated bicyclic RGD peptide for imaging integrin αvβ3 in cancers. Biochem. Biophys. Res. Commun. 2020, 528, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, L.; Kim, Y.S.; Zhai, S.; Liu, Z.; Chen, X.; Liu, S. Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic arginine-glycine-aspartic (RGD) dimers with triglycine linkers. J. Med. Chem. 2008, 51, 7980–7990. [Google Scholar] [CrossRef]
- Zhou, Y.; Kim, Y.S.; Chakraborty, S.; Shi, J.; Gao, H.; Liu, S. 99mTc-labeled cyclic RGD peptides for noninvasive monitoring of tumor integrin αvβ3 expression. Mol. Imaging 2011, 10, 386–397. [Google Scholar] [CrossRef]
- Liang, Y.; Jia, X.; Wang, Y.; Liu, Y.; Yao, X.; Bai, Y.; Han, P.; Chen, S.; Yang, A.; Gao, R. Evaluation of integrin αvβ3-targeted imaging for predicting disease progression in patients with high-risk differentiated thyroid cancer (using 99mTc-3PRGD2). Cancer Imaging 2022, 22, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, J.; Kim, Y.-S.; Zhai, S.; Jia, B.; Zhao, H.; Liu, Z.; Wang, F.; Chen, X.; Liu, S. Improving Tumor-Targeting Capability and Pharmacokinetics of 99mTc-Labeled Cyclic RGD Dimers with PEG4 Linkers. Mol. Pharm. 2009, 6, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Yang, Y.; Fang, W.; Liu, S. Comparison of biological properties of 99mTc-labeled cyclic RGD Peptide trimer and dimer useful as SPECT radiotracers for tumor imaging. Nucl. Med. Biol. 2016, 43, 661–669. [Google Scholar] [CrossRef]
- Bolzati, C.; Salvarese, N.; Carpanese, D.; Seraglia, R.; Meléndez-Alafort, L.; Rosato, A.; Capasso, D.; Saviano, M.; Del Gatto, A.; Comegna, D.; et al. [99mTc][Tc(N)PNP43]-Labeled RGD Peptides As New Probes for a Selective Detection of αvβ3 Integrin: Synthesis, Structure-Activity and Pharmacokinetic Studies. J. Med. Chem. 2018, 61, 9596–9610. [Google Scholar] [CrossRef]
- Decristoforo, C.; Faintuch-Linkowski, B.; Rey, A.; von Guggenberg, E.; Rupprich, M.; Hernandez-Gonzales, I.; Rodrigo, T.; Haubner, R. [99mTc]HYNIC-RGD for imaging integrin αvβ3 expression. Nucl. Med. Biol. 2006, 33, 945–952. [Google Scholar] [CrossRef]
- Echavidre, W.; Durivault, J.; Gotorbe, C.; Blanchard, T.; Pagnuzzi, M.; Vial, V.; Raes, F.; Broisat, A.; Villeneuve, R.; Amblard, R.; et al. Integrin-αvβ3 is a Therapeutically Targetable Fundamental Factor in Medulloblastoma Tumorigenicity and Radioresistance. Cancer Res. Commun. 2023, 3, 2483–2496. [Google Scholar] [CrossRef]
- Sancey, L.; Ardisson, V.; Riou, L.M.; Ahmadi, M.; Marti-Batlle, D.; Boturyn, D.; Dumy, P.; Fagret, D.; Ghezzi, C.; Vuillez, J.-P. In vivo imaging of tumour angiogenesis in mice with the αvβ3 integrin-targeted tracer 99mTc-RAFT-RGD. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2037–2047. [Google Scholar] [CrossRef]
- Liu, S.; Hsieh, W.Y.; Jiang, Y.; Kim, Y.S.; Sreerama, S.G.; Chen, X.; Jia, B.; Wang, F. Evaluation of a 99mTc-labeled cyclic RGD tetramer for noninvasive imaging integrin αvβ3-positive breast cancer. Bioconjug. Chem. 2007, 18, 438–446. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Kim, Y.S.; Zhai, S.; Jia, B.; Wang, F.; Liu, S. 99mTcO(MAG2-3G3-dimer): A new integrin αvβ3-targeted SPECT radiotracer with high tumor uptake and favorable pharmacokinetics. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1874–1884. [Google Scholar] [CrossRef]
- Ji, S.; Czerwinski, A.; Zhou, Y.; Shao, G.; Valenzuela, F.; Sowiński, P.; Chauhan, S.; Pennington, M.; Liu, S. 99mTc-Galacto-RGD2: A novel 99mTc-labeled cyclic RGD peptide dimer useful for tumor imaging. Mol. Pharm. 2013, 10, 3304–3314. [Google Scholar] [CrossRef]
- Fu, J.; Xie, Y.; Fu, T.; Qiu, F.; Yu, F.; Qu, W.; Yao, X.; Zhang, A.; Yang, Z.; Shao, G.; et al. [99mTc]Tc-Galacto-RGD2 integrin αvβ3-targeted imaging as a surrogate for molecular phenotyping in lung cancer: Real-world data. EJNMMI Res. 2021, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.Y.; Abiraj, K.; Frielink, C.; van Dijk, L.K.; Bussink, J.; Oyen, W.J.; Boerman, O.C. Imaging integrin αvβ3 on blood vessels with 111In-RGD2 in head and neck tumor xenografts. J. Nucl. Med. 2014, 55, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Decristoforo, C.; Hernandez Gonzalez, I.; Carlsen, J.; Rupprich, M.; Huisman, M.; Virgolini, I.; Wester, H.J.; Haubner, R. 68Ga- and 111In-labelled DOTA-RGD peptides for imaging of αvβ3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ji, S.; Tomaselli, E.; Yang, Y.; Liu, S. Comparison of biological properties of 111In-labeled dimeric cyclic RGD peptides. Nucl. Med. Biol. 2015, 42, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kim, Y.S.; Chakraborty, S.; Zhou, Y.; Wang, F.; Liu, S. Impact of bifunctional chelators on biological properties of 111In-labeled cyclic peptide RGD dimers. Amino Acids 2011, 41, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, A.; Cortez, A.G.; Yu, J.; Majumdar, S.; Bhise, A.; Hobbs, R.F.; Nedrow, J.R. Evaluation of targeting αvβ3 in breast cancers using RGD peptide-based agents. Nucl. Med. Biol. 2024, 128–129, 108880. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, I.; Kruijtzer, J.A.; Frielink, C.; Corstens, F.H.; Oyen, W.J.; Liskamp, R.M.; Boerman, O.C. αvβ3 integrin-targeting of intraperitoneally growing tumors with a radiolabeled RGD peptide. Int. J. Cancer 2007, 120, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.H.; Jeong, J.M.; Lee, Y.S.; Kim, Y.J.; Lee, B.C.; Lee, D.S.; Chung, J.K.; Lee, M.C.; Jeong, S.Y. Development of a ¹⁷⁷Lu-labeled RGD derivative for targeting angiogenesis. Cancer Biother. Radiopharm. 2010, 25, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fan, D.; Dong, C.; Liu, H.; Jia, B.; Zhao, H.; Jin, X.; Liu, Z.; Li, F.; Wang, F. Anti-tumor effect of integrin targeted 177Lu-3PRGD2 and combined therapy with Endostar. Theranostics 2014, 4, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Bozon-Petitprin, A.; Bacot, S.; Gauchez, A.S.; Ahmadi, M.; Bourre, J.C.; Marti-Batlle, D.; Perret, P.; Broisat, A.; Riou, L.M.; Claron, M.; et al. Targeted radionuclide therapy with RAFT-RGD radiolabelled with 90Y or 177Lu in a mouse model of αvβ3-expressing tumours. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.S.; Chen, Y.; Lee, H.; Liu, D.; Sun, X.; Kweon, J.; Lewis, M.R.; Chong, H.S. Synthesis and evaluation of a new bifunctional NETA chelate for molecular targeted radiotherapy using 90Y or 177Lu. Nucl. Med. Biol. 2015, 42, 242–249. [Google Scholar] [CrossRef]
