Intrapulmonary T Cells Are Sufficient for Schistosoma-Induced Pulmonary Hypertension
Abstract
1. Introduction
2. Results
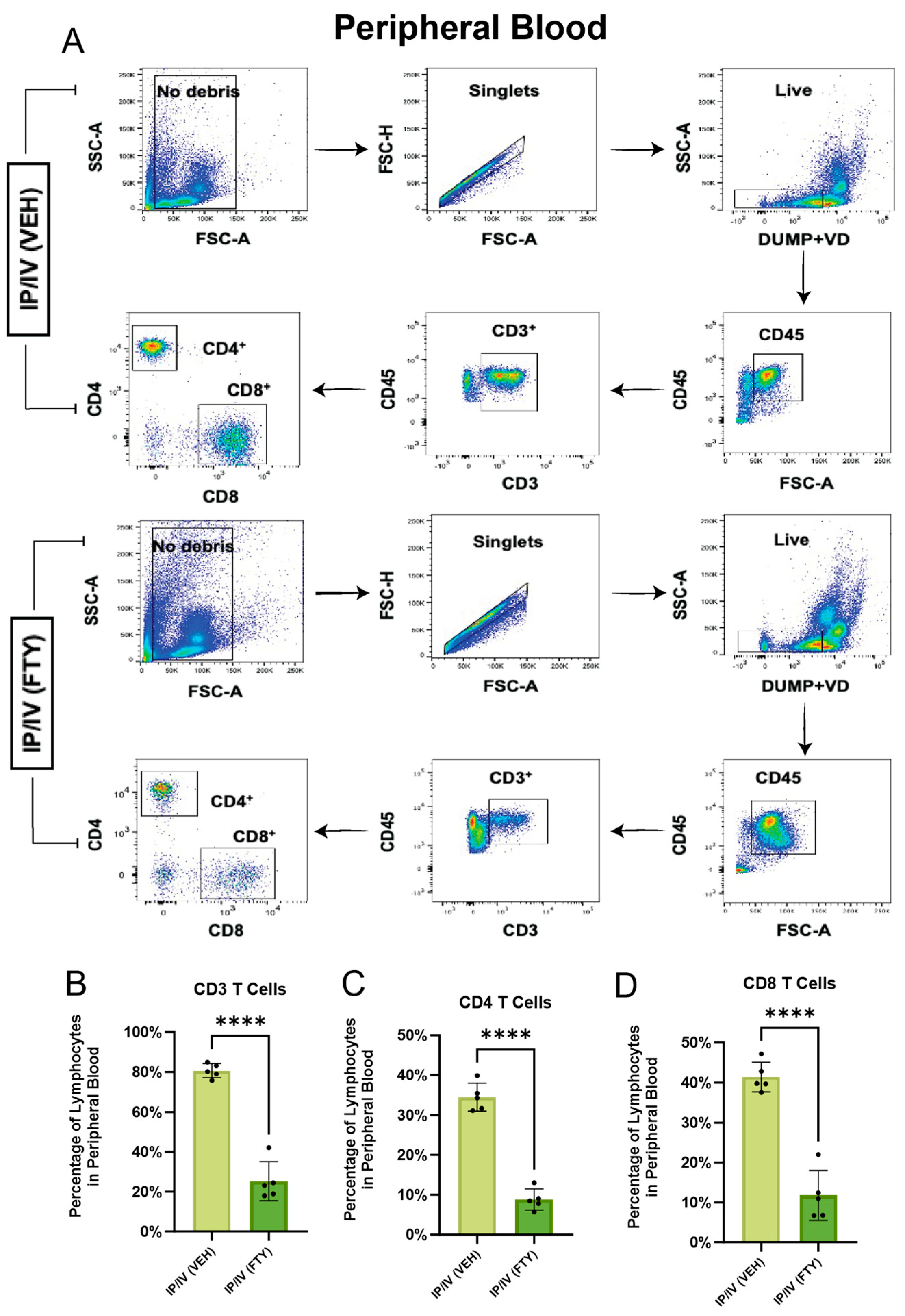
2.1. Effect of FTY720 on T Cells in the Peripheral Blood and Mediastinal Lymph Nodes
2.2. Pulmonary Hypertension Phenotype
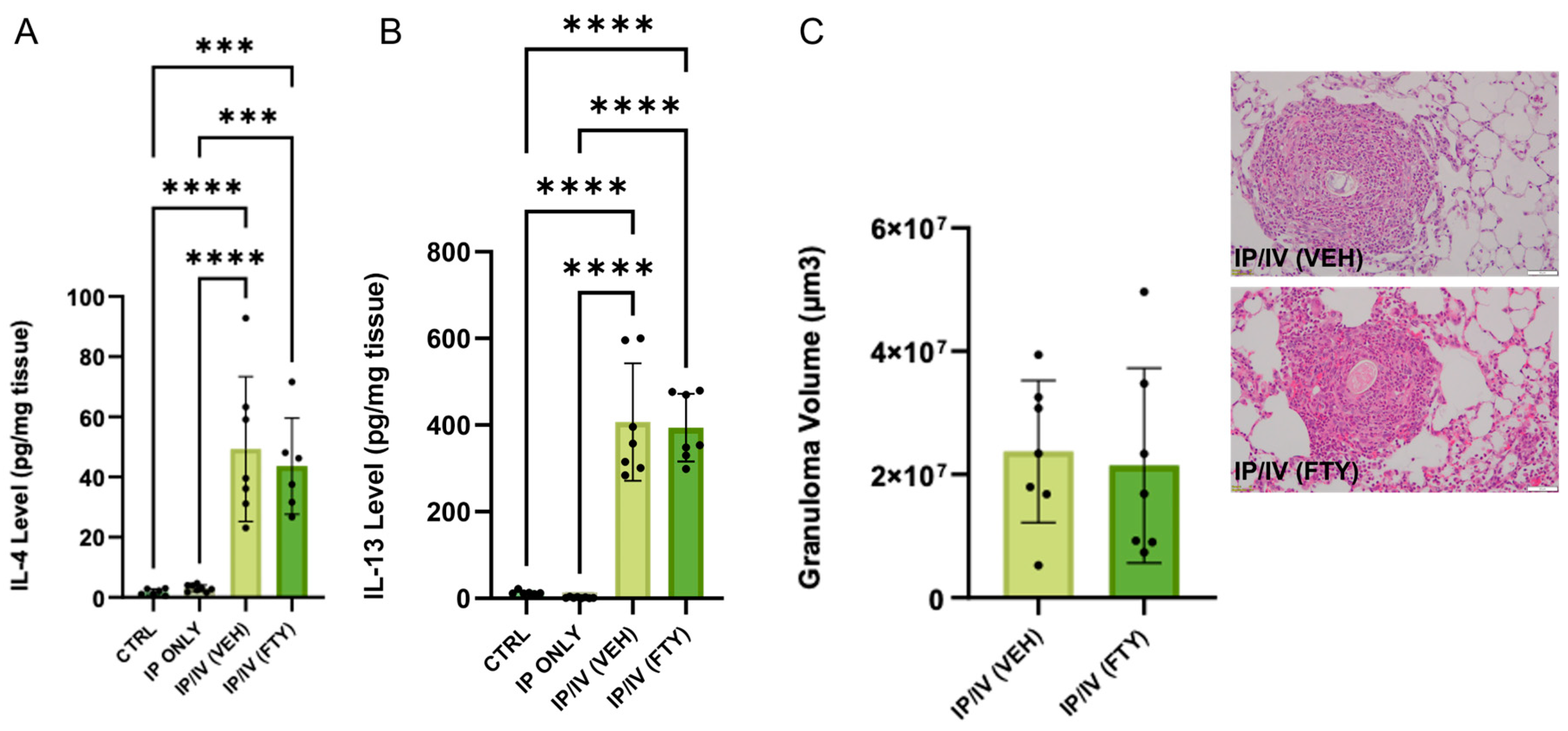
2.3. Pulmonary Inflammation Phenotype
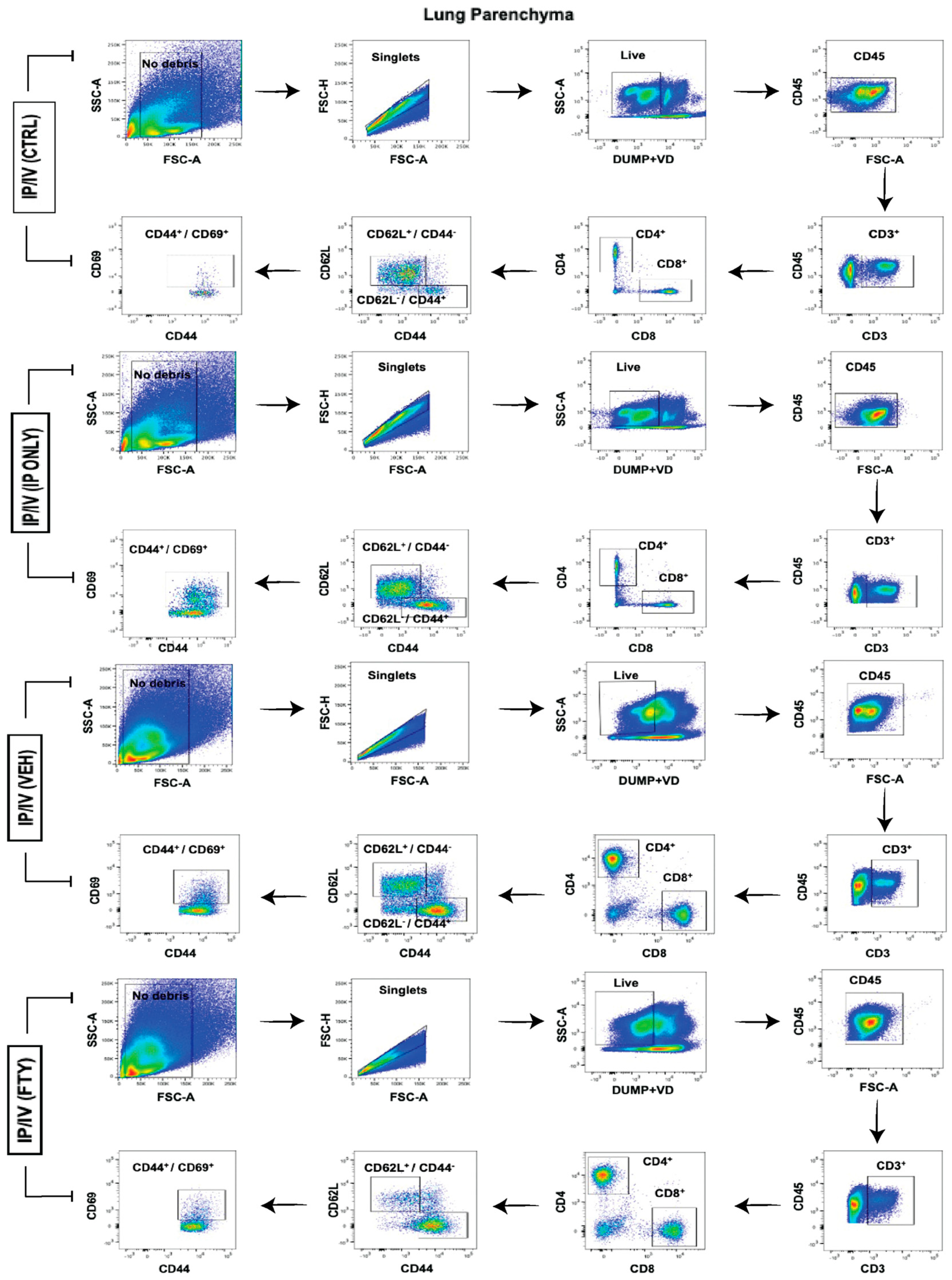
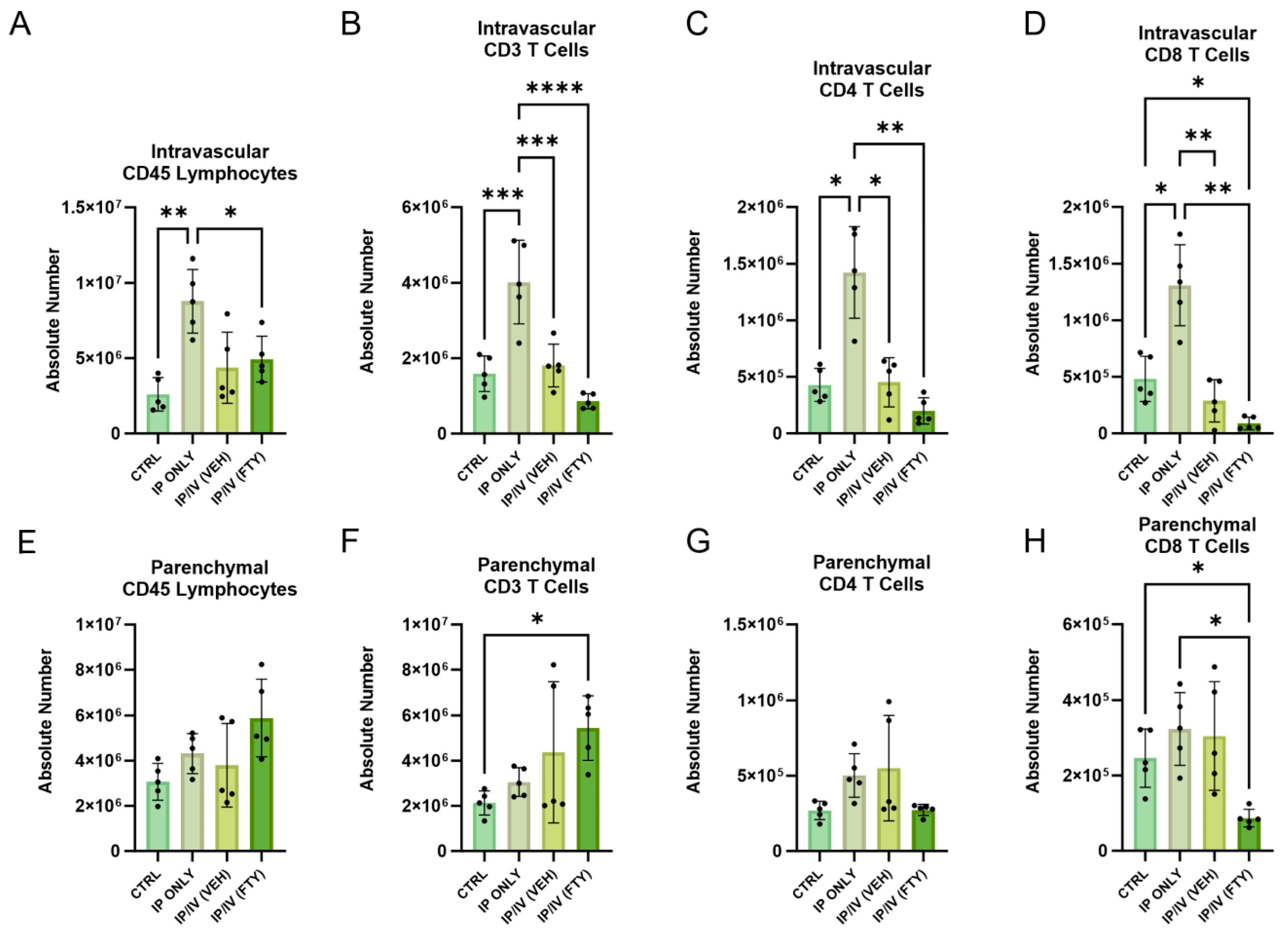
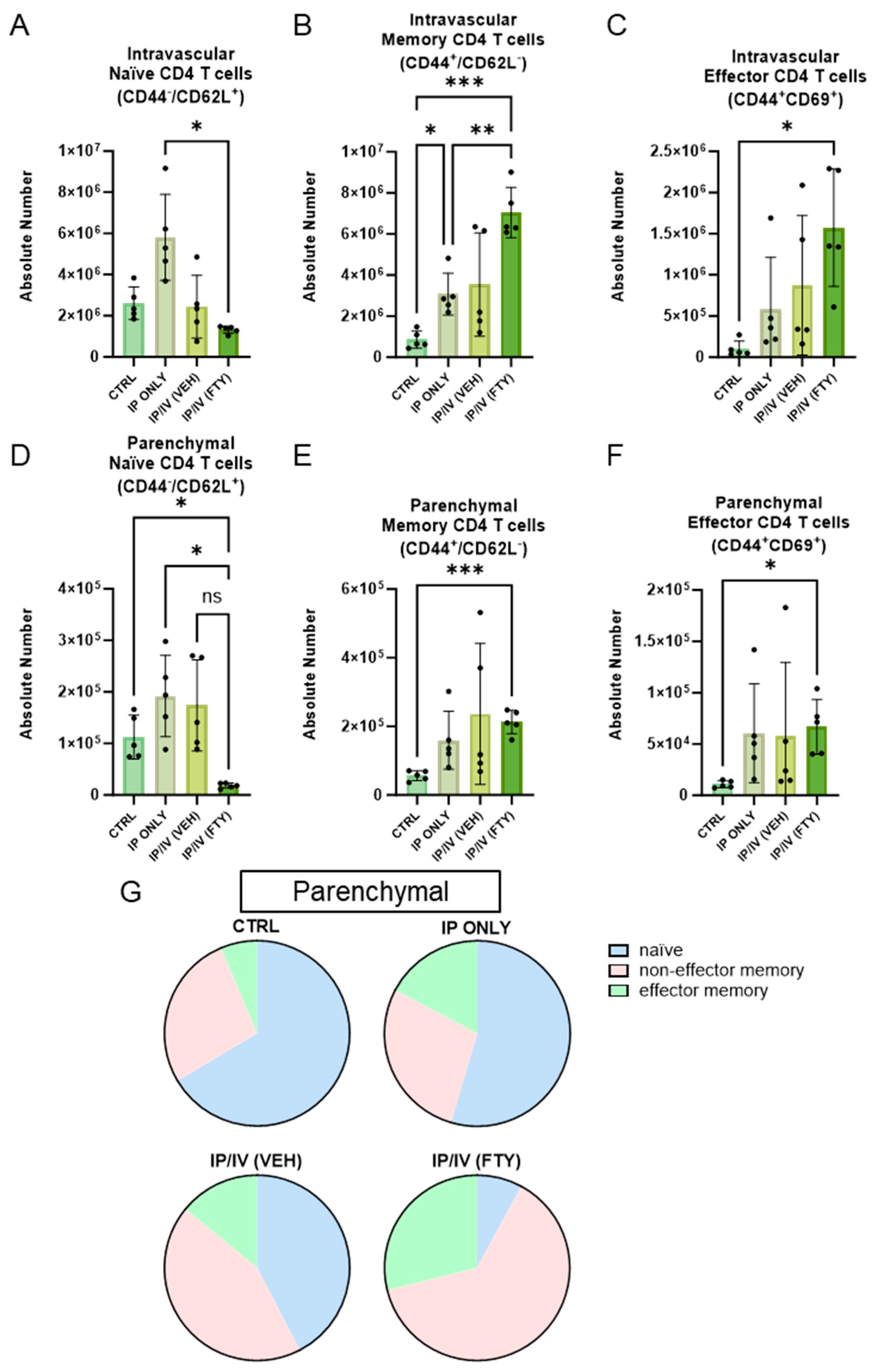
2.4. Pulmonary T Cell Density and Phenotypes
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef]
- Knafl, D.; Gerges, C.; King, C.H.; Humbert, M.; Bustinduy, A.L. Schistosomiasis-Associated Pulmonary Arterial Hypertension: A Systematic Review. Eur. Respir. Rev. 2020, 29, 190089. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mickael, C.; Kassa, B.; Sanders, L.; Koyanagi, D.; Hernandez-Saavedra, D.; Freeman, S.; Morales-Cano, D.; Cogolludo, A.; McKee, A.S.; et al. Th2 CD4+ T Cells Are Necessary and Sufficient for Schistosoma-Pulmonary Hypertension. J. Am. Heart Assoc. 2019, 8, e013111. [Google Scholar] [CrossRef]
