Expression of Autophagy Markers LC3B, LAMP2A, and GRP78 in the Human Kidney during Embryonic, Early Fetal, and Postnatal Development and Their Significance in Diabetic Kidney Disease
Abstract
1. Introduction
2. Results
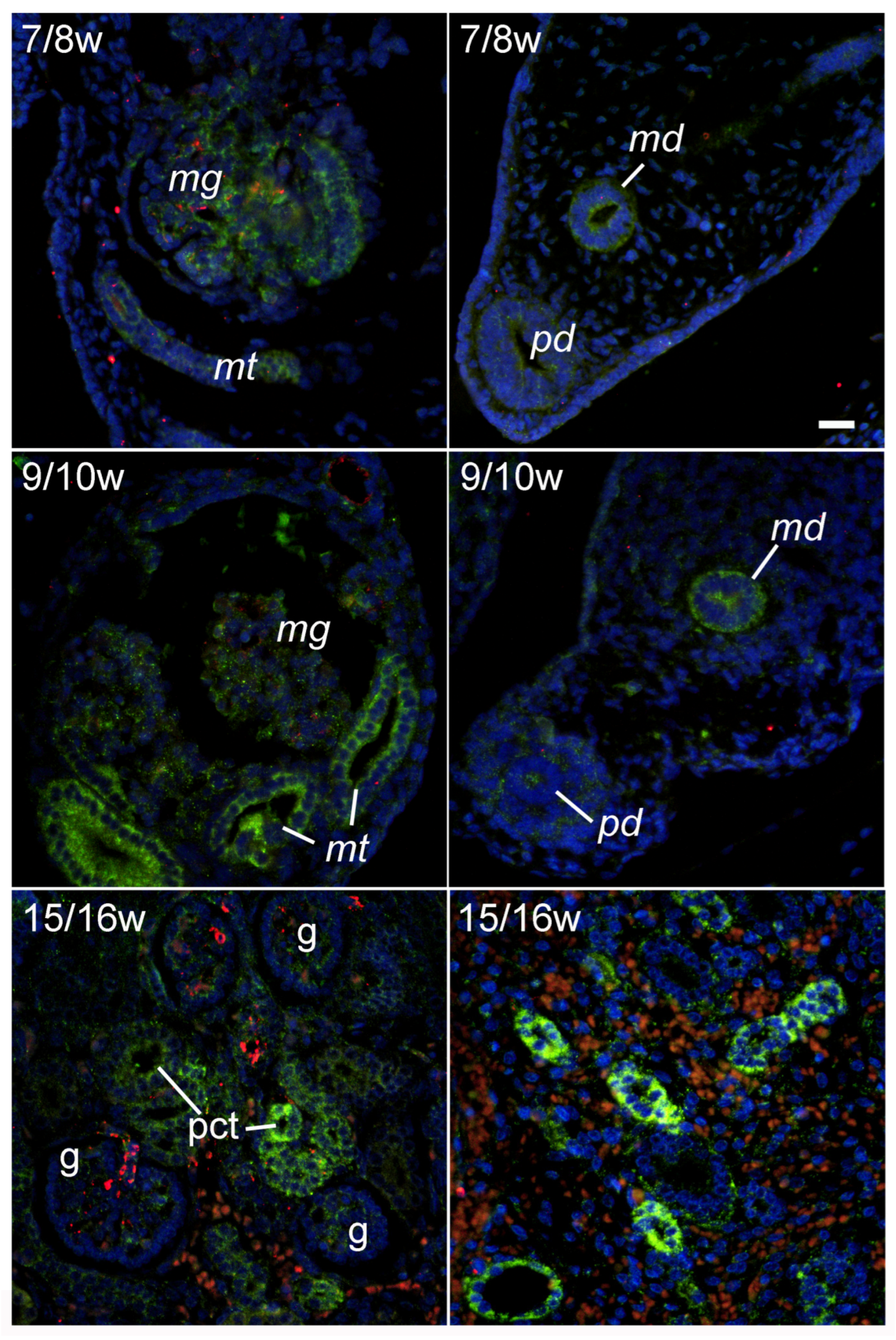
2.1. LC3B Immunoexpression during Early Renal Development
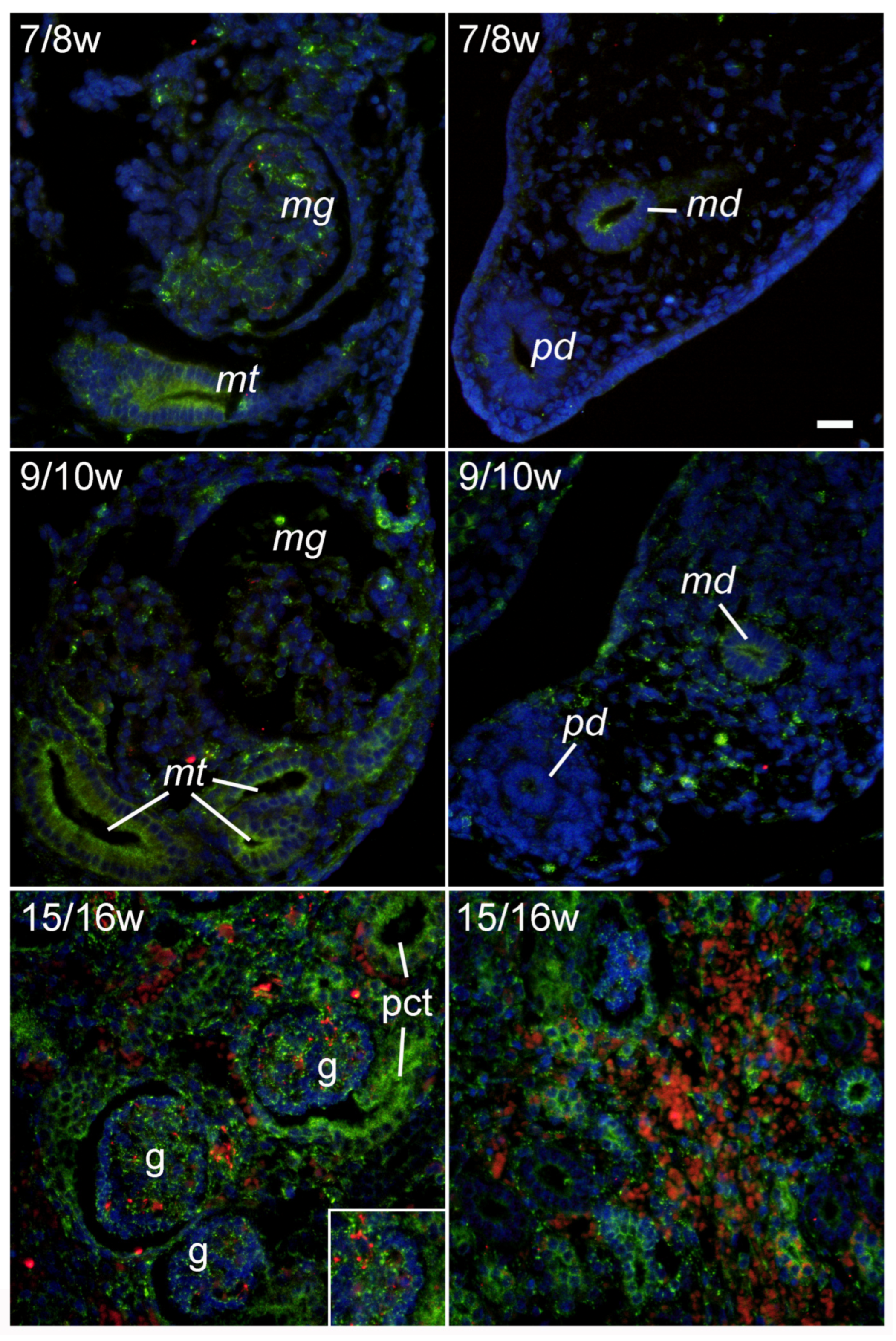
2.2. LAMP2A Immunoexpression during Early Renal Development
2.3. GRP78 Immunoexpression during Early Renal Development
2.4. Expression of LC3B, LAPM2A, and GRP78 in Postnatal Human Kidney
2.5. Expression of LC3B, LAMP2A, and GRP78 in Renal Tissues of Diabetic Patients
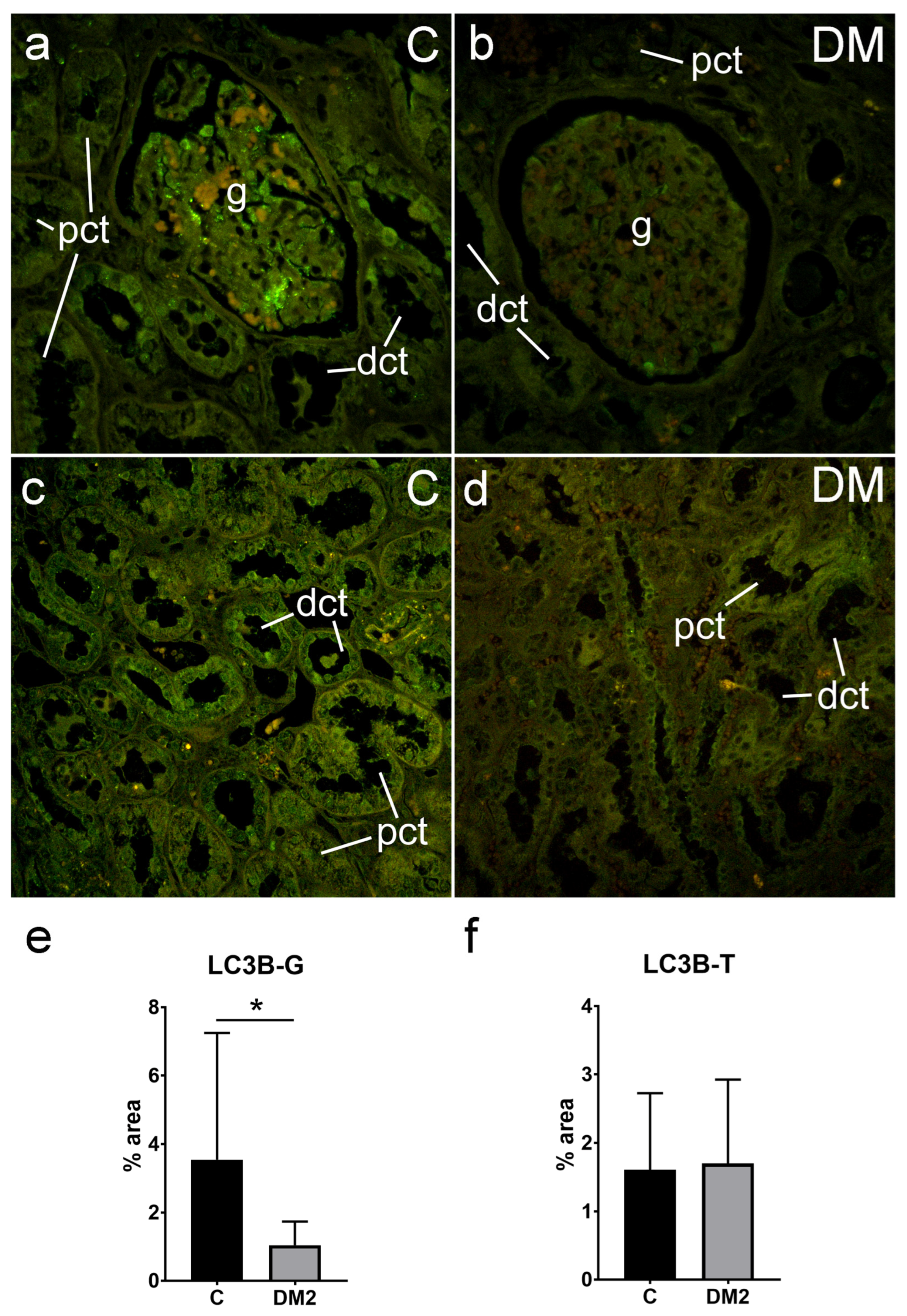
2.5.1. Expression of LC3B in Renal Tissues of Diabetic Patients
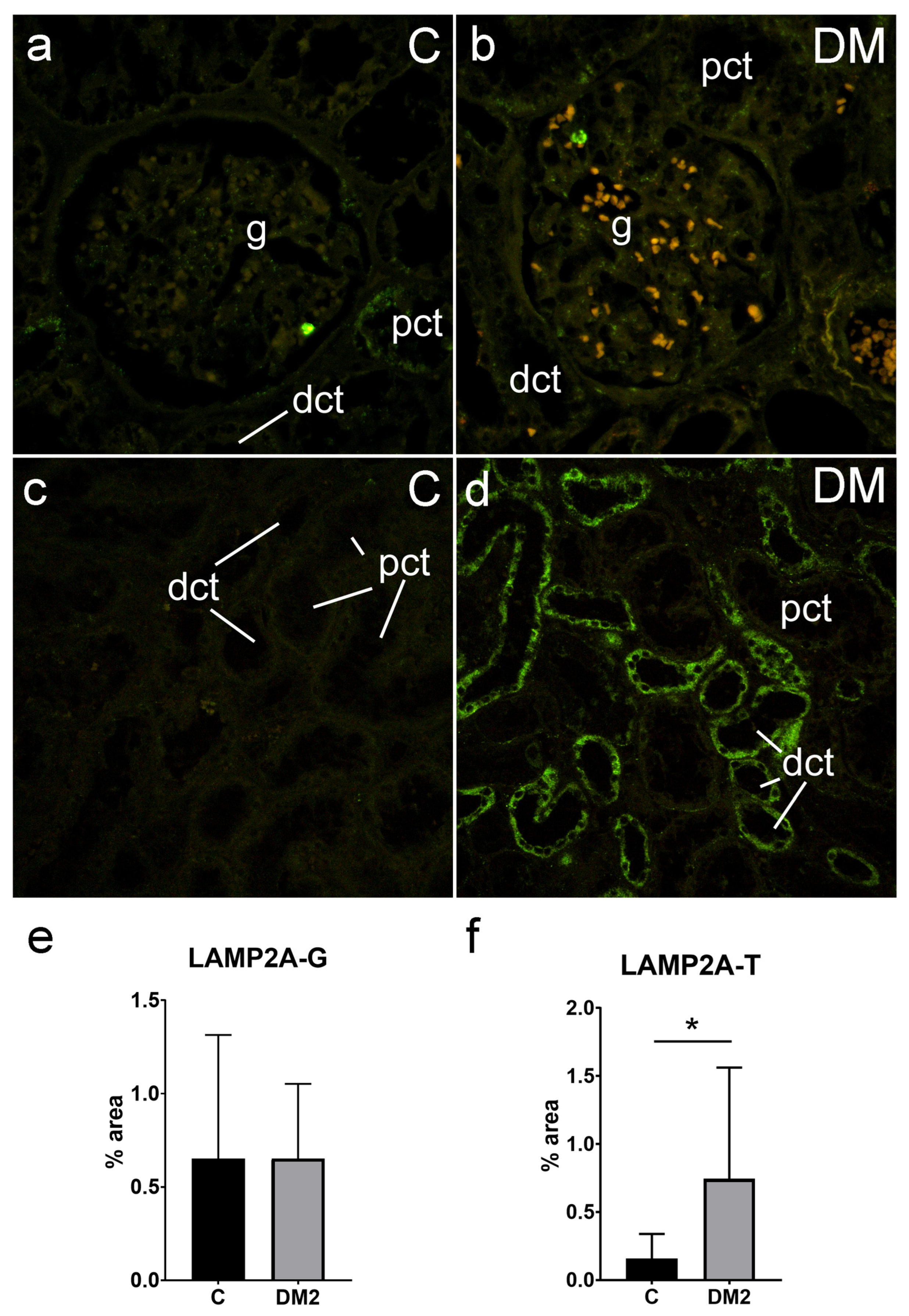
2.5.2. Expression of LAMP2A in Renal Tissues of Diabetic Patients
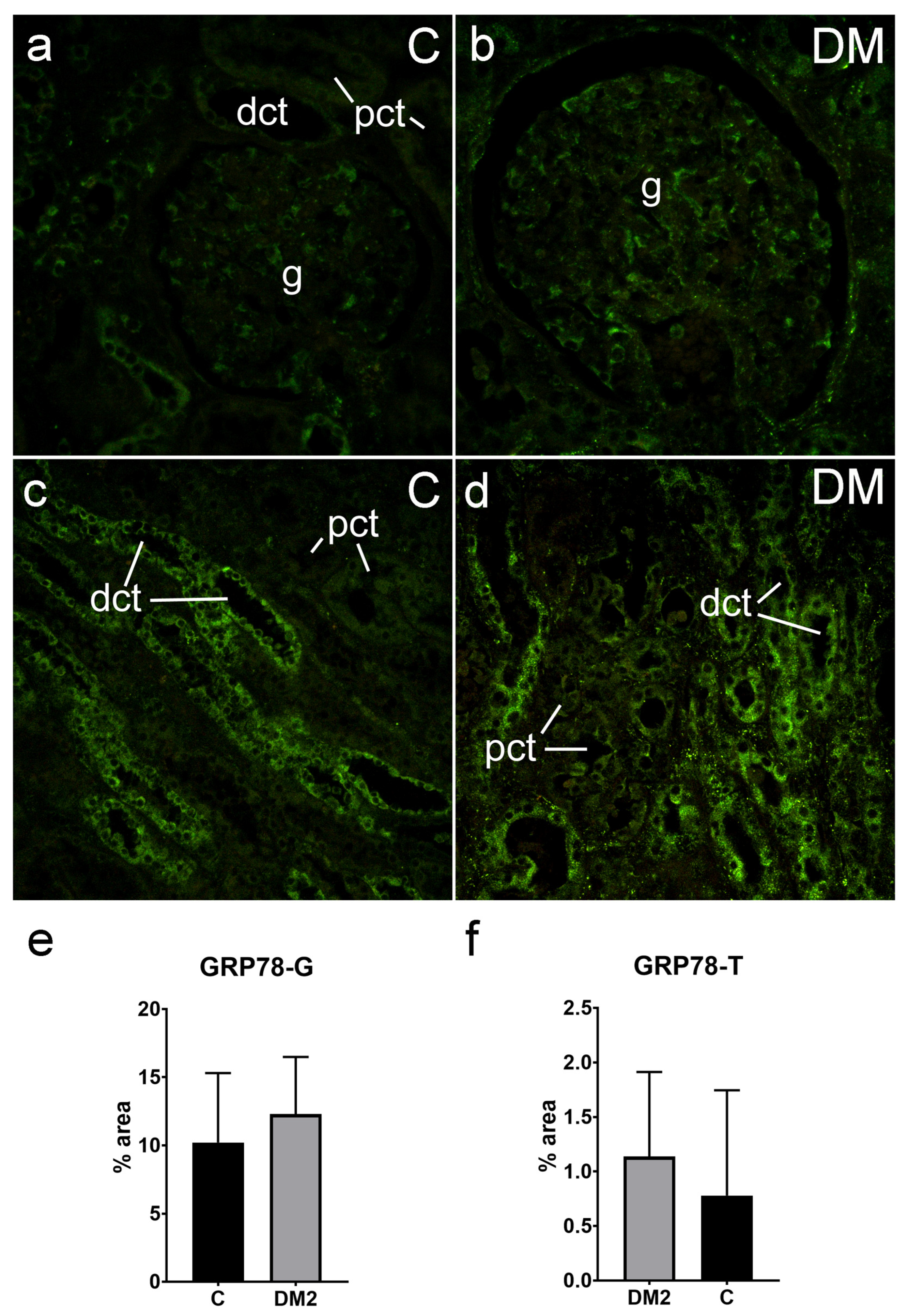
2.5.3. Expression of GRP78 in Renal Tissues of Diabetic Patients
3. Discussion
4. Materials and Methods
4.1. Tissue Procurement and Processing
4.2. Immunohistochemistry Procedure
4.3. Data Acquisition and Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005, 12 (Suppl. S2), 1542–1552. [Google Scholar] [CrossRef]
- Marzella, L.; Ahlberg, J.; Glaumann, H. Autophagy, heterophagy, microautophagy and crinophagy as the means for intracellular degradation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1981, 36, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Lam, H.C.; Jin, Y.; Kim, H.P.; Cao, J.; Lee, S.J.; Ifedigbo, E.; Parameswaran, H.; Ryter, S.W.; Choi, A.M. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc. Natl. Acad. Sci. USA 2010, 107, 18880–18885. [Google Scholar] [CrossRef]
- Bresciani, A.; Spiezia, M.C.; Boggio, R.; Cariulo, C.; Nordheim, A.; Altobelli, R.; Kuhlbrodt, K.; Dominguez, C.; Munoz-Sanjuan, I.; Wityak, J.; et al. Quantifying autophagy using novel LC3B and p62 TR-FRET assays. PLoS ONE 2018, 13, e0194423. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Xin, W.; Li, Z.; Xu, Y.; Yu, Y.; Zhou, Q.; Chen, L.; Wan, Q. Autophagy protects human podocytes from high glucose-induced injury by preventing insulin resistance. Metabolism 2016, 65, 1307–1315. [Google Scholar] [CrossRef]
- Eskelinen, E.L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006, 27, 495–502. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef]