- Chen, H.; Jacobson, O.; Niu, G.; Weiss, I.D.; Kiesewetter, D.O.; Liu, Y.; Ma, Y.; Wu, H.; Chen, X. Novel “Add-On” Molecule Based on Evans Blue Confers Superior Pharmacokinetics and Transforms Drugs to Theranostic Agents. J. Nucl. Med. 2017, 58, 590–597. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, H.; Guo, Z.; Fu, K.; Yao, L.; Fu, L.; Guo, W.; Wen, X.; Jacobson, O.; Zhang, X.; et al. Targeted Radionuclide Therapy in Patient-Derived Xenografts Using 177Lu-EB-RGD. Mol. Cancer Ther. 2020, 19, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, W.; Zhen, Z.; Zhang, W.; Liao, S.; Wu, X.; Wang, L.; Xuan, A.; Gao, Y.; Xu, J. Integrin αvβ3 and EGFR dual-targeted [64Cu]Cu-NOTA-RGD-GE11 heterodimer for PET imaging in pancreatic cancer mouse model. Nucl. Med. Biol. 2023, 124–125, 108364. [Google Scholar] [CrossRef] [PubMed]
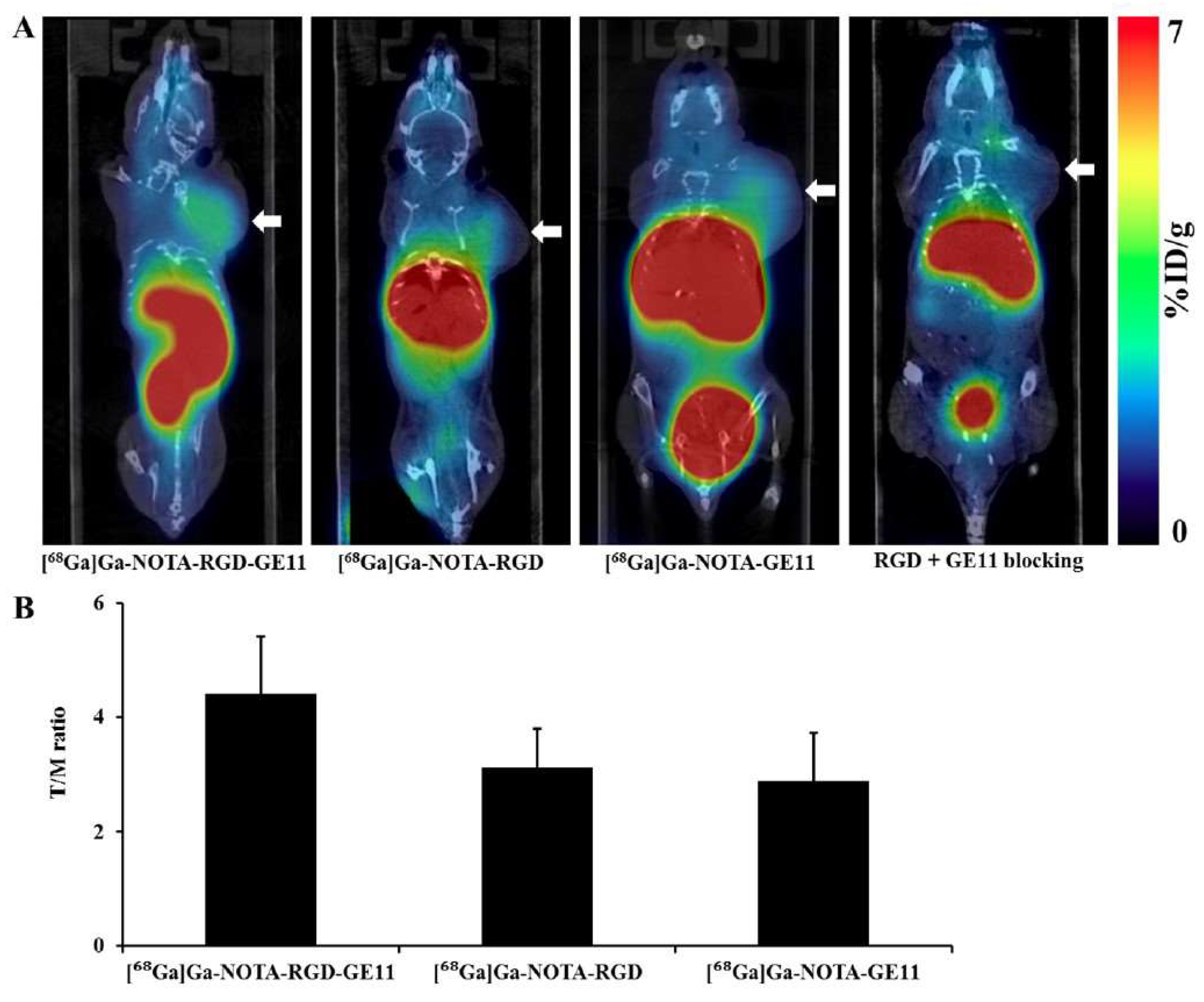
- Yu, H.M.; Chen, J.H.; Lin, K.L.; Lin, W.J. Synthesis of 68Ga-labeled NOTA-RGD-GE11 heterodimeric peptide for dual integrin and epidermal growth factor receptor-targeted tumor imaging. J. Label. Comp. Radiopharm. 2015, 58, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; Fu, K.; Lin, Q.; Wen, X.; Jacobson, O.; Sun, L.; Wu, H.; Zhang, X.; Guo, Z.; et al. Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics 2019, 9, 7948–7960. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Judmann, B.; Cheng, X.; Wangler, B.; Schirrmacher, R.; Fricker, G.; Wangler, C. Synthesis, Radiolabeling, and In Vitro and In Vivo Characterization of Heterobivalent Peptidic Agents for Bispecific EGFR and Integrin αvβ3 Targeting. ACS Omega 2023, 8, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Yu, J.; Yuan, S. Preliminary Clinical Application of RGD-Containing Peptides as PET Radiotracers for Imaging Tumors. Front. Oncol. 2022, 12, 837952. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, F.; Liu, S. Radiolabeled cyclic RGD peptides as radiotracers for tumor imaging. Biophys. Rep. 2016, 2, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, S.; Wang, F.; Liu, S.; Chen, X. Noninvasive imaging of tumor integrin expression using 18F-labeled RGD dimer peptide with PEG4 linkers. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Lang, L.; Li, W.; Kiesewetter, D.O.; Gao, H.; Niu, G.; Xie, Q.; Chen, X. Quantitative Analysis and Comparison Study of [18F]AlF-NOTA-PRGD2, [18F]FPPRGD2 and [68Ga]Ga-NOTA-PRGD2 Using a Reference Tissue Model. PLoS ONE 2012, 7, e37506. [Google Scholar] [CrossRef]
- Haubner, R.; Kuhnast, B.; Mang, C.; Weber, W.A.; Kessler, H.; Wester, H.J.; Schwaiger, M. [18F]Galacto-RGD: Synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug. Chem. 2004, 15, 61–69. [Google Scholar] [CrossRef]
- Eo, J.S.; Jeong, J.M. Angiogenesis Imaging Using 68Ga-RGD PET/CT: Therapeutic Implications. Semin. Nucl. Med. 2016, 46, 419–427. [Google Scholar] [CrossRef]
- Jeong, J.M.; Hong, M.K.; Chang, Y.S.; Lee, Y.S.; Kim, Y.J.; Cheon, G.J.; Lee, D.S.; Chung, J.K.; Lee, M.C. Preparation of a promising angiogenesis PET imaging agent: 68Ga-labeled c(RGDyK)-isothiocyanatobenzyl-1,4,7-triazacyclononane-1,4,7-triacetic acid and feasibility studies in mice. J. Nucl. Med. 2008, 49, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Pohle, K.; Wester, H.-J. Be spoilt for choice with radiolabelled RGD peptides: Preclinical evaluation of 68Ga-TRAP(RGD)3. Nucl. Med. Biol. 2013, 40, 33–41. [Google Scholar] [CrossRef]
- Echavidre, W.; Picco, V.; Faraggi, M.; Montemagno, C. Integrin-αvβ3 as a Therapeutic Target in Glioblastoma: Back to the Future? Pharmaceutics 2022, 14, 1053. [Google Scholar] [CrossRef]
- De Jong, M.; Breeman, W.A.P.; Valkema, R.; Bernard, B.F.; Krenning, E.P. Combination Radionuclide Therapy Using 177Lu- and 90Y-Labeled Somatostatin Analogs. J. Nucl. Med. 2005, 46, 13S–17S. [Google Scholar]
- Sivolapenko, G.B.; Skarlos, D.; Pectasides, D.; Stathopoulou, E.; Milonakis, A.; Sirmalis, G.; Stuttle, A.; Courtenay-Luck, N.S.; Konstantinides, K.; Epenetos, A.A. Imaging of metastatic melanoma utilising a technetium-99m labelled RGD-containing synthetic peptide. Eur. J. Nucl. Med. 1998, 25, 1383–1389. [Google Scholar] [CrossRef]
- Beer, A.J.; Haubner, R.; Goebel, M.; Luderschmidt, S.; Spilker, M.E.; Wester, H.J.; Weber, W.A.; Schwaiger, M. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-galacto-RGD in cancer patients. J. Nucl. Med. 2005, 46, 1333–1341. [Google Scholar]
- Zhao, D.; Jin, X.; Li, F.; Liang, J.; Lin, Y. Integrin αvβ3 imaging of radioactive iodine-refractory thyroid cancer using 99mTc-3PRGD2. J. Nucl. Med. 2012, 53, 1872–1877. [Google Scholar] [CrossRef]
- Iagaru, A.; Mosci, C.; Mittra, E.; Zaharchuk, G.; Fischbein, N.; Harsh, G.; Li, G.; Nagpal, S.; Recht, L.; Gambhir, S.S. Glioblastoma Multiforme Recurrence: An Exploratory Study of 18F FPPRGD2 PET/CT. Radiology 2015, 277, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wu, H.; Li, W.; Zhao, S.; Teng, X.; Lu, H.; Hu, X.; Wang, S.; Yu, J.; Yuan, S. A pilot study imaging integrin αvβ3 with RGD PET/CT in suspected lung cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R.; Finkenstedt, A.; Stegmayr, A.; Rangger, C.; Decristoforo, C.; Zoller, H.; Virgolini, I.J. [68Ga]NODAGA-RGD-Metabolic stability, biodistribution, and dosimetry data from patients with hepatocellular carcinoma and liver cirrhosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2005–2013. [Google Scholar] [CrossRef]
