Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis
Abstract
1. Introduction
2. Approaches for OA Treatment
2.1. Non-Pharmacological Approaches
2.2. Pharmacological Approach
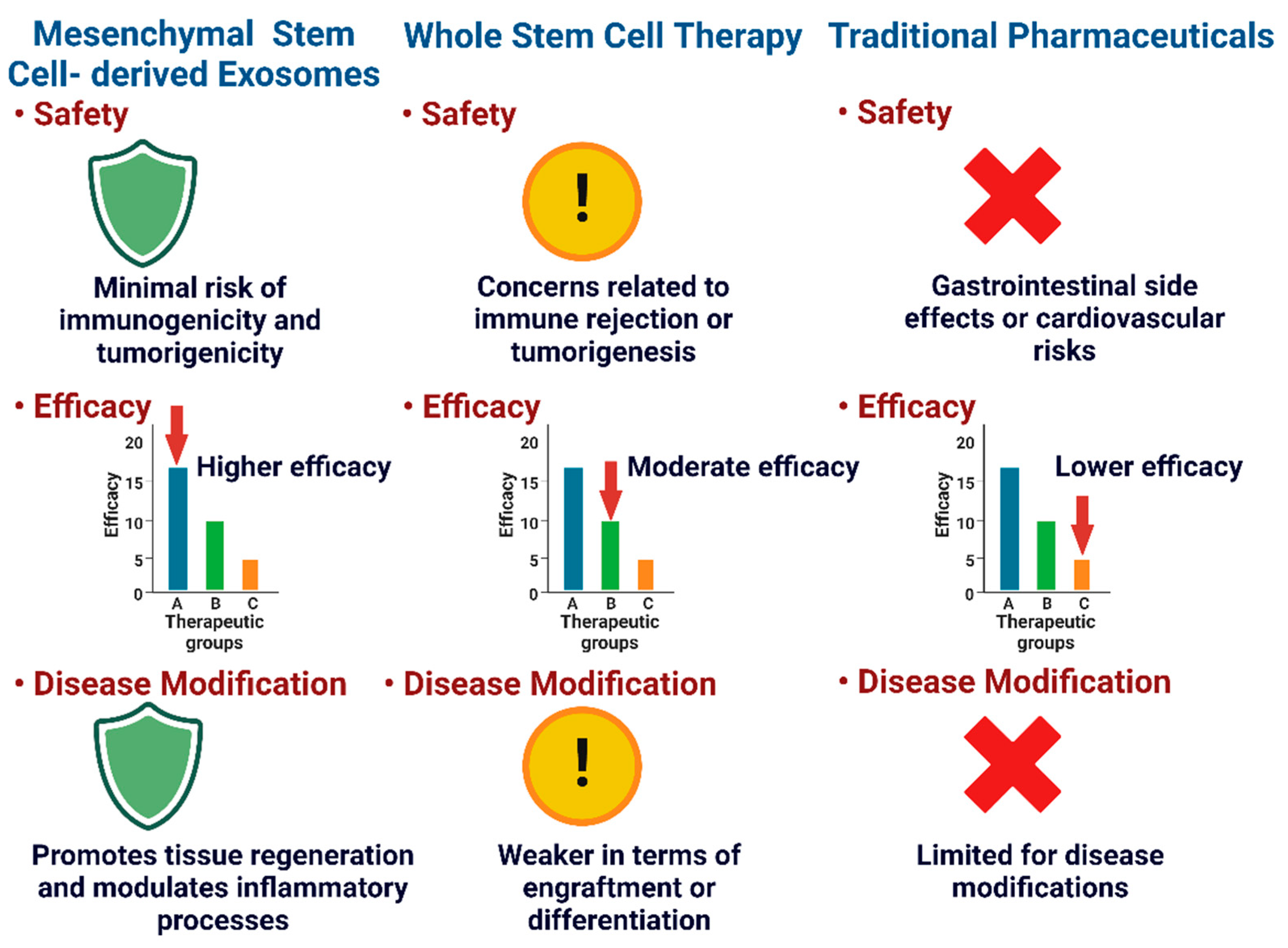
2.3. Regenerative Medicines
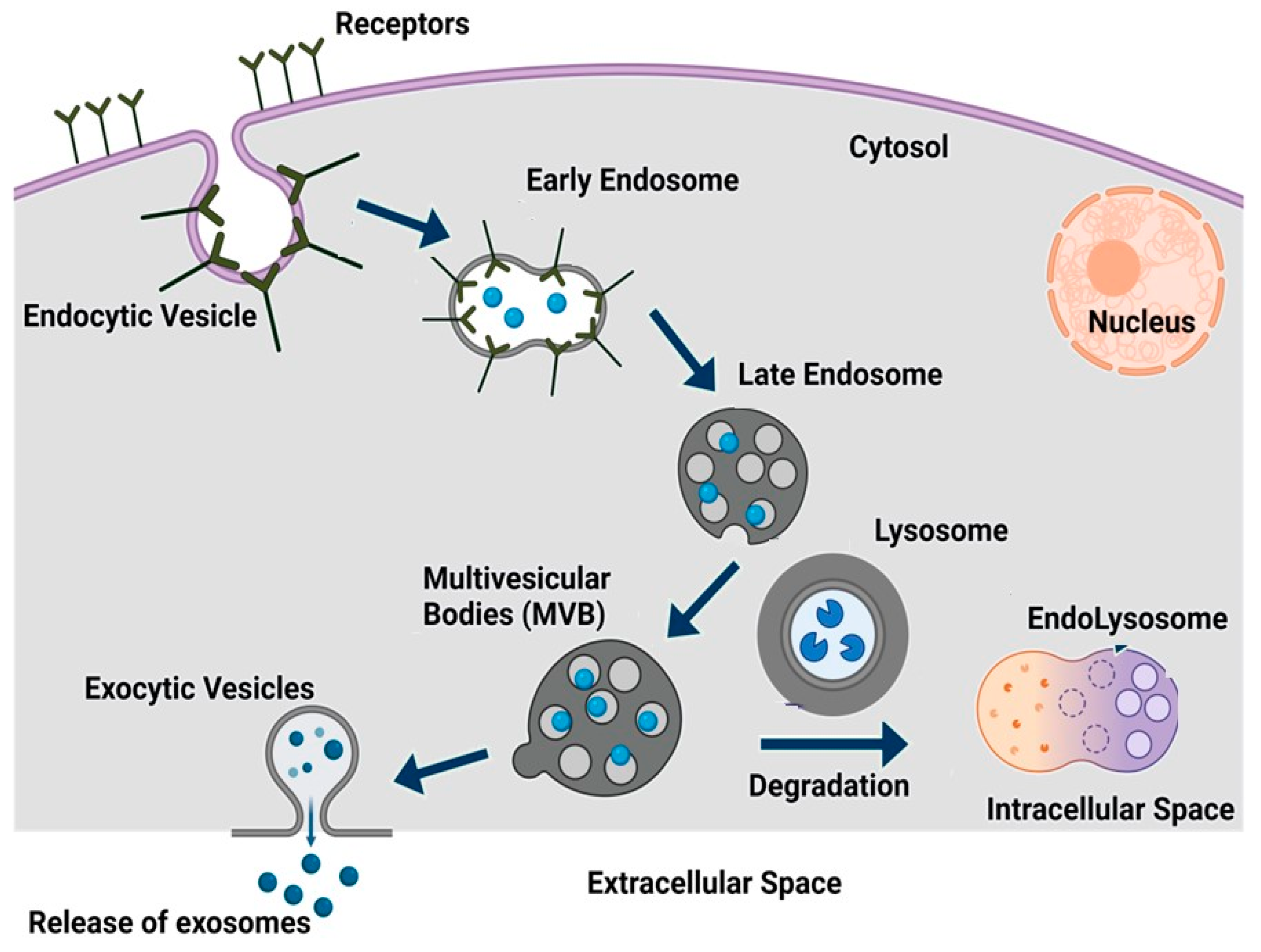
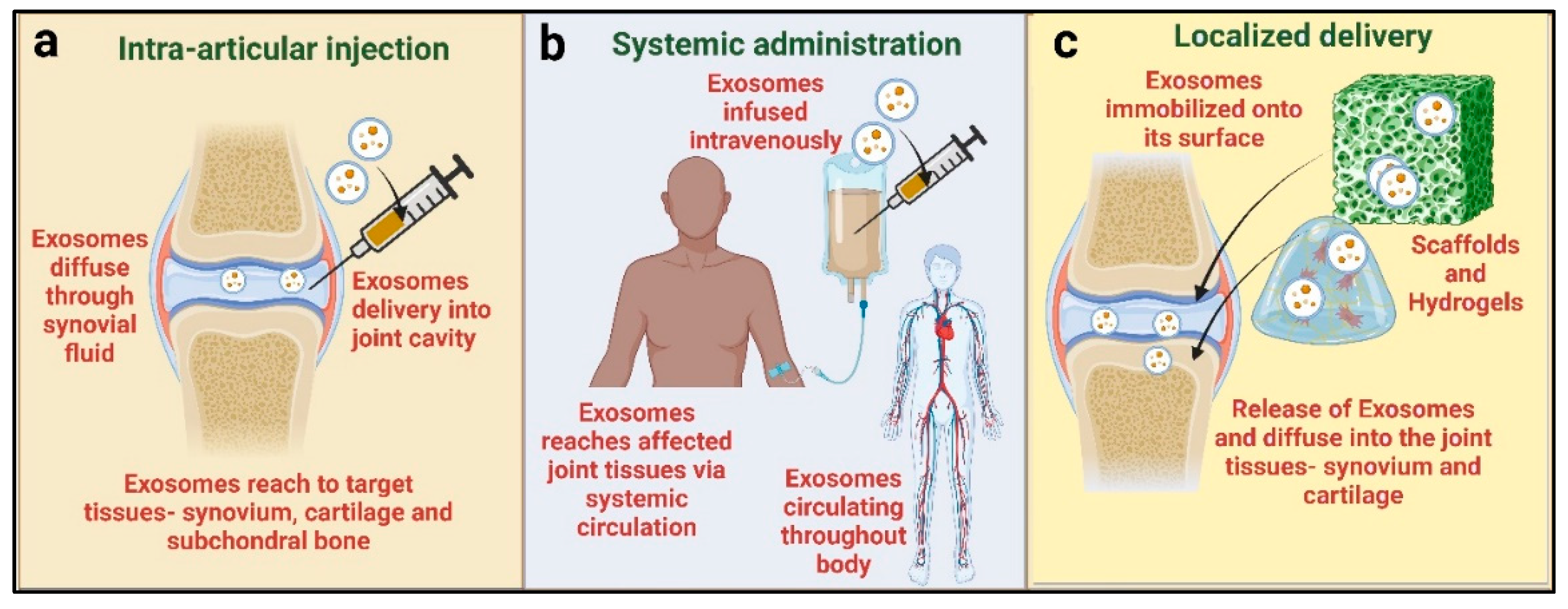
3. Exosomes, Biogenesis, and Routes of Administration
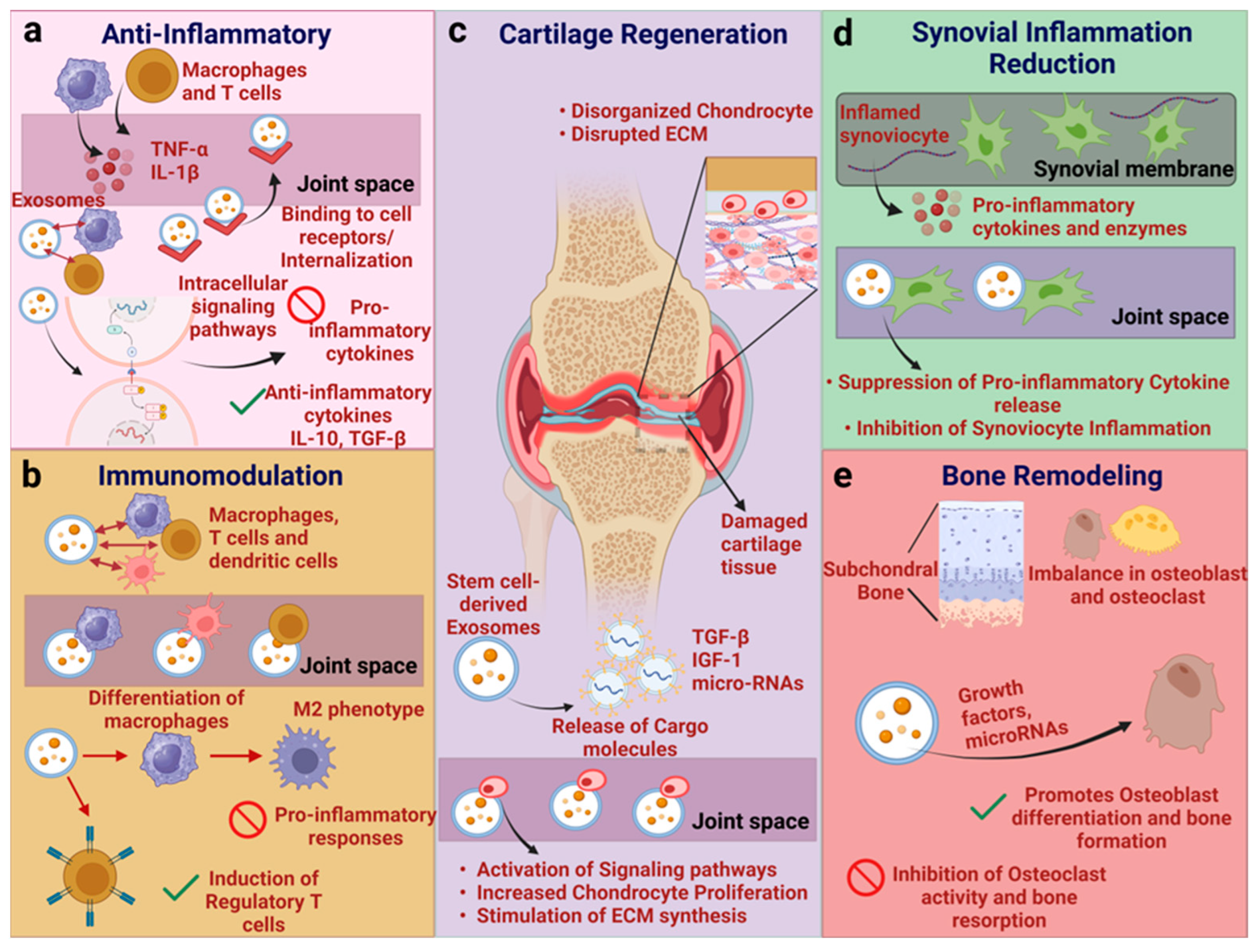
4. Mechanism of Action of Exosomes
4.1. Exosomes Derived from Various Cell Types and Underlying Mechanism
4.1.1. Adipose Mesenchymal Stem Cell (AD-MSC)-Derived Exosomes
4.1.2. Synovial Mesenchymal Stem Cell (SMSC)-Derived Exosomes
4.1.3. Bone Marrow Mesenchymal Stem Cell (BMSC)-Derived Exosomes
4.1.4. Embryonic Mesenchymal Stem Cell (E-MSC)-Derived and Umbilical Cord Mesenchymal Stem Cell (hUCMSC)-Derived Exosomes
4.1.5. Dental Pulp Mesenchymal Stem Cell (DP-MSC)-Derived Exosomes
5. Preclinical Studies: Efficacy and Safety of Mesenchymal Stem Cell-Derived Exosomes in Osteoarthritis Models
| Serial No. | Source of Exosomes | In Vitro/In Vivo Study | Role in Therapeutic Approach | References |
|---|---|---|---|---|
| 1. | hBMSCs | In vitro | Chondrocyte proliferation, the downregulation of MMP-13, and the upregulation of SOX9 | [192] |
| 2. | AD-MSCs | In vitro | Decreased levels of inflammatory cytokines, including IL-6, TNF-α, NO, and PGE2, as well as MMP-13 expression, with increased levels of anti-inflammatory cytokines, including IL-10 and type II collagen expression | [120] |
| 3. | AD-MSCs | In vitro | The downregulation of senescence-associated β-galactosidase and the accumulation of γ-H2AX, with decreased levels of the inflammatory cytokines IL-6 and PGE2 | [139] |
| 4. | SMMSCs and induced pluripotent mesenchymal stem cells (iMSCs) | In vivo (in mice) | Chondrocyte migration and proliferation in both exosomes, but showing a higher therapeutic effect in iMSCs | [42] |
| 5. | MSCs | In vivo (in mice) | Cartilage proliferation and chondrogenic differentiation, and the inhibition of WNT5A expression | [167] |
| 6. | BM-MSCs | In vivo (in mice) | Chondroprotection and the inducement of aggrecan and type II collagen expression, with the inhibition of ADAMTS5, MMP-13, and iNOS | [156] |
| 7. | ESC-MSCs | In vivo (in mice) | Attenuated cartilage damage and increased type II collagen through the decrease in ADAMTS5 expression in the presence of IL-1β | [135] |
| 8. | MSCs | In vivo (in rat) | Increased s-GAG synthesis via IL-1β and inhibited IL-1β-induced MMP-13 and nitric oxide production | [190] |
| 9. | MSCs | In vivo (in rat) | A low dose of MSCs promoted the activation of signaling pathways and chondrogenesis | [193] |
| 10. | BM-MSCs | In vivo (in rat) | The upregulation of COL2A1 and downregulation of MMP-13 in cartilage, and also the upregulation of CGRP and iNOS in dorsal root ganglion | [117] |
| 11. | UC-MSCs | In vivo (in rat) | The downregulation of MMP-13, disintegrin, ADAMTS5, IL-1β, and TNF-α, and the upregulation of type II collagen, ki67, and the IL-1 receptor antagonist | [194] |
| 12. | AD-MSCs | In vivo (in rat) | The downregulation of MMP-13 and collagen X, and the upregulation of collagen type II | [195] |
| 13. | BM-MSCs | In vivo (in rat) | The promotion of KDM6A expression and the activation of SOX9 | [196] |
| 14. | BM-MSCs | In vivo (in rat) | The inhibition of M1 and the promotion of M2, with decreased expression levels of IL-6, TNF-α, and IL-1β, and increased expression levels of IL-10 | [110] |
| 15. | hPMSCs | In vivo (in rat) | Cartilage repair and regeneration | [197] |
6. Clinical Trials: Evaluating the Therapeutic Efficacy of MSC-Exos in OA Patients
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Halsey, G. Osteoarthritis Projected to Affect Nearly 1 Billion by 2050, Obesity a Major Contributing Factor. Patient Care; Gale OneFile, Health and Medicine. 2023. Available online: https://link.gale.com/apps/doc/A777405104/HRCA?u=anon~669bec05&sid=googleScholar&xid=ecf8825a (accessed on 20 July 2024).
- Abhishek, A.; Doherty, M. Diagnosis and clinical presentation of osteoarthritis. Rheum. Dis. Clin. 2013, 39, 45–66. [Google Scholar] [CrossRef]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M. Epidemiology of risk factors for osteoarthritis: Systemic factors. Curr. Opin. Rheumatol. 2001, 13, 447–451. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Brock, T.M.; Heil, K.; Holmes, R.; Weusten, A. A Review on the Management of Hip and Knee Osteoarthritis. Int. J. Chronic. Dis. 2013, 2013, 845015. [Google Scholar] [CrossRef]
- Ng, H.Y.; Lee, K.-X.A.; Shen, Y.-F. Articular cartilage: Structure, composition, injuries and repair. JSM Bone Jt. Dis. 2017, 1, 1010. [Google Scholar]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef]
- Mohammed, A.; Alshamarri, T.; Adeyeye, T.; Lazariu, V.; McNutt, L.A.; Carpenter, D.O. A comparison of risk factors for osteo- and rheumatoid arthritis using NHANES data. Prev. Med. Rep. 2020, 20, 101242. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Ozeki, N.; Koga, H.; Sekiya, I. Degenerative Meniscus in Knee Osteoarthritis: From Pathology to Treatment. Life 2022, 12, 603. [Google Scholar] [CrossRef]
