Enhancing Colorectal Cancer Immunotherapy: The Pivotal Role of Ferroptosis in Modulating the Tumor Microenvironment
Abstract
:1. Introduction
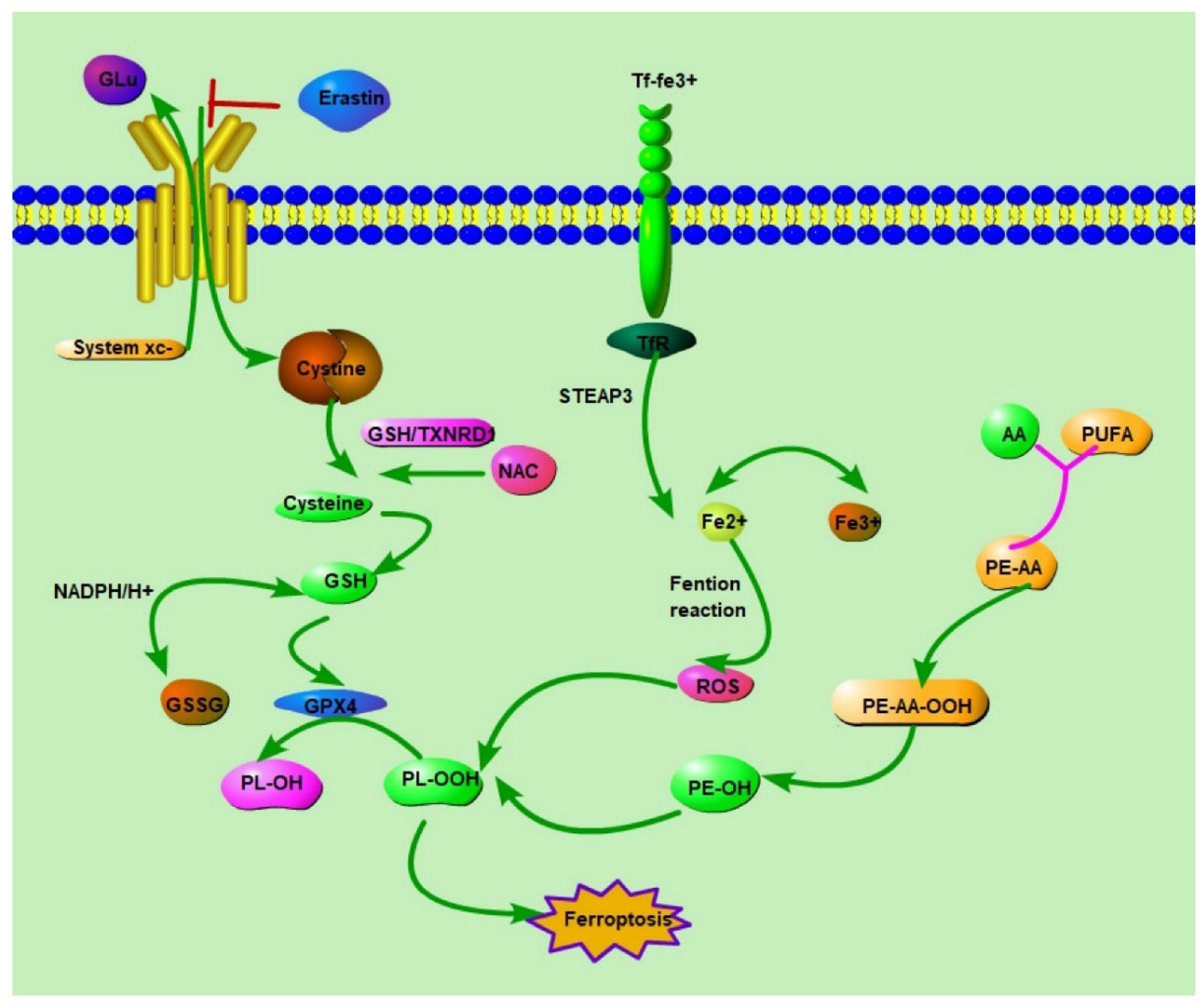
2. Ferroptosis: Understanding Its Mechanism and Impact
3. Effect of Tumor Microenvironment (TME) on Ferroptosis
4. The Role of Ferroptosis in Immunotherapy for Cancer
5. The Challenges of Immunotherapy in CRC
6. The Role of Ferroptosis in Immunotherapy for CRC
7. Global Research Trends
8. Challenges and Limitations
9. Future Prospect
10. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Galandiuk, S.; Fazio, V.W.; Jagelman, D.G.; Lavery, I.C.; Weakley, F.A.; Petras, R.E.; Badhwar, K.; McGonagle, B.; Eastin, K.; Sutton, T. Villous and tubulovillous adenomas of the colon and rectum: A retrospective review, 1964–1985. Am. J. Surg. 1987, 153, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Pischon, T.; Jenab, M.; Bueno-De-Mesquita, H.B.; Fedirko, V.; Norat, T.; Romaguera, D.; Knüppel, S.; Boutron-Ruault, M.-C.; Dossus, L.; et al. Combined impact of healthy lifestyle factors on colorectal cancer: A large European cohort study. BMC Med. 2014, 12, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.H.; Marafie, M.J.; Bitar, M.S.; Al-Dousari, F.; Ismael, S.; Bin Haider, H.; Al-Ali, W.; Jacob, S.P.; Al-Mulla, F. Gender-Associated Genomic Differences in Colorectal Cancer: Clinical Insight from Feminization of Male Cancer Cells. Int. J. Mol. Sci. 2014, 15, 17344–17365. [Google Scholar] [CrossRef]
- Caiazza, F.; Ryan, E.J.; Doherty, G.; Winter, D.C.; Sheahan, K. Estrogen Receptors and Their Implications in Colorectal Carcinogenesis. Front. Oncol. 2015, 5, 19. [Google Scholar] [CrossRef]
- Benslimane, Y.; Amalfi, K.; Lapin, S.; Perrino, S.; Brodt, P. Estrogen receptor blockade potentiates immunotherapy for liver metastases by altering the liver immunosuppressive microenvironment. Cancer Res. Commun. 2024, 4, 1963–1977. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Dhar, R.; Kumar, A.; Karmakar, S. Checkmate with checkpoint inhibitors: New paradigm in immunotherapy. Asian J. Med. Sci. 2023, 14, 1–2. [Google Scholar] [CrossRef]
- Poeta, V.M.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Alard, E.; Butnariu, A.-B.; Grillo, M.; Kirkham, C.; Zinovkin, D.A.; Newnham, L.; Macciochi, J.; Pranjol, Z.I. Advances in Anti-Cancer Immunotherapy: Car-T Cell, Checkpoint Inhibitors, Dendritic Cell Vaccines, and Oncolytic Viruses, and Emerging Cellular and Molecular Targets. Cancers 2020, 12, 1826. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Lin, C.; Chen, C.; Hsueh, C.; Chang, Y.; Wang, C.; Chu, P.; Tai, S.; Yang, M. Ferroptosis Signature Shapes the Immune Profiles to Enhance the Response to Immune Checkpoint Inhibitors in Head and Neck Cancer. Adv. Sci. 2023, 10, 2204514. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, M.; Liao, T.; Kuang, W.; Xia, H.; Yin, Z.; Tan, Q.; Li, Y.; Song, S.; Zhou, E.; et al. Targeting Cancer Cell Ferroptosis to Reverse Immune Checkpoint Inhibitor Therapy Resistance. Front. Cell Dev. Biol. 2022, 10, 818453. [Google Scholar] [CrossRef]
- Fan, F.; Liu, P.; Bao, R.; Chen, J.; Zhou, M.; Mo, Z.; Ma, Y.; Liu, H.; Zhou, Y.; Cai, X.; et al. A Dual PI3K/HDAC Inhibitor Induces Immunogenic Ferroptosis to Potentiate Cancer Immune Checkpoint Therapy. Cancer Res. 2021, 81, 6233–6245. [Google Scholar] [CrossRef]
- Chen, W.M.; Deng, J.M.; Zhou, Y. The construction of a novel ferroptosis-related lncRNA model to predict prognosis in colorectal cancer patients. Medicine 2023, 102, e33114. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2018, 133, 144–152. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Nam, M.; Son, H.Y.; Hyun, K.; Jang, S.Y.; Kim, J.W.; Kim, M.W.; Jung, Y.; Jang, E.; Yoon, S.-J.; et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 32433–32442. [Google Scholar] [CrossRef]