- Napolitano, G.; Johnson, J.L.; He, J.; Rocca, C.J.; Monfregola, J.; Pestonjamasp, K.; Cherqui, S.; Catz, S.D. Impairment of chaperone-mediated autophagy leads to selective lysosomal degradation defects in the lysosomal storage disease cystinosis. EMBO Mol. Med. 2015, 7, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Maglica, M.; Kelam, N.; Perutina, I.; Racetin, A.; Rizikalo, A.; Filipović, N.; Kuzmić Prusac, I.; Mišković, J.; Vukojević, K. Immunoexpression Pattern of Autophagy-Related Proteins in Human Congenital Anomalies of the Kidney and Urinary Tract. Int. J. Mol. Sci. 2024, 25, 6829. [Google Scholar] [CrossRef]
- Zhang, J.; Johnson, J.L.; He, J.; Napolitano, G.; Ramadass, M.; Rocca, C.; Kiosses, W.B.; Bucci, C.; Xin, Q.; Gavathiotis, E.; et al. Cystinosin, the small GTPase Rab11, and the Rab7 effector RILP regulate intracellular trafficking of the chaperone-mediated autophagy receptor LAMP2A. J. Biol. Chem. 2017, 292, 10328–10346. [Google Scholar] [CrossRef]
- Kang, B.R.; Yang, S.H.; Chung, B.R.; Kim, W.; Kim, Y. Cell surface GRP78 as a biomarker and target for suppressing glioma cells. Sci. Rep. 2016, 6, 34922. [Google Scholar] [CrossRef]
- Cook, K.L.; Shajahan, A.N.; Wärri, A.; Jin, L.; Hilakivi-Clarke, L.A.; Clarke, R. Glucose-regulated protein 78 controls cross-talk between apoptosis and autophagy to determine antiestrogen responsiveness. Cancer Res. 2012, 72, 3337–3349. [Google Scholar] [CrossRef]
- Li, J.; Ni, M.; Lee, B.; Barron, E.; Hinton, D.R.; Lee, A.S. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008, 15, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Soomro, A.; Trink, J.; O’Neil, K.; Li, R.; Naiel, S.; Gao, B.; Ask, K.; Krepinsky, J.C. Activin A and Cell-Surface GRP78 Are Novel Targetable RhoA Activators for Diabetic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 2839. [Google Scholar] [CrossRef] [PubMed]
- Van Krieken, R.; Mehta, N.; Wang, T.; Zheng, M.; Li, R.; Gao, B.; Ayaub, E.; Ask, K.; Paton, J.C.; Paton, A.W.; et al. Cell surface expression of 78-kDa glucose-regulated protein (GRP78) mediates diabetic nephropathy. J. Biol. Chem. 2019, 294, 7755–7768. [Google Scholar] [CrossRef] [PubMed]
- Offei, E.B.; Yang, X.; Brand-Saberi, B. The role of autophagy in morphogenesis and stem cell maintenance. Histochem. Cell Biol. 2018, 150, 721–732. [Google Scholar] [CrossRef]