- Sengprasert, P.; Kamenkit, O.; Tanavalee, A.; Reantragoon, R. The immunological facets of chondrocytes in osteoarthritis: A narrative review. J. Rheumatol. 2024, 51, 13–24. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Pettenuzzo, S.; Arduino, A.; Belluzzi, E.; Pozzuoli, A.; Fontanella, C.G.; Ruggieri, P.; Salomoni, V.; Majorana, C.; Berardo, A. Biomechanics of Chondrocytes and Chondrons in Healthy Conditions and Osteoarthritis: A Review of the Mechanical Characterisations at the Microscale. Biomedicines 2023, 11, 1942. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Nohe, A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Liu-Bryan, R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr. Rheumatol. Rep. 2013, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, S.; Li, W. Harnessing Stem Cell-Derived Extracellular Vesicles for the Regeneration of Degenerative Bone Conditions. Int. J. Nanomed. 2023, 18, 5561–5578. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [PubMed]
- Keerthi, N.; Chimutengwende-Gordon, M.; Sanghani, A.; Khan, W. The potential of stem cell therapy for osteoarthritis and rheumatoid arthritis. Curr. Stem Cell Res. Ther. 2013, 8, 444–450. [Google Scholar] [CrossRef]
- Pittenger, M.; Mosca, J.; McIntosh, K. Human mesenchymal stem cells: Progenitor cells for cartilage, bone, fat and stroma. In Lymphoid Organogenesis, Proceedings of the Workshop Held at the Basel Institute for Immunology, Berlin, Germany, 5–6 November 1999; Springer: Berlin/Heidelberg, Germany, 2000; pp. 3–11. [Google Scholar]
- Kong, L.; Zheng, L.Z.; Qin, L.; Ho, K.K.W. Role of mesenchymal stem cells in osteoarthritis treatment. J. Orthop. Translat. 2017, 9, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [PubMed]
- Murphy, S.V.; Atala, A. Amniotic fluid and placental membranes: Unexpected sources of highly multipotent cells. Semin. Reprod. Med. 2013, 31, 62–68. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.-J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory effects of MSCs in bone healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef]
- Herrero, C.; Pérez-Simón, J. Immunomodulatory effect of mesenchymal stem cells. Braz. J. Med. Biol. Res. 2010, 43, 425–430. [Google Scholar] [CrossRef]
- Franquesa, M.; Hoogduijn, M.J.; Bestard, O.; Grinyó, J.M. Immunomodulatory effect of mesenchymal stem cells on B cells. Front. Immunol. 2012, 3, 212. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Chen, M.; Su, W.; Lin, X.; Guo, Z.; Wang, J.; Zhang, Q.; Brand, D.; Ryffel, B.; Huang, J.; Liu, Z. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013, 65, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Rustad, K.C.; Gurtner, G.C. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv. Wound Care 2012, 1, 147–152. [Google Scholar] [CrossRef]
- Diekman, B.O.; Guilak, F. Stem cell-based therapies for osteoarthritis: Challenges and opportunities. Curr. Opin. Rheumatol. 2013, 25, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M. Risk of tumorigenicity in mesenchymal stromal cell–based therapies—Bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.; Lai, R.; Lim, S.; Hui, J.; Toh, W. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Hart, D.A. Osteoarthritis as an umbrella term for different subsets of humans undergoing joint degeneration: The need to address the differences to develop effective conservative treatments and prevention strategies. Int. J. Mol. Sci. 2022, 23, 15365. [Google Scholar] [CrossRef]
- Hasan, M.; Shuckett, R. Clinical features and pathogenetic mechanisms of osteoarthritis of the hip and knee. BC Med. J. 2010, 52, 393–398. [Google Scholar]
- Qi-ping, D.; Min-lei, Q.; Ping, S.; Dong, H. Clinical observation on treatment of 60 cases of osteoarthritis of knee joint by electroacupuncture. J. Acupunct. Tuina Sci. 2003, 1, 38–40. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological Perspective of Osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Moseng, T.; Vlieland, T.P.V.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.; Vaysbrot, E.; Arden, N.; Bennell, K.; Bierma-Zeinstra, S.; Kraus, V.; Lohmander, L.S.; Abbott, J.; Bhandari, M. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Messier, S.P. Diet and exercise for obese adults with knee osteoarthritis. Clin. Geriatr. Med. 2010, 26, 461–477. [Google Scholar] [CrossRef]
- Messier, S.P.; Mihalko, S.L.; Legault, C.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Beavers, D.P.; Hunter, D.J.; Lyles, M.F.; Eckstein, F. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA 2013, 310, 1263–1273. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Dickson, J.; Grant, R.L. Care and management of osteoarthritis in adults: Summary of NICE guidance. BMJ 2008, 336, 502–503. [Google Scholar] [CrossRef]
- Wandel, S.; Jüni, P.; Tendal, B.; Nüesch, E.; Villiger, P.M.; Welton, N.J.; Reichenbach, S.; Trelle, S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: Network meta-analysis. BMJ 2010, 341, c4675. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Campbell, J.; Welch, V.; Gee, T.L.; Bourne, R.; Wells, G.A. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 2006, CD005321. [Google Scholar] [CrossRef] [PubMed]
- Cheng, O.T.; Souzdalnitski, D.; Vrooman, B.; Cheng, J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012, 13, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Jevsevar, D.S. Treatment of osteoarthritis of the knee: Evidence-based guideline. JAAOS-J. Am. Acad. Orthop. Surg. 2013, 21, 571–576. [Google Scholar]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Magni, A.; Agostoni, P.; Bonezzi, C.; Massazza, G.; Menè, P.; Savarino, V.; Fornasari, D. Management of osteoarthritis: Expert opinion on NSAIDs. Pain Ther. 2021, 10, 783–808. [Google Scholar] [CrossRef]
- Madry, H. Surgical therapy in osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.; Catalani, B.; Sharp, C.; Cooper, L. Life-threatening perioperative anesthetic complications: Major issues surrounding perioperative morbidity and mortality. Trauma Surg. Acute Care Open 2017, 2, e000113. [Google Scholar] [CrossRef]
