Innovative Therapeutic Delivery of Metastasis-Associated in Colon Cancer 1-Suppressing miRNA Using High Transmembrane 4 L6 Family Member 5-Targeting Exosomes in Colorectal Cancer Mouse Models
Abstract
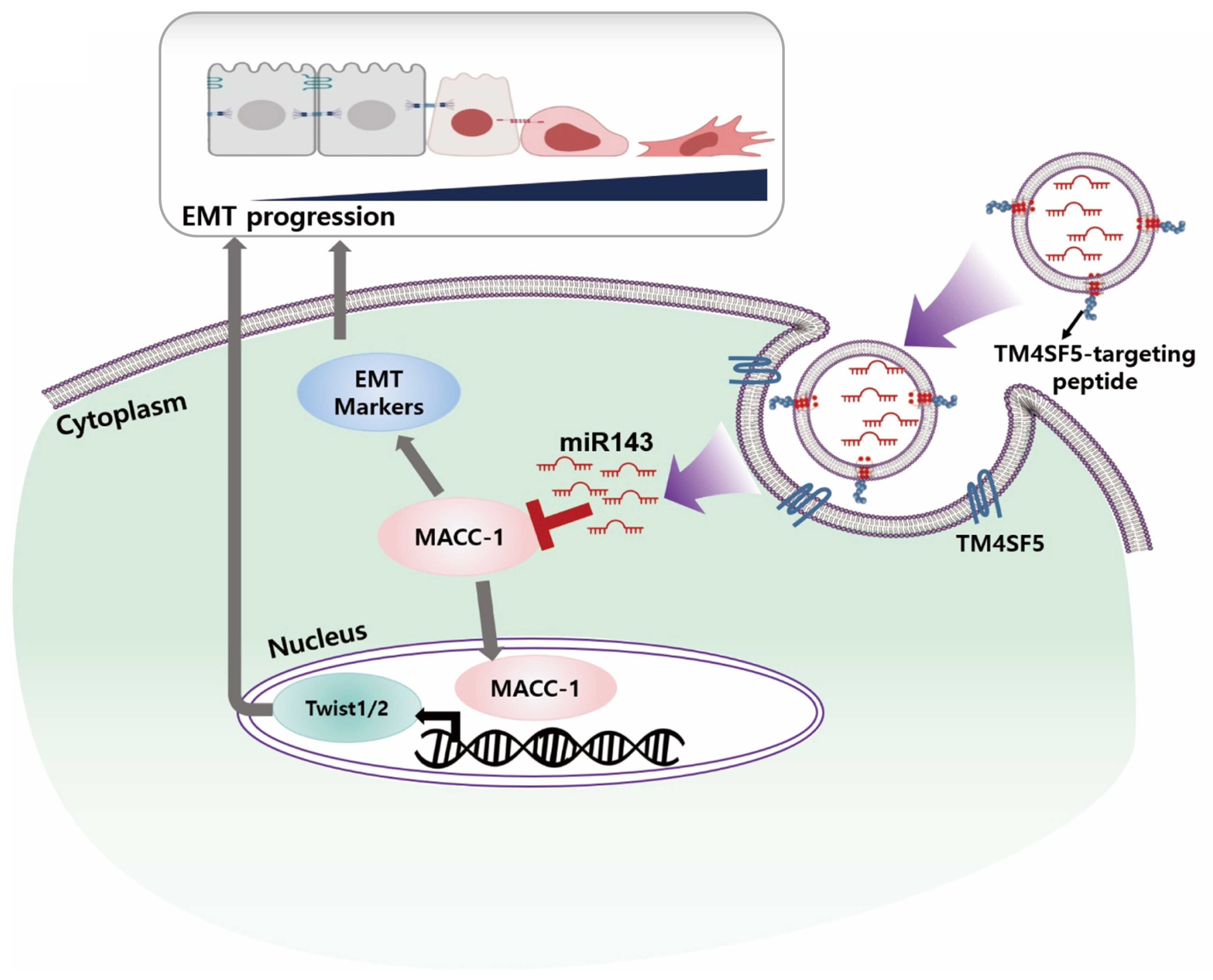
1. Introduction
2. Results
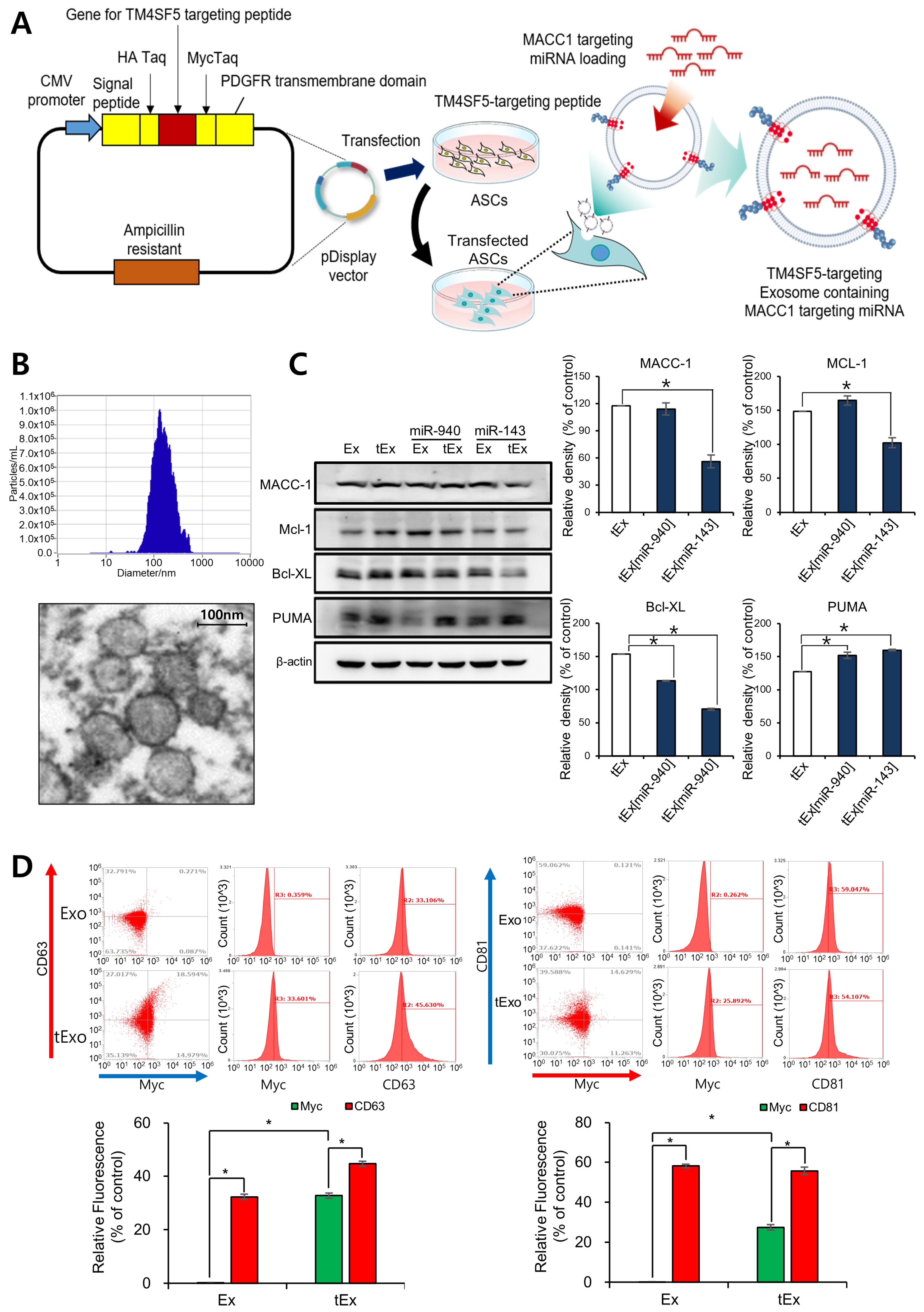
2.1. Anti-Tumor Effects of TM4SF5-Targeting Ex Encapsulating miR-143 in HCT116 Cells
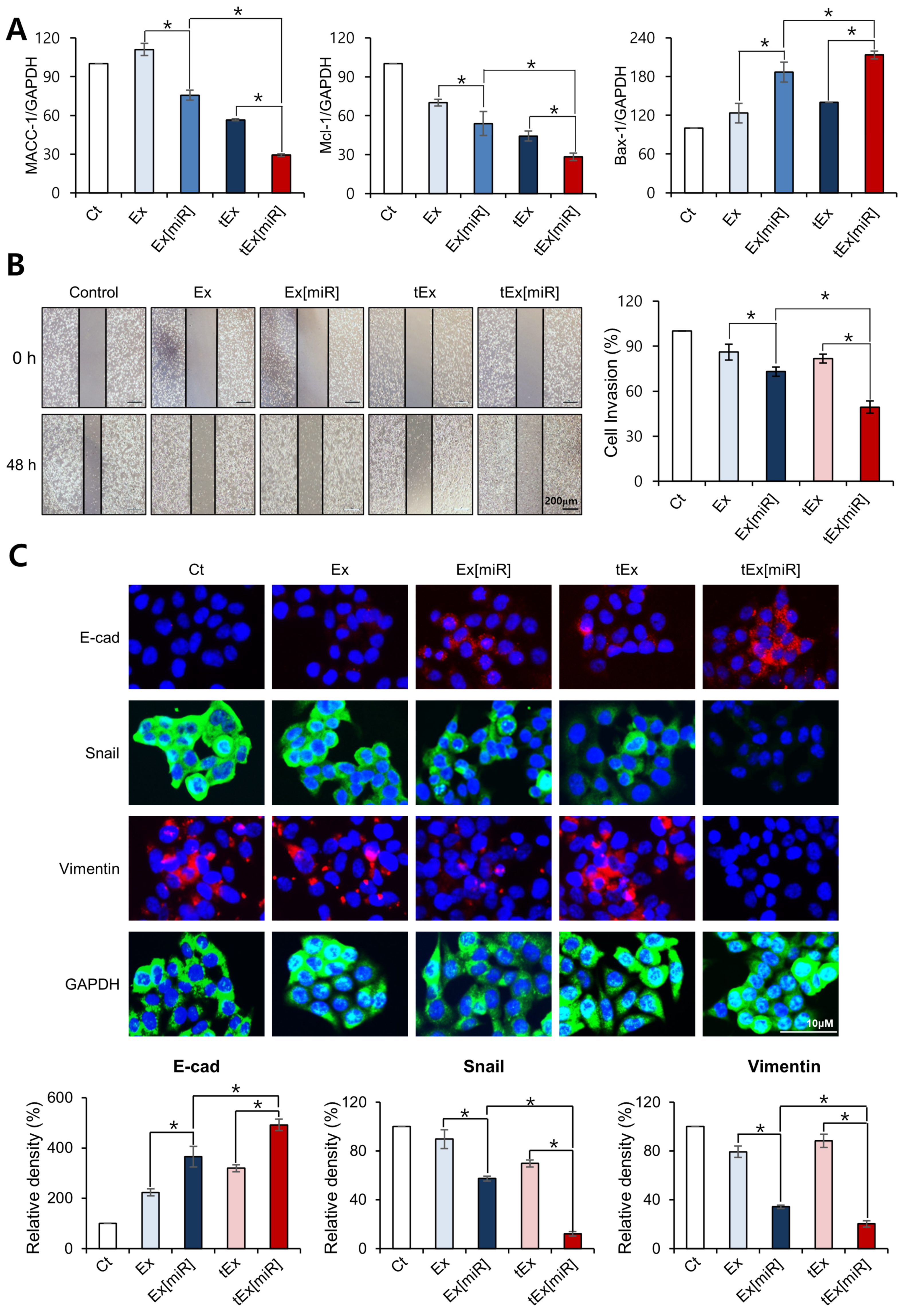
2.2. In Vitro Targetability and Efficacy of Intravenously Administered tEx[miR]
2.3. In Vivo Targetability and Efficacy of Intravenously Administered tEx[miR]
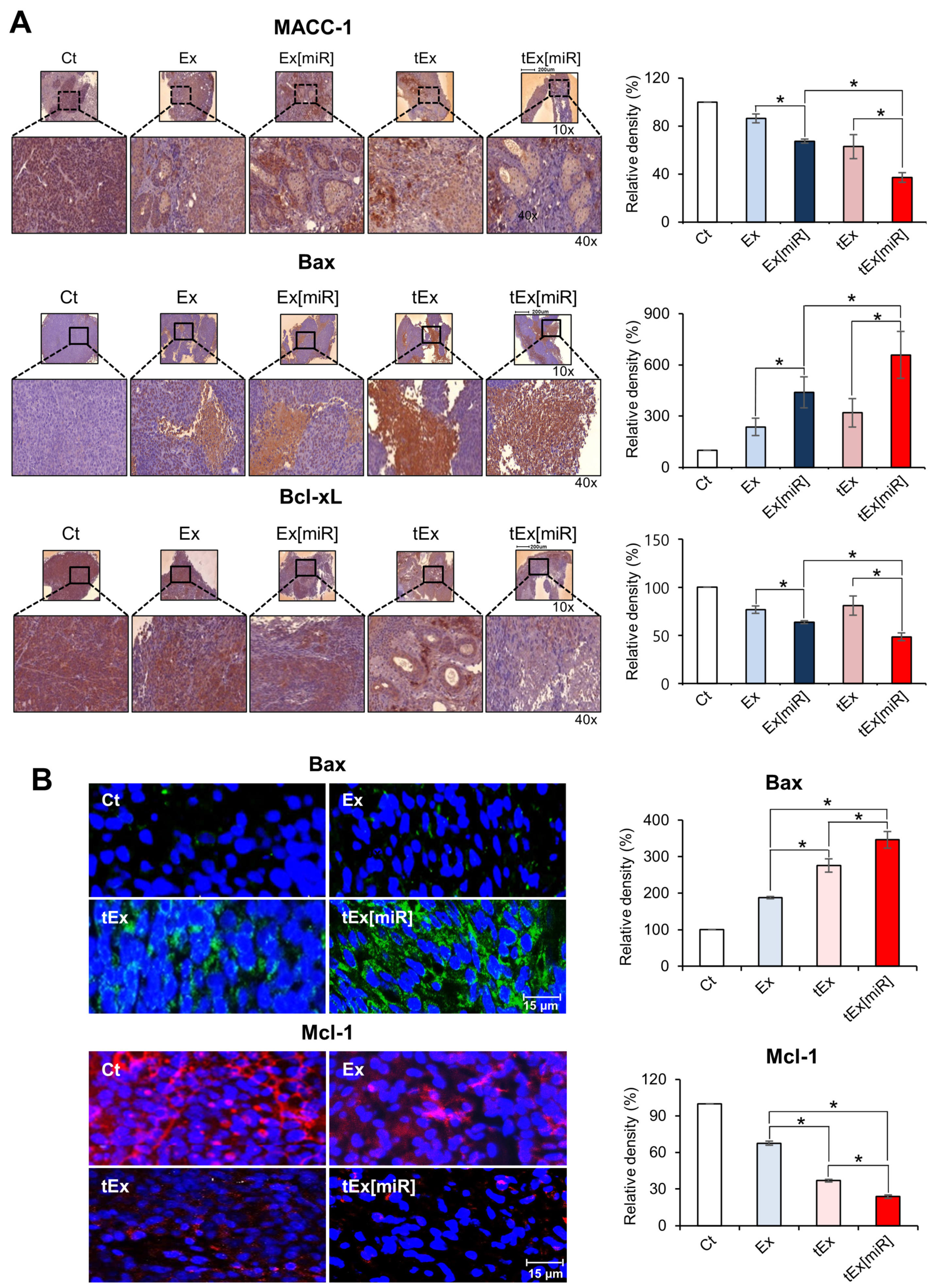
2.4. In Vivo Efficacy of tEx[miR] in Mouse CRC Xenograft Model
2.5. Immunohistochemistry and Immunofluorescence Analyses in Mouse Colorectal Cancer Xenograft Models
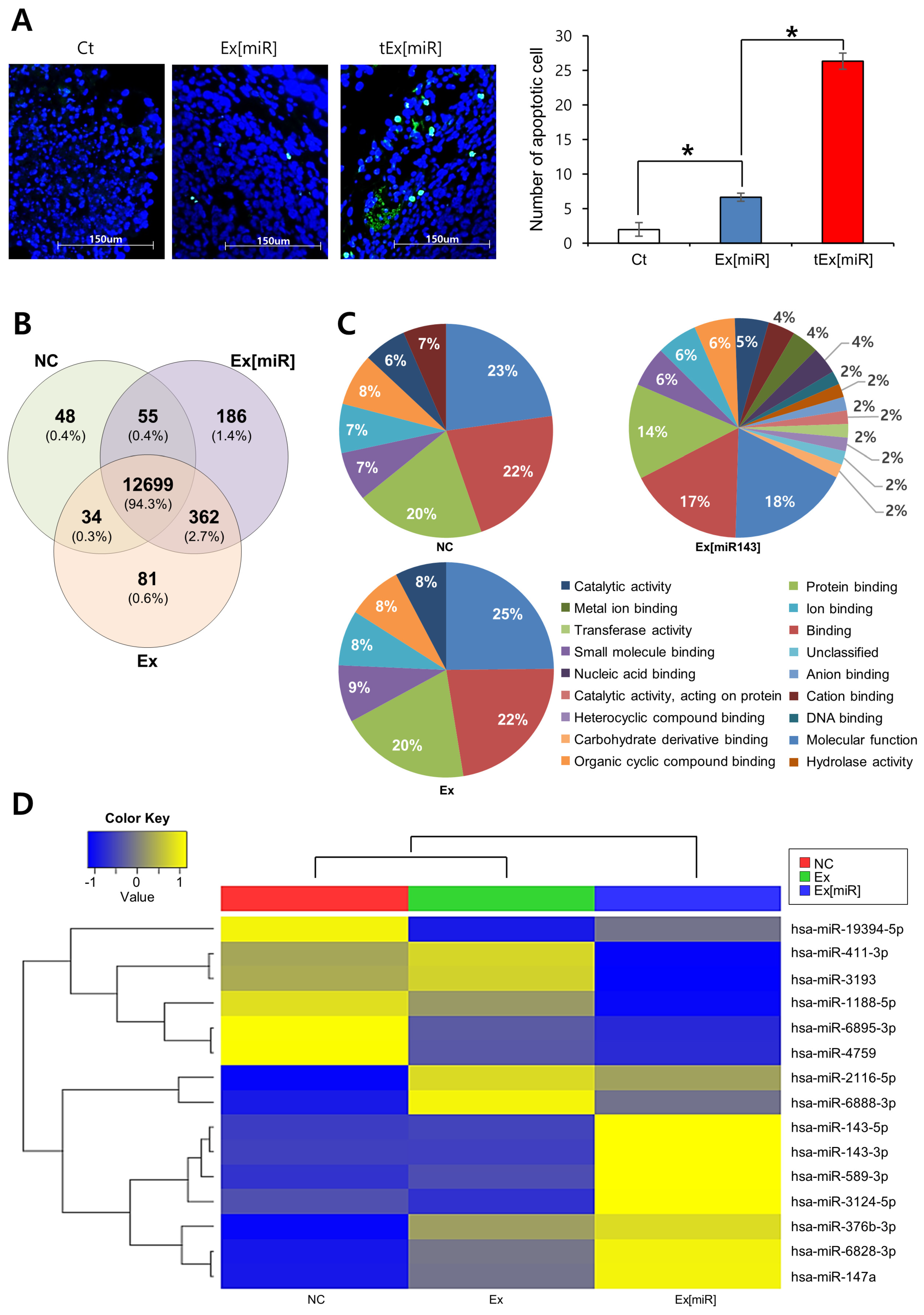
2.6. Analysis of Apoptosis in Mouse Tumor Tissues and miRNA Expression in HCT116 Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Establishment of Doxorubicin-Resistant CRC Cells
4.3. Production of TM4SF5-Targeted Ex (tEx)
4.4. Exosome Characterization
4.5. Transmission Electron Microscopy (TEM)
4.6. Real-Time PCR
4.7. Wound Healing Assay
4.8. Western Blot Analysis
