Effects of Dietary Bio-Fermented Selenium Supplementation on Growth, Immune Performance, and Intestinal Microflora of Chinese Mitten Crabs, Eriocheir sinensis
Abstract
1. Introduction
2. Results
2.1. Growth Performance
2.2. Activities of Antioxidant Enzyme
2.3. Immunity-Related Genes Expression
2.4. Intestinal Morphology
2.5. Intestinal Flora and OTUs
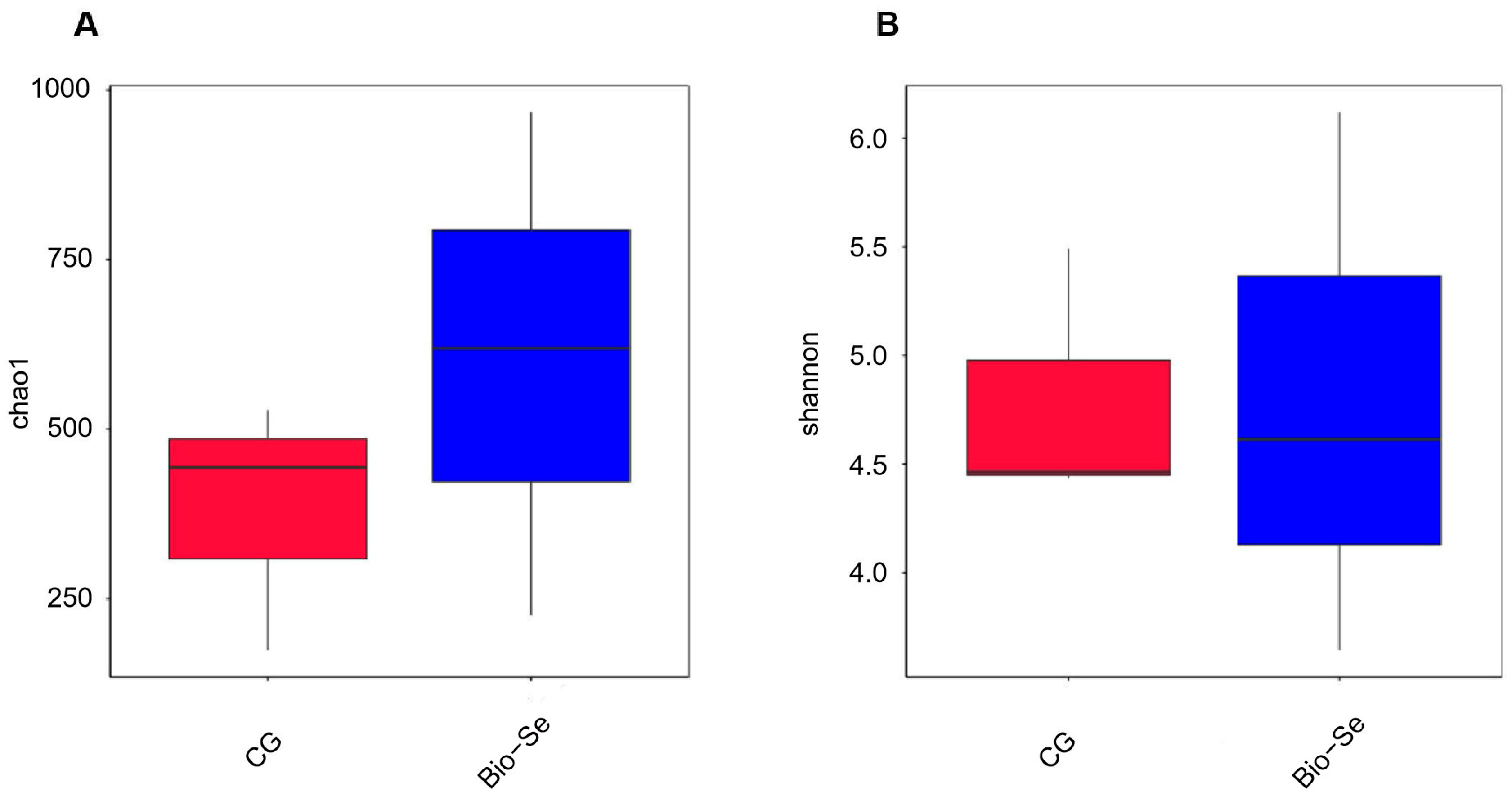
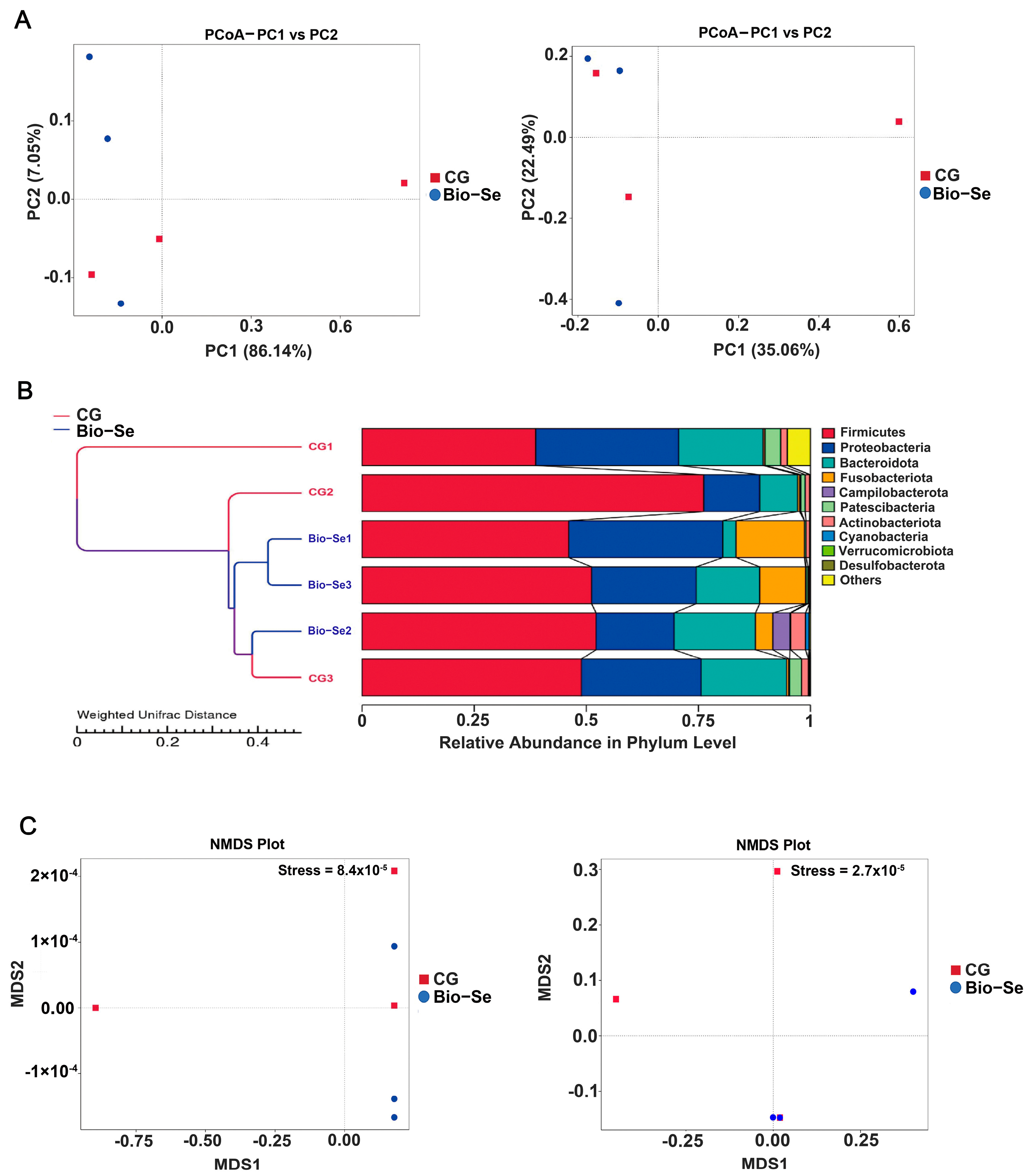
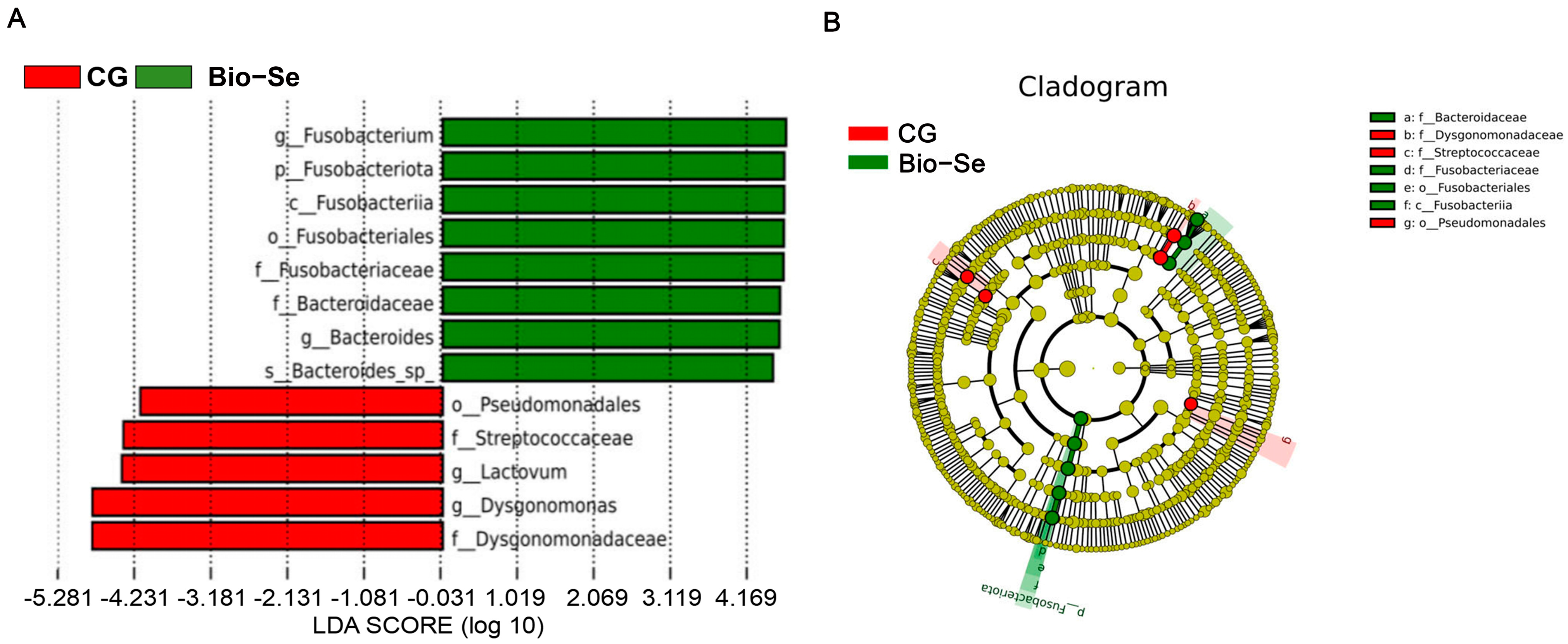
2.6. Intestinal Microbial Diversity and Composition
3. Discussion
4. Materials and Methods
4.1. Diets Preparation
4.2. Experimental Animals and Management Procedure
4.3. Sample Collection
4.4. Growth Parameters Analysis
4.5. Biochemical Analysis
4.6. Quantitative Real-Time PCR Analysis (qRT-PCR)
4.7. Analysis of Intestinal Morphology
4.8. Analysis of Bacterial Diversity in the Intestine
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanda, S.; Paul, B.N.; Ghosh, K.; Giri, S.S. Dietary Essentiality of Trace Minerals in Aquaculture-A Review. Agric. Rev. 2015, 36, 100–112. [Google Scholar] [CrossRef]
- Steven, J.H. Review of Selenium Toxicity in The Aquatic Food Chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar]
- Leonardo, S.T.; Jaqueline Dalbello, B.T.; Cleber Fernando, M.M.; Elisabeth, C.U.; Rodrigo, Y.G.; Marcos, V.S. Long-term Organic Selenium Supplementation Overcomes the Trade-off Between Immune and Antioxidant Systems in Pacu (Piaractus mesopotamicus). Fish Shellfish Immunol. 2017, 60, 311–317. [Google Scholar]
- Silva-Brito, F.; Magnoni, L.J.; Fonseca, S.B.; Peixoto, M.J.; Castro, L.F.; Cunha, I.; Magalhães, F.A.; Gonçalves, J.F. Dietary Oil Source and Selenium Supplementation Modulate Fads2 and Elovl5 Transcriptional Levels in Liver and Brain of Meagre (Argyrosomus regius). Lipids 2016, 51, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Folts, C.M. Selenium as An Integral Part of Factor Against Dietary Necrotic Liver Degeneration. Nutrition 1999, 15, 255. [Google Scholar] [PubMed]
- Khan, K.U.; Zuberi, A.; Nazir, S.; Ullah, I.; Jamil, Z.; Sarwar, H. Synergistic Effects of Dietary Nano Selenium and Vitamin C on Growth, Feeding, and Physiological Parameters of Mahseer Fish (Tor putitora). Aquac. Rep. 2017, 5, 70–75. [Google Scholar] [CrossRef]
- Dawood, M.; Koshio, S.; Zaineldin, A.I.; Van Doan, D.H.; Ahmed, H.A.; Elsabagh, M.; Abdel-Daim, M.M. An Evaluation of Dietary Selenium Nanoparticles for Red Sea Bream (Pagrus major) Aquaculture: Growth, Tissue Bioaccumulation, and Antioxidative Responses. Environ. Sci. Pollut. Res. 2019, 26, 30876–30884. [Google Scholar] [CrossRef]
- Wangkahart, E.; Bruneel, B.; Chantiratikul, A.; Jong, M.D.; Pakdeenarong, N.; Subramani, P.A. Optimum Dietary Sources and Levels of Selenium Improve Growth, Antioxidant Status, and Disease Resistance: Re-Evaluation in A Farmed Fish Species, Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 121, 172–182. [Google Scholar] [CrossRef]
- Kong, Y.; Ding, Z.; Zhang, Y.; Ye, J.; Du, Z. Dietary Selenium Requirement of Juvenile Oriental River Prawn (Macrobrachium nipponense). Aquaculture 2017, 476, 72–78. [Google Scholar] [CrossRef]
