Polθ Inhibitor (ART558) Demonstrates a Synthetic Lethal Effect with PARP and RAD52 Inhibitors in Glioblastoma Cells
Abstract
1. Introduction
2. Results
2.1. Polθ Inhibitor ART558 Used Alone or in Combination with PARP1/RAD52 Inhibitors and the Alkylating Agent TMZ, Lead to Cell Death via Apoptosis in Patient-Derived Glioblastoma Cells
2.1.1. Cell Viability
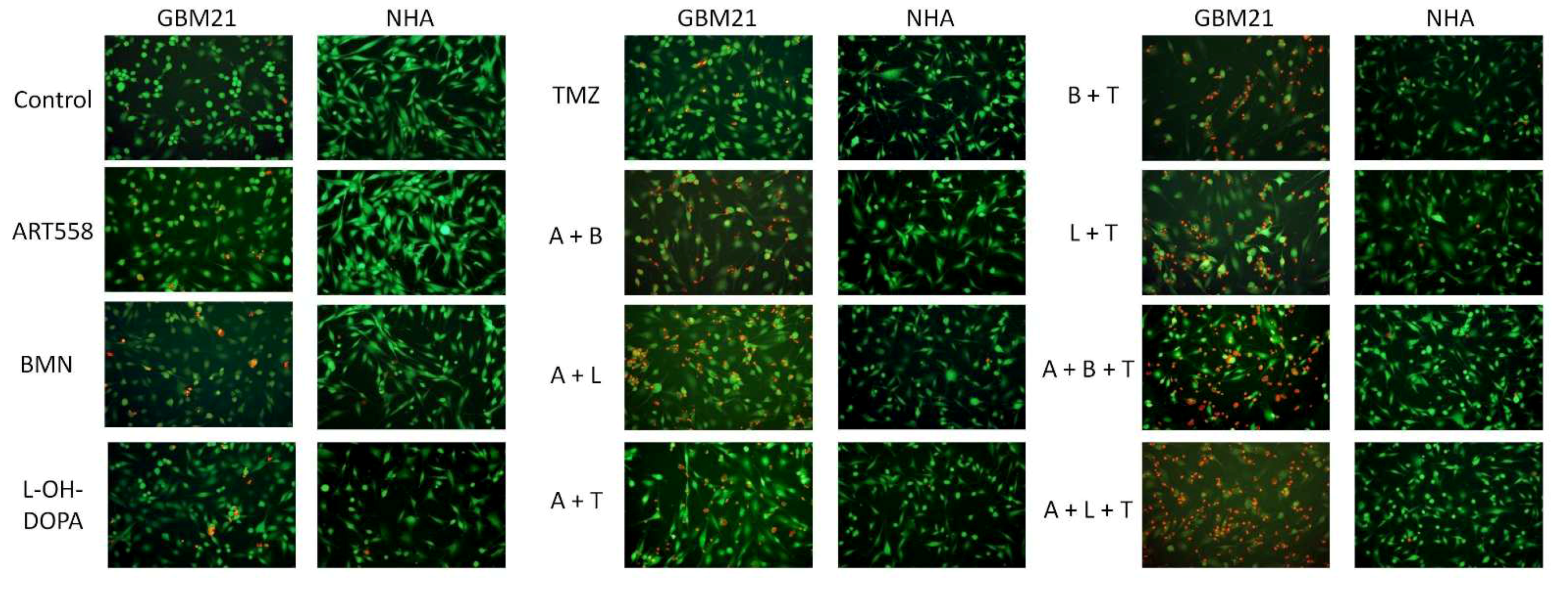
2.1.2. Visualization of Morphological Changes by Double Calcein AM/PI Staining
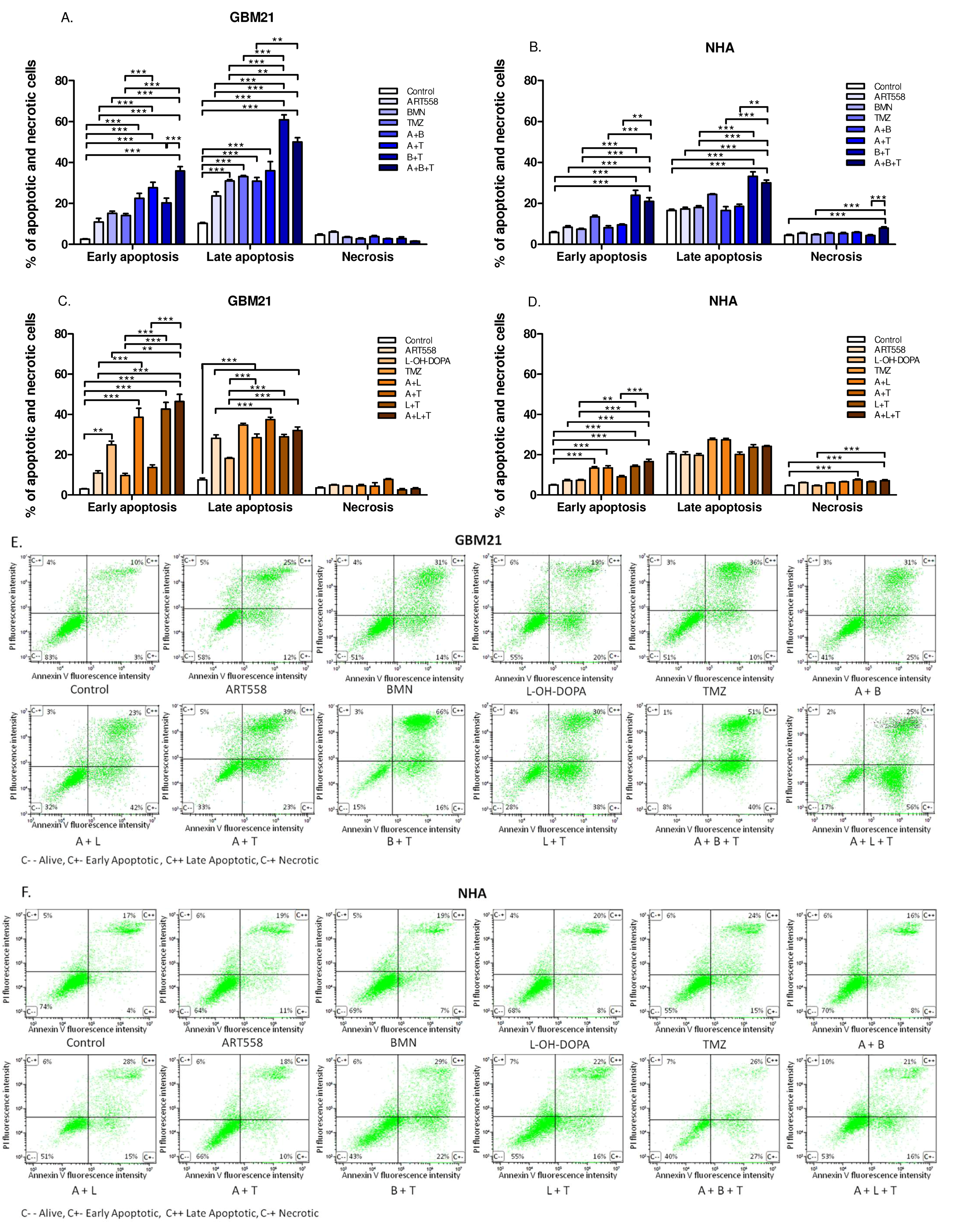
2.1.3. Cell Death Mechanism
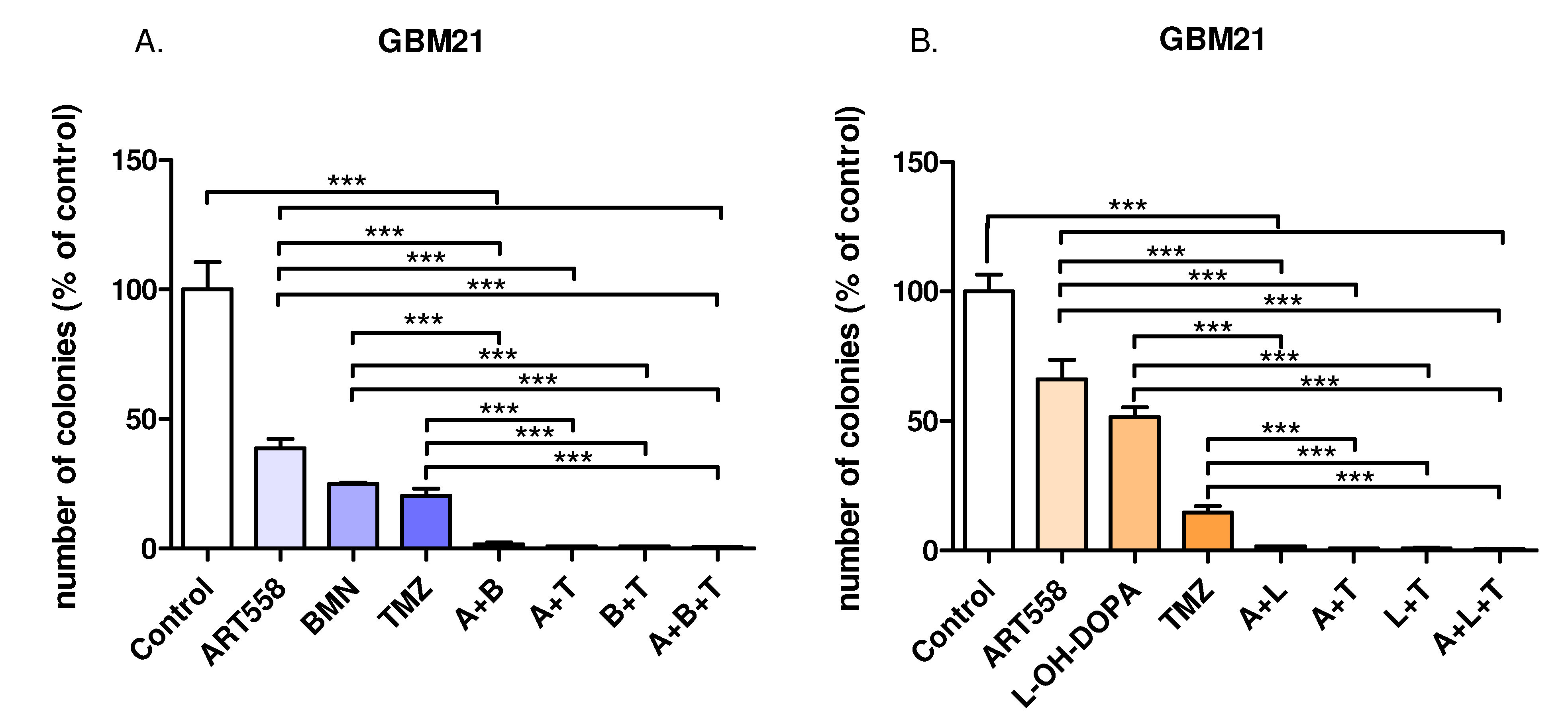
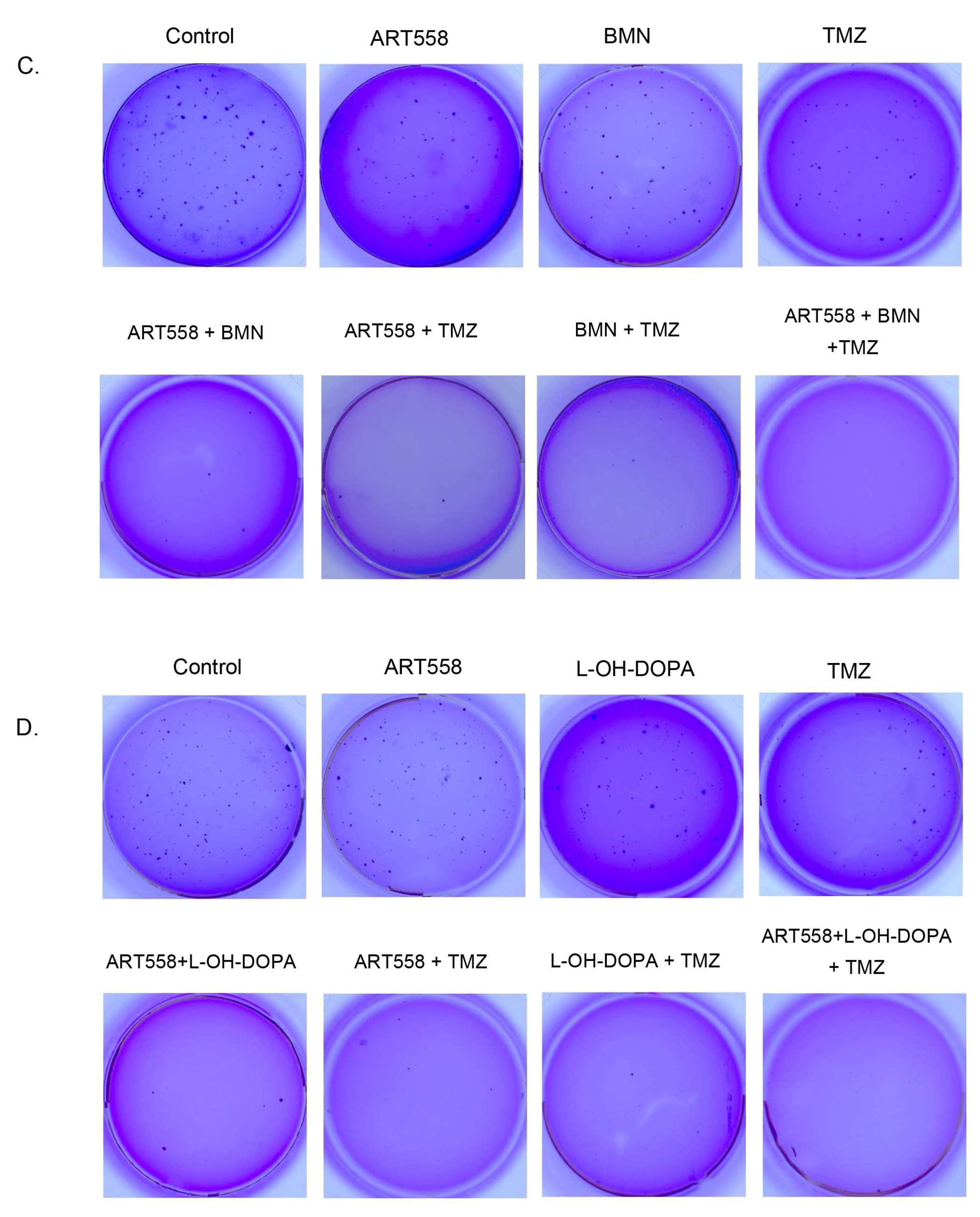
2.2. Inhibition of Polθ and Coinhibiton with Either PARP1 or Rad52 Decreases Proliferation of the Glioblastoma Cells
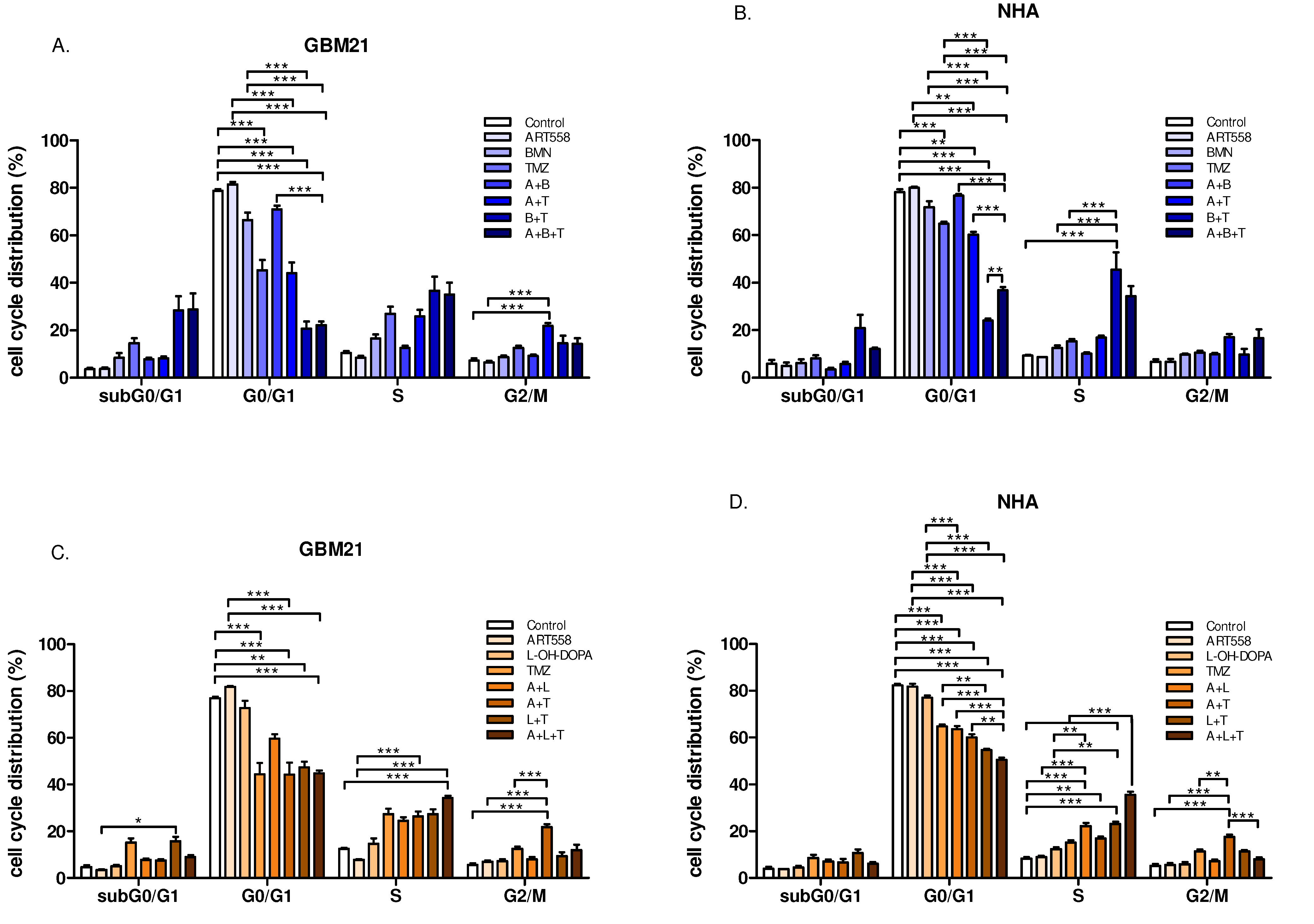
2.3. Combined Inhibition of Polθ and Either PARP1 or Rad52 and Induction of DNA Alkylation by TMZ Leads to a Decrease in G0/G1 and Arrest of Cell Populations in S Phase, in Both Cancer and Normal Cells
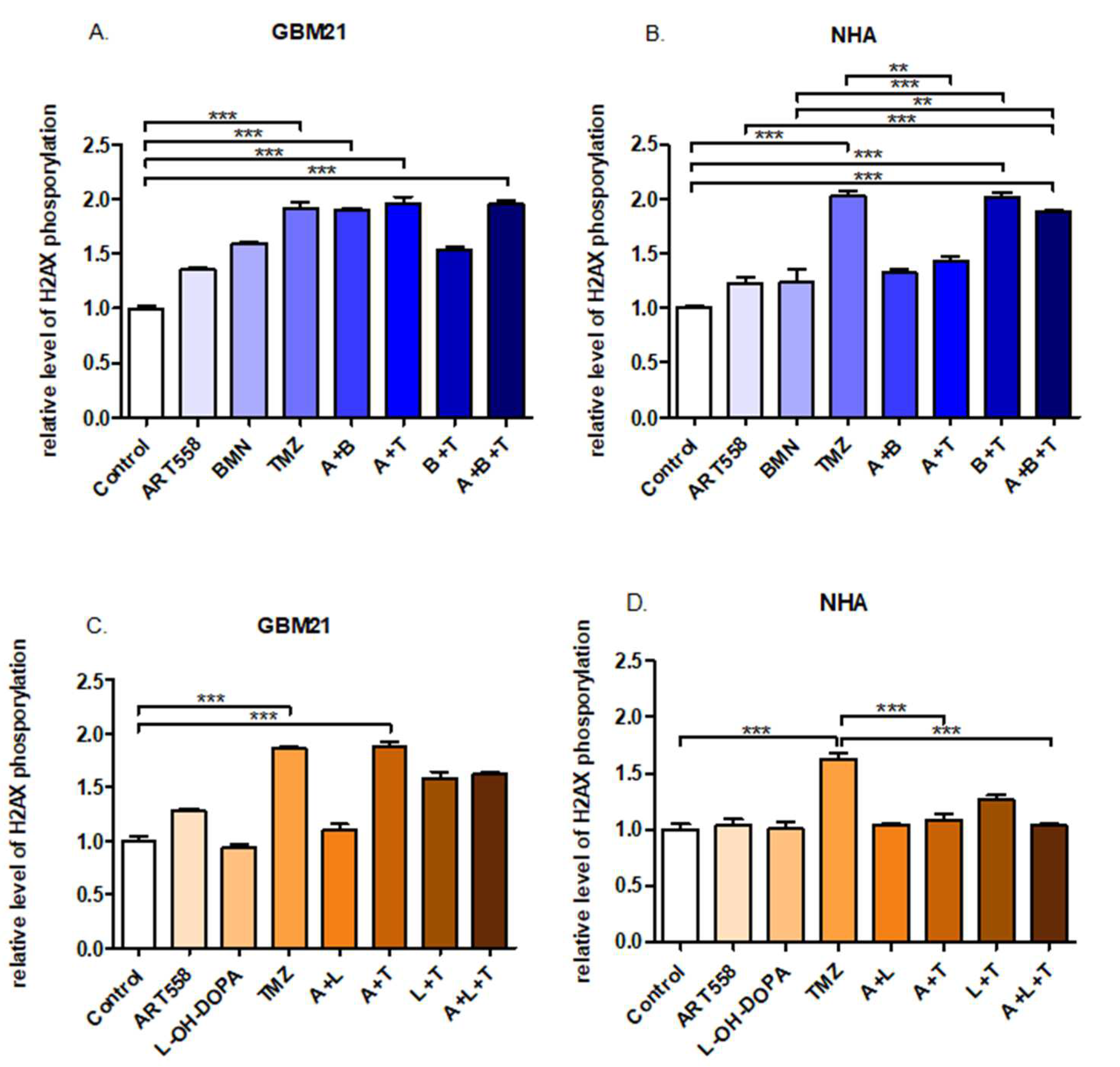
2.4. Coinhibition of Polθ with PARP1 Increases the Number of DSBs in the Glioblastoma Cells
2.5. Coinhibition of Polθ with PARP1 Enhances the Genotoxic Effect Obtained by Gamma Radiation in the Glioblastoma Cells, in Contrast to Normal Cells
3. Discussion
4. Materials and Methods
4.1. In Vitro Cell Culture
4.2. Drug Treatment
4.3. Flow Cytometric Analysis of Apoptosis and Necrosis
4.4. Cell Morphology Visualized by Fluorescence Microscopy
4.5. Clonogenic Assay
4.6. Cell Cycle
4.7. Measurement of Histone H2AX Phosphorylation