4.9. Immunofluorescence and Immunohistochemical Analysis
4.10. Cell Viability Assay
4.11. Flow Cytometry
4.12. RNA Sequencing Library Construction and Validation
4.13. Spheroid Formation and Viability Assessment
4.14. Animal Study Design
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, H.; Cho, H.; Choi, J.; Hwang, K.Y.; Choi, Y.; Kim, S.H.; Yang, Y. Recent Advances in Exosome-Based Drug Delivery for Cancer Therapy. Cancers 2021, 13, 4435. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Saad, M.G.; Beyenal, H.; Dong, W.-J. Exosomes as Powerful Engines in Cancer: Isolation, Characterization and Detection Techniques. Biosensors 2021, 11, 518. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Rahim, N.S.; Wu, Y.S.; Sim, M.S.; Velaga, A.; Bonam, S.R.; Gopinath, S.C.B.; Subramaniyan, V.; Choy, K.W.; Teow, S.-Y.; Fareez, I.M.; et al. Three Members of Transmembrane-4-Superfamily, TM4SF1, TM4SF4, and TM4SF5, as Emerging Anticancer Molecular Targets against Cancer Phenotypes and Chemoresistance. Pharmaceuticals 2023, 16, 110. [Google Scholar] [CrossRef]
- Lorico, A.; Lorico-Rappa, M.; Karbanova, J.; Corbeil, D.; Pizzorno, G. CD9, A tetraspanin target for cancer therapy? Exp. Biol. Med. 2021, 246, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Kimura, T.; Funaki, S.; Ose, N.; Kanou, T.; Fukui, E. Therapeutic Targeting of Cancer-Associated Fibroblasts in the Non-Small Cell Lung Cancer Tumor Microenvironment. Cancers 2023, 15, 335. [Google Scholar] [CrossRef]
- Lee, M.; Lee, J.-S. Exploiting tumor cell senescence in anticancer therapy. BMB Rep. 2014, 47, 51–59. [Google Scholar] [CrossRef]
- Güllü, N.; Smith, J.; Herrmann, P.; Stein, U. MACC1-Dependent Antitumor Effect of Curcumin in Colorectal Cancer. Nutrients 2022, 14, 4792. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Jiao, Y.; Yang, B.; Ye, M.; Di, W.; Su, W.; Zhong, J. MACC1 as a Potential Target for the Treatment and Prevention of Breast Cancer. Biology 2023, 12, 455. [Google Scholar] [CrossRef]
- Ilm, K.; Kemmner, W.; Osterland, M.; Burock, S.; Koch, G.; Herrmann, P.; Schlag, P.M.; Stein, U. High MACC1 expression in combination with mutated KRAS G13 indicates poor survival of colorectal cancer patients. Mol. Cancer 2015, 14, 38. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Lugli, A. The Tumor Border Configuration of Colorectal Cancer as a Histomorphological Prognostic Indicator. Front. Oncol. 2014, 4, 29. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, N.; Zhang, J.; Xie, J.; Liu, F.; Xu, D.; Yu, X.; Tian, Y. Advanced research on vasculogenic mimicry in cancer. J. Cell. Mol. Med. 2015, 19, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Wu, C.; Wang, Y.; Xia, Y.; Chen, L.; Zhu, Q.; Chen, Y. MACC1 overexpression predicts a poor prognosis for non-small cell lung cancer. Med. Oncol. 2013, 31, 790. [Google Scholar] [CrossRef]
- Wu, X.L.; Cheng, B.; Li, P.Y.; Huang, H.J.; Zhao, Q.; Dan, Z.L.; Tian, D.A.; Zhang, P. MicroRNA-143 suppresses gastric cancer cell growth and induces apoptosis by targeting COX-2. World J. Gastroenterol. 2013, 19, 7758–7765. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yuan, X.; Ni, J.; Shen, L.; Cai, M.; Xu, L. Low Serum miR-607 Level as a Potential Diagnostic and Prognostic Biomarker in Patients of Pancreatic Ductal Adenocarcinoma: A Preliminary Study. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8882129. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Walther, W.; Arlt, F.; Schwabe, H.; Smith, J.; Fichtner, I.; Birchmeier, W.; Schlag, P.M. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat. Med. 2008, 15, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zong, Z.; Wu, D.; Sun, K.; Liu, B.; Zhao, Y. The role of metastasis-associated in colon cancer 1 (MACC1) in endometrial carcinoma tumorigenesis and progression. Mol. Carcinog. 