- Kumar, R.; Mickael, C.; Kassa, B.; Gebreab, L.; Robinson, J.C.; Koyanagi, D.E.; Sanders, L.; Barthel, L.; Meadows, C.; Fox, D.; et al. TGF-β Activation by Bone Marrow-Derived Thrombospondin-1 Causes Schistosoma- and Hypoxia-Induced Pulmonary Hypertension. Nat. Commun. 2017, 8, 15494. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Yeager, M.E.; Zaiman, A.; Cool, C.D.; Voelkel, N.F.; Tuder, R.M. Impaired Transforming Growth Factor-Beta Signaling in Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2004, 170, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Muyleart, M.; Konduri, G.G.; Mammoto, A. Twist1 in Hypoxia-Induced Pulmonary Hypertension through Transforming Growth Factor-β-Smad Signaling. Am. J. Respir. Cell Mol. Biol. 2018, 58, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, K.; Reed, N.I.; Atakilit, A.; Ren, X.; Sheppard, D. Transforming Growth Factor-β Plays Divergent Roles in Modulating Vascular Remodeling, Inflammation, and Pulmonary Fibrosis in a Murine Model of Scleroderma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L22–L31. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.-W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A Placebo-Controlled Trial of Oral Fingolimod in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte Egress from Thymus and Peripheral Lymphoid Organs Is Dependent on S1P Receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Napoli, K.L. The FTY720 Story. Ther. Drug Monit. 2000, 22, 47–51. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Schneider, C.; Liao, C.; Lee, J.; Liang, H.-E.; Locksley, R.M. Tissue-Specific Pathways Extrude Activated ILC2s to Disseminate Type 2 Immunity. J. Exp. Med. 2020, 217, e20191172. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Wang, Y.; Jung, H.; Field, R.L.; Zhang, X.; Liu, T.-C.; Ma, C.; Fraser, J.S.; Brestoff, J.R.; Van Dyken, S.J. A Type 2 Immune Circuit in the Stomach Controls Mammalian Adaptation to Dietary Chitin. Science 2023, 381, 1092–1098. [Google Scholar] [CrossRef]