- Sha, W.; Hu, F.; Xi, Y.; Chu, Y.; Bu, S. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9999612. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2021, 289, 7038–7050. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Zimmer, M.; Lamb, J.; Ebert, B.L.; Lynch, M.; Neil, C.; Schmidt, E.; Golub, T.R.; Iliopoulos, O. The Connectivity Map Links Iron Regulatory Protein-1–Mediated Inhibition of Hypoxia-Inducible Factor-2a Translation to the Anti-inflammatory 15-deoxy-Δ12,14-Prostaglandin J2. Cancer Res. 2010, 70, 3071–3079. [Google Scholar] [CrossRef]
- Lou, Y.; McDonald, P.C.; Oloumi, A.; Chia, S.; Ostlund, C.; Ahmadi, A.; Kyle, A.; Keller, U.A.D.; Leung, S.; Huntsman, D.; et al. Targeting Tumor Hypoxia: Suppression of Breast Tumor Growth and Metastasis by Novel Carbonic Anhydrase IX Inhibitors. Cancer Res. 2011, 71, 3364–3376. [Google Scholar] [CrossRef]
- Venkateswaran, G.; McDonald, P.C.; Chafe, S.C.; Brown, W.S.; Gerbec, Z.J.; Awrey, S.J.; Parker, S.J.; Dedhar, S. A Carbonic Anhydrase IX/SLC1A5 Axis Regulates Glutamine Metabolism Dependent Ferroptosis in Hypoxic Tumor Cells. Mol. Cancer Ther. 2023, 22, 1228–1242. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2020, 12, 599–620. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Mondorf, A.; Beifuß, J.; Jung, M.; Brüne, B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020, 36, 101670. [Google Scholar] [CrossRef]
- Li, K.; Xu, K.; He, Y.; Yang, Y.; Tan, M.; Mao, Y.; Zou, Y.; Feng, Q.; Luo, Z.; Cai, K. Oxygen Self-Generating Nanoreactor Mediated Ferroptosis Activation and Immunotherapy in Triple-Negative Breast Cancer. ACS Nano 2023, 17, 4667–4687. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, T.; Yang, Y.; Jiang, L.; Wang, W.; Fu, L.; Zhu, Y.; Hao, Y. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials 2020, 268, 120537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, M.; Yao, X.; Fei, Y.; Lin, Z.; Li, Z.; Cai, K.; Zhao, Y.; Luo, Z. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 2020, 33, 108487. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Chai, W.; Xie, M.; Yang, M.; Yu, Y.; Cao, L.; Yang, L. HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRASQ61L cells. Am. J. Cancer Res. 2019, 9, 730–739. [Google Scholar]
- Xu, Q.; Zhou, L.; Yang, G.; Meng, F.; Wan, Y.; Wang, L.; Zhang, L. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR-541-3p/GPX4 axis. Cell Biol. Int. 2020, 44, 2344–2356. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; Johnson, J.; et al. CD8+ T cells regulate tumor ferroptosis by targeting the system xc− during cancer immunotherapy. J. Immunol. 2019, 202, 137.11. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, R.; Deng, R.; Song, S.; Wang, Y.; Zhang, H. Multi-enzyme Co-expressed Dual-Atom Nanozymes Induce Cascade Immunogenic Ferroptosis via Activating Interferon-γ and Targeting Arachidonic Acid Metabolism. J. Am. Chem. Soc. 2023, 145, 8965–8978. [Google Scholar] [CrossRef]
- Min, K.-J.; Um, H.J.; Cho, K.-H.; Kwon, T.K. Curcumin inhibits oxLDL-induced CD36 expression and foam cell formation through the inhibition of p38 MAPK phosphorylation. Food Chem. Toxicol. 2013, 58, 77–85. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012.e5. [Google Scholar] [CrossRef]
- Xu, S.; Chaudhary, O.; Rodríguez-Morales, P.; Sun, X.; Chen, D.; Zappasodi, R.; Xu, Z.; Pinto, A.F.; Williams, A.; Schulze, I.; et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity 2021, 54, 1561–1577.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, J.; Xiong, Y.; Chen, J.; Du, X.; Liu, Q.; Liu, T. Superparamagnetic Iron Oxide Nanoparticles Induce Ferroptosis of Human Ovarian Cancer Stem Cells by Weakening Cellular Autophagy. J. Biomed. Nanotechnol. 2020, 16, 1612–1622. [Google Scholar] [CrossRef]