- Clausen, M.M.; Carlsen, E.A.; Christensen, C.; Madsen, J.; Brandt-Larsen, M.; Klausen, T.L.; Holm, S.; Loft, A.; Berthelsen, A.K.; Kroman, N.; et al. First-in-Human Study of [68Ga]Ga-NODAGA-E[c(RGDyK)]2 PET for Integrin αvβ3 Imaging in Patients with Breast Cancer and Neuroendocrine Neoplasms: Safety, Dosimetry and Tumor Imaging Ability. Diagnostics 2022, 12, 851. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Loft, M.; Loft, A.; Czyzewska, D.; Andreassen, M.; Langer, S.W.; Knigge, U.; Kjaer, A. Prospective Phase II Trial of [68Ga]Ga-NODAGA-E[c(RGDyK)]2 PET/CT Imaging of Integrin αvβ3 for Prognostication in Patients with Neuroendocrine Neoplasms. J. Nucl. Med. 2023, 64, 252–259. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, H.M.; Lin, C.Y.; Chang, J.T.; Hsieh, C.H.; Liao, C.T.; Kang, C.J.; Yang, L.Y.; Yen, T.C. RGD-K5 PET/CT in patients with advanced head and neck cancer treated with concurrent chemoradiotherapy: Results from a pilot study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1621–1629. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Shang, D.; Liu, Z.; Yu, Q.; Wang, S.; Teng, X.; Zhang, Q.; Hu, X.; Zhao, W.; et al. Pretreatment PET/CT imaging of angiogenesis based on 18F-RGD tracer uptake may predict antiangiogenic response. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 940–947. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, Y.; Miao, W. 99mTc-3PRGD2 for Integrin Receptor Imaging of Esophageal Cancer, Compared Study with 18F-FDG PET/CT. J. Nucl. Med. 2018, 59, 1409. [Google Scholar]
- Lobeek, D.; Rijpkema, M.; Terry, S.Y.A.; Molkenboer-Kuenen, J.D.M.; Joosten, L.; van Genugten, E.A.J.; van Engen-van Grunsven, A.C.H.; Kaanders, J.; Pegge, S.A.H.; Boerman, O.C.; et al. Imaging angiogenesis in patients with head and neck squamous cell carcinomas by [68Ga]Ga-DOTA-E-[c(RGDfK)]2 PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2647–2655. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Q.; Wang, G.; Sui, H.; Wang, R.; Wang, J.; Zhu, Z. A prospective head-to-head comparison of 68Ga-NOTA-3P-TATE-RGD and 68Ga-DOTATATE in patients with gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4218–4227. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, X.; Xu, W.; Pang, Y.; Sun, L.; Wu, X.; Xu, P.; Zhang, J.; Guo, Z.; Lin, Q.; et al. Clinical Evaluation of 68Ga-FAPI-RGD for Imaging of Fibroblast Activation Protein and Integrin αvβ3 in Various Cancer Types. J. Nucl. Med. 2023, 64, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Judmann, B.; Braun, D.; Wangler, B.; Schirrmacher, R.; Fricker, G.; Wangler, C. Current State of Radiolabeled Heterobivalent Peptidic Ligands in Tumor Imaging and Therapy. Pharmaceuticals 2020, 13, 173. [Google Scholar] [CrossRef]
- Chen, C.J.; Chan, C.H.; Lin, K.L.; Chen, J.H.; Tseng, C.H.; Wang, P.Y.; Chien, C.Y.; Yu, H.M.; Lin, W.J. 68Ga-labelled NOTA-RGD-GE11 peptide for dual integrin and EGFR-targeted tumour imaging. Nucl. Med. Biol. 2019, 68–69, 22–30. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ahmadyousefi, Y.; Salimi, Z.; Mirzaei, R.; Najafi, R.; Amirheidari, B.; Rahbarizadeh, F.; Kheshti, J.; Safari, A.; Soleimani, M. Innovative Diagnostic Peptide-Based Technologies for Cancer Diagnosis: Focus on EGFR-Targeting Peptides. ChemMedChem 2023, 18, e202200506. [Google Scholar] [CrossRef]



| Name | Sequence | Source | Ref. |
|---|---|---|---|
| EBP | CMYIEALDKYAC | Experimentally synthesized. | [42] |
| S3 | RCSHGYTGIRCQAVVL | From the third loop structure and linear C-terminal region of vaccinia virus growth factor (VGF). | [43,44] |
| GE11 | YHWYGYTPQNVI | Screened out from a phage display peptide library. | [18,31] |
| P1 | SYPIPDT | [45] | |
| P2 | HTSDQTN | [45] | |
| QRH | QRHKPRE | [46] | |
| D4 | LARLLT | Computer-aided design approach. | [47] |
| P75 | KYFPPLALYNPTEYFY | From one-bead-one-compound (OBOC) library. | [48] |
| Pep 11 | WSGENGPGFYDYEA | Designed using knob-socket model. | [49] |
| --- | EEEEYFELV DEDEYFELV | Experimentally synthesized. | [50] |
| Examples | Comments |
|---|---|
| RGD peptides | |
| RGD, c(RGDfK), c(RGDyK) | RGD peptides can present linear or cyclic formats (cRGD) with different levels of selectivity and specificity. Linear RGD peptides are more sensitive to chemical degradation [51]. Cyclization aims to decrease susceptibility to enzymatic degradation, enhancing tracer stability [52]. c(RGDyK) and c(RGDfK) have been extensively studied, with c(RGDyK) showing superior affinity for integrin αVβ3 [25]. |
| iRGD | iRGD is a 9-amino-acid cRGD derived from phage display screening [53]. |
| LXW7 and LXW64 | LXW7 (cGRGDdvc-NH2) was derived from the OBOC library and LXW64 (cGRGDd-DNaI1-c-NH2) is its optimized version [54]. |
| RGD-4C and NC-100717 | RGD-4 C is a double-cysteine-bridged peptide and the development of NC-100717 was based on the PEGylation of RGD-4C [51,55,56]. |
| c(RGDf[NMe]V (cilenglitide) and RGDechi | Cilenglitide acts as a selective αVβ3 antagonist. Preclinical studies have demonstrated its antiangiogenic and antitumor effects in various cancer models [45]. While cilengitide was well tolerated in phase I/II clinical trials [46,47], it failed to show efficacy in phase III studies [48], likely due to the complexity and plasticity of integrin signaling networks. RGDechi is a designed αVβ3 antagonist based on cilenglitide structures combined with echistatin C-terminal tails [57]. |
| Non-RGD peptides | |
| ATN-161 | ATN-161 (Ac-PHSCN-NH2) is a capped pentapeptide synthesized from the fibronectin—PHSRN sequence and it can be used alone or along with radiotherapy and chemotherapy to prevent metastasis and tumor development [49,50,51]. In phase I of a clinical study, ATN-161 was used for aggressive solid tumors and demonstrated good toleration and safety [53]. |