- Silva, R.R.d.; Santos, A.A.M.; Carvalho Júnior, J.d.S.; Matos, M.A. Quality of life after total knee arthroplasty: Systematic review. Rev. Bras. Ortop. 2014, 49, 520–527. [Google Scholar] [CrossRef]
- Grässel, S.; Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Jeong, S.; Kim, H.; Kang, D.; Lee, J.; Kang, S.-B.; Kim, J.-H. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp. Mol. Med. 2021, 53, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, P.; Chen, T.; Ding, C. Latest insights in disease-modifying osteoarthritis drugs development. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720x231169839. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C. The current role of disease-modifying osteoarthritis drugs. Arch. Bone Jt. Surg. 2023, 11, 11–22. [Google Scholar]
- Vrouwe, J.; Burggraaf, J.; Kloppenburg, M.; Stuurman, F. Challenges and opportunities of pharmacological interventions for osteoarthritis: A review of current clinical trials and developments. Osteoarthr. Cartil. Open 2021, 3, 100212. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, J.; Lee, Y.; Park, K.; Perry, T.A.; Arden, N.K.; Mobasheri, A.; Choi, H. The current state of the osteoarthritis drug development pipeline: A comprehensive narrative review of the present challenges and future opportunities. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221085952. [Google Scholar] [CrossRef]
- Im, G.I. Current status of regenerative medicine in osteoarthritis. Bone Jt. Res. 2021, 10, 134–136. [Google Scholar] [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal stem cell therapy for osteoarthritis: The critical role of the cell secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Riazifar, M.; Pone, E.J.; Lötvall, J.; Zhao, W. Stem cell extracellular vesicles: Extended messages of regeneration. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 125–154. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- D’Arrigo, D.; Roffi, A.; Cucchiarini, M.; Moretti, M.; Candrian, C.; Filardo, G. Secretome and extracellular vesicles as new biological therapies for knee osteoarthritis: A systematic review. J. Clin. Med. 2019, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Compañ, A.; Guillén, M.I. Extracellular vesicles from mesenchymal stem cells as novel treatments for musculoskeletal diseases. Cells 2019, 9, 98. [Google Scholar] [CrossRef]
- Lässer, C.; Jang, S.C.; Lötvall, J. Subpopulations of extracellular vesicles and their therapeutic potential. Mol. Asp. Med. 2018, 60, 1–14. [Google Scholar] [CrossRef]
- Luo, D.; Zhu, H.; Li, S.; Wang, Z.; Xiao, J. Mesenchymal stem cell-derived exosomes as a promising cell-free therapy for knee osteoarthritis. Front. Bioeng. Biotechnol. 2024, 12, 1309946. [Google Scholar] [CrossRef]
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410. [Google Scholar] [CrossRef]
- Tan, S.S.H.; Tjio, C.K.E.; Wong, J.R.Y.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal stem cell exosomes for cartilage regeneration: A systematic review of preclinical in vivo studies. Tissue Eng. Part B Rev. 2021, 27, 1–13. [Google Scholar] [CrossRef]
- Johnny East, D.; Trace Alexander, D.; Dordevic, M. IRB approved pilot safety study of extracellular vesicle isolate product evaluating the treatment of osteoarthritis in combat-related injuries. Stem Cell Res. 2020, 1, 1–10. [Google Scholar]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles. 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Guo, S.-C.; Zhang, C.-Q. Modularized Extracellular Vesicles: The Dawn of Prospective Personalized and Precision Medicine. Adv. Sci. 2018, 5, 1700449. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Zhang, S.; Witwer, K.W.; Mattson, M.P. Extracellular vesicle–depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles 2015, 4, 26373. [Google Scholar] [CrossRef]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Park, H.-K.; Auh, Q.-S.; Nah, H.; Lee, J.S.; Moon, H.-J.; Heo, D.N.; Kim, I.S.; Kwon, I.K. Emerging potential of exosomes in regenerative medicine for temporomandibular joint osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1541. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2009, 38, 215–224. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Guillén, M.I. Cellular and Molecular Targets of Extracellular Vesicles from Mesenchymal Stem/Stromal Cells in Rheumatoid Arthritis. Stem Cells Transl. Med. 2022, 11, 1177–1185. [Google Scholar] [CrossRef]
- Lai, R.C.; Tan, S.S.; Yeo, R.W.Y.; Choo, A.B.H.; Reiner, A.T.; Su, Y.; Shen, Y.; Fu, Z.; Alexander, L.; Sze, S.K. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 2016, 5, 29828. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Muthu, S.; Gulati, A.; Jeyaraman, N.; Prajwal, G.S.; Jain, R. Mesenchymal Stem Cell–Derived Exosomes: A Potential Therapeutic Avenue in Knee Osteoarthritis. Cartilage 2021, 13, 1572S–1585S. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Bajracharya, S.D.; Yuen, P.S.; Zhou, H.; Star, R.A.; Illei, G.G.; Alevizos, I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guo, K.; Jiang, J.; Lin, S. Mesenchymal stem cell-derived exosomes as delivery vehicles for non-coding RNAs in lung diseases. Biomed. Pharmacother. 2024, 170, 116008. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.-J.; Liu, D.; Pan, L.-Y.; Wang, W.-Y.; Ding, Y.-L.; Zhang, Y.-Y.; Ye, R.-X.; Zhou, Y.; An, S.-B.; Xiao, W.-F. Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment. Front. Cell Dev. Biol. 2022, 10, 949690. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-F.; Li, W.-J.; Hu, K.-S.; Gao, J.; Zhai, W.-L.; Yang, J.-H.; Zhang, S.-J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, E.K.; Yim, J.; Lee, M.H.; Lee, E.; Lee, Y.S.; Seo, W. Exosomes: Nomenclature, Isolation, and Biological Roles in Liver Diseases. Biomol. Ther. 2023, 31, 253–263. [Google Scholar] [CrossRef]