- Xu, J.W.; Gong, Y.F.; Sun, Y.; Cai, J.Z.; Liu, Q.; Bao, J.; Yang, J.; Zhang, Z.W. Impact of Selenium Deficiency on Inflammation, Oxidative Stress, and Phagocytosis in Mouse macrophages. Biol. Trace. Elem. Res. 2020, 194, 237–243. [Google Scholar] [CrossRef]
- Khan, K.U.; Zuberi, A.; Nazir, S.; Fernandes, J.B.K.; Jamil, Z.; Sarwar, H. Effects of Dietary Selenium Nanoparticles on Physiological and Biochemical Aspects of Juvenile Tor putitora. Turk. J. Zool. 2016, 40, 704–712. [Google Scholar] [CrossRef]
- Marie-Claire, T.; Boucher, F.; Sylvie, P.; Stephane, T.; Christophe, R.; Drissa, S.; Nicole, T. Effects of Selenium Deficiency on the Response of Cardiac Tissue to Ischemia and Reperfusion. Toxicology 2000, 148, 125–132. [Google Scholar]
- Maier, K.J.; Knight, A.W. Ecotoxicology of Selenium in Freshwater Systems. Rev. Environ. Contam. Toxicol. 1994, 134, 31–48. [Google Scholar]
- Wang, L.; Wu, J.; Wang, W.N.; Cai, D.X.; Liu, D.X.; Wang, A.L. Glutathione Peroxidase from the White Shrimp Litopenaeus vannamei: Characterization and Its Regulation upon pH and Cd Exposure. Ecotoxicology 2012, 21, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, J.C. Oxidative stress, Neurotoxicity, and Non-specific Immune Responses in Juvenile Red Sea Bream, Pagrus major, Exposed to Different Waterborne Selenium Concentrations. Chemosphere 2015, 13, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Huang, Y.H.; Wang, G.X.; Wu, Y.X.; Xian, J.A.; Wang, A.L.; Cao, J.M. Deficient and Excess Dietary Selenium Levels Affect Growth Performance, Blood cells Apoptosis and Liver HSP70 Expression in Juvenile Yellow Catfish Pelteobagrus fulvidraco. Fish. Physiol. Biochem. 2016, 42, 249–261. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, J.; Gao, M.; Zhu, Y.; Hong, Y. Excessive Selenium Affects Neural Development and Locomotor Behavior of Zebrafish embryos. Ecotoxicol. Environ. Saf. 2022, 238, 113611. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Chernick, M.; Li, D.; Ying, G.G.; Yang, J.; Zheng, N.; Xie, L.; Hinton, D.E.; Dong, W. Selenomethionine Exposure Affects Chondrogenic Differentiation and Bone Formation in Japanese Medaka (Oryzias latipes). J. Hazard. Mater. 2020, 387, 121720. [Google Scholar] [CrossRef]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and Seleno proteins: An Overview on Different Biological Systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef]
- Marek, K. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Rider, S.A.; Jha, A.N.; Fisher, A.A.; Knight, J.J.; Sweetman, W. Supra-nutritional Dietary Intake of Selenite and Selenium Yeast in Normal and Stressed Rainbow Trout (Oncorhynchus mykiss): Implications on Selenium Status and Health Responses. Aquaculture 2009, 295, 282–291. [Google Scholar] [CrossRef]
- Bai, Z.A.; Ren, T.J.; Han, Y.Z.; Hu, Y.N.; Jiang, Z.Q. Effect of Dietary Bio-fermented Selenium on Growth Performance, Nonspecific Immune Enzyme, Proximate Composition and Bioaccumulation of Zebrafish (Danio rerio). Aquac. Rep. 2019, 13, 100180. [Google Scholar] [CrossRef]
- Hu, Y.N.; Bai, Z.A.; Jiang, Z.Q.; Ren, T.J. Toxicity Effects of Different Dietary Selenium Forms on Sea Cucumber Apostichopus japonicus. Aquac. Rep. 2019, 15, 100209. [Google Scholar] [CrossRef]