| RWrNK and RWrNM | RWrNK and RWrNM are linear peptides that contains an unnatural d-arginine (r). They present great water solubility and the ability to pass through the blood–brain tumor barrier. They have been investigated for glioblastoma diagnosis [58,59]. |
| EGFR-Targeting | |||||
|---|---|---|---|---|---|
| Radionuclide | Peptide/Sequence | Formulation | RCY * (%) | RCP ** (%) | Ref. |
| 124I | GE11 | [124I]I-GE11 | [31,35] | ||
| [124I]I-GE11 | ≥47 | ≥98 | [79] | ||
| 131I | EEEEYFELV | [131I]I-EEEEYFELV | >84 | >90 | [50] |
| DEDEYFELV | [131I]I-DEDEYFELV | >91 | >90 | ||
| Integrin αVβ3-Targeting | |||||
| 125I | c(RGDyK) | [125I]I-c(RGDyK) | ≥89 | >95 | [80] |
| 77Br | [77Br]Br-c(RGDyK) | ≥73 | >95 | ||
| 131I | GRGDYV | [131I]I-GRGDYV | >95 | >94 | [62] |
| 99mTc | GRGDHV | [99mTc]Tc(CO)3-GRGDHV | >95 | >94 | |
| EGFR-Targeting | |||||||
|---|---|---|---|---|---|---|---|
| Radionuclide | Chelator/ Prosthetic Group | Linker/Spacer | Peptide | Formulation | RCY * (%) | RCP ** (%) | Ref. |
| 64Cu | p-SCN-Bn-NOTA | GE11 | [64Cu]Cu-NOTA-GE11 | 46 | 90 | [81] | |
| β-alanina + ethylene glycol-based linker | [64Cu]Cu-NOTA-linker-β-Ala-GE11 | >99 | [36] | ||||
| [64Cu]Cu-NOTA-linker-β-Ala-GE11-NH2 | >99 | ||||||
| 18F | N-succinimidyl 4-fluorobenzoate (SFB) | -Gly-Gly-Gly-Lys-(GGGK) | [18F]F-SFB-GGGK-GE11 | 95 | [79] | ||
| F-PEG4-propyne | [18F]F-F-PEG4-propyne-GGGK-GE11 | 98 | |||||
| 4-nitrophenyl-2-fluoropropionate (NFP) | GE11/GE11-Lys | [18F]F-NFP-Lys-GE11 | 7 | >99 | [32] | ||
| 68Ga | NODAGA | PEG (different lengths) | GE11 | [68Ga]Ga-NODAGA-PEGn-GE11 | ≥97 | [82] | |
| PEG5 | [68Ga]Ga-NODAGA-PEG5-GE11 | 5–74 | ≥98 | [38] | |||
| D4 | [68Ga]Ga-NODAGA-PEG5-D4 | ||||||
| P1 | [68Ga]-NODAGA-PEG5-P1 | ||||||
| P2 | [68Ga]Ga-NODAGA-PEG5-P2 | ||||||
| CPP | [68Ga]Ga-NODAGA-PEG5-CPP | ||||||
| EGBP | [68Ga]Ga-NODAGA-PEG5-EGPB | ||||||
| QRH | [68Ga]Ga-NODAGA-PEG5-QRH | ||||||
| Pep11 | [68Ga]Ga-NODAGA-PEG5-Pep11 | ||||||
| 124I | N-succinimidyl 4-iodobenzoate (SIB) | GGGK | GE11 | [124I]I-SIB-GGGK-GE11 | 30 | 98 | [79] |
| 111In | p-SCN-Bn-NOTA | [111In]In-NOTA-GGGK-GE11 | 100 | ||||
| 99mTc | HYNIC + tricine | Seryl-seryl-serine residue (SSS) | [99mTc]Tc-tricine-HYNIC-SSS-GE11 | >98 | 95 | [34] | |
| D4 | [99mTc]Tc-tricine-HYNIC-SSS-D4 | 98 | 98 | [78] | |||
| [99mTc]Tc-tricine-HYNIC-D4 | 98 | 98 | [77] | ||||
| HYNIC + tricine + EDDA | SSS | [99mTc]Tc-tricine/EDDA-HYNIC-SSS-D4 | 98 | [76] | |||
| GE11 | [99mTc]Tc-tricine/EDDA-HYNIC-SSS-GE11 | >99 | 99 | [33] | |||
| -Gly-Gly-Gly-Cys-(GGGC) | [99mTc]Tc-GGGC-GE11 | >98 | >90 | [37] | |||
| ECG (tripeptide–several nitrogen atoms and one sulfur atom) | Histidine-containing spacer peptide (GHEG) | P1 | [99mTc]Tc-ECG-SYPIPDT-ECG-TAMRA | >95 | [83] | ||
| Radionuclide | Chelator/Prosthetic Group | Linker/Spacer | Peptide | Formulation | RCY * (%) | RCP ** (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 64Cu | DOTA | E{E[c(RGDfK)]2)2 | [64Cu]Cu-DOTA-E{E[c(RGDfK)]2)2/[64Cu]Cu-DOTA-RGD2 | 75 | 95 | [84] | |
| CB-TE2A (4,11-bis(carboxymethyl)-1,4,8,11-tetraazabicyclo[6.6.2] hexadecane)) | c(RGDyK) | [64Cu]Cu-CB-TE2A-c(RGDyK) | 95 | [75] | |||
| Hexaazamacrobicyclic sarcophagine (Sar) based chelator (diamSar) | c(RGDfD) | [64Cu]Cu-diamSar-c(RGDfD) | 95 | ||||
| DOTA | PEG4 | E[c(RGDfK)]2 | [64Cu]Cu-DOTA-PEG4-E[c(RGDfK)]2 | 95 | 95 | [85] | |
| DOTA | Triglycine (3G) | E[c(RGDfK)]2 | [64Cu]Cu-DOTA-G3-E[c(RGDfK)]2 | 95 | 95 | ||
| Sar-based chelator (BaBaSar) | E[c(RGDfK)]2 | [64Cu]Cu-BaBaSar-E[c(RGDfK)]2/[64Cu]Cu-BaBaSar-RGD2 | 95 | 99 | [86] | ||
| NODAGA | E[c(RGDfK)]2 | [64Cu]Cu-NODAGA-E[c(RGDyK)]2/[64Cu]Cu-RGD2 | 50 | >89 | [69,87] | ||
| Sar-based chelator (AmBaSar) | E[c(RGDfK)]2 | [64Cu]Cu-AmBaSar-RGD2/[64Cu]Cu-AmBaSar-E[c(RGDfK)]2 | >90 | 98 | [88] | ||
| NOTA | PEG4-SAA4 | c(RGDfK) | [64Cu]Cu-NOTA-PEG4-SAA4-c(RGDfK) | >90 | [52] | ||
| NOTA | PEG2 | c(RGDfK) | [64Cu]Cu-NOTA-PEG2-c(RGDfK) | >90 | |||
| 18F | NFP | PEG3 | E{c(RGDyk)2 | [18F]F-FPPRGD2 | >73 | 99 | [70,89,90,91] |
| NFP | PEG2, SAA and 1,2,3-triazole | c(RGDfK) | [18F]F-Galacto-RGD/[18F]F P-SAA-RGD | >24 | 98 | [66] | |
| p-fluorobenzaldeyde | aminooxy-bearing RGD peptide (AH111585) | [18F]F-fluciclatide/[18F]F-AH111585 | >17 | 95 | [92,93] | ||
| pentyne tosylate | c(RGDyK) | [18F]F-RGD-K5 | 35 | 95 | [94,95,96] | ||
| SFB | E[c(RGDyK)]2 | [18F]F-FB-RGD2 | >20 | 99 | [68] | ||
| SFB | c(RGDyK) | [18F]F-FB-RGD | >35 | 99 | [68] | ||
| Al(NOTA) | PEG3 | E[c(RGDyK)]2 | [18F]F-AlF-NOTA-PRGD2 [18F]F-AlF-NOTA-PEG3-E[c(RGDyK)]2/[18F]F-Altatide I | >40 | 95 | [97] | |
| Al(NOTA) | PEG4 | E[c(RGDfk)]2 | [18F]F-NOTA-E[PEG4-c(RGDfk)]2/[18F]F-AlF-NOTA-2P-RGD2/[18F]F-Alfatide II | >40 | 95 | [98] | |
| 68Ga | NODAGA | c(RGDfK) | [68Ga]Ga-RGD/[68Ga]Ga-NODAGA-c(RGDfK) | 96 | 98 | [71,99] | |
| NODAGA | E[c(RGDfK)]2 | [68Ga]Ga-NODAGA-E[c(RGDyK)]2/[68Ga]Ga-RGD2 | 50 | >89 | [87,100,101] | ||
| DOTA | PEG4 | E[c(RGDfK)]2 | [68Ga]Ga-3PRGD2/[68Ga]Ga (PEG4-E[PEG4-c(RGDfK)]2). | 90 | 98 | [71,102] | |
| TRAP | PEG4 | c(RGDfK)3 | [68Ga]Ga-TRAP-(RGD)3 | ≥95 | 99 | [71,103] | |
| p-SCN-Bn-NOTA | 3 PEG4 | E[c(RGDfK)]2 | [68Ga]Ga-NOTA-PRGD2/[68Ga]Ga NOTA-PEG4-E[c(RGDfK)]2 | 42 | 95 | [69,104] | |
| p-SCN-Bn-NOTA | 3G | E[c(RGDfK)]2 | [68Ga]Ga-NOTA-E[G3c(RGDfK)]2 | 43 | 95 | [69,104] | |
| p-SCN-Bn-NOTA | c(RGDyK) | [68Ga]Ga-NOTA-RGD | 98 | 99.5 | [67,73] | ||
| Fusarinine-C (FSC) | c(RGDfK)3 | [68Ga]GaFSC-(RGD)3 | 94 | 95 | [105] | ||
| THP | c(RGDfK) | [68Ga]Ga-HP3-(RGD)3 | >86 | 95 | [72] | ||
| 125I | SIB | c(RGDfK) | [124I]I-bcRGD | 36 | 99 | [106] | |
| 99mTc | HYNIC + tricine +TPPTS | 3G | E{c(RGDFk)2 | [99mTc]Tc-HYNIC-3G-(RGD)2 | >60 | >95 | [107,108] |
| HYNIC + tricine +TPPTS | 3 PEG4 | E{c(RGDFk)2 | [99mTc]Tc-3PRGD2/[99mTc]Tc-HYNIC-PEG4-E[PEG4-c(RGDfK)]2 | >95 | >95 | [109,110,111] | |