- Graham, B.B.; Mentink-Kane, M.M.; El-Haddad, H.; Purnell, S.; Zhang, L.; Zaiman, A.; Redente, E.F.; Riches, D.W.H.; Hassoun, P.M.; Bandeira, A.; et al. Schistosomiasis-Induced Experimental Pulmonary Hypertension: Role of Interleukin-13 Signaling. Am. J. Pathol. 2010, 177, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mickael, C.; Chabon, J.; Gebreab, L.; Rutebemberwa, A.; Garcia, A.R.; Koyanagi, D.E.; Sanders, L.; Gandjeva, A.; Kearns, M.T.; et al. The Causal Role of IL-4 and IL-13 in Schistosoma Mansoni Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2015, 192, 998–1008. [Google Scholar] [CrossRef]
- Anderson, K.G.; Mayer-Barber, K.; Sung, H.; Beura, L.; James, B.R.; Taylor, J.J.; Qunaj, L.; Griffith, T.S.; Vezys, V.; Barber, D.L.; et al. Intravascular Staining for Discrimination of Vascular and Tissue Leukocytes. Nat. Protoc. 2014, 9, 209–222. [Google Scholar] [CrossRef]
- Thawer, S.G.; Horsnell, W.G.; Darby, M.; Hoving, J.C.; Dewals, B.; Cutler, A.J.; Lang, D.; Brombacher, F. Lung-Resident CD4+ T Cells Are Sufficient for IL-4Rα-Dependent Recall Immunity to Nippostrongylus Brasiliensis Infection. Mucosal Immunol. 2014, 7, 239–248. [Google Scholar] [CrossRef] [PubMed]
- de Cassia dos Santos Ferreira, R.; Montenegro, S.M.L.; Domingues, A.L.C.; Bandeira, A.P.; da Mota Silveira, C.A.; Leite, L.A.C.; de Almeida Pereira, C.; Fernandes, I.M.; Mertens, A.B.; Almeida, M.O. TGF Beta and IL13 in Schistosomiasis Mansoni Associated Pulmonary Arterial Hypertension; a Descriptive Study with Comparative Groups. BMC Infect. Dis. 2014, 14, 282. [Google Scholar] [CrossRef][Green Version]
- Mauad, T.; Pozzan, G.; Lanças, T.; Overbeek, M.J.; Souza, R.; Jardim, C.; Dolhnikoff, M.; Mello, G.; Pires-Neto, R.C.; del Carlo Bernardi, F.; et al. Immunopathological Aspects of Schistosomiasis-Associated Pulmonary Arterial Hypertension. J. Infect. 2014, 68, 90–98. [Google Scholar] [CrossRef]