- Teng, Z.L.; Zhang, F.; Wang, L.; Wang, L.; Huang, T.T.; Zhang, X.Z. Dietary selenium requirement of triploid rainbow trout (Oncorhynchus mykiss) based on selenium yeast supplementation. Aquacult. Res. 2022, 53, 3365–3378. [Google Scholar] [CrossRef]
- Chen, F.F.; Zhang, Z.Y.; Wang, L.; Yu, H.D.; Zhang, X.Z.; Rong, K.M. Dietary Selenium Requirement for On-growing Grass carp, Ctenopharyngodon idellus. Aquaculture 2023, 573, 739572. [Google Scholar] [CrossRef]
- Mansour, A.E.; Goda, A.A.; Omar, E.A.; Khalil, H.S.; Esteban, M.Á.J.F. Dietary Supplementation of Organic Selenium Improves Growth, Survival, Antioxidant and Immune Status of Meagre, Argyrosomus regius. Fish Shellfish Immunol. 2017, 68, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.R.; Xia, C.Y.; Han, F.L.; Xu, C.; Rombenso, A.; Qin, J.G.; Chen, L.Q.; Li, E.C. Effect of Different Dietary Selenium Sources on Growth Performance, Antioxidant Capacity, Gut Microbiota, and Molecular Responses in Pacific White Shrimp Litopenaeus vannamei. Aquacult. Nutr. 2022, 2022, 5738008. [Google Scholar] [CrossRef]
- Tian, W.J.; Li, E.C.; Chen, L.Q.; Sun, L.M.; Chen, Y.L.; Li, M.; Jiang, X.; Du, Z.Y. Growth, Body Composition and Anti-oxidative Status of Juvenile Chinese mitten crabs, Eriocheir sinensis Fed Different Dietary Selenium Levels. J. Fish. Sci. China 2014, 21, 92–100. [Google Scholar]
- Nugroho, R.A.; Fotedar, R. Effects of Dietary Organic Selenium on Immune Responses, Total Selenium Accumulation and Digestive System Health of Marron, Cherax cainii. Aquacult. Res. 2015, 46, 1657–1667. [Google Scholar] [CrossRef]
- Wang, D.C.; Wei, L.R.; Liu, T.; Ma, J.B.; Huang, K.Y.; Guo, H.M.; Huang, Y.F.; Zhang, L.; Zhao, J.; Tsuda, K.; et al. Suppression of ETI by PTI Priming to Balance Plant Growth and Defense through an MPK3/MPK6-WRKYs-PP2Cs Module. Mol. Plant 2023, 16, 903–918. [Google Scholar] [CrossRef]
- Wang, X.; Maite, T.P.; Yao, Q.; Zhang, D.M.; Zhang, B.Y.; Chen, Y.K.; Guo, Z.X. Comparison of the Growth Performance and Nutritional Qualities of Chinese mitten crab (Eriocheir sinensis) with Different Stocking Densities in Rice-crab Culture Systems. Aquac. Rep. 2021, 20, 100761. [Google Scholar] [CrossRef]
- Jiang, X.D.; Wang, H.N.; Cheng, Y.X.; Wu, X.G. Growth Performance, Gonad Development and Nutritional Composition of Chinese mitten crab Eriocheir sinensis Selected for Growth and Different Maturity Time. Aquaculture 2020, 523, 735194. [Google Scholar] [CrossRef]
- Pang, Y.Y.; Song, Y.M.; Zhang, L.; Song, X.Z.; Zhang, C.; Lv, J.H. 5-HT2B, 5-HT7, and DA2 Receptors Mediate the Effects of 5-HT and DA on Agonistic Behavior of the Chinese Mitten Crab (Eriocheir sinensis). ACS. Chem. Neurosci. 2019, 10, 4502–4510. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Shen, Z.H.; Wang, C.L.; Li, J.E.; Qin, G.; Chen, L.Q. Dietary Supplementation of Selenium Yeast Enhances the Antioxidant Capacity and Immune Response of Juvenile Eriocheir Sinensis Under Nitrite Stress. Fish Shellfish Immunol. 2019, 87, 22–31. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Wang, Y.; Liu, Y.; Zhu, H.; Li, Z.; Shan, P.; Yuan, Z. Comparative Assessment of Chinese Mitten Crab Aquaculture in China: Spatiotemporal Changes and Trade-offs. Environ. Pollut. 2023, 337, 122544. [Google Scholar] [CrossRef] [PubMed]