- Capone, V.P.; Morello, W.; Taroni, F.; Montini, G. Genetics of Congenital Anomalies of the Kidney and Urinary Tract: The Current State of Play. Int. J. Mol. Sci. 2017, 18, 796. [Google Scholar] [CrossRef]
- Pope, J.C., 4th; Brock, J.W., 3rd; Adams, M.C.; Stephens, F.D.; Ichikawa, I. How they begin and how they end: Classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J. Am. Soc. Nephrol. 1999, 10, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.W. Langman’s Medical Embryology; Wolters Kluwer Health: Philadelphia, PA, USA, 2024. [Google Scholar]
- Jeličić, I.; Vukojević, K.; Racetin, A.; Čarić, D.; Glavina Durdov, M.; Saraga-Babić, M.; Filipović, N. Expression of Pannexin 1 in the Human Kidney during Embryonal, Early Fetal and Postnatal Development and Its Prognostic Significance in Diabetic Nephropathy. Biomedicines 2022, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef]
- Wen-Jin, C.; Xiu-Wu, P.; Jian, C.; Da, X.; Jia-Xin, C.; Wei-Jie, C.; Lin-Hui, W.; Xin-Gang, C. Study of cellular heterogeneity and differential dynamics of autophagy in human embryonic kidney development by single-cell RNA sequencing. Cancer Cell Int. 2021, 21, 460. [Google Scholar] [CrossRef]
- Cecconi, F.; Levine, B. The role of autophagy in mammalian development: Cell makeover rather than cell death. Dev. Cell 2008, 15, 344–357. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Wen, J.; Yang, Z. Autophagy is involved in mouse kidney development and podocyte differentiation regulated by Notch signalling. J. Cell. Mol. Med. 2017, 21, 1315–1328. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Koch, E.A.T.; Nakhoul, R.; Nakhoul, F.; Nakhoul, N. Autophagy in diabetic nephropathy: A review. Int. Urol. Nephrol. 2020, 52, 1705–1712. [Google Scholar] [CrossRef]
- Barbosa Júnior Ade, A.; Zhou, H.; Hültenschmidt, D.; Totovic, V.; Jurilj, N.; Pfeifer, U. Inhibition of cellular autophagy in proximal tubular cells of the kidney in streptozotocin-diabetic and uninephrectomized rats. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1992, 61, 359–366. [Google Scholar] [CrossRef]
- Han, K.; Zhou, H.; Pfeifer, U. Inhibition and restimulation by insulin of cellular autophagy in distal tubular cells of the kidney in early diabetic rats. Kidney Blood Press Res. 1997, 20, 258–263. [Google Scholar] [CrossRef]
