Extracellular Vesicle microRNA: A Promising Biomarker and Therapeutic Target for Respiratory Diseases
Abstract
1. Introduction
2. EV-microRNA
3. EV-microRNAs and Respiratory Diseases
3.1. EVs and EV-microRNAs Maintain Pulmonary Homeostasis
3.2. EV-microRNAs and COPD
3.3. EV-microRNAs and Asthma
3.4. EV-microRNAs and Lung Cancer
3.4.1. EV-microRNAs as Biomarkers for Lung Cancer
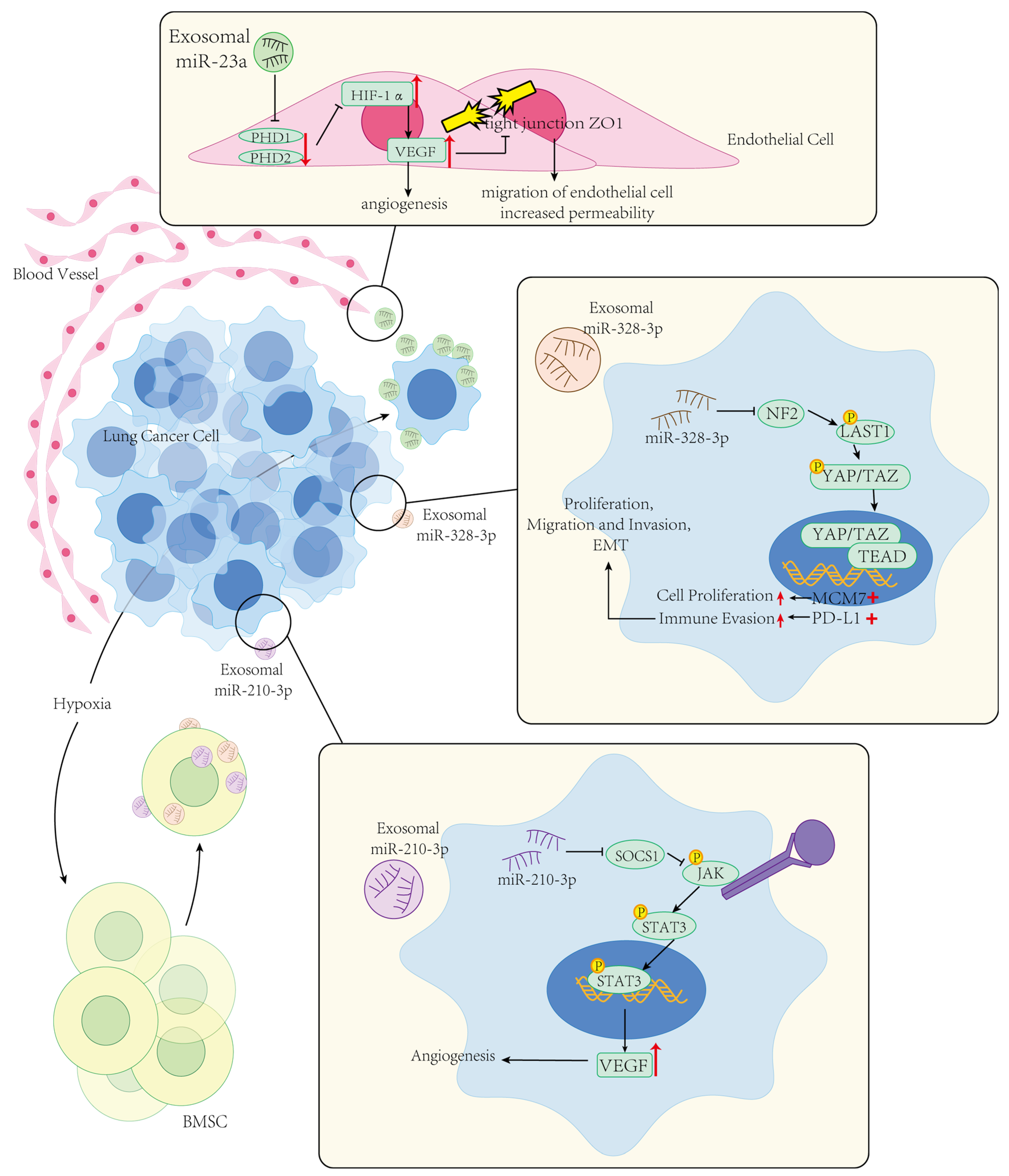
3.4.2. EV-microRNAs in the Development of Lung Cancer
3.4.3. EV-microRNAs in the Treatment of Lung Cancer
3.5. EV-microRNAs and COVID-19
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything-The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Weiz, L.; Burwinkel, B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem. Sci. 2012, 37, 460–465. [Google Scholar] [CrossRef]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Moghaddam, S.S.; Ghamari, S.H.; Rad, E.M.; Rezaei, N.; Shobeiri, P.; Aali, A.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abdelmasseh, M.; et al. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, Y.M.; Huang, J.; Jin, Y.Z.; Jiang, W.; Liu, P.L.; Liu, F.J.; Ma, J.X.; Ma, J.Y.; Wang, Y.; et al. COVID-19 pandemic: Global epidemiological trends and China’s subsequent preparedness and responses. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 642–647. [Google Scholar] [CrossRef]
- Ukwishaka, J.; Ndayishimiye, Y.; Destine, E.; Danwang, C.; Kirakoya-Samadoulougou, F. Global prevalence of coronavirus disease 2019 reinfection: A systematic review and meta-analysis. BMC Public Health 2023, 23, 778. [Google Scholar] [CrossRef]
- Lin, C.H.; Cheng, S.L.; Chen, C.Z.; Chen, C.H.; Lin, S.H.; Wang, H.C. Current Progress of COPD Early Detection: Key Points and Novel Strategies. Int. J. Chron. Obs. Pulmon Dis. 2023, 18, 1511–1524. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Kayser, V.; Ramzan, I. Vaccines and vaccination: History and emerging issues. Hum. Vaccin. Immunother. 2021, 17, 5255–5268. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Stout-Delgado, H.W. Aging and Lung Disease. Annu. Rev. Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xue, Y.; Duan, Y.; Mao, C.; Wan, M. Extracellular vesicles and their engineering strategies, delivery systems, and biomedical applications. J. Control Release 2024, 365, 1089–1123. [Google Scholar] [CrossRef]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Baruah, H.; Sarma, A.; Basak, D.; Das, M. Exosome: From biology to drug delivery. Drug Deliv. Transl. Res. 2024, 14, 1480–1516. [Google Scholar] [CrossRef]
- Schwager, S.C.; Reinhart-King, C.A. Mechanobiology of microvesicle release, uptake, and microvesicle-mediated activation. Curr. Top Membr. 2020, 86, 255–278. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.J.; Tang, Y.C.; Hao, X.Y.; Xu, W.J.; Xiang, D.X.; Wu, J.Y. Apoptotic bodies for advanced drug delivery and therapy. J. Control Release 2022, 351, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Rhim, W.K.; Kim, J.Y.; Lee, S.Y.; Cha, S.G.; Park, J.M.; Park, H.J.; Park, C.G.; Han, D.K. Recent advances in extracellular vesicle engineering and its applications to regenerative medicine. Biomater. Res. 2023, 27, 130. [Google Scholar] [CrossRef] [PubMed]
- Agbu, P.; Carthew, R.W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- van den Berg, A.; Mols, J.; Han, J. RISC-target interaction: Cleavage and translational suppression. Biochim. Biophys. Acta 2008, 1779, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Yaylak, B.; Akgül, B. Experimental MicroRNA Detection Methods. Methods Mol. Biol. 2022, 2257, 33–55. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandao, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in plasma exosome is stable under different storage conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- Fu, J.; Song, W.; Hao, Z.; Fan, M.; Li, Y. Research trends and hotspots of exosomes in respiratory diseases. Medicine 2023, 102, e35381. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Struman, I.; Louis, E.; Louis, R.; Malaise, M.; Njock, M.S. Exosomal miRNAs in Lung Diseases: From Biologic Function to Therapeutic Targets. J. Clin. Med. 2019, 8, 1345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, Z.; Fan, J.; Zhang, S.; Yang, W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct. Target. Ther. 2019, 4, 47. [Google Scholar] [CrossRef]