- Long, X.W.; Pan, J.; Wade, N.M.; Sun, Y.F.; Liu, Y.M.; Cheng, Y.X.; Wu, X.G. Effects of Three Feeding Modes on the Metabolism, Antioxidant Capacity, and Metabolome of the Adult Male Chinese mitten crab Eriocheir sinensis. Aquacult. Int. 2022, 30, 1101–1119. [Google Scholar] [CrossRef]
- Ghaffarizadeh, A.; Sotoudeh, E.; Mozanzadeh, M.T.; Sanati, A.M.; Ghasemi, A. Supplementing Dietary Selenium Nano-particles Increased Growth, Antioxidant Capacity and Immune-related Genes Transcription in Pacific whiteleg shrimp (Penaeus vannamei) juveniles. Aquac. Rep. 2022, 25, 101215. [Google Scholar] [CrossRef]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as Feed: Using Economic Allocation to Quantify the Fish In: Fish Out Ratio of Major Fed Aquaculture Species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Yu, Q.R.; Fu, Z.Q.; Huang, M.X.; Xu, C.; Wang, X.D.; Qin, J.G.; Chen, L.Q.; Han, F.L.; Li, E.C. Growth, Physiological, Biochemical, and Molecular Responses of Pacific White Shrimp Litopenaeus vannamei fed different levels of dietary selenium. Aquaculture 2021, 535, 736393. [Google Scholar] [CrossRef]
- Yuan, L.X.; Zhang, R.; Ma, X.Z.; Yang, L.; Zheng, Q.; Chen, D.; Li, M.; Fan, T.; Liu, Y.X.; Pan, L.P.; et al. Selenium Accumulation, Antioxidant Enzyme Levels, and Amino Acids Composition in Chinese mitten crab (Eriocheir sinensis) fed Selenium-biofortified Corn. Nutrients 2018, 10, 318. [Google Scholar] [CrossRef]
- Qin, F.; Shi, M.; Yuan, H.; Yuan, L.; Lu, W.; Zhang, J.; Tong, J.; Song, X. Dietary Nano-selenium Relieves Hypoxia Stress and, Improves Immunity and Disease Resistance in the Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2016, 54, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Mai, K.S.; Zhang, W.B.; Xu, W.; Ai, Q.H.; Liu, Z.G.; Li, H.T. Dietary Selenium Requirement and Its Toxicity in Juvenile Abalone Haliotis discus hannai Ino. Aquaculture 2012, 330, 42–46. [Google Scholar] [CrossRef]
- Dawit Moges, F.; Hamdi, H.; Al-Barty, A.; Zaid, A.A.; Sundaray, M.; Parashar, S.K.S.; Gubale, A.G.; Das, B. Effects of Selenium Nanoparticle on the Growth Performance and Nutritional Quality in Nile Tilapia, Oreochromis niloticus. PLoS ONE 2022, 17, e0268348. [Google Scholar] [CrossRef] [PubMed]
- Contempre, B.; Le Moine, O.; Dumont, J.E.; Denef, J.F.; Many, M.C. Selenium Deficiency and Thyroid Fibrosis. A key Role for Macrophages and Transforming Growth Factor beta (TGF-beta). Mol. Cell. Endocrinol. 1996, 124, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Selenium, Iodine and Iron-essential Trace Elements for Thyroid Hormone Synthesis and Metabolism. Int. J. Mol. Sci. 2023, 24, 3393. [Google Scholar] [CrossRef]
- Hofstee, P.; Bartho, L.A.; McKeating, D.R.; Radenkovic, F.; McEnroe, G.; Fisher, J.J.; Holland, O.J.; Vanderlelie, J.J.; Perkins, A.V.; Cuffe, J.S.M. Maternal Selenium Deficiency During Pregnancy in Mice Increases Thyroid Hormone Concentrations, Alters Placental Function and Reduces Fetal Growth. J. Physiol. 2019, 597, 5597–5617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Chen, X.Y.; Shao, M.L.; Zhang, J.J.; Wang, K.; Lin, G.; Li, M.Z. Hepatopancreatic Transcriptome Profiles Reveal the Effects of Toxic Dietary Concentrations of Selenium on the Immunity and Growth of Juvenile Abalone Haliotis discus hannai. Aquac. Rep. 2023, 28, 101449. [Google Scholar] [CrossRef]