- Yamahara, K.; Kume, S.; Koya, D.; Tanaka, Y.; Morita, Y.; Chin-Kanasaki, M.; Araki, H.; Isshiki, K.; Araki, S.; Haneda, M.; et al. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J. Am. Soc. Nephrol. 2013, 24, 1769–1781. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Li, H.; Zhang, P. Pathophysiology of diabetic kidney disease and autophagy: A review. Medicine 2023, 102, e33965. [Google Scholar] [CrossRef]
- Vukojevic, K.; Raguz, F.; Saraga, M.; Filipovic, N.; Bocina, I.; Kero, D.; Glavina Durdov, M.; Martinovic, V.; Saraga-Babic, M. Glomeruli from patients with nephrin mutations show increased number of ciliated and poorly differentiated podocytes. Acta Histochem. 2018, 120, 748–756. [Google Scholar] [CrossRef]
- Filipović, N.; Vukojevic, K.; Bočina, I.; Saraga, M.; Glavina Durdov, M.; Kablar, B.; Saraga-Babic, M. Immunohistochemical and electronmicroscopic features of mesenchymal-to-epithelial transition in human developing, postnatal and nephrotic podocytes. Histochem. Cell Biol. 2017, 147, 481–495. [Google Scholar] [CrossRef]
- Chevalier, R.L. Evolution, kidney development, and chronic kidney disease. Semin. Cell Dev. Biol. 2019, 91, 119–131. [Google Scholar] [CrossRef]
- Maglica, M.; Kelam, N.; Haque, E.; Perutina, I.; Racetin, A.; Filipović, N.; Katsuyama, Y.; Vukojević, K. Immunoexpression Pattern of Autophagy Markers in Developing and Postnatal Kidneys of Dab1−/−(yotari) Mice. Biomolecules 2023, 13, 402. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, A.I.; Arias, E.; Díaz-Carretero, A.; Cuervo, A.M.; Cuadrado, A. Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A. Autophagy 2018, 14, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, S.; Tan, X.; Wang, D. New Insights into the Mechanisms of Chaperon-Mediated Autophagy and Implications for Kidney Diseases. Cells 2022, 11, 406. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. Regulation of lamp2a levels in the lysosomal membrane. Traffic 2000, 1, 570–583. [Google Scholar] [CrossRef]
- Kaushik, S.; Massey, A.C.; Cuervo, A.M. Lysosome membrane lipid microdomains: Novel regulators of chaperone-mediated autophagy. EMBO J. 2006, 25, 3921–3933. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Mann, L.; Bonten, E.J.; d’Azzo, A.; Dice, J.F. Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J. 2003, 22, 47–59. [Google Scholar] [CrossRef]