| Cysteine (Cys) | PNP = [(CH3)2P(CH2)2N(C2H4OCH3)(CH2)2P(CH3)2] | RGDechi | [99mTc]Tc(RGDechi-Cys)PNP43 | >82 | 98 | [112] | |
| HYNIC + tricine +EDDA | c(RGDyK) | [99mTc]Tc-HYNIC-RGD | >94 | >95 | [113] | ||
| DOTA | RAFT-E[c(RGDfK)]2 | [99mTc]Tc-RAFT-RGD | >90 | [114,115] | |||
| HYNIC + tricine +TPPTS | E[c(RGDfK)]2 | [99mTc]Tc(HYNIC-E E[c(RGDfK) 2]2)(tricine)(TPPTS)] | 65 | 95 | [116] | ||
| MAG2 | 3G | E{c(RGDFk)2 | [99mTc]TcO(MAG2-3G3-E{c(RGDFk)2 | 95 | [117] | ||
| HYNIC + tricine +TPPTS | PEG2, SAA and 1,2,3-triazole | E{c(RGDFk)2 | [99mTc]Tc-Galacto-RGD2 | >95 | [118,119] | ||
| 111In | DOTA | E-[c(RGDfK)]2 | [111In]In-RGD2/[111In]InDOTA-E-[c(RGDfK)]2 | >95 | [120] | ||
| DOTA | c(RGDfK) | [111In]In-RGD | 90 | [121] | |||
| DOTA | PEG2, SAA and 1,2,3-triazole | E-[c(RGDfK)]2 | [111In]In-DOTA-Galacto-RGD2 | 95 | [122] | ||
| DOTA | PEG4 | E-[c(RGDfK)]2 | [111In]In-3P-RGD2 | 33 | >95 | [122] | |
| DTPA | PEG4 | E-[c(RGDfK)]2 | [111In]In-DTPA-3PRGD2 | 37 | >95 | [123] | |
| DOTA | E-[c(RGDfK)]2 | [111In]In-DOTA-RGD2 | >95 | [124] |
| Radionuclide | Chelator | Linker | Peptide | Formulation | Radiolabeling Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| 177Lu | DOTA | E-c(RGDfK) | [177Lu]Lu-DOTA-E-c(RGDfK) | >93 | [125] | |
| NOTA-SCN | c(RGDyK) | [177Lu]Lu-NOTA-SCN-c(RGDyK) | 99 | [126] | ||
| DOTA | 3 PEG4 | E[c(RGDfK)]2 | [177Lu]Lu-DOTA-(PEG4)3-E[c(RGDfK)]2/ [177Lu]Lu-3PRGD2 | >99 | [127] | |
| 90Y | RAFT(c[-RGDfK-])4 | [90Y]Y-DOTA-RAFT-(c[-RGDfK-])4/[90Y]Y-RAFT-RGD | >90 | [128] | ||
| 177Lu | [177Lu]Lu-DOTA-RAFT-(c[-RGDfK-])4/ [177Lu]Lu -RAFT-RGD | >97 | ||||
| 5p-C-NETA | c(RGDyK) | [177Lu]Lu-5p-C-NETA-c(RGDyK) | 99 | [129] | ||
| 90Y | [90Y]Y-5p-C-NETA-c(RGDyK) | 99 | ||||
| NOTA | Evans blue (EB) | c(RGDfK) | [90Y]Y-NOTA-EB- c(RGDfK)/[90Y]Y-NMEB-RGD | >90 | [130] | |
| 177Lu | [177Lu]Lu-NOTA-c(RGDfK)/[177Lu]Lu -RGD | >95 | [131] | |||
| EB | [177Lu]Lu-NOTA-EB-c(RGDfK)/[177Lu]Lu-EB-RGD | >95 | ||||
| 225Ac | DOTA | E[c(RGDfK)]2 | [225Ac]Ac-DOTA- E[c(RGDfK)]2/[225Ac]Ac-DOTA-RGD2 | >95 | [124] |
| Formulation | Cell Line | In Vitro Investigation | Animal Model | In Vivo Investigation | Biodistribution Tumor Uptake (%ID/g) | Imaging Tumor Uptake | Ref. |
|---|---|---|---|---|---|---|---|
| [124I]I-GE11 | SMMC-7721 | Binding affinity, internalization, MTT | Hepatocellular carcinoma | Biodistribution (at 0.5 and 4 h p.i.), gene delivery and transfection | 3.2 (4 h p.i.) | N/A | [31] |
| [124I]I -GE11 | A431 | Binding affinity (no inhibition) | Epidermal and glioblastoma | Efficiency of treatment | N/A | N/A | [35] |
| [64Cu]Cu-NOTA-linker-β-Ala-GE11-NH2 and [64Cu]Cu-NOTA-linker-β-Ala-GE11 | A431, MDA-MB-435 and FaDu | Human serum stability, cell medium and buffer viability, binding saturation, displacement assays on intact cells and homogenates, immunoblotting | Head and neck squamous cell carcinoma (HNSC) | Biodistribution (at 5 and 60 min p.i.), PET (at 1 h and 36 h p.i.) | <1 (5 min p.i.) | Some [64Cu]Cu-NOTA-linker-β-Ala-GE11 accumulation was detected 5 min p.i. | [36] |
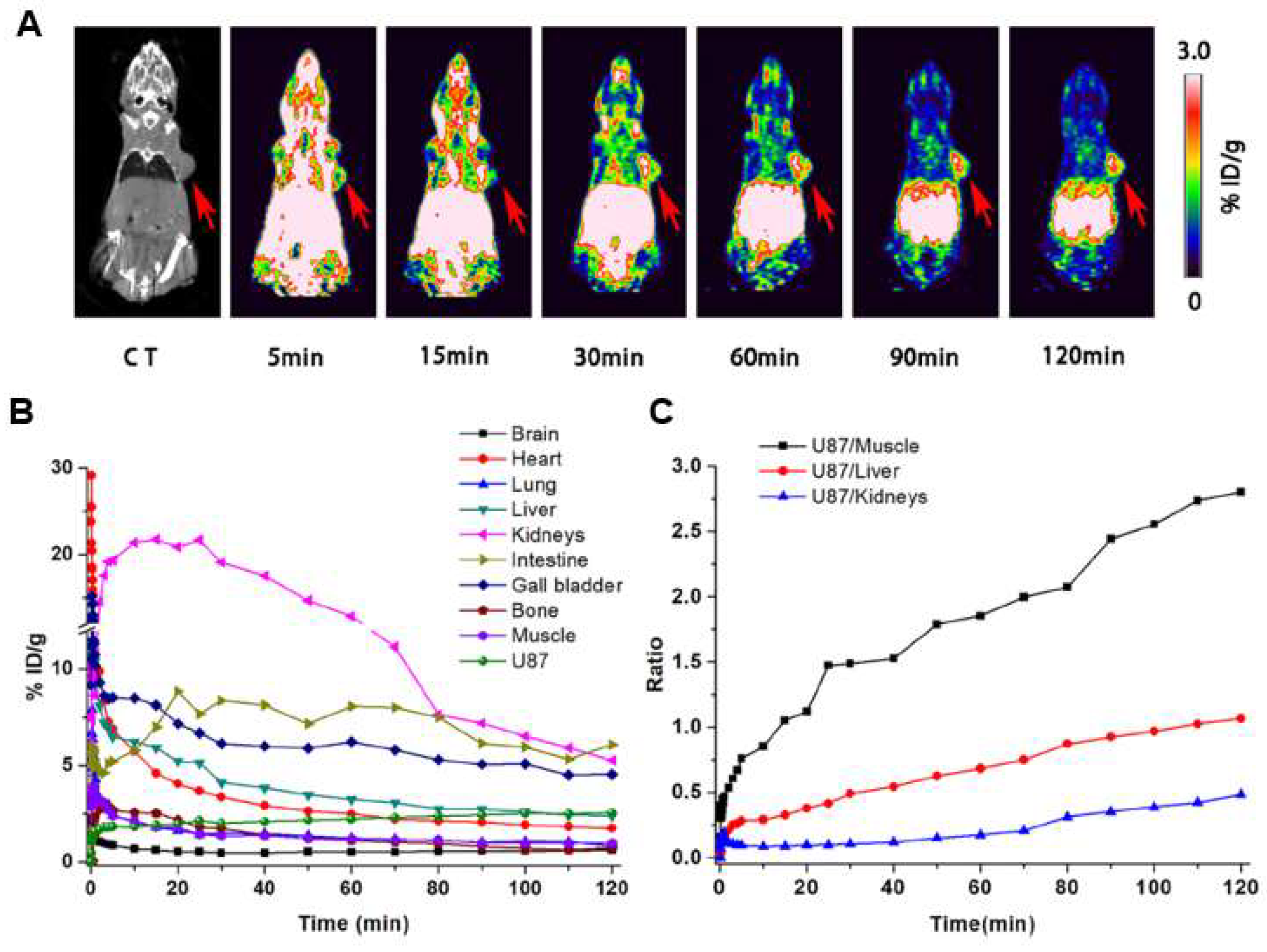
| [18F]FP-Lys-GE11 | A431, U87MGand PC3 | Receptor binding, partition coefficient, stability, cell uptake and blocking assays | Glioblastoma and prostate cancer | metabolism, immunohistochemistry, biodistribution at 2 h p.i., and 2 h dynamic PET/CT imaging | N/A | 3.5 ± 0.4 (U87) and 3.7 ± 0.8 (PC-3) | [32] |
| [99mTc]Tc-tricine-HYNIC-SSS-GE11 | SKOV3 | Plasma stability, receptor binding, internalization and, blocking assay | Ovarian cancer | Biodistribution at 1 and, 4 h p.i. | ~2.3 (1 h p.i.) | N/A | [34] |
| [99mTc]Tc-tricine-HYNIC/EDDA-SSS-GE11 | Receptor binding and, internalization | Biodistribution at 1 and, 4 h p.i., gamma camera imaging at 1 h p.i. | 3.6 ± 0.7 (1 h p.i.) | Good A549 tumor visualization at 1 h p.i. | [33] | ||
| [99mTc]Tc-GGGC-GE11 | A549 | Cellular uptake, retention kinetics, internalization and blocking assays | Non-small-cell lung cancer (NSCLC) | Biodistribution at 1 and, 4 h p.i., SPECT scans at 1, 2, and 4 h p.i. | 3.4 ± 0.5 (2 h p.i.) | The signal of A549 tumors reached its strongest at 2 h p.i. | [37] |
| [99mTc]Tc-tricine-HYNIC-SSS-D4 | Stability in solution and human serum, receptor binding, internalization assays | Biodistribution at 1 and, 4 h p.i., gamma camera imaging at 1 h p.i. | ~7.6 (1 h p.i.) | Good A549 tumor visualization at 1 h p.i. | [78] | ||
| [99mTc]Tc-tricine-HYNIC-D4 | Stability, receptor binding | Biodistribution at 1 and, 4 h p.i. | ~8.1 (1 h p.i.) | N/A | [77] | ||
| [99mTc]Tc-tricine/EDDA-HYNIC-SSS-D4 | Cellular uptake and blocking test | Biodistribution at 1 and, 4 h p.i. | ~2.5 (1 h p.i.) | N/A | [76] | ||
| [99mTc]Tc-SYPIPDT-ECG-TAMRA | NCI-H460 | Receptor binding affinity, cellular uptake by microscopy | NSCLC | Gamma camera imaging and biodistribution at 1, 2, and 3 h p.i., fluorescent imaging and immunohistochemical staining | 1.9 ± 0.1 (1 h p.i.) | 2.7 ± 0.6 at 1 h p.i. | [83] |
| [131I]I-EEEEYFELV and [131I}I-DEDEYFELV | C6 | Stability, partition coefficient, serum protein binding | Glioblastoma allografts | Biodistribution at 0.25, 1, and 2 h p.i.), binding, and internalization studies in brain homogenates | 2.3 ± 0.2 ([131I]I-EEEEYFELV) and 0.6 ± 0.0 ([131I]I-DEDEYFELV) at 1 h p.i. | N/A | [50] |
| Formulation | Cell Line | In Vitro Investigation | Murine Model | In Vivo Investigation | Biodistribution Tumor Uptake (%ID/g) | PET Imaging Tumor Uptake | Ref. |
|---|---|---|---|---|---|---|---|
| [18F]F-FPPRGD2 | U-87 MG | Receptor-binding assay (IC50 = 51.8 ± 4.6 nM) | Glioblastoma | Biodistribution at 1 h p.i., and static microPET scans at 20 min, 1 and 2 h p.i. | 2.3 ± 0.2 | 4.2 ± 0.2 (20 min) | [70] |
| [18F]F-Galacto-RGD | Receptor-binding assay (IC50 = 404 ± 38 nM) | Static microPET scans at 20 min, 1 and 2 h p.i. | N/A | 2.1 ± 0.2 (20 min) | [70] | ||