- Canesi, L. Pro-oxidant and Antioxidant Processes in Aquatic Invertebrates. Ann. N. Y. Acad. Sci. 2015, 1340, 1–7. [Google Scholar] [CrossRef]
- Johansson, M.W.; Holmblad, T.; Thornqvist, P.O.; Cammarata, M.; Parrinello, N.; Soderhall, K. A Cell-surface Superoxide Dismutase Is a Binding Protein for Peroxinectin, a Cell-adhesive Peroxidase in Crayfish. Cell. Sci. 1999, 112, 917–925. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Z.; Guo, Y.; Lu, S.; Du, H.; Cao, Y. Antioxidant Capacity of Flavonoids from Folium Artemisiae Argyi and the Molecular Mechanism in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 279, 114398. [Google Scholar] [CrossRef]
- Missirlis, F.; Rahlfs, S.; Dimopoulos, N.; Bauer, H.; Becker, K.; Hilliker, A.; Phillips, J.P.; Jäckle, H.A. Putative Glutathione Peroxidase of Drosophila Encodes a Thioredoxin Peroxidase that Provides Resistance Against Oxidative Stress but Fails to Complement a Lack of Catalase Activity. Biol. Chem. 2003, 384, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The Role of Glutathione Peroxidase-1 in Health and Disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Toppo, S.; Orian, L. The Glutathione Peroxidase Family: Discoveries and Mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dotan, Y.; Lichtenberg, D.; Pinchuk, I. Lipid Peroxidation cannot be Used as a Universal Criterion of Oxidative Stress. Prog. Lipid Res. 2004, 43, 200–227. [Google Scholar] [CrossRef] [PubMed]
- Egessa, R. Antimicrobial Peptides from Freshwater Invertebrate Species: Potential for Future Applications. Mol. Biol. Rep. 2022, 249, 9797–9811. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Yuan, J.; Xiang, F. Chitin Synthesis and Degradation in Crustaceans: A Genomic View and Application. Mar. Drugs. 2021, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, F. The Anti-lipopolysaccharide Factors in Crustaceans. Subcell. Biochem. 2020, 94, 63–80. [Google Scholar]
- Barreto, C.; Matos, G.M.; Rosa, R.D. On the Wave of the Crustin Antimicrobial Peptide Family: From Sequence Diversity to Function. Fish Shellfish Immunol. Rep. 2022, 3, 100069. [Google Scholar] [CrossRef]
- Vanderkelen, L.; Van Herreweghe, J.M.; Michiels, C.W. Lysozyme Inhibitors as Tools for Lysozyme Profiling: Identification and Antibacterial Function of Lysozymes in the Hemolymph of the Blue Mussel. Molecules 2023, 28, 7071. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, S.; Liu, L.; Li, R.; Jin, P.; Li, S. Drosophila Relish Activating lncRNA-CR33942 Transcription Facilitates Antimicrobial Peptide Expression in Imd Innate Immune Response. Front. Immunol. 2022, 13, 905899. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B. ERK/MAPK Signalling Pathway and Tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.R.; Miao, Z.Y.; Shi, X.X.; Wu, H.; Yao, Y.J.; Xu, S.W. The Antagonistic Effect of Selenium on Lead-induced Apoptosis and Necroptosis via P38/JNK/ERK Pathway in Chicken Kidney. Ecotoxicol. Environ. Saf. 2022, 231, 113176. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Cheng, Y.; Song, D.; Li, X.; Hu, Y.; Lu, Z.; Wang, F.; Wang, Y. Selenium-enriched Bacillus paralicheniformis SR14 Attenuates H2O2-induced Oxidative Damage in Porcine Jejunum Epithelial Cells via the MAPK Pathway. Appl. Microbiol. Biotechnol. 2019, 103, 6231–6243. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Q.; Xu, C.; Wang, S.; Li, J.; Liu, Z.; Li, S. Methionine Selenium Antagonizes LPS-induced Necroptosis in the Chicken Liver via the miR-155/TRAF3/MAPK Axis. J. Cell. Physiol. 2021, 236, 4024–4035. [Google Scholar]