- Festa, B.; Berquez, M.; Nieri, D.; Luciani, A. Endolysosomal Disorders Affecting the Proximal Tubule of the Kidney: New Mechanistic Insights and Therapeutics; Springer Nature: Cham, Switzerland, 2021; pp. 1–25. [Google Scholar]
- Festa, B.P.; Chen, Z.; Berquez, M.; Debaix, H.; Tokonami, N.; Prange, J.A.; Hoek, G.V.; Alessio, C.; Raimondi, A.; Nevo, N.; et al. Impaired autophagy bridges lysosomal storage disease and epithelial dysfunction in the kidney. Nat. Commun. 2018, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, J.; Johnson, J.L.; Rahman, F.; Gavathiotis, E.; Cuervo, A.M.; Catz, S.D. Chaperone-Mediated Autophagy Upregulation Rescues Megalin Expression and Localization in Cystinotic Proximal Tubule Cells. Front. Endocrinol. 2019, 10, 21. [Google Scholar] [CrossRef]
- Guo, X.; Kong, Y.; Kwon, T.H.; Li, C.; Wang, W. Autophagy and regulation of aquaporins in the kidneys. Kidney Res. Clin. Pract. 2023, 42, 676–685. [Google Scholar] [CrossRef]
- Guo, X.; Xu, L.; Kong, Y.; Li, M.; Zhai, Q.; Liang, B.; Zhao, X.; Long, L.; Huang, M.; Wang, W.; et al. Autophagy is involved in degradation of AQP1 in response to an acute decrement in tonicity. iScience 2023, 26, 108485. [Google Scholar] [CrossRef]
- Mak, A.; Sung, C.C.; Pisitkun, T.; Khositseth, S.; Knepper, M.A. ‘Aquaporin-omics’: Mechanisms of aquaporin-2 loss in polyuric disorders. J. Physiol. 2023, 602, 3191–3206. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Severs, D. Autophagy and renal epithelial transport: Eat to survive. Kidney Int. 2017, 91, 1003–1005. [Google Scholar] [CrossRef]
- Tagawa, A.; Yasuda, M.; Kume, S.; Yamahara, K.; Nakazawa, J.; Chin-Kanasaki, M.; Araki, H.; Araki, S.; Koya, D.; Asanuma, K.; et al. Impaired Podocyte Autophagy Exacerbates Proteinuria in Diabetic Nephropathy. Diabetes 2016, 65, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, Y.S.; Sun, L.; Xie, P.; Liu, F.-Y.; Chen, S. A Glimpse of Various Pathogenetic Mechanisms of Diabetic Nephropathy. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 395–423. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, M.-Y.; Han, Y.-P.; Liu, L.-J.; Yan, J.-L.; Qian, L.-B. The protection of luteolin against diabetic cardiomyopathy in rats is related to reversing JNK-suppressed autophagy. Food Funct. 2023, 14, 2740–2749. [Google Scholar] [CrossRef]