- Kepp, O.; Kroemer, G. Is ferroptosis immunogenic? The devil is in the details! Oncoimmunology 2022, 11, 2127273. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Bai, L.; Qu, C.; Dai, E.; Liu, J.; Kang, R.; Zhou, D.; Tang, D.; Zhao, Y. PPARG-mediated ferroptosis in dendritic cells limits antitumor immunity. Biochem. Biophys. Res. Commun. 2021, 576, 33–39. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2020, 17, 2054–2081. [Google Scholar] [CrossRef]
- He, J.; Ding, H.; Li, H.; Pan, Z.; Chen, Q. Intra-Tumoral Expression of SLC7A11 Is Associated with Immune Microenvironment, Drug Resistance, and Prognosis in Cancers: A Pan-Cancer Analysis. Front. Genet. 2021, 12, 770857. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhu, J.; Li, J.; Fan, K.; Gao, Y.; Cheng, S.; Kong, C.; Zheng, L.; Wu, F.; Weng, Q.; et al. The ferroptosis and iron-metabolism signature robustly predicts clinical diagnosis, prognosis and immune microenvironment for hepatocellular carcinoma. Cell Commun. Signal. 2020, 18, 174. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Dahal, S.; Yurkovich, J.T.; Xu, H.; Palsson, B.O.; Yang, L. Synthesizing Systems Biology Knowledge from Omics Using Genome-Scale Models. Proteomics 2020, 20, e1900282. [Google Scholar] [CrossRef]
- Buetti-Dinh, A.; Herold, M.; Christel, S.; El Hajjami, M.; Delogu, F.; Ilie, O.; Bellenberg, S.; Wilmes, P.; Poetsch, A.; Sand, W.; et al. Reverse engineering directed gene regulatory networks from transcriptomics and proteomics data of biomining bacterial communities with approximate Bayesian computation and steady-state signalling simulations. BMC Bioinform. 2020, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B.; Langefeld, C.D.; Olivier, M.; Cox, L.A. Integrated Omics: Tools, Advances, and Future Approaches. J. Mol. Endocrinol. 2019, 62, R21–R45. [Google Scholar] [CrossRef] [PubMed]
- Henao, J.D.; Lauber, M.; Azevedo, M.; Grekova, A.; Theis, F.; List, M.; Ogris, C.; Schubert, B. Multi-omics regulatory network inference in the presence of missing data. Briefings Bioinform. 2023, 24, bbad309. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, J.; Kang, R.; Zhou, B.; Tang, D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019, 510, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Wu, S.; Duan, Q.; Liu, L.; Li, L.; Luo, Y.; Wang, A. Ferroptosis-dependent breast cancer cell-derived exosomes inhibit migration and invasion of breast cancer cells by suppressing M2 macrophage polarization. PeerJ 2023, 11, e15060. [Google Scholar] [CrossRef] [PubMed]
- Efimova, I.; Catanzaro, E.; Van der Meeren, L.; Turubanova, V.D.; Hammad, H.; Mishchenko, T.A.; Vedunova, M.V.; Fimognari, C.; Bachert, C.; Coppieters, F.; et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer 2020, 8, e001369. [Google Scholar] [CrossRef]
- Gu, X.; Liu, Y.; Dai, X.; Yang, Y.-G.; Zhang, X. Deciphering the potential roles of ferroptosis in regulating tumor immunity and tumor immunotherapy. Front. Immunol. 2023, 14, 1137107. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhou, Z.; Wu, R.; Chen, X.; Yu, C.; Stockwell, B.; Kroemer, G.; Kang, R.; Tang, D. Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer. Sci. Transl. Med. 2023, 15, eadg3049. [Google Scholar] [CrossRef]
- Ghoochani, A.; Hsu, E.-C.; Aslan, M.; Rice, M.A.; Nguyen, H.M.; Brooks, J.D.; Corey, E.; Paulmurugan, R.; Stoyanova, T. Ferroptosis Inducers Are a Novel Therapeutic Approach for Advanced Prostate Cancer. Cancer Res. 2021, 81, 1583–1594. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Li, H.; Han, L. FIN56, a novel ferroptosis inducer, triggers lysosomal membrane permeabilization in a TFEB-dependent manner in glioblastoma. J. Cancer 2021, 12, 6610–6619. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Li, Z.; Xu, C.; Wang, H.; Jia, B.; Gong, A.; Xu, M. Vitamin C Sensitizes Pancreatic Cancer Cells to Erastin-Induced Ferroptosis by Activating the AMPK/Nrf2/HMOX1 Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 5361241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lian, J.; Lan, Z.; Zou, K.; Wang, W.; Yu, G. Ferroptosis promotes anti-tumor immune response by inducing immunogenic exposure in HNSCC. Oral Dis. 2021, 29, 933–941. [Google Scholar] [CrossRef]