- Tran, N.T.; Zhang, J.; Xiong, F.; Wang, G.T.; Li, W.X.; Wu, S.G. Altered Gut Microbiota Associated with Intestinal Disease in Grass Carp (Ctenopharyngodon idellus). World J. Microbiol. Biotechnol. 2018, 34, 71. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of Environmental Pollutants on Gut Microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Long, Y.; Li, B.; Zhao, L.; Luo, J.; Xu, L.; Luo, W.; Du, Z.; Zhou, J.; Yang, S. Rice-shrimp Culture: A Better Intestinal microbiota, Immune Enzymatic Activities, and Muscle Relish of Crayfish (Procambarus clarkii) in Sichuan Province. Appl. Microbiol. Biotechnol. 2020, 104, 9413–9420. [Google Scholar] [CrossRef]
- Guo, K.; Ruan, G.; Fan, W.; Wang, Q.; Fang, L.; Luo, J.; Liu, Y. Immune Response to Acute Heat Stress in the Intestine of the Red Swamp Crayfish, Procambarus clarkii. Fish Shellfish Immunol. 2020, 100, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Wu, Q.; Mo, B.; Zhou, J.; Li, J.; Zou, J.; Fang, L. Intestinal Morphology and Microflora to Vibrio alginolyticus in Pacific White Shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2022, 121, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Krogdahl, A.; Saele, O.; Chikwati, E.; Lokka, G.; Kortner, T.M. Digestive and Immune Functions in the Intestine of Wild Ballan Wrasse (Labrus bergylta). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2021, 260, 111011. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Wang, L.L.; Song, L.S. An Insulin-like Peptide Serves as a Regulator of Glucose Metabolism in the Immune Response of Chinese mitten crab Eriocheir sinensis. Dev. Comp. Immunol. 2020, 108, 103686. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, X.; Fu, L. Effect of Selenium Nanoparticles with Different Sizes in Primary Cultured Intestinal Epithelial Cells of Crucian Carp, Carassius auratus gibelio. Int. J. Nanomed. 2013, 8, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, H.; Li, P.; Wang, C.; Liu, G.; Zhang, X.; Zhang, C.; Qi, M.; Ji, H. Dietary Nano-selenium Alleviated Intestinal Damage of Juvenile Grass Carp (Ctenopharyngodon idella) Induced by High-fat Diet: Insight from Intestinal Morphology, Tight Junction, Inflammation, Anti-oxidization and Intestinal Microbiota. Anim. Nutr. 2022, 8, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction Between the Gut Microbiome and Mucosal Immune System. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Pushpanathan, P.; Mathew, G.S.; Selvarajan, S.; Seshadri, K.G.; Srikanth, P. Gut Microbiota and its Mysteries. Indian J. Med. Microbiol. 2019, 37, 268–277. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; Rinaldis, E.D.; Bell, J.T.; Venter, J.C.; et al. Interplay Between the Human Gut Microbiome and Host Metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef]