- Barros, F.M.; Carneiro, F.; Machado, J.C.; Melo, S.A. Exosomes and Immune Response in Cancer: Friends or Foes? Front. Immunol. 2018, 9, 730. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int. J. Pharm. 2019, 566, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Sun, F.; Liu, J.; Ding, T.; She, J.; Mao, F.; Xu, W.; Qian, H.; Yan, Y. Emerging Role of Mesenchymal Stem Cell-derived Exosomes in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2019, 14, 482–494. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Lötvall, J.; Lässer, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23111. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Yakubovich, E.I.; Polischouk, A.G.; Evtushenko, V.I. Principles and Problems of Exosome Isolation from Biological Fluids. Biochem. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 115–126. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, Y.; Luo, C.; Cui, W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging 2020, 12, 25138. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Yang, X.; Zhao, H. Harnessing exosomes as cutting-edge drug delivery systems for revolutionary osteoarthritis therapy. Biomed. Pharmacother. 2023, 165, 115135. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yang, W.; Cui, W.; Li, C.; Ma, C.; Ji, X.; Hong, J.; Qu, Z.; Chen, J.; Liu, A.; et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon–bone healing. J. Nanobiotechnol. 2023, 21, 14. [Google Scholar] [CrossRef]
- Sang, X.; Zhao, X.; Yan, L.; Jin, X.; Wang, X.; Wang, J.; Yin, Z.; Zhang, Y.; Meng, Z. Thermosensitive hydrogel loaded with primary chondrocyte-derived exosomes promotes cartilage repair by regulating macrophage polarization in osteoarthritis. Tissue Eng. Regen. Med. 2022, 19, 629–642. [Google Scholar] [CrossRef]
- Tao, S.-C.; Huang, J.-Y.; Gao, Y.; Li, Z.-X.; Wei, Z.-Y.; Dawes, H.; Guo, S.-C. Small extracellular vesicles in combination with sleep-related circRNA3503: A targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021, 6, 4455–4469. [Google Scholar] [CrossRef] [PubMed]
- Tevlin, R.; Huber, J.; DiIorio, S.; Longaker, M.; Wan, D. Musculoskeletal tissue engineering: Adipose derived stromal cell implementation for the treatment of osteoarthritis. Biomaterials 2022, 286, 121544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-X.; Liu, P.; Ding, W.; Meng, Q.-B.; Su, D.-H.; Zhang, Q.-C.; Lian, R.-X.; Yu, B.-Q.; Zhao, M.-D.; Dong, J. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.Y.; Peng, W.M.; Yuan, B.; Bi, Q.; Xu, Y.J. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020, 21, 1881–1889. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell. Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef]
- Guillén, M.I.; Tofiño-Vian, M.; Silvestre, A.; Castejón, M.A.; Alcaraz, M.J. Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J. Orthop. Transl. 2021, 30, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Whitty, C.; Pernstich, C.; Marris, C.; McCaskie, A.; Jones, M.; Henson, F. Sustained delivery of the bone morphogenetic proteins BMP-2 and BMP-7 for cartilage repair and regeneration in osteoarthritis. Osteoarthr. Cartil. Open 2022, 4, 100240. [Google Scholar] [CrossRef]
- Vonk, L.A.; van Dooremalen, S.F.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 2018, 8, 906. [Google Scholar] [CrossRef]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wang, Q.; Hao, L.; Wei, G.; Wang, T.; Lu, W.W.; Xiao, G.; Tong, L.; Zhao, X.; Chen, D. miR-204 ameliorates osteoarthritis pain by inhibiting SP1-LRP1 signaling and blocking neuro-cartilage interaction. Bioact. Mater. 2023, 26, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, S.; Sun, Z.; Zhai, C.; Xia, J.; Wen, C.; Zhang, Y.; Zhang, Y. Enhancement of the therapeutic efficacy of mesenchymal stem cell-derived exosomes in osteoarthritis. Cell. Mol. Biol. Lett. 2023, 28, 75. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Muthu, S.; Shehabaz, S.; Jeyaraman, N.; Rajendran, R.L.; Hong, C.M.; Nallakumarasamy, A.; Packkyarathinam, R.P.; Sharma, S.; Ranjan, R. Current understanding of MSC-derived exosomes in the management of knee osteoarthritis. Exp. Cell Res. 2022, 418, 113274. [Google Scholar] [CrossRef]
- Maqsood, M.; Kang, M.; Wu, X.; Chen, J.; Teng, L.; Qiu, L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020, 256, 118002. [Google Scholar] [CrossRef]
- Tanimoto, T.; Endo, K.; Sakamaki, Y.; Ozeki, N.; Katano, H.; Mizuno, M.; Koga, H.; Sekiya, I. Human synovial mesenchymal stem cells show time-dependent morphological changes and increased adhesion to degenerated porcine cartilage. Sci. Rep. 2022, 12, 16619. [Google Scholar] [CrossRef]
- Cai, J.; Wu, J.; Wang, J.; Li, Y.; Hu, X.; Luo, S.; Xiang, D. Extracellular vesicles derived from different sources of mesenchymal stem cells: Therapeutic effects and translational potential. Cell Biosci. 2020, 10, 69. [Google Scholar] [CrossRef]
- Lee, S.; Chae, D.-S.; Song, B.-W.; Lim, S.; Kim, S.W.; Kim, I.-K.; Hwang, K.-C. ADSC-based cell therapies for musculoskeletal disorders: A review of recent clinical trials. Int. J. Mol. Sci. 2021, 22, 10586. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Yang, X.; Zhang, D.; Li, B.; Zhang, D.; Li, Q.; Xiong, Y. Analysis of gene expression and methylation datasets identified ADAMTS9, FKBP5, and PFKBF3 as biomarkers for osteoarthritis. J. Cell. Physiol. 2019, 234, 8908–8917. [Google Scholar] [CrossRef]