- Gon, Y.; Shimizu, T.; Mizumura, K.; Maruoka, S.; Hikichi, M. Molecular techniques for respiratory diseases: MicroRNA and extracellular vesicles. Respirology 2020, 25, 149–160. [Google Scholar] [CrossRef]
- Soccio, P.; Moriondo, G.; Lacedonia, D.; Tondo, P.; Quarato, C.M.I.; Foschino Barbaro, M.P.; Scioscia, G. EVs-miRNA: The New Molecular Markers for Chronic Respiratory Diseases. Life 2022, 12, 1544. [Google Scholar] [CrossRef]
- Fujita, Y.; Araya, J.; Ito, S.; Kobayashi, K.; Kosaka, N.; Yoshioka, Y.; Kadota, T.; Hara, H.; Kuwano, K.; Ochiya, T. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J. Extracell. Vesicles 2015, 4, 28388. [Google Scholar] [CrossRef]
- Xu, H.; Ling, M.; Xue, J.; Dai, X.; Sun, Q.; Chen, C.; Liu, Y.; Zhou, L.; Liu, J.; Luo, F.; et al. Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium-fibroblast cross-talk in COPD induced by cigarette smoking. Theranostics 2018, 8, 5419–5433. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wu, Y.; Zhao, J.; Li, W.; Lu, L.; Ma, H.; Cheng, C.; Sun, J.; Xiang, Q.; Bian, T.; et al. The aberrant cross-talk of epithelium-macrophages via METTL3-regulated extracellular vesicle miR-93 in smoking-induced emphysema. Cell Biol. Toxicol. 2022, 38, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Serban, K.A.; Rezania, S.; Petrusca, D.N.; Poirier, C.; Cao, D.; Justice, M.J.; Patel, M.; Tsvetkova, I.; Kamocki, K.; Mikosz, A.; et al. Structural and functional characterization of endothelial microparticles released by cigarette smoke. Sci. Rep. 2016, 6, 31596. [Google Scholar] [CrossRef] [PubMed]
- Zhuansun, Y.; Du, Y.; Huang, F.; Lin, L.; Chen, R.; Jiang, S.; Li, J. MSCs exosomal miR-1470 promotes the differentiation of CD4(+)CD25(+)FOXP3(+) Tregs in asthmatic patients by inducing the expression of P27KIP1. Int. Immunopharmacol. 2019, 77, 105981. [Google Scholar] [CrossRef]
- Li, C.; Deng, C.; Zhou, T.; Hu, J.; Dai, B.; Yi, F.; Tian, N.; Jiang, L.; Dong, X.; Zhu, Q.; et al. MicroRNA-370 carried by M2 macrophage-derived exosomes alleviates asthma progression through inhibiting the FGF1/MAPK/STAT1 axis. Int. J. Biol. Sci. 2021, 17, 1795–1807. [Google Scholar] [CrossRef]
- Fang, H.; Liu, Y.; He, Y.; Jiang, Y.; Wei, Y.; Liu, H.; Gong, Y.; An, G. Extracellular vesicle-delivered miR-505-5p, as a diagnostic biomarker of early lung adenocarcinoma, inhibits cell apoptosis by targeting TP53AIP1. Int. J. Oncol. 2019, 54, 1821–1832. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, F.; Wang, B.; Li, H.; Xu, Y.; Liu, X.; Shi, L.; Lu, X.; Xu, W.; Lu, L.; et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016, 370, 125–135. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Hung, J.Y.; Chang, W.A.; Lin, Y.S.; Pan, Y.C.; Tsai, P.H.; Wu, C.Y.; Kuo, P.L. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 40. [Google Scholar] [CrossRef]
- He, S.; Li, Z.; Yu, Y.; Zeng, Q.; Cheng, Y.; Ji, W.; Xia, W.; Lu, S. Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Exp. Cell Res. 2019, 379, 203–213. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, F.; Wang, Z.; Tang, L.; Zou, B.; Xu, P.; Yu, T. Hypoxic bone marrow mesenchymal cell-extracellular vesicles containing miR-328-3p promote lung cancer progression via the NF2-mediated Hippo axis. J. Cell Mol. Med. 2021, 25, 96–109. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Jiang, X.M.; Guo, J.; Fu, Z.; Zhou, Z.; Yang, P.; Guo, H.; Guo, X.; Liang, G.; et al. Decreased inhibition of exosomal miRNAs on SARS-CoV-2 replication underlies poor outcomes in elderly people and diabetic patients. Signal Transduct. Target. Ther. 2021, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.L.; Liu, H.J.; Chen, D.Y.; Tang, K.T.; Chen, Y.M.; Liu, P.Y. SARS-CoV-2 primed platelets-derived microRNAs enhance NETs formation by extracellular vesicle transmission and TLR7/8 activation. Cell Commun. Signal 2023, 21, 304. [Google Scholar] [CrossRef]
- Kesimer, M.; Scull, M.; Brighton, B.; DeMaria, G.; Burns, K.; O’Neal, W.; Pickles, R.J.; Sheehan, J.K. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: A possible role in innate defense. FASEB J. 2009, 23, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Radicioni, G.; Abdelwahab, S.; Dang, H.; Carpenter, J.; Chua, M.; Mieczkowski, P.A.; Sheridan, J.T.; Randell, S.H.; Kesimer, M. Intercellular Communication between Airway Epithelial Cells Is Mediated by Exosome-Like Vesicles. Am. J. Respir. Cell Mol. Biol. 2019, 60, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef]