- Lee, N.; Carlisle, A.E.; Peppers, A.; Park, S.J.; Doshi, M.B.; Spears, M.E.; Kim, D. xCT-Driven Expression of GPX4 Determines Sensitivity of Breast Cancer Cells to Ferroptosis Inducers. Antioxidants 2021, 10, 317. [Google Scholar] [CrossRef]
- Lizardo, D.Y.; Kuang, C.; Hao, S.; Yu, J.; Huang, Y.; Zhang, L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1874, 188447. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Ding, K.; Mou, P.; Wang, Z.; Liu, S.; Liu, J.; Lu, H.; Yu, G. The next bastion to be conquered in immunotherapy: Microsatellite stable colorectal cancer. Front. Immunol. 2023, 14, 1298524. [Google Scholar] [CrossRef] [PubMed]
- Mulet-Margalef, N.; Linares, J.; Badia-Ramentol, J.; Jimeno, M.; Monte, C.S.; Mozo, J.L.M.; Calon, A. Challenges and Therapeutic Opportunities in the dMMR/MSI-H Colorectal Cancer Landscape. Cancers 2023, 15, 1022. [Google Scholar] [CrossRef]
- Fan, A.; Wang, B.; Wang, X.; Nie, Y.; Fan, D.; Zhao, X.; Lu, Y. Immunotherapy in colorectal cancer: Current achievements and future perspective. Int. J. Biol. Sci. 2021, 17, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, L.; Kong, F.; Wu, D.; Hu, B.; Yang, J.; He, J.; Liu, L. Case report: A combined immunotherapy strategy as a promising therapy for MSI-H colorectal carcinomas with multiple HPD risk factors. Front. Med. 2023, 10, 1051034. [Google Scholar] [CrossRef]
- Li, D.-D.; Tang, Y.-L.; Wang, X. Challenges and exploration for immunotherapies targeting cold colorectal cancer. World J. Gastrointest. Oncol. 2023, 15, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Thibaudin, M.; Ghiringhelli, F. Chemoimmunotherapy triggers immune responses targeting microsatellite stable colorectal cancer. Oncoimmunology 2023, 12, 2257098. [Google Scholar] [CrossRef]
- Ros, J.; Balconi, F.; Baraibar, I.; Gonzalez, N.S.; Salva, F.; Tabernero, J.; Elez, E. Advances in immune checkpoint inhibitor combination strategies for microsatellite stable colorectal cancer. Front. Oncol. 2023, 13, 1112276. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, W.; He, X.; Xu, J.; Xu, R.; Wan, T.; Wu, Y. Synergistic Therapeutic Effects of Low Dose Decitabine and NY-ESO-1 Specific TCR-T Cells for the Colorectal Cancer With Microsatellite Stability. Front. Oncol. 2022, 12, 895103. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, S.; Asadzadeh, Z.; Hemmat, N.; Baghbanzadeh, A.; Sgambato, A.; Ghorbaninezhad, F.; Safarpour, H.; Argentiero, A.; Brunetti, O.; Bernardini, R.; et al. Immune Checkpoint Inhibitors in Colorectal Cancer: Challenges and Future Prospects. Biomedicines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Shi, G.; Yang, Q.; Zhang, Y.; Jiang, Q.; Lin, Y.; Yang, S.; Wang, H.; Cheng, L.; Zhang, X.; Li, Y.; et al. Modulating the Tumor Microenvironment via Oncolytic Viruses and CSF-1R Inhibition Synergistically Enhances Anti-PD-1 Immunotherapy. Mol. Ther. 2019, 27, 244–260. [Google Scholar] [CrossRef]
- Luo, W.; Dai, W.; Li, Q.; Mo, S.; Han, L.; Xiao, X.; Gu, R.; Xiang, W.; Ye, L.; Wang, R.; et al. Ferroptosis-associated molecular classification characterized by distinct tumor microenvironment profiles in colorectal cancer. Int. J. Biol. Sci. 2022, 18, 1773–1794. [Google Scholar] [CrossRef]
- Yang, C.; Huang, S.; Cao, F.; Zheng, Y. Role of ferroptosis-related genes in prognostic prediction and tumor immune microenvironment in colorectal carcinoma. PeerJ 2021, 9, e11745. [Google Scholar] [CrossRef]