4.8. Neutral Comet Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Yabo, Y.A.; Niclou, S.P.; Golebiewska, A. Cancer cell heterogeneity and plasticity: A paradigm shift in glioblastoma. Neuro-Oncology 2022, 24, 669–682. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Odia, Y.; Khosla, A.A.; Ahluwalia, M.S. Key Clinical Principles in the Management of Glioblastoma. JCO Oncol. Pract. 2023, 19, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Badkas, A.; De Landtsheer, S.; Sauter, T. Expanding the Disease Network of Glioblastoma Multiforme via Topological Analysis. Int. J. Mol. Sci. 2023, 24, 3075. [Google Scholar] [CrossRef]
- Kreatsoulas, D.; Bolyard, C.; Wu, B.X.; Cam, H.; Giglio, P.; Li, Z. Translational landscape of glioblastoma immunotherapy for physicians: Guiding clinical practice with basic scientific evidence. J. Hematol. Oncol. 2022, 15, 80. [Google Scholar] [CrossRef]
- Wick, W.; Platten, M.; Weller, M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro-Oncology 2009, 11, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Neyns, B.; Tosoni, A.; Hwu, W.-J.; Reardon, D.A. Dose-Dense Temozolomide Regimens Antitumor Activity, Toxicity, and Immunomodulatory Effects. Cancer 2010, 116, 2868–2877. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. ScienceDirect Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Stupp, R.; Brada, M.; Van Den Bent, M.J.; Tonn, J.; Pentheroudakis, G. Clinical practice guidelines High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii93–iii101. [Google Scholar] [CrossRef]
- Pismataro, M.C.; Astolfi, A.; Barreca, M.L.; Pacetti, M.; Schenone, S.; Bandiera, T.; Carbone, A.; Massari, S. Small Molecules Targeting DNA Polymerase Theta (POL θ) as Promising Synthetic Lethal Agents for Precision Cancer Therapy. J. Med. Chem. 2023, 66, 6498–6522. [Google Scholar] [CrossRef]
- Huang, A.; Garraway, L.A.; Ashworth, A.; Weber, B. Synthetic lethality as an engine for cancer drug target discovery. Nat. Rev. Drug Discov. 2020, 19, 23–38. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, N.J.; Bailey, M.L.; Hieter, P. Synthetic lethality and cancer. Nat. Rev. Genet. 2017, 18, 613–623. [Google Scholar] [CrossRef]