2016, 56, 1361–1371. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Zhao, S.; Shi, Y.; Yao, J.; Zhang, Y.; Guo, H.; Liu, X. Overexpression of MACC1 and the association with hepatocyte growth factor/c-Met in epithelial ovarian cancer. Oncol. Lett. 2015, 9, 1989–1996. [Google Scholar] [CrossRef]
- Zhen, T.; Dai, S.; Li, H.; Yang, Y.; Kang, L.; Shi, H.; Zhang, F.; Yang, D.; Cai, S.; He, Y.; et al. MACC1 promotes carcinogenesis of colorectal cancer via beta-catenin signaling pathway. Oncotarget 2014, 5, 3756–3769. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Z.-M.; Jiang, C.-F.; Liu, X.; Chen, Q.-D.; Qian, X.; Li, D.-M.; Ge, X.; Wang, X.-F.; Liu, L.-Z.; et al. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget 2014, 5, 5416–5427. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.Z.; Xu, L.; Han, T.; Luan, J.; Li, X.; Liu, Y.; Wang, Z.; Liu, Q.; Kong, X.; et al. Exploring new frontiers: Cell surface vimentin as an emerging marker for circulating tumor cells and a promising therapeutic target in advanced gastric Cancer. J. Exp. Clin. Cancer Res. 2024, 43, 129. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 2016, 15, 18. [Google Scholar] [CrossRef]