- Shang, X.C.; Wang, B.; Sun, Q.S.; Zhang, Y.; Lu, Y.T.; Liu, S.J.; Li, Y.H. Selenium-enriched Bacillus Subtilis Reduces the Effects of Mercury-induced on Inflammation and Intestinal Microbes in Carp (Cyprinus carpio var. specularis). Fish Physiol. Biochem. 2022, 48, 215–226. [Google Scholar] [CrossRef]
- Hong, Y.; Huang, Y.; Wu, S.; Yang, X.; Dong, Y.; Xu, D.; Huang, Z. Effects of Imidacloprid on the Oxidative Stress, Detoxification and Gut Microbiota of Chinese Mitten Crab, Eriocheir sinensis. Sci. Total. Environ. 2020, 729, 138276. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, Z.G.; Luo, L.; Wang, S.H.; Zhang, R.; Xu, W.; Qiao, G. Immune and Intestinal Microbiota Responses to Aerial Exposure Stress in Chinese Mitten crab (Eriocheir sinensis). Aquaculture 2021, 541, 736833. [Google Scholar] [CrossRef]
- Feng, W.R.; Feng, W.; Ge, J.C.; Li, J.L.; Su, S.; Jia, R.; Yu, J.; Xu, P.; Tang, Y. Alterations of Amino Acid Metabolism and Intestinal Microbiota in Chinese Mitten Crab (Eriocheir sinensis) Fed on Formulated Diet and Iced Trash Fish. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2021, 40, 100924. [Google Scholar] [CrossRef]
- Potrykus, J.; Mahaney, B.; White, R.L.; Bearne, S.L. Proteomic Investigation of Glucose Metabolism in the Butyrate-producing Gut Anaerobe Fusobacterium varium. Proteomics 2007, 7, 1839–1853. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Xie, Y.L.; Xiao, X.Y.; Kang, Z.R.; Lin, X.L.; Zhang, L.; Li, C.S.; Qian, Y.; Xu, P.P.; Leng, X.X.; et al. Fusobacterium Nucleatum-derived Succinic Acid Induces Tumor Resistance to Immunotherapy in Colorectal Cancer. Cell Host Microbe 2023, 31, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, Y.; Wang, L.; Ren, G.; Zou, E. Effects of Cadmium Alone and in Combination with Low Molecular Weight Chitosan on Metallothionein, Glutathione-S-transferase, Acid Phosphatase, and ATPase of Freshwater Cab Sinopotamon yangtsekiense. Environ. Toxicol. 2014, 29, 298–309. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Effects of Saline-alkali Stress on the Tissue Structure, Antioxidation, Immunocompetence and Metabolomics of Eriocheir sinensis. Sci. Total Environ. 2023, 871, 162109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, X.; Wang, J.; Li, C. Effect of Pure Microcystin-LR on Activity and Transcript Level of Immune-related Enzymes in the White Shrimp (Litopenaeus vannamei). Ecotoxicology 2017, 26, 702–710. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yi, Q.L.; Zhao, D.; Wang, L.L.; Song, L.S. Interval Feeding of CpG ODN Supplemental Diets Enhanced Growth in Juvenile Chinese Mitten Crab, Eriocheir sinensis. Aquac. Res. 2022, 53, 5559–5571. [Google Scholar] [CrossRef]
- Chi, T.; Zhao, Q.; Wang, P. Fecal 16S rRNA Gene Sequencing Analysis of Changes in the Gut Microbiota of Rats with Low-dose Aspirin-related Intestinal Injury. Biomed. Res. Int. 2021, 1, 8848686. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Knight, R. QIIME Allows Analysis of High-throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
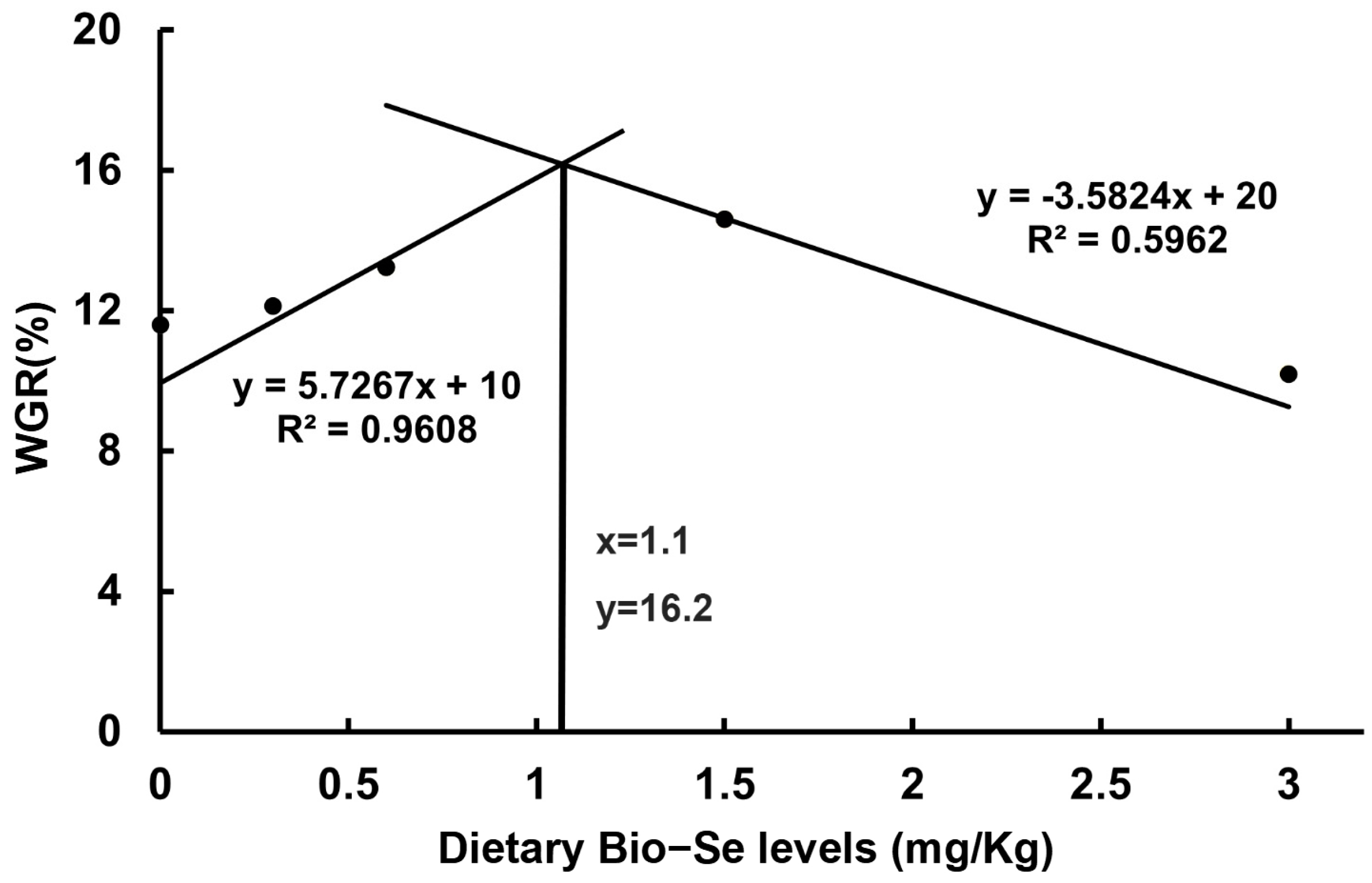
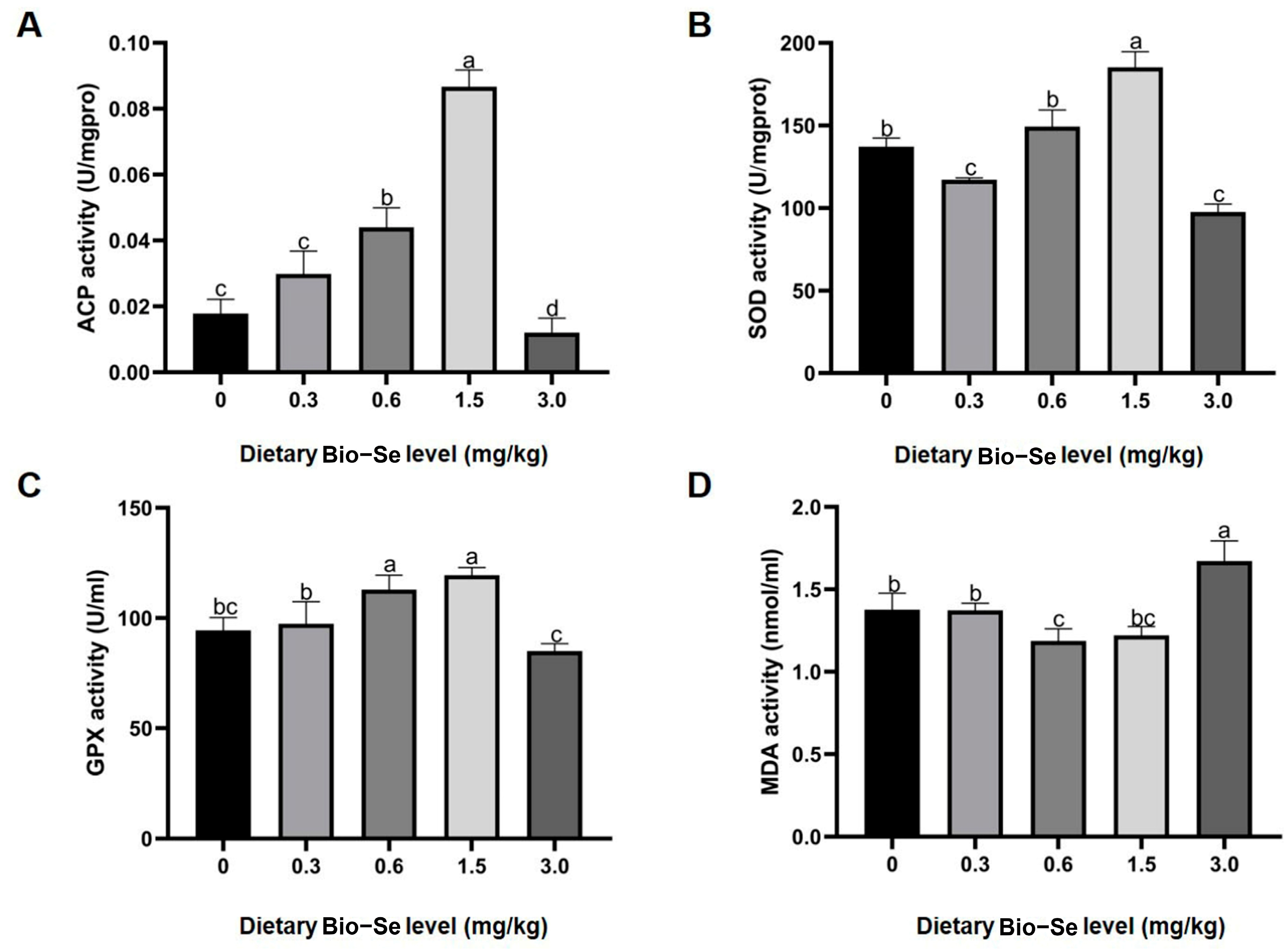

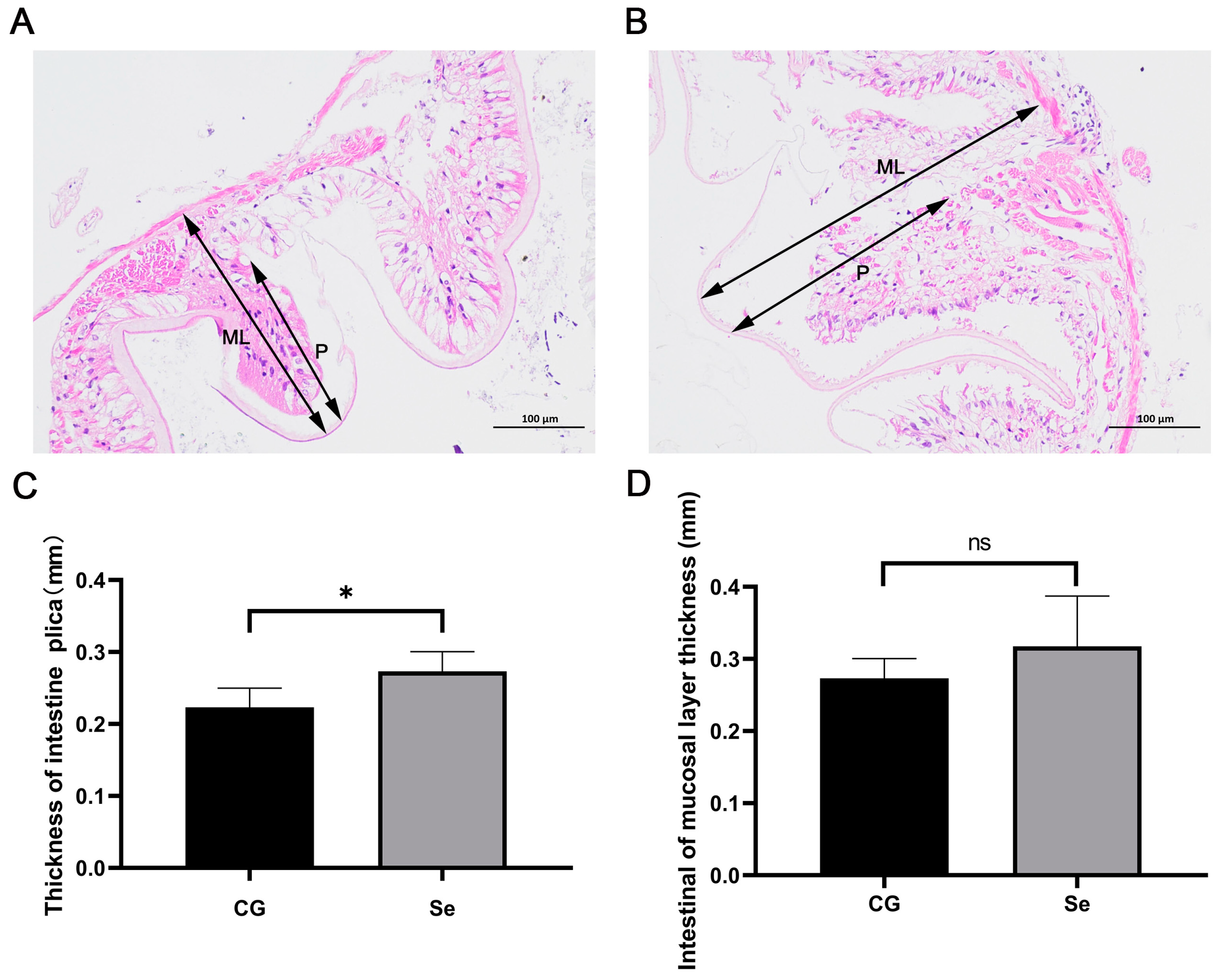
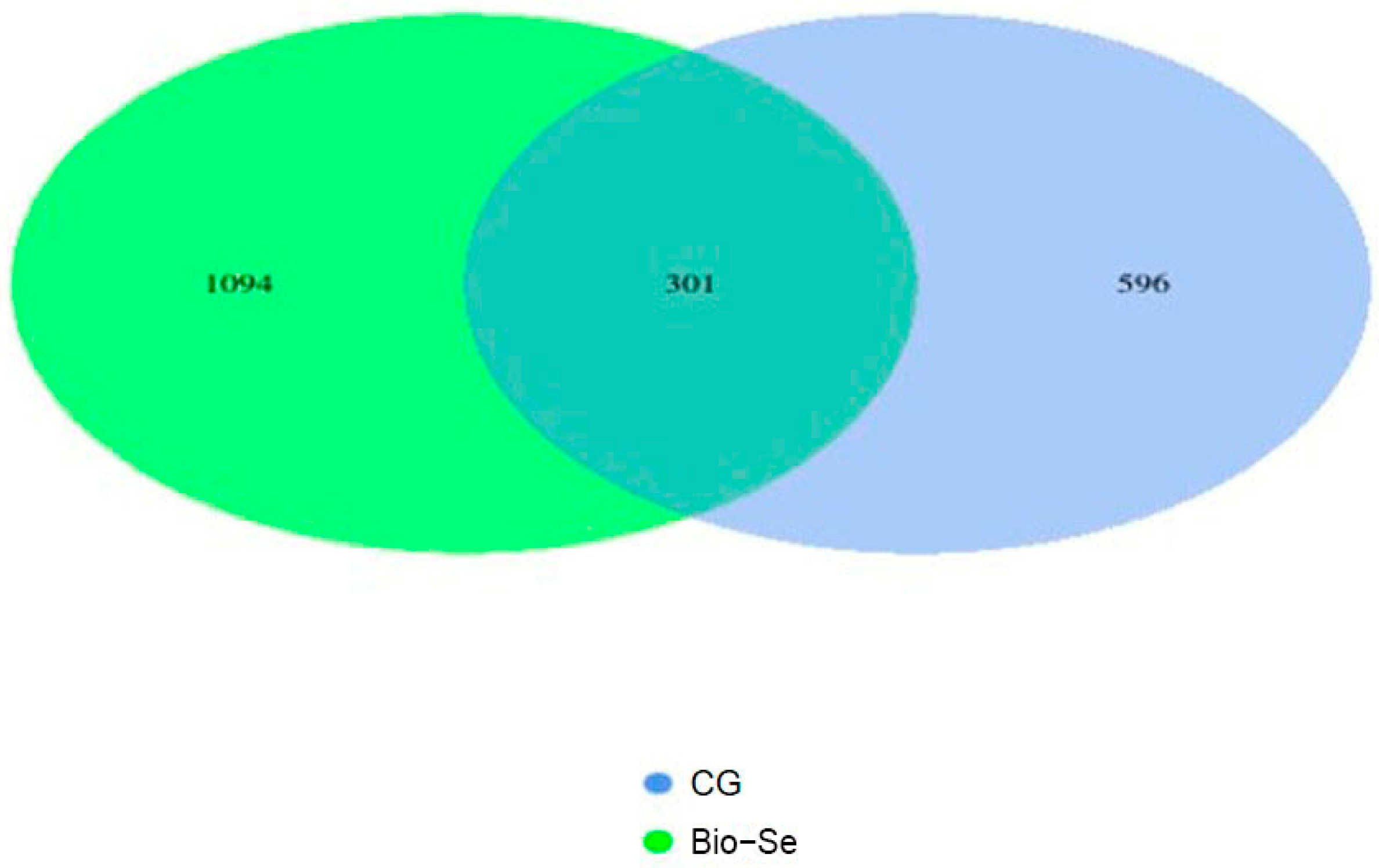

| Bio−Se (mg/kg) | Wt (g) | WGR (%) | SGR (%/day) | SR (%) |
|---|---|---|---|---|
| 0 | 18.14 ± 0.34 c | 11.81 ± 0.27 d | 0.38 ± 0.03 bc | 63.33 ± 6.67 b |