- Patel, P.S.; Algouneh, A.; Hakem, R. Exploiting synthetic lethality to target BRCA1/2-deficient tumors: Where we stand. Oncogene 2021, 3001–3014. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, M.; Barszczewska-Pietraszek, G.; Czarny, P.; Skorski, T.; Śliwiński, T. Synthetic Lethality Targeting Polθ. Genes 2022, 13, 1101. [Google Scholar] [CrossRef]
- Livraghi, L.; Garber, J.E. PARP inhibitors in the management of breast cancer: Current data and future prospects. BMC Med. 2015, 13, 188. [Google Scholar] [CrossRef]
- Rajawat, J.; Shukla, N.; Mishra, D.P. Therapeutic Targeting of Poly(ADP-Ribose) Polymerase-1 in Cancer: Current Developments, Therapeutic Strategies, and Future. Med. Res. Rev. 2017, 37, 1461–1491. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, D.; Riillo, C.; Teresa, M.; Martino, D.; Tagliaferri, P. Alternative Non-Homologous End-Joining: Error-Prone DNA Repair as Cancer’s Achilles’ Heel. Cancers 2021, 13, 1392. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.S.; Zatreanu, D.; Pettitt, S.J.; Lord, C.J. Resistance to DNA repair inhibitors in cancer. Mol. Oncol. 2022, 16, 3811–3827. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Wood, R.D. Mini review DNA polymerase POLQ and cellular defense against DNA damage. DNA Repair 2013, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.D.; Doublié, S. DNA polymerase θ (POLQ), double-strand break repair, and cancer. DNA Repair 2016, 44, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Zatreanau, D.; Robinson, H.M.R.; Alkhatib, O.; Boursier, M.; Finch, H.; Geo, L.; Grande, D.; Grinkevich, V.; Heald, R.A.; Langdon, S.; et al. Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Nat. Commun. 2021, 12, 36. [Google Scholar] [CrossRef]