- Hohmann, T.; Hohmann, U.; Dehghani, F. MACC1-induced migration in tumors: Current state and perspective. Front. Oncol. 2023, 13, 1165676. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.-X.; Wen, J.-G.; Zhou, H.-H. Metastasis-associated in colon cancer 1: A promising biomarker for the metastasis and prognosis of colorectal cancer. Oncol. Lett. 2017, 14, 3899–3908. [Google Scholar] [CrossRef]
- Xu, B.; Lv, W.; Li, X.; Zhang, L.; Lin, J. Clinicopathological significance of TM4SF5 expression in human hepatocellular carcinoma tissues. Oncol. Lett. 2019, 17, 5187–5192. [Google Scholar] [CrossRef]
- Li, B.; Jin, J.; Guo, D.; Tao, Z.; Hu, X. Immune Checkpoint Inhibitors Combined with Targeted Therapy: The Recent Advances and Future Potentials. Cancers 2023, 15, 2858. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.; Leahy, T.P.; Ray, J.; Wilkinson, S.; Sammon, C.; Subbiah, V. The value of innovation: Association between improvements in survival of advanced and metastatic non-small cell lung cancer and targeted and immunotherapy. BMC Med. 2021, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Zhan, J.; Zhao, X.; Chen, X.; Xiao, L.; Wu, K.; Ma, Y.; Li, M.; Yang, Y.; et al. Utility of comprehensive genomic profiling in directing treatment and improving patient outcomes in advanced non-small cell lung cancer. BMC Med. 2021, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Kim, H.; Griffith, T.S.; Panyam, J. Poly (d,l-lactide-co-glycolide) Nanoparticles as Delivery Platforms for TLR7/8 Agonist-Based Cancer Vaccine. J. Pharmacol. Exp. Ther. 2019, 370, 715–724. [Google Scholar] [CrossRef]
- Nanni, P.; Gatta, V.; Menotti, L.; De Giovanni, C.; Ianzano, M.; Palladini, A.; Grosso, V.; Dall’Ora, M.; Croci, S.; Nicoletti, G.; et al. Preclinical Therapy of Disseminated HER-2+ Ovarian and Breast Carcinomas with a HER-2-Retargeted Oncolytic Herpesvirus. PLoS Pathog. 2013, 9, e1003155. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.-J.; Lee, D.; Park, J.H.; Hong, T.H.; Kim, O.-H.; Lee, S.C.; Kim, K.-H.; Choi, H.J.; Kim, S.-J. Innovative Therapeutic Delivery of Metastasis-Associated in Colon Cancer 1-Suppressing miRNA Using High Transmembrane 4 L6 Family Member 5-Targeting Exosomes in Colorectal Cancer Mouse Models. Int. J. Mol. Sci. 2024, 25, 9232. https://doi.org/10.3390/ijms25179232