- Lv, Y.; Tang, W.T.; Xu, Y.Q.; Chang, W.J.; Zhang, Z.Y.; Lin, Q.; Ji, M.L.; Feng, Q.Y.; He, G.D.; Xu, J.M. Apolipoprotein L3 enhances CD8+ T cell antitumor immunity of colorectal cancer by promoting LDHA-mediated ferroptosis. Int. J. Biol. Sci. 2023, 19, 1284–1298. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, H.; Huang, L.; Li, S.; Wang, C.; Aikemu, B.; Yang, G.; Hong, H.; Yang, X.; Zhang, S.; et al. An Original Ferroptosis-Related Gene Signature Effectively Predicts the Prognosis and Clinical Status for Colorectal Cancer Patients. Front. Oncol. 2021, 11, 711776. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Liu, L.; Wu, Y.; Fu, P.; Cao, Y.; Xiong, J.; Tu, Y.; Li, Z.; Liu, Y.; et al. Comprehensive Analysis of Immune Infiltrates of Ferroptosis-Related Long Noncoding RNA and Prediction of Colon Cancer Patient Prognoses. J. Immunol. Res. 2022, 2022, 9480628. [Google Scholar] [CrossRef]
- Feng, S.; Rao, Z.; Zhang, J.; She, X.; Chen, Y.; Wan, K.; Li, H.; Zhao, C.; Feng, Y.; Wang, G.; et al. Inhibition of CARM1-Mediated Methylation of ACSL4 Promotes Ferroptosis in Colorectal Cancer. Adv. Sci. 2023, 10, e2303484. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Peng, F.; Song, B.; Wang, L.; Chen, J.; Shang, B. Heat Shock Protein Beta 1 is a Prognostic Biomarker and Correlated with Immune Infiltrates in Hepatocellular Carcinoma. Int. J. Gen. Med. 2021, 14, 5483–5492. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, T.; Wang, X.; Xiong, F.; Hu, Z.; Qiao, X.; Yuan, X.; Wang, D. ACSL3 and ACSL4, Distinct Roles in Ferroptosis and Cancers. Cancers 2022, 14, 5896. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, W.; Wang, Y.; Zhu, J.; Wang, H.; Tu, J.; Weng, Q.; Kong, C.; Yang, Y.; Qiu, R.; et al. Identification of critical ferroptosis regulators in lung adenocarcinoma that RRM2 facilitates tumor immune infiltration by inhibiting ferroptotic death. Clin. Immunol. 2021, 232, 108872. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Su, R.; Bao, X.; Cao, K.; Du, Y.; Wang, N.; Wang, J.; Xing, F.; Yan, F.; Huang, K.; et al. Glycyrrhetinic acid nanoparticles combined with ferrotherapy for improved cancer immunotherapy. Acta Biomater. 2022, 144, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Ren, Y.; Chen, S.; Wang, Y.; Chu, L. Ferroptosis and tumor immunotherapy: A promising combination therapy for tumors. Front. Oncol. 2023, 13, 1119369. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Xie, L.; Chen, W.; Xu, Z.; Song, E.; Zhu, X.; Song, Y. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale 2021, 13, 4855–4870. [Google Scholar] [CrossRef]
- Wang, W.; Ling, Y.; Zhong, Y.; Li, Z.; Tan, C.; Mao, Z. Ferroptosis-Enhanced Cancer Immunity by a Ferrocene-Appended Iridium(III) Diphosphine Complex. Angew. Chem. Int. Ed. 2021, 61, e202115247. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Dai, L.; Yu, Z.; Wang, M.; Chan, S.; Sun, R.; Han, Q.; Chen, J.; Zuo, X.; et al. A novel oxidative stress- and ferroptosis-related gene prognostic signature for distinguishing cold and hot tumors in colorectal cancer. Front. Immunol. 2022, 13, 1043738. [Google Scholar] [CrossRef]
- Lv, Y.; Zheng, P.; Mao, Y.; Xu, Y.; Chang, W.; Lin, Q.; Ji, M.; Ye, L.; Tang, W.; Xu, J. Intratumor APOL3 delineates a distinctive immunogenic ferroptosis subset with prognosis prediction in colorectal cancer. Cancer Sci. 2023, 115, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhao, F.; Ke, B.; Chen, D.; Liu, F. Harnessing Ferroptosis to Overcome Drug Resistance in Colorectal Cancer: Promising Therapeutic Approaches. Cancers 2023, 15, 5209. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Liu, Z.; Wang, J.; Wang, R.; Zhu, L.; Zhu, Z.; Wang, X. Microenvironment immune response induced by tumor ferroptosis—The application of nanomedicine. Front. Oncol. 2022, 12, 1019654. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, C.; Luo, M.; Cen, C.; Qiu, J.; Zhang, S.; Hu, K. Ferroptosis in Cancer Treatment: Another Way to Rome. Front. Oncol. 2020, 10, 571127. [Google Scholar] [CrossRef]