- Zhou, J.; Gelot, C.; Pantelidou, C.; Li, A.; Yücel, H.; Davis, R.E.; Färkkilä, A.; Kochupurakkal, B.; Syed, A.; Shapiro, G.I.; et al. A first-in-class polymerase theta inhibitor selectively targets homologous-recombination-deficient tumors. Nat. Cancer 2021, 2, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, M.; Mader, P.; Mochirian, P.; Vallée, F.; Clark, J.; Truchon, J.F.; Perryman, A.L.; Pau, V.; Kurinov, I.; Zahn, K.E.; et al. Identification of RP-6685, an Orally Bioavailable Compound that Inhibits the DNA Polymerase Activity of Polθ. J. Med. Chem. 2022, 65, 13198–13215. [Google Scholar] [CrossRef]
- Sullivan, K.; Cramer-Morales, K.; Mcelroy, D.L.; Ostrov, D.A.; Haas, K.; Childers, W.; Hromas, E.; Skorski, T. Identification of a Small Molecule Inhibitor of RAD52 by Structure-Based Selection. PLoS ONE 2016, 11, e0147230. [Google Scholar] [CrossRef] [PubMed]
- Toma, M.; Sullivan-Reed, K.; Sliwinski, T.; Skorksi, T. RAD52 as a Potential Target for Synthetic Lethality-Based Anticancer Therapies. Cancers 2019, 11, 1561. [Google Scholar] [CrossRef]
- Drzewiecka, M.; Jaśniak, D.; Barszczewska-Pietraszek, G.; Czarny, P.; Kobrzycka, A.; Wieczorek, M.; Radek, M.; Szemraj, J.; Skorski, T.; Śliwiński, T. Class I HDAC Inhibition Leads to a Downregulation of FANCD2 and RAD51, and the Eradication of Glioblastoma Cells. J. Pers. Med. 2023, 13, 1315. [Google Scholar] [CrossRef]
- Toma, M.; Witusik-Perkowska, M.; Szwed, M.; Stawski, R.; Szemraj, J.; Drzewiecka, M.; Nieborowska-Skorska, M.; Radek, M.; Kolasa, P.; Matlawska-Wasowska, K.; et al. Eradication of LIG4-deficient glioblastoma cells by the combination of PARP inhibitor and alkylating agent. Oncotarget 2018, 9, 36867–36877. [Google Scholar] [CrossRef][Green Version]