- Long, L.; Zou, G.; Cheng, Y.; Li, F.; Wu, H.; Shen, Y. MATN3 delivered by exosome from synovial mesenchymal stem cells relieves knee osteoarthritis: Evidence from in vitro and in vivo studies. J. Orthop. Transl. 2023, 41, 20–32. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, Q.; Lin, F.; Wang, J. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered 2021, 12, 11225–11238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, W.; Lv, S.; Sun, Z.; Si, L.; Hu, J.; Yang, Y.; Qiu, D.; Liu, X.; Zhu, S. Human umbilical cord mesenchymal stem cells-derived exosomes exert anti-inflammatory effects on osteoarthritis chondrocytes. Aging 2023, 15, 9544. [Google Scholar] [CrossRef]
- Fu, Y.; Cui, S.; Zhou, Y.; Qiu, L. Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. Int. J. Mol. Sci. 2023, 24, 4926. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Pan, S.; Shi, C.; Wu, G.; Feng, Y.; Qin, C.; Zhang, J.; Yu, Z.; Liang, B.; Gui, J. Sustained released of microRNA-99b-3p abundant exosomes derived from adipose stem cell encapsulated with hydrogel microparticles (HMPs) for long-term osteoarthritis treatment. Res. Square 2023. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez del Caz, M.D.; Castejón, M.A.; Alcaraz, M.J. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxidative Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, J.; Lin, W.; Liu, J. Exosomal miR-93-5p regulated the progression of osteoarthritis by targeting ADAMTS9. Open Med. 2023, 18, 20230668. [Google Scholar] [CrossRef]
- Li, F.; Xu, Z.; Xie, Z.; Sun, X.; Li, C.; Chen, Y.; Xu, J.; Pi, G. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA-376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis 2023, 28, 362–378. [Google Scholar] [CrossRef]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Saad, S.; Dharmapatni, A.; Crotti, T.; Cantley, M.; Algate, K.; Findlay, D.; Atkins, G.; Haynes, D. Semaphorin-3a, neuropilin-1 and plexin-A1 in prosthetic-particle induced bone loss. Acta Biomater. 2016, 30, 311–318. [Google Scholar] [CrossRef]
- Li, X.; Liao, Z.; Deng, Z.; Chen, N.; Zhao, L. Combining bulk and single-cell RNA-sequencing data to reveal gene expression pattern of chondrocytes in the osteoarthritic knee. Bioengineered 2021, 12, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Xie, Y.; Tan, G.; Wang, X.; Huang, P.; Hong, L. Synovial mesenchymal stem cell-derived exosomal miR-485-3p relieves cartilage damage in osteoarthritis by targeting the NRP1-mediated PI3K/Akt pathway: Exosomal miR-485-3p relieves cartilage damage. Heliyon 2024, 10, e24042. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Lou, C.; Jiang, H.; Lin, Z.; Xia, T.; Wang, W.; Lin, C.; Zhang, Z.; Fu, H.; Iqbal, S.; Liu, H.; et al. MiR-146b-5p enriched bioinspired exosomes derived from fucoidan-directed induction mesenchymal stem cells protect chondrocytes in osteoarthritis by targeting TRAF6. J. Nanobiotechnol. 2023, 21, 486. [Google Scholar] [CrossRef]
- Sun, W.; Qu, S.; Ji, M.; Sun, Y.; Hu, B. BMP-7 modified exosomes derived from synovial mesenchymal stem cells attenuate osteoarthritis by M2 polarization of macrophages. Heliyon 2023, 9, e19934. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, K.; Ge, G.; Zhang, D.; Bai, J.; Guo, X.; Zhou, J.; Xu, T.; Xu, M.; Long, X.; et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021, 37, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, M.; Wagener, R. Chapter 23—Matrilins. In Methods in Cell Biology; Mecham, R.P., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 143, pp. 429–446. [Google Scholar]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cao, Y.; Guo, Z.; Wang, L.; Xiang, C. Biological roles and therapeutic potential of circular RNAs in osteoarthritis. Mol. Ther. Nucleic Acids 2021, 24, 856–867. [Google Scholar] [CrossRef]
- Kong, R.; Zhang, J.; Ji, L.; Yu, Y.; Gao, J.; Zhao, D. Synovial mesenchymal stem cell-derived exosomal microRNA-320c facilitates cartilage damage repair by targeting ADAM19-dependent Wnt signalling in osteoarthritis rats. Inflammopharmacology 2023, 31, 915–926. [Google Scholar] [CrossRef]
- Zheng, T.; Li, Y.; Zhang, X.; Xu, J.; Luo, M. Exosomes Derived From miR-212-5p Overexpressed Human Synovial Mesenchymal Stem Cells Suppress Chondrocyte Degeneration and Inflammation by Targeting ELF3. Front. Bioeng. Biotechnol. 2022, 10, 816209. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, B. BMSC-Derived Exosomes Ameliorate Osteoarthritis by Inhibiting Pyroptosis of Cartilage via Delivering miR-326 Targeting HDAC3 and STAT1//NF-κB p65 to Chondrocytes. Mediat. Inflamm. 2021, 2021, 9972805. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Qi, H.; Liu, D.P.; Xiao, D.W.; Tian, D.C.; Su, Y.W.; Jin, S.F. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Z.; Li, Y.; Wang, W.; Wang, J.; Yu, H.; Liu, A.; Miao, J.; Chen, S.; Wu, T.; et al. BMSCs-Derived Exosomes Ameliorate Pain Via Abrogation of Aberrant Nerve Invasion in Subchondral Bone in Lumbar Facet Joint Osteoarthritis. J. Orthop. Res. 2020, 38, 670–679. [Google Scholar] [CrossRef]
- Jin, Z.; Ren, J.; Qi, S. Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 2020, 381, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Salminen-Mankonen, H.; Säämänen, A.M.; Jalkanen, M.; Vuorio, E.; Pirilä, L. Syndecan-1 expression is upregulated in degenerating articular cartilage in a transgenic mouse model for osteoarthritis. Scand. J. Rheumatol. 2005, 34, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, S.; Chang, X. E2F2 stimulates CCR4 expression and activates synovial fibroblast-like cells in rheumatoid arthritis. Cent. Eur. J. Immunol. 2021, 46, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, H.X.; Xu, D.; Xue, X.; Xu, X. The Anti-Inflammatory Effect of miR-140-3p in BMSCs-Exosomes on Osteoarthritis. Acta Chir. Orthop. Traumatol. Cech. 2023, 90, 267–276. [Google Scholar] [CrossRef]