- Li, Y.; Wei, C.; Yan, J.; Li, F.; Chen, B.; Sun, Y.; Luo, K.; He, B.; Liang, Y. The application of nanoparticles based on ferroptosis in cancer therapy. J. Mater. Chem. B 2023, 12, 413–435. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core–Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Xia, G.; Zhou, S.; Liu, Z.; Zhang, N.; Wang, H.; Guo, Z.; et al. Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4 @C@MIL-100(Fe) nanoparticles. Biomaterials 2016, 107, 88–101. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Tsai, H.-I.; Liu, Y.; Gao, J.; Wang, M.; Song, L.; Cao, X.; Xu, Z.; Chen, H.; et al. Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ. 2020, 28, 1222–1236. [Google Scholar] [CrossRef]
- Hu, Q.; Wei, W.; Wu, D.; Huang, F.; Li, M.; Li, W.; Yin, J.; Peng, Y.; Lu, Y.; Zhao, Q.; et al. Blockade of GCH1/BH4 Axis Activates Ferritinophagy to Mitigate the Resistance of Colorectal Cancer to Erastin-Induced Ferroptosis. Front. Cell Dev. Biol. 2022, 10, 810327. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, X.; Shui, S.; Wang, B.; Cui, Y.; Dong, S.; Yuwen, T.; Liu, G. PDTAC: Targeted Photodegradation of GPX4 Triggers Ferroptosis and Potent Antitumor Immunity. J. Med. Chem. 2022, 65, 12176–12187. [Google Scholar] [CrossRef]
- Feng, Y.; Dai, Y. APOL3-LDHA axis related immunity activation and cancer ferroptosis induction. Int. J. Biol. Sci. 2023, 19, 1401–1402. [Google Scholar] [CrossRef]
- Dennie, T.W.; Fleming, R.A.; Bowen, C.J.; Dar, M.M.; Alberti, D.; Oliver, K.; Loconte, N.; Mulkerin, D.; Holen, K.D. A Phase I Study of Capecitabine, Oxaliplatin, and Lapatinib in Metastatic or Advanced Solid Tumors. Clin. Colorectal Cancer 2011, 10, 57–62. [Google Scholar] [CrossRef]
- von Moos, R.; Koeberle, D.; Schacher, S.; Hayoz, S.; Winterhalder, R.C.; Roth, A.; Bodoky, G.; Samaras, P.; Berger, M.D.; Rauch, D.; et al. Neoadjuvant radiotherapy combined with capecitabine and sorafenib in patients with advanced KRAS-mutated rectal cancer: A phase I/II trial (SAKK 41/08). Eur. J. Cancer 2018, 89, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Samalin, E.; Bouché, O.; Thézenas, S.; Francois, E.; Adenis, A.; Bennouna, J.; Taieb, J.; Desseigne, F.; Seitz, J.F.; Conroy, T.; et al. Sorafenib and irinotecan (NEXIRI) as second- or later-line treatment for patients with metastatic colorectal cancer and KRAS-mutated tumours: A multicentre Phase I/II trial. Br. J. Cancer 2014, 110, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Samalin, E.; de la Fouchardière, C.; Thézenas, S.; Boige, V.; Senellart, H.; Guimbaud, R.; Taïeb, J.; François, E.; Galais, M.-P.; Lièvre, A.; et al. Sorafenib Plus Irinotecan Combination in Patients With RAS-mutated Metastatic Colorectal Cancer Refractory To Standard Combined Chemotherapies: A Multicenter, Randomized Phase 2 Trial (NEXIRI-2/PRODIGE 27). Clin. Colorectal Cancer 2020, 19, 301–310.e1. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lafky, J.M.; Morlan, B.W.; Stella, P.J.; Dakhil, S.R.; Gross, G.G.; Loui, W.S.; Hubbard, J.M.; Alberts, S.R.; Grothey, A. Dual VEGF inhibition with sorafenib and bevacizumab as salvage therapy in metastatic colorectal cancer: Results of the phase II North Central Cancer Treatment Group study N054C (Alliance). Ther. Adv. Med. Oncol. 2020, 12, 1758835920910913. [Google Scholar] [CrossRef] [PubMed]
- Hendlisz, A.; Deleporte, A.; Delaunoit, T.; Maréchal, R.; Peeters, M.; Holbrechts, S.; Eynde, M.V.D.; Houbiers, G.; Filleul, B.; Van Laethem, J.-L.; et al. The Prognostic Significance of Metabolic Response Heterogeneity in Metastatic Colorectal Cancer. PLoS ONE 2015, 10, e0138341. [Google Scholar] [CrossRef]
- Kim, R.; Prithviraj, G.K.; Shridhar, R.; Hoffe, S.E.; Jiang, K.; Zhao, X.; Chen, D.-T.; Almhanna, K.; Strosberg, J.; Campos, T.; et al. Phase I study of pre-operative continuous 5-FU and sorafenib with external radiation therapy in locally advanced rectal adenocarcinoma. Radiother. Oncol. 2016, 118, 382–386. [Google Scholar] [CrossRef]