- Maston, L.D.; Jones, D.T.; Giermakowska, W.; Howard, T.A.; Cannon, J.L.; Wang, W.; Wei, Y.; Xuan, W.; Resta, T.C.; Gonzalez Bosc, L.V. Central Role of T Helper 17 Cells in Chronic Hypoxia-Induced Pulmonary Hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L609–L624. [Google Scholar] [CrossRef]
- Hashimoto-Kataoka, T.; Hosen, N.; Sonobe, T.; Arita, Y.; Yasui, T.; Masaki, T.; Minami, M.; Inagaki, T.; Miyagawa, S.; Sawa, Y.; et al. Interleukin-6/Interleukin-21 Signaling Axis Is Critical in the Pathogenesis of Pulmonary Arterial Hypertension. Proc. Natl. Acad. Sci. USA 2015, 112, E2677–E2686. [Google Scholar] [CrossRef]
- Takenaka, E.; Van Vo, A.; Yamashita-Kanemaru, Y.; Shibuya, A.; Shibuya, K. Selective DNAM-1 Expression on Small Peritoneal Macrophages Contributes to CD4+ T Cell Costimulation. Sci. Rep. 2018, 8, 15180. [Google Scholar] [CrossRef] [PubMed]
- Macrophages Transfer Antigens to Dendritic Cells by Releasing Exosomes Containing Dead-Cell-Associated Antigens Partially through a Ceramide-Dependent Pathway to Enhance CD4+ T-cell Responses—Xu—2016—Immunology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/imm.12630 (accessed on 2 April 2024).