| [18F]F-fluciclatide | EA-Hy926 | Biding affinity (Ki = 2.3 nM) | Lewis lung Ccarcinoma (LLC) | Biodistribution and PET at 2 h p.i., blocking study | 1.6 ± 0.1 | 1.5 ± 0.3 | [92] |
| [18F]F-RGD-K5 | U-87 MG | x | Glioblastoma | Metabolic stability, biodistribution, PET, blocking study | 4.2 ± 0.6 | 1.4 ± 0.2 (U-87 MG); 1.2 ± 0.4 (A549) (SUVmax) | [99] |
| [18F]F-RGD2 | Receptor-binding assay IC50 = 2.3 ± 0.7 nM | Biodistribution at 0.5, 1 h, 2 h, and 4 h p.i., PET 1 h p.i. | 4.3 ± 1.0 (2 h p.i.) | 4.4 ± 0.6 (1 h p.i.) | [68] | ||
| [18F]F-RGD | Receptor-binding assay IC50 = 3.5 ± 0.3 nM | Biodistribution at 0.5, 1 h, 2 h, and 4 h p.i., PET 1 h p.i. | 1.6 ± 0.4 (2 h p.i.) | [68] | |||
| [18F]F-Alfatide I | Receptor-binding assay IC50 = 46 ± 4.4 nM | Serum stability, biodistribution at 2 h p.i. and dynamic (2 -35 min) and static PET 1 and 2 h p.i., blocking study | 2.3 ± 0.9 (2 h p.i.) | 5.3 ± 1.2% (peak at 3 min p.i); 0.3 ± 0.1 (60 min p.i.) | [97] | ||
| [18F]F-Alfatide II | U-87 MG and MDA-MB-435 | N/A | Glioblastoma or breast cancer | Dual PET imaging (18F-alfatide and 18F-FDG) of mice treated with doxorubicin or paclitaxel, computational modeling (ROI, time–activity curves, dual-tracer input function and tumor time–activity curve separation and kinetics. | N/A | 4.7 ± 1.0%ID/g at 40 min | [98] |
| [68Ga]Ga-RGD | U-87 MG and A549 | N/A | Glioblastoma and NSCLC | Biodistribution at 80 min p.i. and statistic PET at 1 h p.i. | 2.9 ± 0.8 (U-87 MG) and 3.9 ± 1.2 (A549) | 0.9 ± 0.3 (U-87 MG); 0.9 ± 0.3 (A549) (SUVmax) | [99] |
| [68Ga]Ga-RGD2 | U-87 MG and H727 | N/A | Glioblastoma and neuroendocrine tumors | PET/CT scans, blocking and biodistribution (in major organs) at 1, 2 and 4 h p.i., dosimetry | N/A | 2.2 ± 0.1 (U-87 MG); 1.5 ± 0.1 (A549) 1 h p.i. | [87] |
| B16-F10 | In vitro stability | Melanoma | Biodistribution at 10 min, 0.5 and 1 h p.i. and blocking studies at 0.5 h and clinical study | 4.1 ± 0.9 | N/A | [101] | |
| [68Ga]Ga-3PRGD2 | U-87 MG and LCC | N/A | LLC | PET (1 h p.i.), immunofluorescence, western blot | N/A | 4.9 ± 1.2 | [102] |
| [68Ga]Ga-TRAP-(RGD)3 | MDA-MB-231 | N/A | Breast cancer | Immunohistochemistry analysis, PET/CT at 1 h p.i., autoradiography and immunofluorescence imaging | N/A | 3.0 ± 0.9 | [103] |
| [68Ga]Ga-NOTA-PRGD2 | U-87 MG | Receptor-binding assay (IC50 = 88.8 ± 5.4 nM) | Glioblastoma | Biodistribution at 1 h p.i. and PET imaging at 0.5, 1 and 2 h p.i. | 7.0 ± 1.1 | 9.0 ± 2.0 (30 min) | [104] |
| [68Ga]Ga-NOTA-E[G3c(RGDfK)]2 | Receptor-binding assay (IC50 = 61.6 ± 3.3 nM) | Biodistribution at 1 h p.i. and PET imaging at 0.5, 1, and 2 h p.i. | 8.0 ± 0.9 | 10.1 ± 1.8 (0.5 h) | [104] | ||
| [68Ga]Ga-NOTA-RGD | SNUC4 | Binding assay with human serum (Ki = 1.9 nM) | Colon cancer | Biodistribution 1 h p.i., blocking study and PET imaging at 1 and 2 h p.i. | 1.6 ± 0.2 | 5.1 ± 1.0 | [142] |
| [68Ga]Ga FSC-(RGD)3 | M21 | Stability, protein binding, binding affinity, internalization | Melanoma | Immunohistochemistry and PET/CT at 1 h p.i. | N/A | 3.0 ± 0.9 | [105] |
| [68Ga]Ga-HP3-(RGD)3 | Binding assay (IC50 = 73 ± 22 nM) | Biodistribution 1 and 2 h p.i., blocking, and dynamic PET imaging for 1.5 h p.i., stability | 6.1 ± 0.6 (1 h) | qualitative | [143] | ||
| [64Cu]Cu-RGD2 | U-87 MG and H727 | N/A | Glioblastoma and neuroendocrine tumors | PET/CT scans, blocking and biodistribution in major organs 1, 4, and 18 h p.i., dosimetry | N/A | 2.3 ± 0.2 (U-87 MG); 1.5 ± 0.1 (H727) 1 h p.i. | [87] |
| [64Cu]Cu-DOTA- E[c(RGDfK)]2 | U-87 MG | Binding assay (IC50 = 73 ± 22 nM) | Glioblastoma | Biodistribution 1 h p.i., PET imaging at 15, 30 min, 1, 2, 4, and 18 h p.i., dosimetry | 9.9 ± 1.1 (0.5 h) | 8.7 ± 1.5 (1 h p.i.) | [84] |
| [64Cu]Cu-CB-TE2A-c(RGDyK) | M21 | Binding assay (IC50 = 6.0 ± 3.6 nM) | Melanoma | Biodistribution, blocking and PET imaging at 1, 2, 4 and 24 h pi | 3.0 ± 0.9 (1 h p.i.) | qualitative | [75] |
| [64Cu]Cu-diamSar-c(RGDfD) | Binding assay (IC50 = 4.8 ± 0.9 nM) | Biodistribution, blocking and PET imaging at 1, 2, 4 and 24 h pi | 1.5 ± 0.5 (1 h p.i.) | qualitative | |||
| [64Cu]Cu-DOTA-PEG4-E[PEG4-c(RGDfK)]2 | U-87 MG | Binding assay (IC50 = 74 ± 3 nM) | Glioblastoma | Biodistribution at 0.5, 1 and 2 h p.i., blocking and PET imaging at 1 h p.i. | 8.2 ± 2.0 (0.5 h p.i.) | qualitative | [85] |
| [64Cu]Cu-DOTA-G3-E[G3-c(RGDfK)]2 | Binding assay (IC50 = 62 ± 6 nM) | Biodistribution at 0.5, 1 and 2 h p.i., blocking and PET imaging at 1 h pi | 8.5 ± 1.4 (0.5 h p.i.) | qualitative | |||
| [64Cu]Cu-AmBaSar-RGD2 | Binding assay (IC50 = 10.0 ± 0.5 nM) | PET imaging at 1, 2, 4, and, 20 h p.i., blocking and biodistribution at 20 h | 1.8 ± 0.4 | ~2.5 (20 h) | [88] | ||
| [64Cu]Cu-NOTA-PEG4-SAA4-c(RGDfK) | Binding assay (IC50 = 444 ± 41 nM) | PET imaging at 0.5, and 2 and 4 h p.i, blocking and biodistribution at 4 h p.i | 2.5 ± 0.2 (0.5 h p.i.) | 1.1 ± 0.3 | [52] | ||
| [64Cu]Cu-NOTA-(PEG)2-c(RGDfK) | Binding assay (IC50 = 288 ± 66 nM) | PET imaging at 0.5, and 2 and 4 h p.i, blocking and biodistribution at 4 p.i. | ~4 (0.5 h p.i.) | 2.4 ± 0.3 | |||
| [125I]I-bcRGD | U-87 MG and A549 | Selective (2.1 ± 0.6) and binding assay (1.6% and 0.3% dose/mg for U-87 MG and A549) | Biodistribution at 10 min, 0.5, 1 and 2 h p.i. | 3.8 ± 0.4 (U-87 MG); 2.1 ± 0.6 (A549) (0.5 h p.i.) | N/A | [106] |
| Formulation | Cell Line | In Vitro Investigation | Murine Model | In Vivo Investigation | Biodistribution Tumor Uptake (%ID/g) | Ref. |
|---|---|---|---|---|---|---|
| [99mTc]Tc-HYNIC-3G-RGD2 | U-87 MG | Partition coefficient, binding assay U-87 MG (IC50 = 61.1 ± 2.1) | Glioblastoma and breast cancer | Biodistribution at 0.5, 1 and 2 h p.i., blocking, SPECT/CT and, metabolism | 9.1 ± 1.8 (MDA-MB-435); 7.7 ± 1.2 (U-87 MG) (2 h p.i.) | [107] |
| [99mTc]Tc-3PRGD2 | Binding assay (IC50 = 2.4 ± 0.7 nM) | Glioblastoma | Biodistribution at 0.5, 1 and 2 h p.i., blocking, SPECT/CT (0.5, 1, 2 and 4 h p.i.) and, metabolism | 9.7 ± 3.2 (2 h p.i.) | [110] | |