- Shen, X.; Qin, J.; Wei, Z.; Liu, F. Bone marrow mesenchymal stem cell exosome-derived lncRNA TUC339 influences the progression of osteoarthritis by regulating synovial macrophage polarization and chondrocyte apoptosis. Biomed. Pharmacother. 2023, 167, 115488. [Google Scholar] [CrossRef]
- Li, B.; Shen, E.; Wu, Z.; Qi, H.; Wu, C.a.; Liu, D.; Jiang, X. BMSC-Derived Exosomes Attenuate Rat Osteoarthritis by Regulating Macrophage Polarization through PINK1/Parkin Signaling Pathway. Cartilage 2024, 20, 19476035241245805. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021, 384, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Tang, T.; Gao, G.; Wei, Q.; Sun, K.; Huang, W. Bone marrow mesenchymal stem cell-derived exosomes inhibit chondrocyte apoptosis and the expression of MMPs by regulating Drp1-mediated mitophagy. Acta Histochem. 2021, 123, 151796. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Hosseini-Farahabadi, S.; Geetha-Loganathan, P.; Fu, K.; Nimmagadda, S.; Yang, H.J.; Richman, J.M. Dual functions for WNT5A during cartilage development and in disease. Matrix Biol. 2013, 32, 252–264. [Google Scholar] [CrossRef]
- Huang, G.; Chubinskaya, S.; Liao, W.; Loeser, R. Wnt5a induces catabolic signaling and matrix metalloproteinase production in human articular chondrocytes. Osteoarthr. Cartil. 2017, 25, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Man, Z.; Li, W.; Sun, S.; Zhang, W. Silencing of Wnt5a prevents interleukin-1β-induced collagen type II degradation in rat chondrocytes. Exp. Ther. Med. 2016, 12, 3161–3166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, L.; Zou, R.; Wen, C.; Wang, Z.; Lin, F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 2018, 17, 2411–2422. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Zhao, G.-Z.; Xu, X.-Y.; Fang, J.; Chen, J.-M.; Li, J.-W.; Gao, X.-J.; Hao, L.-J.; Chen, Y.-Z. Integrin-β1 regulates chondrocyte proliferation and apoptosis through the upregulation of GIT1 expression. Int. J. Mol. Med. 2015, 35, 1074–1080. [Google Scholar] [CrossRef]
- Zhao, G.Z.; Zhang, L.Q.; Liu, Y.; Fang, J.; Li, H.Z.; Gao, K.H.; Chen, Y.Z. Effects of platelet-derived growth factor on chondrocyte proliferation, migration and apoptosis via regulation of GIT1 expression. Mol. Med. Rep. 2016, 14, 897–903. [Google Scholar] [CrossRef]
- Gu, Y.L.; Rong, X.X.; Wen, L.T.; Zhu, G.X.; Qian, M.Q. miR-195 inhibits the proliferation and migration of chondrocytes by targeting GIT1. Mol. Med. Rep. 2017, 15, 194–200. [Google Scholar] [CrossRef]
- Wen, C.; Lin, L.; Zou, R.; Lin, F.; Liu, Y. Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell Cycle 2022, 21, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Jiang, T.; Chen, Y.; Mao, X. Mesenchymal Stem Cell-Derived Exosomes Modulate Chondrocyte Glutamine Metabolism to Alleviate Osteoarthritis Progression. Mediat. Inflamm. 2021, 2021, 2979124. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Sun, C.; Lai, C.; Zhang, L. Exosomes derived from mesenchymal stem cells rescue cartilage injury in osteoarthritis through Ferroptosis by GOT1/CCR2 expression. Int. Immunopharmacol. 2023, 122, 110566. [Google Scholar] [CrossRef]
- Li, P.; Lv, S.; Jiang, W.; Si, L.; Liao, B.; Zhao, G.; Xu, Z.; Wang, L.; Zhang, J.; Wu, H.; et al. Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann. Transl. Med. 2022, 10, 976. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Cai, Z.; Zhou, Q.; Li, L.; Fu, P. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol. Int. 2021, 45, 2096–2106. [Google Scholar] [CrossRef]
- Kim, M.; Shin, D.I.; Choi, B.H.; Min, B.H. Exosomes from IL-1β-Primed Mesenchymal Stem Cells Inhibited IL-1β- and TNF-α-Mediated Inflammatory Responses in Osteoarthritic SW982 Cells. Tissue Eng. Regen. Med. 2021, 18, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Ham, O.; Lee, C.Y.; Kim, R.; Lee, J.; Oh, S.; Lee, M.Y.; Kim, J.; Hwang, K.-C.; Maeng, L.-S.; Chang, W. Therapeutic potential of differentiated mesenchymal stem cells for treatment of osteoarthritis. Int. J. Mol. Sci. 2015, 16, 14961–14978. [Google Scholar] [CrossRef]
- Lee, W.Y.-W.; Wang, B. Cartilage repair by mesenchymal stem cells: Clinical trial update and perspectives. J. Orthop. Transl. 2017, 9, 76–88. [Google Scholar] [CrossRef]
- Yubo, M.; Yanyan, L.; Li, L.; Tao, S.; Bo, L.; Lin, C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS ONE 2017, 12, e0175449. [Google Scholar] [CrossRef]
- Wyles, C.C.; Houdek, M.T.; Behfar, A.; Sierra, R.J. Mesenchymal stem cell therapy for osteoarthritis: Current perspectives. Stem Cells Cloning Adv. Appl. 2015, 8, 117–124. [Google Scholar]
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng. Part B Rev. 2017, 23, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Jing, H.; He, X.; Zheng, J. Exosomes and regenerative medicine: State of the art and perspectives. Transl. Res. 2018, 196, 1–16. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Wang, R.; Xu, B.; Xu, H. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 2018, 17, 2756–2765. [Google Scholar] [CrossRef]
- Sun, H.; Hu, S.; Zhang, Z.; Lun, J.; Liao, W.; Zhang, Z. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J. Cell. Biochem. 2019, 120, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, W.; Li, J.J.; Chai, S.; Xing, D.; Yu, H.; Zhang, Y.; Yan, W.; Xu, Z.; Zhao, B. A low dose cell therapy system for treating osteoarthritis: In vivo study and in vitro mechanistic investigations. Bioact. Mater. 2022, 7, 478–490. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, E.; Rao, W.; Zhang, Y.Q.; Xiao, C.H.; Li, C.Y.; Han, B.; Wu, D. Intra-articular injection of human umbilical cord mesenchymal stem cells ameliorates monosodium iodoacetate-induced osteoarthritis in rats by inhibiting cartilage degradation and inflammation. Bone Jt. Res. 2021, 10, 226–236. [Google Scholar] [CrossRef]