- Bourdonnay, E.; Zaslona, Z.; Penke, L.R.; Speth, J.M.; Schneider, D.J.; Przybranowski, S.; Swanson, J.A.; Mancuso, P.; Freeman, C.M.; Curtis, J.L.; et al. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J. Exp. Med. 2015, 212, 729–742. [Google Scholar] [CrossRef]
- Njock, M.S.; Cheng, H.S.; Dang, L.T.; Nazari-Jahantigh, M.; Lau, A.C.; Boudreau, E.; Roufaiel, M.; Cybulsky, M.I.; Schober, A.; Fish, J.E. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood 2015, 125, 3202–3212. [Google Scholar] [CrossRef]
- Dhar, R.; Mukherjee, S.; Mukerjee, N.; Mukherjee, D.; Devi, A.; Ashraf, G.M.; Alserihi, R.F.; Tayeb, H.H.; Hashem, A.M.; Alexiou, A.; et al. Interrelation between extracellular vesicles miRNAs with chronic lung diseases. J. Cell Physiol. 2022, 237, 4021–4036. [Google Scholar] [CrossRef]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Petrache, I. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Investig. 2012, 122, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Agusti, A.; Hogg, J.C. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019, 381, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef]
- Araya, J.; Kuwano, K. Cellular senescence-an aging hallmark in chronic obstructive pulmonary disease pathogenesis. Respir. Investig. 2022, 60, 33–44. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxid 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef]
- Carpi, S.; Polini, B.; Nieri, D.; Dubbini, N.; Celi, A.; Nieri, P.; Neri, T. Expression Analysis of Muscle-Specific miRNAs in Plasma-Derived Extracellular Vesicles from Patients with Chronic Obstructive Pulmonary Disease. Diagn 2020, 10, 502. [Google Scholar] [CrossRef]
- O’Farrell, H.E.; Bowman, R.V.; Fong, K.M.; Yang, I.A. Plasma Extracellular Vesicle miRNA Profiles Distinguish Chronic Obstructive Pulmonary Disease Exacerbations and Disease Severity. Int. J. Chron. Obs. Pulmon Dis. 2022, 17, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, B.; Qiao, J.; Bai, L.; Li, Z.; Sun, W.; Liu, Q.; Yang, S.; Cui, L. Serum exosomal microRNA-1258 may as a novel biomarker for the diagnosis of acute exacerbations of chronic obstructive pulmonary disease. Sci. Rep. 2023, 13, 18332. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, H.E.; Bowman, R.V.; Fong, K.M.; Yang, I.A. Plasma Extracellular Vesicle miRNAs Can Identify Lung Cancer, Current Smoking Status, and Stable COPD. Int. J. Mol. Sci. 2021, 22, 5803. [Google Scholar] [CrossRef] [PubMed]
- Burke, H.; Cellura, D.; Freeman, A.; Hicks, A.; Ostridge, K.; Watson, A.; Williams, N.P.; Spalluto, C.M.; Staples, K.J.; Wilkinson, T.M.A. Pulmonary EV miRNA profiles identify disease and distinct inflammatory endotypes in COPD. Front. Med. 2022, 9, 1039702. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Jenkins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022, 10, 497–511. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Rabe, K.F. From COPD to chronic systemic inflammatory syndrome? Lancet 2007, 370, 797–799. [Google Scholar] [CrossRef]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef]
- Huang, K.; Yang, T.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet 2019, 394, 407–418. [Google Scholar] [CrossRef]
- Porsbjerg, C.; Melen, E.; Lehtimaki, L.; Shaw, D. Asthma. Lancet 2023, 401, 858–873. [Google Scholar] [CrossRef]
- King-Biggs, M.B. Asthma. Ann. Intern. Med. 2019, 171, ITC49–ITC64. [Google Scholar] [CrossRef]
- Harker, J.A.; Lloyd, C.M. T helper 2 cells in asthma. J. Exp. Med. 2023, 220, e20221094. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Gosens, R.; Gross, N. The mode of action of anticholinergics in asthma. Eur. Respir. J. 2018, 52, 1701247. [Google Scholar] [CrossRef] [PubMed]
- Meurs, H.; Gosens, R.; Zaagsma, J. Airway hyperresponsiveness in asthma: Lessons from in vitro model systems and animal models. Eur. Respir. J. 2008, 32, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 2022, 59, 2102730. [Google Scholar] [CrossRef]
- Ray, A.; Camiolo, M.; Fitzpatrick, A.; Gauthier, M.; Wenzel, S.E. Are We Meeting the Promise of Endotypes and Precision Medicine in Asthma? Physiol. Rev. 2020, 100, 983–1017. [Google Scholar] [CrossRef]
- Holt, P.G.; Sly, P.D. Viral infections and atopy in asthma pathogenesis: New rationales for asthma prevention and treatment. Nat. Med. 2012, 18, 726–735. [Google Scholar] [CrossRef]