- Han, Y.P.; Liu, L.J.; Yan, J.L.; Chen, M.Y.; Meng, X.F.; Zhou, X.R.; Qian, L.B. Autophagy and its therapeutic potential in diabetic nephropathy. Front. Endocrinol. 2023, 14, 1139444. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, N.F.; Elbaset, M.A.; Moustafa, P.E.; Ibrahim, S.M. Empagliflozin mitigates type 2 diabetes-associated peripheral neuropathy: A glucose-independent effect through AMPK signaling. Arch. Pharm. Res. 2022, 45, 475–493. [Google Scholar] [CrossRef]
- Su, P.P.; Liu, D.W.; Zhou, S.J.; Chen, H.; Wu, X.M.; Liu, Z.S. Down-regulation of Risa improves podocyte injury by enhancing autophagy in diabetic nephropathy. Mil. Med. Res. 2022, 9, 23. [Google Scholar] [CrossRef]
- Koya, D.; Hayashi, K.; Kitada, M.; Kashiwagi, A.; Kikkawa, R.; Haneda, M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J. Am. Soc. Nephrol. 2003, 14, S250–S253. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Meng, L.; Cao, G.; Wu, Y. Sirtuin 6 overexpression relieves sepsis-induced acute kidney injury by promoting autophagy. Cell Cycle 2019, 18, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cuervo, A.M. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat. Med. 2008, 14, 959–965. [Google Scholar] [CrossRef]
- Liu, C.; Gidlund, E.K.; Witasp, A.; Qureshi, A.R.; Söderberg, M.; Thorell, A.; Nader, G.A.; Barany, P.; Stenvinkel, P.; von Walden, F. Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease. Am. J. Physiol. Renal Physiol. 2019, 317, F1122–F1131. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, J.A.; Kaushik, S.; Koga, H.; Dall’Armi, C.; Shui, G.; Wenk, M.R.; Di Paolo, G.; Cuervo, A.M. Inhibitory effect of dietary lipids on chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA 2012, 109, E705–E714. [Google Scholar] [CrossRef]
- Kiffin, R.; Kaushik, S.; Zeng, M.; Bandyopadhyay, U.; Zhang, C.; Massey, A.C.; Martinez-Vicente, M.; Cuervo, A.M. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J. Cell Sci. 2007, 120, 782–791. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Dice, J.F. Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. 2000, 275, 31505–31513. [Google Scholar] [CrossRef]