- Syed, A.; Filandr, F.; Patterson-Fortin, J.; Bacolla, A.; Ravindranathan, R.; Zhou, J.; McDonald, D.T.; Albuhluli, M.E.; Verway-Cohen, A.; Newman, J.A.; et al. Novobiocin blocks nucleic acid binding to Polθ and inhibits stimulation of its ATPase activity. Nucleic Acids Res. 2023, 51, 9920–9937. [Google Scholar] [CrossRef]
- Schrempf, A.; Bernardo, S.; Arasa Verge, E.A.; Ramirez Otero, M.A.; Wilson, J.; Kirchhofer, D.; Timelthaler, G.; Ambros, A.M.; Kaya, A.; Wieder, M.; et al. POLθ processes ssDNA gaps and promotes replication fork progression in BRCA1-deficient cells. Cell Rep. 2022, 41, 111716. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Liu, J.; Sun, D.; Meng, F.; Zhang, M.; Aliper, A.; Ren, F.; Zhavoronkov, A.; Ding, X. Discovery of 3-hydroxymethyl-azetidine derivatives as potent polymerase theta inhibitors. Bioorg. Med. Chem. 2024, 103, 117662. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wang, J.; Ma, X.; Shao, N.; Ye, K.; Zhang, D.; Shi, C.; Luo, L. A Progressively Disassembled DNA Repair Inhibitors Nanosystem for the Treatment of BRCA Wild-Type Triple-Negative Breast Cancer. Int. J. Nanomed. 2023, 18, 6001–6019. [Google Scholar] [CrossRef] [PubMed]
- Sullivan-Reed, K.; Toma, M.M.; Drzewiecka, M.; Nieborowska-Skorska, M.; Nejati, R.; Karami, A.; Wasik, M.A.; Sliwinski, T.; Skorski, T. Simultaneous Targeting of DNA Polymerase Theta and PARP1 or RAD52 Triggers Dual Synthetic Lethality in Homologous Recombination–Deficient Leukemia Cells. Mol. Cancer Res. 2023, 21, 1017–1022. [Google Scholar] [CrossRef]
- Starowicz, K.; Ronson, G.; Anthony, E.; Clarke, L.; Garvin, A.J.; Beggs, A.D.; Whalley, C.M.; Edmonds, M.; Beesley, J.; Morris, J.R. RAD52 underlies the synthetic-lethal relationship between BRCA1/2 and 53BP1 deficiencies and DNA polymerase theta loss. bioRxiv 2022, 485027. [Google Scholar] [CrossRef]