- Gutierrez, M.J.; Gomez, J.L.; Perez, G.F.; Pancham, K.; Val, S.; Pillai, D.K.; Giri, M.; Ferrante, S.; Freishtat, R.; Rose, M.C.; et al. Airway Secretory microRNAome Changes during Rhinovirus Infection in Early Childhood. PLoS ONE 2016, 11, e0162244. [Google Scholar] [CrossRef]
- Gon, Y.; Maruoka, S.; Inoue, T.; Kuroda, K.; Yamagishi, K.; Kozu, Y.; Shikano, S.; Soda, K.; Lötvall, J.; Hashimoto, S. Selective release of miRNAs via extracellular vesicles is associated with house-dust mite aller-gen-induced airway inflammation. Clin. Exp. Allergy 2017, 47, 1586–1598. [Google Scholar] [CrossRef]
- Zheng, R.; Du, M.; Tian, M.; Zhu, Z.; Wei, C.; Chu, H.; Gan, C.; Liang, J.; Xue, R.; Gao, F.; et al. Fine Particulate Matter Induces Childhood Asthma Attacks via Extracellular Vesicle-Packaged Let-7i-5p-Mediated Modulation of the MAPK Signaling Pathway. Adv. Sci. 2022, 9, e2102460. [Google Scholar] [CrossRef]
- Pua, H.H.; Happ, H.C.; Gray, C.J.; Mar, D.J.; Chiou, N.T.; Hesse, L.E.; Ansel, K.M. Increased Hematopoietic Extracellular RNAs and Vesicles in the Lung during Allergic Airway Responses. Cell Rep. 2019, 26, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Yadav, A.K.; Chakraborty, S.; Kabra, S.K.; Lodha, R.; Kumar, M.; Kulshreshtha, A.; Sethi, T.; Pandey, R.; Malik, G.; et al. Exosome-enclosed microRNAs in exhaled breath hold potential for biomarker discovery in patients with pulmonary diseases. J. Allergy Clin. Immunol. 2013, 132, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Levanen, B.; Bhakta, N.R.; Torregrosa Paredes, P.; Barbeau, R.; Hiltbrunner, S.; Pollack, J.L.; Skold, C.M.; Svartengren, M.; Grunewald, J.; Gabrielsson, S.; et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J. Allergy Clin. Immunol. 2013, 131, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, R.P.; Wang, Y.; Howrylak, J.; Chinchilli, V.M.; Craig, T.J.; August, A.; Ishmael, F.T. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J. Allergy Clin. Immunol. 2016, 137, 1423–1432. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.P.; Geng, X.R.; Zhao, M.; Ma, S.B.; Yang, Y.H.; Deng, Z.H.; Luo, L.M.; Pan, X.Q. Expression Level of MiRNA-126 in Serum Exosomes of Allergic Asthma Patients and Lung Tissues of Asthmatic Mice. Curr. Drug Metab. 2019, 20, 799–803. [Google Scholar] [CrossRef]
- Zhao, M.; Juanjuan, L.; Weijia, F.; Jing, X.; Qiuhua, H.; Hua, Z.; Fuhe, L.; Hao, P. Expression Levels of MicroRNA-125b in Serum Exosomes of Patients with Asthma of Different Severity and its Diagnostic Significance. Curr. Drug Metab. 2019, 20, 781–784. [Google Scholar] [CrossRef]
- Rostami Hir, S.; Alizadeh, Z.; Mazinani, M.; Mahlooji Rad, M.; Fazlollahi, M.R.; Kazemnejad, A.; Zavaran Hosseini, A.; Moin, M. Exosomal MicroRNAs as Biomarkers in Allergic Asthma. Iran. J. Allergy Asthma Immunol. 2021, 20, 160–168. [Google Scholar] [CrossRef]
- Soccio, P.; Moriondo, G.; Lacedonia, D.; Tondo, P.; Pescatore, D.; Quarato, C.M.I.; Carone, M.; Foschino Barbaro, M.P.; Scioscia, G. MiRNA and Exosomal miRNA as New Biomarkers Useful to Phenotyping Severe Asthma. Biomolecules 2023, 13, 1542. [Google Scholar] [CrossRef]
- Sim, S.; Lee, D.H.; Kim, K.S.; Park, H.J.; Kim, Y.K.; Choi, Y.; Park, H.S. Micrococcus luteus-derived extracellular vesicles attenuate neutrophilic asthma by regulating miRNAs in airway epithelial cells. Exp. Mol. Med. 2023, 55, 196–204. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Luo, X.; Gao, X.; Gu, W.; Ma, Y.; Xu, L.; Yu, M.; Liu, X.; Liu, J.; et al. A non-invasive strategy for suppressing asthmatic airway inflammation and remodeling: Inhalation of nebulized hypoxic hUCMSC-derived extracellular vesicles. Front. Immunol. 2023, 14, 1150971. [Google Scholar] [CrossRef]
- Tu, W.; Hu, X.; Wan, R.; Xiao, X.; Shen, Y.; Srikaram, P.; Avvaru, S.N.; Yang, F.; Pi, F.; Zhou, Y.; et al. Effective delivery of miR-511-3p with mannose-decorated exosomes with RNA nanoparticles confers protection against asthma. J. Control Release 2024, 365, 602–616. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Hanna, N. Advances in systemic therapy for non-small cell lung cancer. BMJ 2021, 375, n2363. [Google Scholar] [CrossRef] [PubMed]
- Megyesfalvi, Z.; Gay, C.M.; Popper, H.; Pirker, R.; Ostoros, G.; Heeke, S.; Lang, C.; Hoetzenecker, K.; Schwendenwein, A.; Boettiger, K.; et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J. Clin. 2023, 73, 620–652. [Google Scholar] [CrossRef]