- Sooparb, S.; Price, S.R.; Shaoguang, J.; Franch, H.A. Suppression of chaperone-mediated autophagy in the renal cortex during acute diabetes mellitus. Kidney Int. 2004, 65, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Benigni, A.; Remuzzi, G. Proteinuria and Tubulotoxicity: Pathophysiology and Clinical Aspects; Springer: Cham, Switzerland, 2019; pp. 197–214. [Google Scholar]
- D’Amico, G.; Bazzi, C. Pathophysiology of proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Xu, N.; Yin, D.; Zheng, P.; Liu, W.; Wang, G.; Hui, Y.; Han, G.; Yang, C.; Cheng, X. Levels of circulating GRP78 and CHOP in endoplasmic reticulum stress pathways in Chinese type 2 diabetic kidney disease patients. Medicine 2021, 100, e26879. [Google Scholar] [CrossRef]
- Liu, G.; Sun, Y.; Li, Z.; Song, T.; Wang, H.; Zhang, Y.; Ge, Z. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem. Biophys. Res. Commun. 2008, 370, 651–656. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- O’Rahilly, R.; Gardner, E. The timing and sequence of events in the development of the human nervous system during the embryonic period proper. Z. Fur Anat. Entwicklungsgeschichte 1971, 134, 1–12. [Google Scholar] [CrossRef]
- Hills, G.W.P.; Wall, M.J.; Kaufmann, T.J.; Tang, S.C.-W.; Yiu, W.H.; Squires, P.E. Cellular physiology and biochemistry. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 45, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Wang, M.; Hou, Y.; Huang, W.; Zhou, D.; Wang, Z.; Yang, S.; Tang, W.; Zhen, J.; Li, Y.; et al. Elevation of JAML Promotes Diabetic Kidney Disease by Modulating Podocyte Lipid Metabolism. Cell Metab. 2020, 32, 1052–1062.e1058. [Google Scholar] [CrossRef] [PubMed]
- Rizikalo, A.; Maglica, M.; Kelam, N.; Perutina, I.; Ogorevc, M.; Racetin, A.; Filipović, N.; Katsuyama, Y.; Zovko, Z.; Mišković, J.; et al. Unraveling the Impact of Dab1 Gene Silencing on the Expression of Autophagy Markers in Lung Development. Life 2024, 14, 316. [Google Scholar] [CrossRef]
- Racetin, A.; Filipović, N.; Lozić, M.; Ogata, M.; Gudelj Ensor, L.; Kelam, N.; Kovačević, P.; Watanabe, K.; Katsuyama, Y.; Saraga-Babić, M.; et al. A Homozygous Dab1(-/-) Is a Potential Novel Cause of Autosomal Recessive Congenital Anomalies of the Mice Kidney and Urinary Tract. Biomolecules 2021, 11, 609. [Google Scholar] [CrossRef]


| N Male | N Female | Age—Years (Range) | Creatinine—μmol/L (Range) | |
|---|---|---|---|---|
| All | 14 | 6 | 65.9 ± 7.83 (44–78) | 96.74 ± 35.48 (50–214) |