| 0.3 | 18.47 ± 0.32 b | 12.54 ± 0.41 c | 0.42 ± 0.03 bc | 85.93 ± 2.97 a |
| 0.6 | 18.70 ± 0.49 ab | 13.71 ± 0.48 b | 0.45 ± 0.03 ab | 80.96 ± 0.94 a |
| 1.5 | 18.87 ± 0.45 a | 14.74 ± 0.48 a | 0.50 ± 0.03 a | 87.43 ± 1.73 a |
| 3.0 | 18.11 ± 0.32 c | 10.32 ± 0.13 d | 0.36 ± 0.04 c | 69.83 ± 0.17 b |
| Group | Chao1 | Observed_Features | Pielou_e | Shannon | Simple | Coverage |
|---|---|---|---|---|---|---|
| CG | 362.04 ± 27.95 a | 317.06 ± 51.27 a | 0.63 ± 0.12 a | 4.78 ± 0.00 a | 0.91 ± 0.02 a | 0.999 ± 0.00 a |
| Bio−Se | 552.06 ± 62.94 b | 438.17 ± 94.29 b | 0.59 ± 0.13 a | 4.77 ± 0.02 a | 0.90 ±0.02 a | 0.997 ± 0.01 a |
| Taxonomy | Firmicutes | Proteobacteria | Bacteroidota | Fusobacteriota | Campilobacterota |
|---|---|---|---|---|---|
| CG | 54.62 | 23.66 | 15.47 | 0.45 | 0.16 |
| Bio−Se | 49.81 | 25.02 | 11.77 | 9.80 | 1.32 |
| Ingredient | (%) |
|---|---|
| Fish meal 1 | 35.0 |
| Bean meal | 25.0 |
| Peanut meal | 16.0 |
| Corn starch | 5.7 |
| Soybean oil 2 | 4.0 |
| yeast | 5.0 |
| Cellulose | 4.7 |
| Vitamin mix 3 | 2.0 |
| Se-free mineral mix 4 | 2.0 |
| Cholesterol | 0.3 |
| Choline chloride | 0.2 |
| Ethopabate | 0.01 |
| Calcium propionate | 0.10 |
| Crude protein | 44.8 |
| Crude lipid | 7.8 |
| Primer | Sequence (5′-3′) |
|---|---|
| EsERK-F | TTCAGCAACAGGCTCATC |
| EsERK-R | TGTTCAGGAGGAGGTTTGATGGC |
| EsRelish-F | TCTCCCTACTCTGACCATTCC |
| EsRelish-R | TTCCCACCATCTCACTCTTGT |
| EsALF-1-F | GACGCAGGAGGATGCTAAC |
| EsALF-1-R | TGATGGCAGATGAAGGACAC |
| EsCrus-1-F | GCTCTATGGCGGAGGATGTCA |
| EsCrus-1-R | CGGGCTTCAGACCCACTTTAC |
| EsLYS-F | CTGGGATGATGTGGAGAAGTGC |
| EsLYS-R | TTATTCGGTGTGTTATGAGGGGT |
| Esβ-actin-F | GCATCCACGAGACCACTTACA |
| Esβ-actin-R | CTCCTGCTTGCTGATCCACATC |
| EsV3-V4-F EsV3-V4-R | CCTACGGGAGGCAGCAG GACTACCAGGGTATCTAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Li, J.; Ma, Y.; Wu, Z.; Li, J.; Wang, F.; Xi, Y.; Jiang, Y.; Huang, S.; Yi, Q. Effects of Dietary Bio-Fermented Selenium Supplementation on Growth, Immune Performance, and Intestinal Microflora of Chinese Mitten Crabs, Eriocheir sinensis. Int. J. Mol. Sci. 2024, 25, 9219. https://doi.org/10.3390/ijms25179219
Yang Z, Li J, Ma Y, Wu Z, Li J, Wang F, Xi Y, Jiang Y, Huang S, Yi Q. Effects of Dietary Bio-Fermented Selenium Supplementation on Growth, Immune Performance, and Intestinal Microflora of Chinese Mitten Crabs, Eriocheir sinensis. International Journal of Molecular Sciences. 2024; 25(17):9219. https://doi.org/10.3390/ijms25179219
Chicago/Turabian StyleYang, Zhichao, Jialin Li, Yuhan Ma, Zihao Wu, Jiaming Li, Fengchi Wang, Yuting Xi, Yusheng Jiang, Shu Huang, and Qilin Yi. 2024. "Effects of Dietary Bio-Fermented Selenium Supplementation on Growth, Immune Performance, and Intestinal Microflora of Chinese Mitten Crabs, Eriocheir sinensis" International Journal of Molecular Sciences 25, no. 17: 9219. https://doi.org/10.3390/ijms25179219
APA StyleYang, Z., Li, J., Ma, Y., Wu, Z., Li, J., Wang, F., Xi, Y., Jiang, Y., Huang, S., & Yi, Q. (2024). Effects of Dietary Bio-Fermented Selenium Supplementation on Growth, Immune Performance, and Intestinal Microflora of Chinese Mitten Crabs, Eriocheir sinensis. International Journal of Molecular Sciences, 25(17), 9219. https://doi.org/10.3390/ijms25179219