- The National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.B.; Hou, L.K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.M.; Sun, F.; Lu, H.M.; Deng, J.; et al. Liquid biopsy in lung cancer: Significance in diagnostics, prediction, and treatment monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2017, 8, 13048–13058. [Google Scholar] [CrossRef]
- Grimolizzi, F.; Monaco, F.; Leoni, F.; Bracci, M.; Staffolani, S.; Bersaglieri, C.; Gaetani, S.; Valentino, M.; Amati, M.; Rubini, C.; et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci. Rep. 2017, 7, 15277. [Google Scholar] [CrossRef]
- Jin, X.; Chen, Y.; Chen, H.; Fei, S.; Chen, D.; Cai, X.; Liu, L.; Lin, B.; Su, H.; Zhao, L.; et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non-Small Cell Lung Cancer Using Next-Generation Sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.M.; Zou, Y.Q.; Lin, J.; Huang, B.; Liu, J.; Li, J.; Zhang, J.; Yang, W.M.; Min, Q.H.; et al. Identification of differential expressed PE exosomal miRNA in lung adenocarcinoma, tuberculosis, and other benign lesions. Medicine 2017, 96, e8361. [Google Scholar] [CrossRef]
- Poroyko, V.; Mirzapoiazova, T.; Nam, A.; Mambetsariev, I.; Mambetsariev, B.; Wu, X.; Husain, A.; Vokes, E.E.; Wheeler, D.L.; Salgia, R. Exosomal miRNAs species in the blood of small cell and non-small cell lung cancer patients. Oncotarget 2018, 9, 19793–19806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Yin, Y.; Li, S. Detection of circulating exosomal miR-17-5p serves as a novel non-invasive diagnostic marker for non-small cell lung cancer patients. Pathol. Res. Pr. 2019, 215, 152466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Qin, H.; Man Cheung, F.K.; Su, J.; Zhang, D.D.; Liu, S.Y.; Li, X.F.; Qin, J.; Lin, J.T.; Jiang, B.Y.; et al. Plasma extracellular vesicle microRNAs for pulmonary ground-glass nodules. J. Extracell. Vesicles 2019, 8, 1663666. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Xie, H.; Wei, T.; Zhu, D.; Zhang, C.; Yang, Y. Diagnostic potential of extracellular vesicle-associated microRNA-10b and tumor markers for lung adenocarcinoma. Oncol. Lett. 2021, 22, 614. [Google Scholar] [CrossRef]
- Zheng, D.; Zhu, Y.; Zhang, J.; Zhang, W.; Wang, H.; Chen, H.; Wu, C.; Ni, J.; Xu, X.; Nian, B.; et al. Identification and evaluation of circulating small extracellular vesicle microRNAs as diagnostic biomarkers for patients with indeterminate pulmonary nodules. J. Nanobiotechnol. 2022, 20, 172. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Shimada, Y.; Yoshioka, Y.; Kudo, Y.; Mimae, T.; Miyata, Y.; Adachi, H.; Ito, H.; Okada, M.; Ohira, T.; Matsubayashi, J.; et al. Extracellular vesicle-associated microRNA signatures related to lymphovascular invasion in early-stage lung adenocarcinoma. Sci. Rep. 2023, 13, 4823. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.T.; Huang, Y.Y.; Li, J.H.; Qin, S.H.; Xu, Y.; An, T.X.; Liu, C.C.; Wang, Q.; Zheng, L. Alterations in exosomal miRNA profile upon epithelial-mesenchymal transition in human lung cancer cell lines. BMC Genom. 2018, 19, 802. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xu, Y.; Zhao, J.; Chen, M.; Wang, M. Hippo pathway in non-small cell lung cancer: Mechanisms, potential targets, and biomarkers. Cancer Gene Ther. 2024, 31, 652–666. [Google Scholar] [CrossRef]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Han, B.; Molins, L.; He, Y.; Vinolas, N.; Sanchez-Lorente, D.; Boada, M.; Guirao, A.; Diaz, T.; Martinez, D.; Ramirez, J.; et al. Characterization of the MicroRNA Cargo of Extracellular Vesicles Isolated from a Pulmonary Tumor-Draining Vein Identifies miR-203a-3p as a Relapse Biomarker for Resected Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 7138. [Google Scholar] [CrossRef] [PubMed]
- Cartei, G.; Sacco, C.; Sibau, A.; Pella, N.; Iop, A.; Tabaro, G. Cisplatin and gemcitabine in non-small-cell lung cancer. Ann. Oncol. 1999, 10, S57–S62. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, R.; Zhu, Y.; Hao, R. Exosome-derived microRNA-433 inhibits tumorigenesis through incremental infiltration of CD4 and CD8 cells in non-small cell lung cancer. Oncol. Lett. 2021, 22, 607. [Google Scholar] [CrossRef]