| Non- diabetic | 8 | 3 | 66.27 ± 8.83 (44–75) | 91.27 ± 22.12 (50–121) |
| Diabetic | 6 | 3 | 65.44 ± 6.89 (55–78) | 104.25 ± 49.25 (63–214) |
| Antibody | Code No. | Host | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | Anti-LC3B | ab48394 | Rabbit | 1:100 | Abcam, Cambridge, UK |
| Anti-LAMP2A | ab18528 | Rabbit | 1:100 | Abcam, Cambridge, UK | |
| Anti-GRP78 | PA5-19503 | Rabbit | 1:300 | Thermo Fisher Scientific, Waltham, MA, USA | |
| Anti-CD31/PECAM-1 (H3), Alexa fluor 546 conjugated | sc-376764 | Mouse | 1:50 | Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA | |
| Secondary | Alexa Fluor®488 AffiniPure Anti- Rabbit lgG (H + L) | 711-545-152 | Donkey | 1:400 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brdar, I.; Racetin, A.; Jeličić, I.; Vukojević, K.; Vučković, L.; Ljutić, D.; Saraga-Babić, M.; Filipović, N. Expression of Autophagy Markers LC3B, LAMP2A, and GRP78 in the Human Kidney during Embryonic, Early Fetal, and Postnatal Development and Their Significance in Diabetic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 9152. https://doi.org/10.3390/ijms25179152
Brdar I, Racetin A, Jeličić I, Vukojević K, Vučković L, Ljutić D, Saraga-Babić M, Filipović N. Expression of Autophagy Markers LC3B, LAMP2A, and GRP78 in the Human Kidney during Embryonic, Early Fetal, and Postnatal Development and Their Significance in Diabetic Kidney Disease. International Journal of Molecular Sciences. 2024; 25(17):9152. https://doi.org/10.3390/ijms25179152
Chicago/Turabian StyleBrdar, Ivan, Anita Racetin, Ivo Jeličić, Katarina Vukojević, Ljiljana Vučković, Dragan Ljutić, Mirna Saraga-Babić, and Natalija Filipović. 2024. "Expression of Autophagy Markers LC3B, LAMP2A, and GRP78 in the Human Kidney during Embryonic, Early Fetal, and Postnatal Development and Their Significance in Diabetic Kidney Disease" International Journal of Molecular Sciences 25, no. 17: 9152. https://doi.org/10.3390/ijms25179152
APA StyleBrdar, I., Racetin, A., Jeličić, I., Vukojević, K., Vučković, L., Ljutić, D., Saraga-Babić, M., & Filipović, N. (2024). Expression of Autophagy Markers LC3B, LAMP2A, and GRP78 in the Human Kidney during Embryonic, Early Fetal, and Postnatal Development and Their Significance in Diabetic Kidney Disease. International Journal of Molecular Sciences, 25(17), 9152. https://doi.org/10.3390/ijms25179152