| [99mTc]Tc (RGDechi-Cys)(PNP)43 | Flow cytometry, cell uptake | Biodistribution at 0.5 and 2 h p.i. and metabolism | ~0.4 (2 h p.i.) | [112] | ||
| [99mTc]Tc-HYNIC-RGD | M21 | Protein binding in fresh human plasma, binding affinity | Melanoma | Biodistribution at 1 and 4 p.i. and planar γ-camera (10 min) imaging | 2.1 ± 0.4 (M21); 1.5 ± 0.3 (A549) (4 h p.i.) | [113] |
| [99mTc]Tc-RAFT-RGD | B16F0- and TS/A-pc | Immunoprecipitation, western blot, blood distribution | Melanoma and mammary cancer | Biodistribution, immunohistochemistry, autoradiography, planar γ-camera and SPECT imaging | 2.4 ± 0.5 (B16F0); 2.7 ± 0.8 (TS/A-pc) (1 h p.i.) | [115] |
| [99mTc]Tc-RAFT-RGD | DAOY-Luc spheroids or HD-MB03-Luc | Experiments involving the generation of radioresistant medulloblastoma cell lines | Intracranial orthotopic | SPECT and bioluminescence | N/A | [114] |
| [99mTc]Tc (HYNIC-E E[c(RGDfK) 2] 2)(tricine)(TPPTS)] | MDA-MB-435 | Solution stability, partition coefficient, binding assay (IC50 = 51 ± 11 nM) | Breast cancer | Biodistribution at 5 min, 0.5, 1 and 2 h p.i., SPECT at 1, 2 and 4 h p.i. and metabolism | 7.3 ± 1.3 (2 h p.i.) | [116] |
| [99mTc]TcO(MAG2-3G3-E{c(RGDFk)2 | U-87 MG | Binding assay (IC50 = 3.6 ± 0.6 nM), partition coefficient | Glioblastoma | Biodistribution at 0.5 and 2 h p.i., planar imaging and metabolic stability | 8.3 ± 1.5 (2 h p.i.) | [117] |
| [99mTc]Tc-Galacto-RGD2 | Binding assay (IC50 = 20 ± 2 nM) | Biodistribution at 5, 30, 60 and 120 min p.i., blocking, SPECT/CT at 1 h p.i. and metabolism, immunostaining, immunohistochemistry | 5.6 ± 1.5 (2 h p.i.) | [118] | ||
| [111In]In-RGD2/[111In]In-DOTA-E-[c(RGDfK)]2 | FaDu, SCCNij3, and SCCNij20 | N/A | HNSCC | Biodistribution at 1 h p.i., immunohistochemistry, autoradiography and SPECT/CT (3 frames of 20 min) | 2.2 ± 0.0 (FaDu), 1.9 ± 0.5 (SCCNij3), and 1.2 ± 0.0 (SCCNij20) (1 h p.i.) | [120] |
| [111In]In (DOTA-Galacto-RGD2) | U-87 MG and MDA-MB-435 | Binding assay U-87 MG (27 ± 2 nM) | Breast cancer | Biodistribution at 1, 4, 24 and 72 h, planar imaging at 1, 4 and 24 h p.i., blocking, immunostaining, immunohistochemistry | 6.8 ± 1.0 (1 h p.i.) | [122] |
| [111In]In-3P-RGD2 | Binding assay U-87 MG (29 ± 4 nM) | Biodistribution at 1, 4, 24 and 72 h, planar imaging at 1, 4 and 24 h p.i., blocking, immunostaining, immunohistochemistry | 6.2 ± 1.6 (1 h p.i.) | [122] | ||
| [111In]In-DOTA-RGD2 | NT2.5 and MDA-MB-231 | Serum stability | Biodistribution at 0.5, 1.5, 3, 6 and 24 h | 4.8 ± 0.7(NT2.5), 2.2 ± 0.9 (MDA-MB-231) (3 h p.i.) | [124] |
| Formulation | Therapy Dose (MBq) | Murine Model | In Vivo Investigation | Main Findings | Ref. |
|---|---|---|---|---|---|
| [177Lu]Lu-DOTA-E-c(RGDfK) | 37 | OVCAR-3 ovarian cancer | Survival study | Treated mice survived 16 weeks more than the untreated group. | [125] |
| [177Lu]Lu-NOTA-SCN-c(RGDyK) | 0.37 | CT-26 colon cancer | Biodistribution (at 1, 2, 4, 12 and 24 h p.i.) | The tumor uptake and the tumor/muscle ratio at 1 h p.i. was 1.7 ± 0.3 and 2.1 ± 0.4%ID/g. The highest uptake was observed in kidneys 7.6 ± 0.7%ID/g. | [126] |
| [177Lu]Lu-3PRGD2 | 0.37, 37.74 and 111 | LCC tumor model | Biodistribution (at 1, 4, 24 and 72 h p.i.), gamma imaging (at 4 and 24 h p.i.) and maximum tolerated dose (MTD), immunohistochemistry and hematoxylin-eosin staining | The tumor uptake at 1 h p.i. was 6.0 ± 0.6%ID/g and remained at 1.2 ± 0.2%ID/g 72 h p.i. Highest uptake was observed in the intestine (5.2 ± 0.5%ID/g) and kidney (4.2 ± 1.1) at 1 h p.i. The MTD was greater than 111 MBq per mouse. | [127] |
| [90Y]Y-RAFT-RGD | 30–37 (1× or fractionated 2×) | U-87 MG glioblastoma | Biodistribution at 1, 4, 24 and 48 h p.i. of 0.37 MBq of [90Y]Y-RAFT-RGD, toxicity and, dosimetry | The tumor uptake of [90Y]Y-RAFT-RGD 1 h p.i. was quick and high (9.0 ± 4.3% ID/g) and remained at 1.8 ± 0.7% ID/g 48 h p.i. The highest kidney uptake was 13.9 ± 3.5%ID/g at 1 h p.i. The toxicity findings were as follows: reduction in leukocyte and platelet counts and higher serum creatinine levels in the treated groups (compared to control). Radiation dosimetry extrapolation to humans: the whole-body effective dose was estimated at 0.11 mSv/MBq. | [128] |
| [177Lu]Lu-RAFT-RGD | 30–37 | SPECT/CT (at 1 and 4 h p.i.), toxicity | The tumor uptake at 1 and 4 h p.i. was 3.3 ± 0.5%ID/g and 3.8 ± 0.9%ID/g, and remained at 1.6 ± 0.0%ID/g 48 h p.i. The tumor/muscle ratio was ~10 at 1 h p.i.The highest activity levels (~6%ID/g) were detected in the kidneys and the bladder. Toxicity findings: reduction in leukocyte and platelet counts in treated groups (compared to control). | ||
| [177Lu]Lu-5p-C-NETA-c(RGDyK) | 2.22 | Biodistribution at 1, 4 and, 24 h p.i | Tumor uptake was 1.4 ± 0.6% ID/g 1 h p.i. The kidneys presented the highest uptake 1 h p.i. (3.2 ± 0.5%ID/g) | [129] | |
| [90Y]Y-NMEB-RGD | 7.4, 3.7 and 1.75 MBq | Antitumoral radiotherapy efficacy, TUNEL and hematoxilin-eosin staining | The treated group presented lower tumor volumes. The tumor vasculature was lower in the group receiving a medium dose of 90Y-NMEB-RGD. Also, this group presented more cell apoptosis. | [130] | |
| [177Lu]Lu-RGD | 18.5 and 29.6 | Patient-derived xenografts (PDX) from NSCLC | SPECT imaging (at 4, 24, 48, 72, and 96 h p.i.) and biodistribution (at 4, 24, 48 and, 72 h p.i.), therapy regimen, CD31 immunohistochemistry | [177Lu]Lu-EB-RGD demonstrated higher tumor uptake than [177Lu]Lu-RGD (13.4 ± 1.0 vs. 2.6 ± 1.6%ID/g). The [177Lu]Lu-EB-RGD group showed more cell apoptosis than control and [177Lu]Lu-RGD. A therapy dose of 18.5 MBq [177Lu]Lu-EB-RGD might be strong enough. | [131] |
| [177Lu]Lu-EB-RGD | |||||