- Wei, F.; Ma, C.; Zhou, T.; Dong, X.; Luo, Q.; Geng, L.; Ding, L.; Zhang, Y.; Zhang, L.; Li, N.; et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol. Cancer 2017, 16, 132. [Google Scholar] [CrossRef]
- Shukuya, T.; Ghai, V.; Amann, J.M.; Okimoto, T.; Shilo, K.; Kim, T.K.; Wang, K.; Carbone, D.P. Circulating MicroRNAs and Extracellular Vesicle-Containing MicroRNAs as Response Biomarkers of Anti-programmed Cell Death Protein 1 or Programmed Death-Ligand 1 Therapy in NSCLC. J. Thorac. Oncol. 2020, 15, 1773–1781. [Google Scholar] [CrossRef]
- Inoue, A. Progress in individualized treatment for EGFR-mutated advanced non-small cell lung cancer. Proc. Jpn. Acad. Ser. B 2020, 96, 266–272. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, C.Y.; Tseng, J.T.; Hung, C.H.; Wu, S.Y.; Huang, Y.T.; Chang, W.Y.; Su, P.L.; Su, W.C. Extracellular Vesicle miR-200c Enhances Gefitinib Sensitivity in Heterogeneous EGFR-Mutant NSCLC. Biomedicines 2021, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Hu, C. Exosomal transfer of miR-214 mediates gefitinib resistance in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018, 507, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.P.; Barton, M.B. Evidence-based estimates of the demand for radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2015, 27, 70–76. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, R.; Guo, X.; Zhao, P.; Zhang, R.; Zhao, Z.; Zhou, H. Exosome miR-101-3p derived from bone marrow mesenchymal stem cells promotes radiotherapy sensitivity in non-small cell lung cancer by regulating DNA damage repair and autophagy levels through EZH2. Pathol. Res. Pr. 2024, 256, 155271. [Google Scholar] [CrossRef]
- Tang, Y.; Cui, Y.; Li, Z.; Jiao, Z.; Zhang, Y.; He, Y.; Chen, G.; Zhou, Q.; Wang, W.; Zhou, X.; et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 7. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Chen, C.; Ming, P.; Huang, Q.; Li, C.; Cao, D.; Xu, X.; Ge, W. The extracellular vesicles secreted by lung cancer cells in radiation therapy promote endothelial cell angiogenesis by transferring miR-23a. PeerJ 2017, 5, e3627. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. Jama 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Marks, K.M.; Gulick, R.M. COVID-19. Ann. Intern. Med. 2023, 176, ITC145–ITC160. [Google Scholar] [CrossRef] [PubMed]
- Meidert, A.S.; Hermann, S.; Brandes, F.; Kirchner, B.; Buschmann, D.; Billaud, J.N.; Klein, M.; Lindemann, A.; Aue, E.; Schelling, G.; et al. Extracellular Vesicle Associated miRNAs Regulate Signaling Pathways Involved in COVID-19 Pneumonia and the Progression to Severe Acute Respiratory Corona Virus-2 Syndrome. Front. Immunol. 2021, 12, 784028. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Bulut, Ö.; GÜrsel, İ. Mesenchymal stem cell derived extracellular vesicles: Promising immunomodulators against autoimmune, autoinflammatory disorders and SARS-CoV-2 infection. Turk. J. Biol. 2020, 44, 273–282. [Google Scholar] [CrossRef]
- Dos Santos, C.C.; Lopes-Pacheco, M.; English, K.; Rolandsson Enes, S.; Krasnodembskaya, A.; Rocco, P.R.M. The MSC-EV-microRNAome: A Perspective on Therapeutic Mechanisms of Action in Sepsis and ARDS. Cells 2024, 13, 122. [Google Scholar] [CrossRef]
- Schultz, I.C.; Bertoni, A.P.S.; Wink, M.R. Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying miRNA as a Potential Multi Target Therapy to COVID-19: An In Silico Analysis. Stem Cell Rev. Rep. 2021, 17, 341–356. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168. [Google Scholar] [CrossRef]
- Cetin, Z. Targeting the PANoptosome with miRNA Loaded Mesenchymal Stem Cell Derived Extracellular Vesicles; a New Path to Fight Against the COVID-19? Stem Cell Rev. Rep. 2021, 17, 1074–1077. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Yi, Q.; Xu, Z.; Thakur, A.; Zhang, K.; Liang, Q.; Liu, Y.; Yan, Y. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol. Res. 2023, 190, 106733. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef] [PubMed]
- Kalarikkal, S.P.; Sundaram, G.M. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021, 414, 115425. [Google Scholar] [CrossRef] [PubMed]