- Yan, B.; Lv, S.; Tong, P.; Yan, L.; Chen, Z.; Zhou, L.; Yuan, Q.; Guo, L.; Shan, L. Intra-articular injection of adipose-derived stem cells ameliorates pain and cartilage anabolism/catabolism in osteoarthritis: Preclinical and clinical evidences. Front. Pharmacol. 2022, 13, 854025. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Zhang, C.; Kang, J.; Wang, Y.; Liu, J.; Wu, F.; Xu, G. The therapeutic effect of bone marrow–derived mesenchymal stem cells on osteoarthritis is improved by the activation of the KDM6A/SOX9 signaling pathway caused by exposure to hypoxia. J. Cell. Physiol. 2020, 235, 7173–7182. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.J.P.; Kotikalapudi, N.; Venkatesan, V. A novel therapeutic combination of mesenchymal stem cells and stigmasterol to attenuate osteoarthritis in rodent model system—A proof of concept study. Stem Cell Investig. 2021, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.A.; Jones, I.A.; Han, B.; Vangsness, C.T., Jr. Intra-articular mesenchymal stem cell therapy for the human joint: A systematic review. Am. J. Sports Med. 2018, 46, 3550–3563. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Arthurs, J.R.; Heckman, M.G.; Bestic, J.M.; Kazmerchak, S.E.; Diehl, N.N.; Zubair, A.C.; O’Connor, M.I. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage 2019, 10, 432–443. [Google Scholar] [CrossRef]
- Hernigou, P.; Bouthors, C.; Bastard, C.; Flouzat Lachaniette, C.H.; Rouard, H.; Dubory, A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: What better postpone knee arthroplasty at fifteen years? A randomized study. Int. Orthop. 2021, 45, 391–399. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: A double-blinded prospective randomized controlled clinical trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.K.; Forogh, B.; Ahmadbeigi, N.; Kharazi, H.H.; Fallahzadeh, K.; Kashani, L.; Karami, M.; Kheyrollah, Y.; Vasei, M. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: A pilot study. Cytotherapy 2019, 21, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Akgun, I.; Unlu, M.C.; Erdal, O.A.; Ogut, T.; Erturk, M.; Ovali, E.; Kantarci, F.; Caliskan, G.; Akgun, Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: A 2-year randomized study. Arch. Orthop. Trauma Surg. 2015, 135, 251–263. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Therapeutic Application of Mesenchymal Stem Cells Derived Extracellular Vesicles for Immunomodulation. Front. Immunol. 2019, 10, 2663. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Harmati, M.; Tarnai, Z.; Decsi, G.; Kormondi, S.; Szegletes, Z.; Janovak, L.; Dekany, I.; Saydam, O.; Gyukity-Sebestyen, E.; Dobra, G. Stressors alter intercellular communication. J. Oral Pathol. Med. 2017, 46, 259–266. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, M.; Mohammadi, M.R.; Pone, E.J.; Yeri, A.; Lasser, C.; Segaliny, A.I.; McIntyre, L.L.; Shelke, G.V.; Hutchins, E.; Hamamoto, A. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for separation and characterization of extracellular vesicles: Results of a worldwide survey performed by the ISEV rigor and standardization subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Domenis, R.; Zanutel, R.; Caponnetto, F.; Toffoletto, B.; Cifù, A.; Pistis, C.; Di Benedetto, P.; Causero, A.; Pozzi, M.; Bassini, F. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediat. Inflamm. 2017, 2017, 4814987. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xiao, K.; Xiang, S.; Li, Z.; Weng, X. Emerging role of exosomes in the joint diseases. Cell. Physiol. Biochem. 2018, 47, 2008–2017. [Google Scholar] [CrossRef]
- Kolhe, R.; Hunter, M.; Liu, S.; Jadeja, R.N.; Pundkar, C.; Mondal, A.K.; Mendhe, B.; Drewry, M.; Rojiani, M.V.; Liu, Y. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017, 7, 2029. [Google Scholar] [CrossRef]
- Ni, Z.; Kuang, L.; Chen, H.; Xie, Y.; Zhang, B.; Ouyang, J.; Wu, J.; Zhou, S.; Chen, L.; Su, N. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zheng, M.; Yu, M.; Bai, W.; Zuo, L.; Bu, X.; Liu, Y.; Xia, L.; Hu, J.; Liu, L. Transplantation of CRISPRa system engineered IL10-overexpressing bone marrow-derived mesenchymal stem cells for the treatment of myocardial infarction in diabetic mice. J. Biol. Eng. 2019, 13, 49. [Google Scholar] [CrossRef]
- Ji, Y.; Mi, L.; Zhao, M.; He, X.; Hu, Y.; Gao, Y.; Yin, C.; Xu, K. Innovative Diagnosis and Therapeutic Modalities: Engineered Exosomes in Autoimmune Disease. Int. J. Nanomed. 2024, 19, 3943–3956. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Alahdal, M. Exosomes loaded with chondrogenic stimuli agents combined with 3D bioprinting hydrogel in the treatment of osteoarthritis and cartilage degeneration. Biomed. Pharmacother. 2023, 168, 115715. [Google Scholar] [CrossRef] [PubMed]
| Type of MSC-Derived Exosomes | Source of Exosomes | Mechanism of Action | References |
|---|---|---|---|
| Adipose mesenchymal stem cell (AD-MSC)-derived exosomes | Adipose tissue (subcutaneous fat mostly from the abdomen) | Prevent ECM degradation and the reduction in inflammatory mediators | [132] |
| Synovial mesenchymal stem cell (SMSC)-derived exosomes | Synovial fluid | Inhibit chondrocyte apoptosis and ECM degradation | [133] |