- Zheng, J.; Sato, M.; Mishima, E.; Sato, H.; Proneth, B.; Conrad, M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 2021, 12, 698. [Google Scholar] [CrossRef]
- Leung, W.-H.; Shih, J.-W.; Chen, J.-S.; Mokgautsi, N.; Wei, P.-L.; Huang, Y.-J. Preclinical Identification of Sulfasalazine’s Therapeutic Potential for Suppressing Colorectal Cancer Stemness and Metastasis through Targeting KRAS/MMP7/CD44 Signaling. Biomedicines 2022, 10, 377. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Xie, S.; Wang, J.; Li, Z.; Chen, L.; Mao, M.; Chen, C.; Huang, A.; Chen, Y.; et al. Metformin induces Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 206. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Guo, Q.; Shen, Z.; Yang, W.; Zhou, Y.; Sun, Z.; Yao, X.; Wu, H. Frontiers of ferroptosis research: An analysis from the top 100 most influential articles in the field. Front. Oncol. 2022, 12, 948389. [Google Scholar] [CrossRef]
- Xu, H.; Ye, D.; Ren, M.; Zhang, H.; Bi, F. Ferroptosis in the tumor microenvironment: Perspectives for immunotherapy. Trends Mol. Med. 2021, 27, 856–867. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, D.; Wang, X.; Chen, X. Ferroptosis-Driven Nanotherapeutics to Reverse Drug Resistance in Tumor Microenvironment. ACS Appl. Bio Mater. 2022, 5, 2481–2506. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, D.; Yu, Q.; Li, Z.; Lenahan, C.; Dong, Y.; Wei, Q.; Shao, A. A Promising Future of Ferroptosis in Tumor Therapy. Front. Cell Dev. Biol. 2021, 9, 629150. [Google Scholar] [CrossRef] [PubMed]
- Chafe, S.C.; McDonald, P.C.; Saberi, S.; Nemirovsky, O.; Venkateswaran, G.; Burugu, S.; Gao, D.; Delaidelli, A.; Kyle, A.H.; Baker, J.H.E.; et al. Targeting Hypoxia-Induced Carbonic Anhydrase IX Enhances Immune-Checkpoint Blockade Locally and Systemically. Cancer Immunol. Res. 2019, 7, 1064–1078. [Google Scholar] [CrossRef]
- Hou, J.; Wang, B.; Li, J.; Liu, W. Ferroptosis and its role in gastric and colorectal cancers. Korean J. Physiol. Pharmacol. 2024, 28, 183–196. [Google Scholar] [CrossRef]
- Eaton, J.K.; Furst, L.; Ruberto, R.A.; Moosmayer, D.; Hilpmann, A.; Ryan, M.J.; Zimmermann, K.; Cai, L.L.; Niehues, M.; Badock, V.; et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat. Chem. Biol. 2020, 16, 497–506. [Google Scholar] [CrossRef]
- Yang, Y.-B.; Zhou, J.-X.; Qiu, S.-H.; He, J.-S.; Pan, J.-H.; Pan, Y.-L. Identification of a Novel Ferroptosis-Related Gene Prediction Model for Clinical Prognosis and Immunotherapy of Colorectal Cancer. Dis. Mark. 2021, 2021, 4846683. [Google Scholar] [CrossRef]
- Yu, B.; Choi, B.; Li, W.; Kim, D.-H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat. Commun. 2020, 11, 3637. [Google Scholar] [CrossRef]
- Lei, H.; Li, Q.; Pei, Z.; Liu, L.; Yang, N.; Cheng, L. Nonferrous Ferroptosis Inducer Manganese Molybdate Nanoparticles to Enhance Tumor Immunotherapy. Small 2023, 19, e2303438. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-L.; Linghu, D.-L.; Hung, M.-C. Ferroptosis: A promising target for cancer immunotherapy. Am. J. Cancer Res. 2021, 11, 5856–5863. [Google Scholar] [PubMed]
- Lang, X.; Green, M.D.; Wang, W.; Yu, J.; Choi, J.E.; Jiang, L.; Liao, P.; Zhou, J.; Zhang, Q.; Dow, A.; et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019, 9, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Tang, H.; Chen, H.; Li, M.; Xiong, D. Ferroptosis: A new approach for immunotherapy. Cell Death Discov. 2020, 6, 122. [Google Scholar] [CrossRef]
- Lachaier, E.; Louandre, C.; Godin, C.; Saidak, Z.; Baert, M.; Diouf, M.; Chauffert, B.; Galmiche, A. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res. 2014, 34, 6417–6422. [Google Scholar] [PubMed]