- Ao, D.; He, X.; Liu, J.; Xu, L. Strategies for the development and approval of COVID-19 vaccines and therapeutics in the post-pandemic period. Signal Transduct. Target. Ther. 2023, 8, 466. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Barriere, J.; Frank, F.; Besancon, L.; Samuel, A.; Saada, V.; Billy, E.; Al-Ahmad, A.; Florens, N.; Seitz-Polski, B.; Robert, J. Letter to Editor “Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs”: Important concerns on the validity of this article. Food Chem. Toxicol. 2023, 178, 113897. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Lee, J.; Woodruff, M.C.; Kim, E.H.; Nam, J.H. Knife’s edge: Balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 2023, 55, 1305–1313. [Google Scholar] [CrossRef]
- Miyashita, Y.; Yoshida, T.; Takagi, Y.; Tsukamoto, H.; Takashima, K.; Kouwaki, T.; Makino, K.; Fukushima, S.; Nakamura, K.; Oshiumi, H. Circulating extracellular vesicle microRNAs associated with adverse reactions, proinflammatory cytokine, and antibody production after COVID-19 vaccination. NPJ Vaccines 2022, 7, 16. [Google Scholar] [CrossRef]
- Morissette, M.; Godbout, K.; Cote, A.; Boulet, L.P. Asthma COPD overlap: Insights into cellular and molecular mechanisms. Mol. Asp. Med. 2022, 85, 101021. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, M.; Zhao, Y.; Kabir, M.A.; Peng, X. Exosomal microRNA/miRNA Dysregulation in Respiratory Diseases: From Mycoplasma-Induced Respiratory Disease to COVID-19 and Beyond. Cells 2023, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Li, N.; Feng, Z.; Zheng, Y.; Zhu, C.; Zhang, F. Exosomal microRNAs as novel diagnostic biomarkers in breast cancer: A systematic evaluation and meta-analysis. Asian J. Surg. 2023, 46, 4727–4736. [Google Scholar] [CrossRef]
- Manea, I.; Iacob, R.; Iacob, S.; Cerban, R.; Dima, S.; Oniscu, G.; Popescu, I.; Gheorghe, L. Liquid biopsy for early detection of hepatocellular carcinoma. Front. Med. 2023, 10, 1218705. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Sachkova, A.; Panciani, P.P.; Rohde, V.; Fontanella, M.M.; Schatlo, B. Liquid Biopsy in Low-Grade Glioma: A Systematic Review and a Proposal for a Clinical Utility Score. Cell Mol. Neurobiol. 2023, 43, 3833–3845. [Google Scholar] [CrossRef]
- Crocetto, F.; Falcone, A.; Mirto, B.F.; Sicignano, E.; Pagano, G.; Dinacci, F.; Varriale, D.; Machiella, F.; Giampaglia, G.; Calogero, A.; et al. Unlocking Precision Medicine: Liquid Biopsy Advancements in Renal Cancer Detection and Monitoring. Int. J. Mol. Sci. 2024, 25, 3867. [Google Scholar] [CrossRef]
- Li, Y.; Sui, S.; Goel, A. Extracellular vesicles associated microRNAs: Their biology and clinical significance as biomarkers in gastrointestinal cancers. Semin. Cancer Biol. 2024, 99, 5–23. [Google Scholar] [CrossRef]
- Liu, J.; Zhijin, Z.; Zhang, W.; Niraj, M.; Yang, F.; Changcheng, G.; Shen, L.; Xu, T.; Liu, S.; Junfeng, Z.; et al. Urinary exosomes: Potential diagnostic markers and application in bladder cancer. Heliyon 2024, 10, e32621. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular vesicles in cancer nanomedicine. Semin. Cancer Biol. 2021, 69, 212–225. [Google Scholar] [CrossRef]
| Vesicle | Size(nm) | Biogenesis | Possible Function |
|---|---|---|---|
| Exosome | 30–150 | endocytosis→cargo sorting→MVB maturation and secretion | Cellular interactions and signaling |
| Microvesicle | 100–1000 | plasma membrane budding and shedding | Cellular interactions and signaling |
| Apoptotic body | 50–5000 | chromatin and nucleus condense in cell apoptosis | Clearance of apoptotic debris |
| Isolation Technique | Principle | Advantage | Disadvantage |
|---|---|---|---|
| Ultracentrifugation | High-speed centrifugal force to sediment EVs | Suitable for large volumes and various samples; yields relatively pure EVs | Time-consuming; complex procedures; impact on EV activity |
| Size-based separation | Physical barriers (chromatography, filtration, etc.) | Efficient; easy to perform; little impact on EV activity | Relatively low purity; limited sample volume |
| Immunoaffinity Capture | Antibody-based binding of specific EV markers | High specificity; high purity | Expensive; limited to specific EV subtypes |
| Polymer Precipitation | Use of polymers to alter the solubility of EVs | Efficient; easy to perform | May co-precipitate non-EV components; relatively low purity |
| Microfluidics | Manipulation of fluids at microscale through microchannel of microfluidic chip | High precision; integrates multiple steps; efficient; little impact on EV activity | Requires specialized equipment; limited throughput |
| Kit Method | Commercially available kits for EV isolation | User-friendly | Often expensive; variable purity and yield |