| Bone marrow mesenchymal stem cell (BMSC)-derived exosomes | Bone marrow | Support the restoration of cartilage, and inhibit apoptosis and cartilage degradation | [134] |
| Embryonic mesenchymal stem cell (E-MSC)-derived exosomes | Embryo (inner mass of the blastocyst) | Balance cartilage extracellular matrix synthesis and degradation | [135] |
| Umbilical cord mesenchymal stem cell (hUCMSC)-derived exosomes | Wharton’s jelly of the umbilical cord | Promote anti-inflammation, the proliferation of chondrocytes, and immunomodulation | [136] |
| Dental pulp mesenchymal stem cell (DP-MSC)-derived exosomes | Dental pulp of the teeth | Inhibition of cell apoptosis, increase in matrix synthesis, and decrease in catabolic factor expression | [137] |
| Stem Cell-Derived Exosomes | Gender | Age Group | Clinical Phase Trial | Participants Enrolled | Country | Dosage of Exosomes | Site of Injection | Completion Status | Results | Clinical Trial ID Number |
|---|---|---|---|---|---|---|---|---|---|---|
| MSCs | All | 30–70 years | Phase I | 10 | Chile | 3–5 × 1011 cells | Intra-articular | Not completed | Not disclosed | NCT05060107 |
| BM-MSCs, UC-MSCs, and AD-MSCs | All | 40–70 years | Phase III | 475 | United States | 2 × 107 cells | Intra-articular | Completed | No adverse events and no significant change in the magnetic resonance imaging OA score compared to the baseline | NCT03818737 |
| UC-MSCs | All | 30–75 years | Phase I | 24 | Chile | 2 × 106 cells for the low dose, 20 × 106 cells for the medium dose, and 80 × 106 cells for the high dose | Intra-articular | Completed | Decreased inflammation and degenerative response and significant pain improvement; all of the doses were safe and no severe adverse events were reported; improvements were high in the medium- and low-dose treatments | NCT03810521 |
| BM-MSCs | All | 48–66 years | Phase II | 30 | Spain | 40 × 106 cells | Intra-articular | Completed | T2 relaxation measurements showed a decrease in poor cartilage areas and pain relief; feasible and safe | NCT01586312 |
| BM-MSCs | All | 18–76 years | Phase I/II | 12 | Spain | 2 × 107 cells | Intra-articular | Completed | T2 relaxation measurement showed a significant decrease in poor cartilage areas, the improvement of algo-functional indices from 65% to 78% by 1 year, and pain relief; feasible and safe | NCT01183728 |
| BM-MSCs | All | 40–80 years | Phase I/II | 38 | Spain | 100 × 106 cells | Intra-articular | Completed | Clinical improvement at the end of the follow-up; viable therapeutic option | NCT02365142 |
| AD-MSCs | All | 40–75 years | Phase II | 106 | China | Not mentioned | Intra-articular | Completed | Not disclosed | NCT04208646 |
| AD-MSCs | All | 45–65 years | Phase II | 18 | Saudi Arabia | 1 × 108 cells | Intra-articular | Completed | Not disclosed | NCT03308006 |
| AD-MSCs | All | 42–75 years | Phase I | 10 | Jordan | 5 × 107 cells | Intra-articular | Not completed | Not disclosed | NCT02966951 |
| UC-MSCs (Wharton Jelly-derived) | All | 42–75 years | Phase I | 10 | Jordan | 5 × 107 cells | Intra-articular | Not completed | Not disclosed | NCT02963727 |
| AD-MSCs | All | 18–70 years | Phase I | 18 | China | 1 × 107 cells for the low dose, 2 × 107 cells for the medium dose, and 5 × 107 cells for the high dose | Intra-articular | Completed | Reduction in WOMAC and SF-36 scores; multi-compositional MRI is an effective tool for evaluating cartilage repair | NCT02641860 |
| BM-MSCs | All | 18–65 years | Phase I | 6 | Iran | Not mentioned | Intra-articular | Completed | Not disclosed | NCT01436058 |
| BM-MSCs | All | 18–65 years | Phase II | 40 | Iran | Not mentioned | Intra-articular | Completed | Not disclosed | NCT01504464 |
| AD-MSCs | All | 40–70 years | Phase I/II | 18 | China | 1 × 107 cells for the low dose, 2 × 107 cells for the medium dose, 5 × 107 cells for the high dose | Intra-articular | Completed | Safe, alleviates pain, improves cartilage volume and function; 5 × 107 cells exhibited the highest improvement | NCT01809769 |
| BM-MSCs | All | 30–70 years | Phase I/II | 10 | India | Not mentioned | Intra-articular | Not completed | Not disclosed | NCT01152125 |
| UC-MSCs | All | Up to 70 years | Phase II | 60 | China | 1 × 107 cells for the low dose and 2 × 107 cells for the high dose | Intra-articular | Not completed | Not disclosed | NCT03383081 |
| AD-MSCs | All | 50–70 years | Phase I | 4 | Taiwan | 8–10 × 106 cells | Intra-articular | Completed | Not disclosed | NCT02544802 |
| BM-MSCs | All | 40–75 years | Phase I/II | 24 | India | 10 × 106 cells | Intra-articular | Not completed | Not disclosed | NCT01985633 |
| DP-MSCs | All | 40–70 years | Phase I | 60 | China | Not mentioned | Intra-articular | Not completed | Not disclosed | NCT04130100 |
| BM-MSCs | All | 18–70 years | Phase II | 50 | Malaysia | Not mentioned | Intra-articular | Not completed | Not disclosed | NCT01459640 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadhan, A.; Gupta, T.; Hsu, W.-L. Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 9149. https://doi.org/10.3390/ijms25179149
Vadhan A, Gupta T, Hsu W-L. Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis. International Journal of Molecular Sciences. 2024; 25(17):9149. https://doi.org/10.3390/ijms25179149
Chicago/Turabian StyleVadhan, Anupama, Tanvi Gupta, and Wen-Li Hsu. 2024. "Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis" International Journal of Molecular Sciences 25, no. 17: 9149. https://doi.org/10.3390/ijms25179149
APA StyleVadhan, A., Gupta, T., & Hsu, W.-L. (2024). Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis. International Journal of Molecular Sciences, 25(17), 9149. https://doi.org/10.3390/ijms25179149