- Ronson, G.E.; Starowicz, K.; Anthony, E.J.; Piberger, A.L.; Clarke, L.C.; Garvin, A.J.; Beggs, A.D.; Whalley, C.M.; Edmonds, M.J.; Beesley, J.F.; et al. Mechanisms of synthetic lethality between BRCA1/2 and 53BP1 deficiencies and DNA polymerase theta targeting. Nat. Commun. 2023, 14, 7834. [Google Scholar] [CrossRef]
- Schrempf, A.; Slyskova, J.; Loizou, J.I. Targeting the DNA Repair Enzyme Polymerase θ in Cancer Therapy. Trends Cancer 2021, 7, 98–111. [Google Scholar] [CrossRef]
- Rodriguez-Berriguete, G.; Ranzani, M.; Prevo, R.; Puliyadi, R.; Machado, N.; Bolland, H.R.; Millar, V.; Ebner, D.; Boursier, M.; Cerutti, A.; et al. Small-molecule Polθ inhibitors provide safe and effective tumor radiosensitization in preclinical models. Clin. Cancer Res. 2023, 29, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.S.; Prevo, R.; Lee, Y.; Helleday, T.; Muschel, R.J.; Taylor, S.; Yoshimura, M.; Hickson, I.D.; Bernhard, E.J.; McKenna, W.G. A siRNA Screen of Genes Involved in DNA Repair Identifies Tumour Specific Radiosensitisation by POLQ Knockdown. Cancer Res. 2010, 70, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Ukai, A.; Maruyama, T.; Mochizuki, S.; Ouchida, R.; Masuda, K. Role of DNA polymerase θ in tolerance of endogenous and exogenous DNA damage in mouse B cells. Genes Cells 2006, 11, 111–121. [Google Scholar] [CrossRef]
- Brambati, A.; Barry, R.; Sfeir, A. DNA polymerase theta (Polθ)—An error-prone polymerase necessary for genome stability. Curr. Opin. Genet. Dev. 2020, 60, 119–126. [Google Scholar] [CrossRef]