Choi B-J, Lee D, Park JH, Hong TH, Kim O-H, Lee SC, Kim K-H, Choi HJ, Kim S-J. Innovative Therapeutic Delivery of Metastasis-Associated in Colon Cancer 1-Suppressing miRNA Using High Transmembrane 4 L6 Family Member 5-Targeting Exosomes in Colorectal Cancer Mouse Models. International Journal of Molecular Sciences. 2024; 25(17):9232. https://doi.org/10.3390/ijms25179232
Chicago/Turabian StyleChoi, Byung-Jo, Dosang Lee, Jung Hyun Park, Tae Ho Hong, Ok-Hee Kim, Sang Chul Lee, Kee-Hwan Kim, Ho Joong Choi, and Say-June Kim. 2024. "Innovative Therapeutic Delivery of Metastasis-Associated in Colon Cancer 1-Suppressing miRNA Using High Transmembrane 4 L6 Family Member 5-Targeting Exosomes in Colorectal Cancer Mouse Models" International Journal of Molecular Sciences 25, no. 17: 9232. https://doi.org/10.3390/ijms25179232
APA StyleChoi, B.-J., Lee, D., Park, J. H., Hong, T. H., Kim, O.-H., Lee, S. C., Kim, K.-H., Choi, H. J., & Kim, S.-J. (2024). Innovative Therapeutic Delivery of Metastasis-Associated in Colon Cancer 1-Suppressing miRNA Using High Transmembrane 4 L6 Family Member 5-Targeting Exosomes in Colorectal Cancer Mouse Models. International Journal of Molecular Sciences, 25(17), 9232. https://doi.org/10.3390/ijms25179232