- Lyons-Cohen, M.R.; Shamskhou, E.A.; Gerner, M.Y. Site-Specific Regulation of Th2 Differentiation within Lymph Node Microenvironments. J. Exp. Med. 2024, 221, e20231282. [Google Scholar] [CrossRef]
- Peterson, W.P.; Von Lichtenberg, F. Studies on Granuloma Formation. IV. In Vivo Antigenicity of Schistosome Egg Antigen in Lung Tissue. J. Immunol. 1965, 95, 959–965. [Google Scholar] [CrossRef]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J.; Jacobson, J. Helminth Infections: The Great Neglected Tropical Diseases. J. Clin. Investig. 2008, 118, 1311–1321. [Google Scholar] [CrossRef]
- Kumar, R.; Lee, M.H.; Kassa, B.; Fonseca Balladares, D.C.; Mickael, C.; Sanders, L.; Andruska, A.; Kumar, M.; Spiekerkoetter, E.; Bandeira, A.; et al. Repetitive Schistosoma Exposure Causes Perivascular Lung Fibrosis and Persistent Pulmonary Hypertension. Clin. Sci. 2023, 137, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Crompton, D.W.T.; Nesheim, M.C. Nutritional Impact of Intestinal Helminthiasis During the Human Life Cycle. Annu. Rev. Nutr. 2002, 22, 35–59. [Google Scholar] [CrossRef]
- Miguel, E.; Kremer, M. Worms: Identifying Impacts on Education and Health in the Presence of Treatment Externalities. Econometrica 2004, 72, 159–217. [Google Scholar] [CrossRef]
- Bleakley, H. Disease and Development: Evidence from Hookworm Eradication in the American South. Q. J. Econ. 2007, 122, 73–117. [Google Scholar] [CrossRef]
- Hotez, P.J.; Ferris, M.T. The Antipoverty Vaccines. Vaccine 2006, 24, 5787–5799. [Google Scholar] [CrossRef]
- Connor, L.M.; Harvie, M.C.; Rich, F.J.; Quinn, K.M.; Brinkmann, V.; Gros, G.L.; Kirman, J.R. A Key Role for Lung-Resident Memory Lymphocytes in Protective Immune Responses after BCG Vaccination. Eur. J. Immunol. 2010, 40, 2482–2492. [Google Scholar] [CrossRef]
- Bošnjak, B.; Kazemi, S.; Altenburger, L.M.; Mokrović, G.; Epstein, M.M. Th2-TRMs Maintain Life-Long Allergic Memory in Experimental Asthma in Mice. Front. Immunol. 2019, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, C.-L.; Yang, B.; Du, T.; Li, X.-L.; Zhang, P.; Ge, M.-R.; Lian, Y.; Li, H.; Liu, Y.-D.; et al. Prophylactic Administration of Fingolimod (FTY720) Ameliorated Experimental Autoimmune Myasthenia Gravis by Reducing the Number of Dendritic Cells, Follicular T Helper Cells and Antibody-Secreting Cells. Int. Immunopharmacol. 2021, 96, 107511. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, L. Revisiting the Role of the Granuloma in Tuberculosis. Nat. Rev. Immunol. 2012, 12, 352–366. [Google Scholar] [CrossRef]
- Alves, J.L.; Gavilanes, F.; Jardim, C.; dos Santos Fernandes, C.J.C.; Morinaga, L.T.K.; Dias, B.; Hoette, S.; Humbert, M.; Souza, R. Pulmonary Arterial Hypertension in the Southern Hemisphere. Chest 2015, 147, 495–501. [Google Scholar] [CrossRef]