- Lemée, F.; Bergoglio, V.; Fernandez-vidal, A.; Machado-silva, A.; Pillaire, M. DNA polymerase θ up-regulation is associated with poor survival in breast cancer, perturbs DNA replication, and promotes genetic instability. Proc. Natl. Acad. Sci. USA 2010, 107, 13390–13395. [Google Scholar] [CrossRef] [PubMed]
- Liddiard, K.; Aston-Evans, A.N.; Cleal, K.; Baird, D.M.; Hendrickson, E.A. POLQ suppresses genome instability and alterations in DNA repeat tract lengths. NAR Cancer 2022, 4, zcac020. [Google Scholar] [CrossRef] [PubMed]
- Lavudi, K.; Banerjee, A.; Li, N.; Yang, Y.; Cai, S.; Bai, X.; Zhang, X.; Li, A.; Wani, E.; Yang, S.M.; et al. ALDH1A1 promotes PARP inhibitor resistance by enhancing retinoic acid receptor-mediated DNA polymerase θ expression. NPJ Precis. Oncol. 2023, 7, 66. [Google Scholar] [CrossRef]
- Oh, G.; Wang, A.; Wang, L.; Li, J.; Werba, G.; Weissinger, D.; Zhao, E.; Dhara, S.; Hernandez, R.E.; Ackermann, A.; et al. POLQ inhibition elicits an immune response in homologous recombination-deficient pancreatic adenocarcinoma via cGAS/STING signaling. J. Clin. Investig. 2023, 133, e165934. [Google Scholar] [CrossRef]
- Fried, W.; Tyagi, M.; Minakhin, L.; Chandramouly, G.; Tredinnick, T.; Ramanjulu, M.; Auerbacher, W.; Calbert, M.; Rusanov, T.; Hoang, T.; et al. Discovery of a small-molecule inhibitor that traps Polθ on DNA and synergizes with PARP inhibitors. Nat. Commun. 2024, 15, 2862. [Google Scholar] [CrossRef]
- Czyż, M.; Toma, M.; Gajos-Michniewicz, A.; Majchrzak, K.; Hoser, G.; Szemraj, J.; Nieborowska-Skorska, M.; Cheng, P.; Gritsyuk, D.; Levesque, M.; et al. PARP1 inhibitor olaparib (Lynparza) exerts synthetic lethal effect against ligase 4-deficient melanomas. Oncotarget 2016, 7, 75551–75560. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, M.; Gajos-Michniewicz, A.; Hoser, G.; Jaśniak, D.; Barszczewska-Pietraszek, G.; Sitarek, P.; Czarny, P.; Piekarski, J.; Radek, M.; Czyż, M.; et al. Histone Deacetylases (HDAC) Inhibitor—Valproic Acid Sensitizes Human Melanoma Cells to Dacarbazine and PARP Inhibitor. Genes 2023, 14, 1295. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barszczewska-Pietraszek, G.; Czarny, P.; Drzewiecka, M.; Błaszczyk, M.; Radek, M.; Synowiec, E.; Wigner-Jeziorska, P.; Sitarek, P.; Szemraj, J.; Skorski, T.; et al. Polθ Inhibitor (ART558) Demonstrates a Synthetic Lethal Effect with PARP and RAD52 Inhibitors in Glioblastoma Cells. Int. J. Mol. Sci. 2024, 25, 9134. https://doi.org/10.3390/ijms25179134
Barszczewska-Pietraszek G, Czarny P, Drzewiecka M, Błaszczyk M, Radek M, Synowiec E, Wigner-Jeziorska P, Sitarek P, Szemraj J, Skorski T, et al. Polθ Inhibitor (ART558) Demonstrates a Synthetic Lethal Effect with PARP and RAD52 Inhibitors in Glioblastoma Cells. International Journal of Molecular Sciences. 2024; 25(17):9134. https://doi.org/10.3390/ijms25179134
Chicago/Turabian StyleBarszczewska-Pietraszek, Gabriela, Piotr Czarny, Małgorzata Drzewiecka, Maciej Błaszczyk, Maciej Radek, Ewelina Synowiec, Paulina Wigner-Jeziorska, Przemysław Sitarek, Janusz Szemraj, Tomasz Skorski, and et al. 2024. "Polθ Inhibitor (ART558) Demonstrates a Synthetic Lethal Effect with PARP and RAD52 Inhibitors in Glioblastoma Cells" International Journal of Molecular Sciences 25, no. 17: 9134. https://doi.org/10.3390/ijms25179134
APA StyleBarszczewska-Pietraszek, G., Czarny, P., Drzewiecka, M., Błaszczyk, M., Radek, M., Synowiec, E., Wigner-Jeziorska, P., Sitarek, P., Szemraj, J., Skorski, T., & Śliwiński, T. (2024). Polθ Inhibitor (ART558) Demonstrates a Synthetic Lethal Effect with PARP and RAD52 Inhibitors in Glioblastoma Cells. International Journal of Molecular Sciences, 25(17), 9134. https://doi.org/10.3390/ijms25179134