- Sato, M.; Kusumi, R.; Hamashima, S.; Kobayashi, S.; Sasaki, S.; Komiyama, Y.; Izumikawa, T.; Conrad, M.; Bannai, S.; Sato, H. The ferroptosis inducer erastin irreversibly inhibits system xc− and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci. Rep. 2018, 8, 968. [Google Scholar] [CrossRef]

| TME Factor/ Regulator | Mechanism of Impact | Effect on Ferroptosis | Reference Numbers |
|---|---|---|---|
| Hypoxia | Induction of IRP2 post-translational upregulation<br>- Regulation of iron transporter and storage protein expression | Promotes ferroptosis | [26] |
| Carbonic Anhydrase IX (CAIX) | Modulation of the cystine/glutamate antiporter xCT | Prevents ferroptosis | [27,28] |
| HIF-1α/lncRNA-PMAN | Regulation of key genes involved in iron metabolism | Protects cancer cells from ferroptosis | [29] |
| CBSLR/CBS Signal Axis | Same as above | Protects cancer cells from ferroptosis | [29] |
| NCOA4 Expression Reduction | Reduced expression in macrophages, leading to increased ferritin levels and decreased ferroptosis susceptibility | Decreases ferroptosis susceptibility | [22,30] |
| Polymeric Nanocarriers | Depleting NADPH, GSH, and Trx to sensitize hypoxic tumor cells to ferroptosis | Sensitizes hypoxic tumor cells to ferroptosis | [31,32] |
| Lactate Accumulation | Upregulation of HCAR1 and MCT1 reducing lipid peroxidation | Inhibits ferroptosis | [33] |
| Inflammatory Factors | Regulation of ferroptosis through various cancer types and signaling pathways, such as JAK2/STAT3 and RAS-JNK/p38 | May promote or inhibit ferroptosis | [34] |
| Non-coding RNAs | Modulation of ferroptosis by targeting key regulators like xCT and GPX4 | Influences cancer progression and ferroptosis | [35] |
| CD8 T Cells | Secretion of cytokines like IFNγ inhibiting the expression of SLC3A2 and SLC7A11 | Promotes ferroptosis in tumor cells | [36,37] |
| oxLDL and CD36 | Inducing ferroptosis and p38 phosphorylation in CD8+ T cells via CD36-dependent mechanisms | Affects T cell function and antitumor immunity | [40,41,42] |
| NK Cells | Cytotoxic effects on tumor cells through perforin, granzyme, and IFNγ release | Enhances NK cell activity and promotes ferroptosis | [42,43] |
| Ferroptosis and Immune Response | Release of cytokines and DAMPs without activating antitumor immune responses, inhibiting dendritic cell functions | May not represent an immunogenic cell death form | [44,45] |
| Drug/Molecule | Target | Clinical Trial ID | Phase | Combination/Specific Aim | Reference |
|---|---|---|---|---|---|
| Sulfasalazine | SLC7A11 | NCT06134388 | Phase I | Metastatic CRC | - |
| CNSI-Fe (II) | Iron ions | NCT06048367 | Phase I | Advanced solid tumors including CRC | - |
| Neratinib | Iron ions | NCT03457896 | Phase II | Combination with Trastuzumab or Cetuximab | - |
| Lapatinib | Iron ions | NCT00536809 NCT00574171 NCT01184482 NCT04831528 NCT03418558 | Phase I/II | Combination with various drugs | [102] |
| Sorafenib | SLC7A11 | NCT00780169 NCT00869570 NCT00989469 NCT01715441 NCT00826540 NCT01290926 NCT00134069 NCT00865709 NCT00703638 NCT01376453 | Phase I/II | Combination with chemotherapy | [103,104,105,106,107,108] |
| Simvastatin | HMGCR | NCT01238094 | Phase I | Combination with XELIRI/FOLFIRI | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cheng, X. Enhancing Colorectal Cancer Immunotherapy: The Pivotal Role of Ferroptosis in Modulating the Tumor Microenvironment. Int. J. Mol. Sci. 2024, 25, 9141. https://doi.org/10.3390/ijms25179141
Li Y, Cheng X. Enhancing Colorectal Cancer Immunotherapy: The Pivotal Role of Ferroptosis in Modulating the Tumor Microenvironment. International Journal of Molecular Sciences. 2024; 25(17):9141. https://doi.org/10.3390/ijms25179141
Chicago/Turabian StyleLi, Yanqing, and Xiaofei Cheng. 2024. "Enhancing Colorectal Cancer Immunotherapy: The Pivotal Role of Ferroptosis in Modulating the Tumor Microenvironment" International Journal of Molecular Sciences 25, no. 17: 9141. https://doi.org/10.3390/ijms25179141