- Lapa, M.; Dias, B.; Jardim, C.; Fernandes, C.J.C.; Dourado, P.M.M.; Figueiredo, M.; Farias, A.; Tsutsui, J.; Terra-Filho, M.; Humbert, M.; et al. Cardiopulmonary Manifestations of Hepatosplenic Schistosomiasis. Circulation 2009, 119, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Tandrup, T.; Gundersen, H.J.; Jensen, E.B. The Optical Rotator. J. Microsc. 1997, 186, 108–120. [Google Scholar] [CrossRef]


| Antibody Specificity | Fluorochrome | Clone | Final Concentration (μg) | Manufacturer |
|---|---|---|---|---|
| anti-mouse CD16/32 (blocking Fc domain) | - | 93 | 1 | BioLegend |
| anti-mouse CD19 | eFluo450 | 1D3 | 0.5 | eBioscience |
| anti-mouse NK1.1 | eFluo450 | PK136 | 0.5 | eBioscience |
| anti-mouse Ly6G | eFluo450 | 1A8 | 0.5 | eBioscience |
| Fixable Viability dye | eFluo450 | 0.0625 | eBioscience | |
| anti-mouse CD45 | AF700 | 30 F11 | 1 | eBioscience |
| BV570 | 30 F11 | 2 | BioLegend | |
| anti-mouse CD3 | FITC | 17A2 | 0.5 | BioLegend |
| anti-mouse CD4 | APC | RM4-5 | 0.5 | BioLegend |
| anti-mouse CD8 | PECy7 | 53-5.8 | 0.5 | BioLegend |
| anti-mouse CD62L | BV650 | MEL-14 | 0.8 | BioLegend |
| anti-mouse CD45R/B220 | BV605 | RA3-6B2 | 1 | BioLegend |
| anti-mouse CD44 | PE | IM7 | 0.5 | BioLegend |
| anti-mouse CD69 | BV785 | H1.2F3 | 0.5 | BioLegend |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca Balladares, D.C.; Kassa, B.; Mickael, C.; Kumar, R.; Nolan, K.; Menezes, T.C.F.; Lee, M.H.; Lau-Xiao, A.M.; Molofsky, A.B.; Wells, E.; et al. Intrapulmonary T Cells Are Sufficient for Schistosoma-Induced Pulmonary Hypertension. Int. J. Mol. Sci. 2024, 25, 9202. https://doi.org/10.3390/ijms25179202
Fonseca Balladares DC, Kassa B, Mickael C, Kumar R, Nolan K, Menezes TCF, Lee MH, Lau-Xiao AM, Molofsky AB, Wells E, et al. Intrapulmonary T Cells Are Sufficient for Schistosoma-Induced Pulmonary Hypertension. International Journal of Molecular Sciences. 2024; 25(17):9202. https://doi.org/10.3390/ijms25179202
Chicago/Turabian StyleFonseca Balladares, Dara C., Biruk Kassa, Claudia Mickael, Rahul Kumar, Kevin Nolan, Thais C. F. Menezes, Michael H. Lee, Anthony M. Lau-Xiao, Ari B. Molofsky, Elina Wells, and et al. 2024. "Intrapulmonary T Cells Are Sufficient for Schistosoma-Induced Pulmonary Hypertension" International Journal of Molecular Sciences 25, no. 17: 9202. https://doi.org/10.3390/ijms25179202
APA StyleFonseca Balladares, D. C., Kassa, B., Mickael, C., Kumar, R., Nolan, K., Menezes, T. C. F., Lee, M. H., Lau-Xiao, A. M., Molofsky, A. B., Wells, E., & Graham, B. B. (2024). Intrapulmonary T Cells Are Sufficient for Schistosoma-Induced Pulmonary Hypertension. International Journal of Molecular Sciences, 25(17), 9202. https://doi.org/10.3390/ijms25179202