| Method | Principle | Advantage | Disadvantage |
|---|---|---|---|
| Northern blot | Separation of specific-sized RNA fragments by electrophoresis, followed by membrane transfer and probe hybridization detection | Reliable results without amplification; can detect both miRNAs and their precursors | Low sensitivity; time-consuming; requires large number of RNA samples; RNA is prone to degradation |
| Microarray | Hybridization of labeled miRNAs with DNA probes fixed on a solid surface | High throughput; can simultaneously detect multiple samples | High cost; complex operation; sensitivity and specificity are limited; requires standardized probe sets |
| qRT-PCR | Reverse transcription of miRNA into cDNA; followed by real-time monitoring with fluorescently labeled probes and primers | Gold standard for miRNA detection; highly sensitive and accurate; suitable for quantitative analysis | Complex primer design; requires consideration of RNA integrity; cDNA synthesis; primer design; and other parameters |
| NGS | High-throughput sequencing analysis of multiple small RNA fragments | No need for whole genome sequence; extensive coverage of miRNAs at different expression levels | Complex data processing; relatively high cost |
| miRNAs | Samples | Target | Expression | Function | Pathogenesis | References | |
|---|---|---|---|---|---|---|---|
| COPD | miR210 | HBEC | ATG7 | upregulated | Control autophagy process | Promote airway remodeling | [31] |
| miR21 | HBEC | VHL | upregulated | Promote myoblast differentiation | Promote airway remodeling | [32] | |
| miR93 | Lung tissue | DUSP2 | upregulated | Activate JNK pathway in macrophages | Contribute to emphysema | [33] | |
| Let-7d, miR-191, miR-126, miR-125a | plasma | upregulated | Compromise the phagocytic function of macrophages | Escalate endothelial damage and an inflammatory response | [34] | ||
| Asthma | miR-1470 | MSC | P27KIP1 | upregulated | Induce CD4+CD25+FOXP3+ Tregs differentiation | Regulate inflammatory response | [35] |
| miR-370 | Lung tissue | FGF1 | downregulated | Suppress the FGF1/MAPK/STAT1 axis | Reduce inflammation, inhibit airway remodeling | [36] | |
| Lung Cancer | miR-505-5p | Plasma and tumor tissue | TP53AIP1 | upregulated | Inhibits cell apoptosis and promotes cell proliferation | Contribute to an abnormal cell cycle | [37] |
| miR-21 | HBEC | VEGF | upregulated | Form new blood vessels | Contribute to angiogenesis | [38] | |
| miR-23a | lung cancer cells | HIF-1α | upregulated | Form new blood vessels | Contribute to angiogenesis | [39] | |
| miR-193a-3p, miR-210-3p, miR-5100 | Plasma and Lung tissue | STAT3 | upregulated | Activate STAT3 signaling-induced EMT | Promote metastasis of cancer cells | [40] | |
| miR-499a-5p | Lung tumor cells | mTORC1 | upregulated | Activate the mTOR signaling pathway | Promote cell proliferation, migration, and EMT | [41] | |
| miR-328-3p | BMSC | NF2 | upregulated | Inhibit the Hippo signaling pathway | Promote proliferation, invasion, migration, and EMT | [42] | |
| COVID19 | miR-7-5p, miR-24-3p, miR-145-5p, miR-223-3p | plasma | S protein | downregulated | Inhibit S protein expression and SARS-CoV-2 replication | Act as endogenous antiviral molecules | [43] |
| miR-21, let-7b | plasma | TLR7/8 | upregulated | Promote the production of ROS and the formation of NETs | Form immune thrombi | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Xiong, X. Extracellular Vesicle microRNA: A Promising Biomarker and Therapeutic Target for Respiratory Diseases. Int. J. Mol. Sci. 2024, 25, 9147. https://doi.org/10.3390/ijms25179147
Lv J, Xiong X. Extracellular Vesicle microRNA: A Promising Biomarker and Therapeutic Target for Respiratory Diseases. International Journal of Molecular Sciences. 2024; 25(17):9147. https://doi.org/10.3390/ijms25179147
Chicago/Turabian StyleLv, Jiaxi, and Xianzhi Xiong. 2024. "Extracellular Vesicle microRNA: A Promising Biomarker and Therapeutic Target for Respiratory Diseases" International Journal of Molecular Sciences 25, no. 17: 9147. https://doi.org/10.3390/ijms25179147
APA StyleLv, J., & Xiong, X. (2024). Extracellular Vesicle microRNA: A Promising Biomarker and Therapeutic Target for Respiratory Diseases. International Journal of Molecular Sciences, 25(17), 9147. https://doi.org/10.3390/ijms25179147