| [225Ac]Ac-DOTA-cRGDfK | 0.037 | HER2 (NT2.5 and MDA-MB-231) breast cancer | The estimation of maximum tolerated activity (eMTA), biodistribution in normal tissues, α-Camera | The organs receiving the highest mean absorbed dose were kidneys (2.5 Gy) > spleen (1.8 Gy) > liver (1.2 Gy). The eMTA was 150 kBq (kidneys as the limiting tissue). The decay of daughters (213Bi and 221Fr) was also monitored. All the free 221Fr and the majority of the 213Bi decayed at 3 h. | [124] |
| Agent | Clinical Trials | SUVmax | Cancer Type | No. of Patients | Clinical Phase/Study Period | Radio-Dose | Toxicity | Imaging Tool | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| [99mTc]Tc-RGD-SCRGDSY/[99mTc]Tc-αP2) | N/A | N/A | Metastatic melanoma | 14 (10 men and 4 women) | Up to 12 months | 185 to 1222 MBq | No adverse effect | SPECT/CT | Tumor detection. | [146] |
| [18F]F-Galacto-RGD | N/A | Ranged in tumors (1.2 to 9.0) | Malignant melanoma, sarcomas, and osseous metastases | 19 (9 men, 10 women) | Phase I/II | 133–200 MBq | N/A | PET | Demonstrate a highly favorable biodistribution in humans with specific receptor binding, and visualization of αvβ3 expression in tumors with high contrast. | [147] |
| [99mTc]Tc-3 PRGD2 | N/A | N/A | Differentiated thyroid cancer patients with radioactive iodine refractory lesions (RAIR-DTC) | 10 (2 men and 8 women) | N/A | 11.1 MBq/kg | N/A | SPECT/CT | [99mTc]Tc-3PRGD2 with SPECT is a promising modality for diagnosing and guiding further treatment of RAIR DTC. | [148] |
| [18F]F-FPPRGD2 | NCT01806675 | 0.8–5.8 | Glioblastoma multiforme recurrence | 17 (8 men and 9 women) | Phase I/II | The [18F]F-FPPRGD2 doses at injection ranged from 3.8 to 9.9 mCi (mean, 8.1 mCi ± 1.7) | Safe | PET/CT | Function as increase uptake of glioblastoma. | [149] |
| [18F]F-alfatide | N/A | 5.4 ± 2.2 | Lung cancer | 26 | N/A | (213.3 ± 29.8 MBq) | Safe | PET/CT | Effective in the diagnosis of NSCL. | [150] |
| [68Ga]Ga-NODAGA-RGD | N/A | N/A | Hepatocellular carcinoma | 9 men | Phase I | 154–184 MBq | Well tolerated and metabolically stable in humans | PET/CT | Uptake in HCC tumors was not enough. | [151] |
| [68Ga]Ga-NODAGA-RGD2 | N/A | >10 | Locally advanced breast cancer | 5 women (33 to 68 years old) | N/A | 111–185 MBq | N/A | PET/CT | Good uptake in tumor site. | [101] |
| NCT02970786 | 4.5–17.7 | Neuroendocrine neoplasms and breast cancer | 10 (5 with neoplasm and 5 with breast cancer) | Phase I | 97.3–220 MBq | Safe. | PET/CT | Good image contrast and stable retention in tumor | [152] | |
| NCT03271281 | 1.4–14.1 | Neuroendocrine neoplasms | 113 | Phase II | 104–226 MBq | No grade 3–5 adverse events | PET/CT | Great tumor uptake | [153] | |
| [18F]F-RGD-K5 | NCT01447134 | For main tumor (5.3–6.0) For nodal (3.3–4.7) | Head and neck carcinoma (HNC) | 11 | Phase II | 10 mCi | N/A | PET/CT | Assessing response to concurrent chemoradiotherapy (CCRT) in patients with advanced HNC. | [154] |
| [18F]F-FPPRGD2 | NA | 3.7 ± 1.3 | Cervical and ovarian tumors | 6 women | NA | Ranged from 196 to 344 MBq | N/A | PET/CT | Have potential for early prediction of response to treatment. | [89] |
| [18F]F-ALF-NOTA-PRGD2 | NCT03384511 | 5.4 ± 2.6 | Lung cancer, stomach cancer, cervical cancer, gall bladder cancer, breast cancer, nasopharyngeal carcinoma, soft tissue carcinoma, esophageal cancer | 38 (only 25 met criteria) | Phase IV | 224.6 ± 38.2 MBq | N/A | PET/CT | Predictive for treatment response of antiangiogenic treatment (apatinib). | [155] |
| [99mTc]Tc-3PRGD2 | NCT02744729 | Malignant vs. benign lesions (5.1 ± 2.0 vs. 2.0 ± 0.7) | Esophageal cancer | 29 (24 men and 5 women) | Early phase I | 11.1 MBq/kg | N/A | SPECT/CT | Valuable for the diagnosis and staging of esophageal cancer. | [156] |
| [68Ga]Ga-RGD | NCT04222543 | Ranged between 4.0 and 12.7 | Oral squamous cell carcinoma (OSCC). | 10 | Phase II | 214 ± 9 MBq | N/A | PET/CT | Provide insight in angiogenesis as a hallmark of the head and neck squamous cell carcinomas’ tumor microenvironment. | [157] |
| [68Ga]Ga-NOTA-3P-TATE-RGD | NCT02817945 | 27.2 ± 13.6 | Gastroenteropancreatic–neuroendocrine tumors (GEP-NETs) | 35 | Early phase I | 74–148 MBq | N/A | PET/CT | Detection of liver metastases. | [158] |
| [68Ga]Ga-FAPI-RGD | NCT05543317 | Primary tumors: SUVmax 18.0 and lymph node metastases: SUVmax 12.1 | Nasopharyngeal carcinoma, small lung cancer, pancreatic cancer; lymph node, brain, lung, liver, bone, and subcutaneous metastasis. | 22 | NA | 3.0–3.7 MBq/kg | Safe and well tolerated | PET/CT | Imaging of various cancer types and functions to increase tumor uptake. | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Toledo, C.; Tantawy, A.A.; Lima Fuscaldi, L.; Malavolta, L.; de Aguiar Ferreira, C. EGFR- and Integrin αVβ3-Targeting Peptides as Potential Radiometal-Labeled Radiopharmaceuticals for Cancer Theranostics. Int. J. Mol. Sci. 2024, 25, 8553. https://doi.org/10.3390/ijms25158553
Rodrigues Toledo C, Tantawy AA, Lima Fuscaldi L, Malavolta L, de Aguiar Ferreira C. EGFR- and Integrin αVβ3-Targeting Peptides as Potential Radiometal-Labeled Radiopharmaceuticals for Cancer Theranostics. International Journal of Molecular Sciences. 2024; 25(15):8553. https://doi.org/10.3390/ijms25158553
Chicago/Turabian StyleRodrigues Toledo, Cibele, Ahmed A. Tantawy, Leonardo Lima Fuscaldi, Luciana Malavolta, and Carolina de Aguiar Ferreira. 2024. "EGFR- and Integrin αVβ3-Targeting Peptides as Potential Radiometal-Labeled Radiopharmaceuticals for Cancer Theranostics" International Journal of Molecular Sciences 25, no. 15: 8553. https://doi.org/10.3390/ijms25158553
APA StyleRodrigues Toledo, C., Tantawy, A. A., Lima Fuscaldi, L., Malavolta, L., & de Aguiar Ferreira, C. (2024). EGFR- and Integrin αVβ3-Targeting Peptides as Potential Radiometal-Labeled Radiopharmaceuticals for Cancer Theranostics. International Journal of Molecular Sciences, 25(15), 8553. https://doi.org/10.3390/ijms25158553









