Mesenchymal Stem Cell Therapy in Alopecia Areata: Visual and Molecular Evidence from a Mouse Model
Abstract
1. Introduction
2. Results
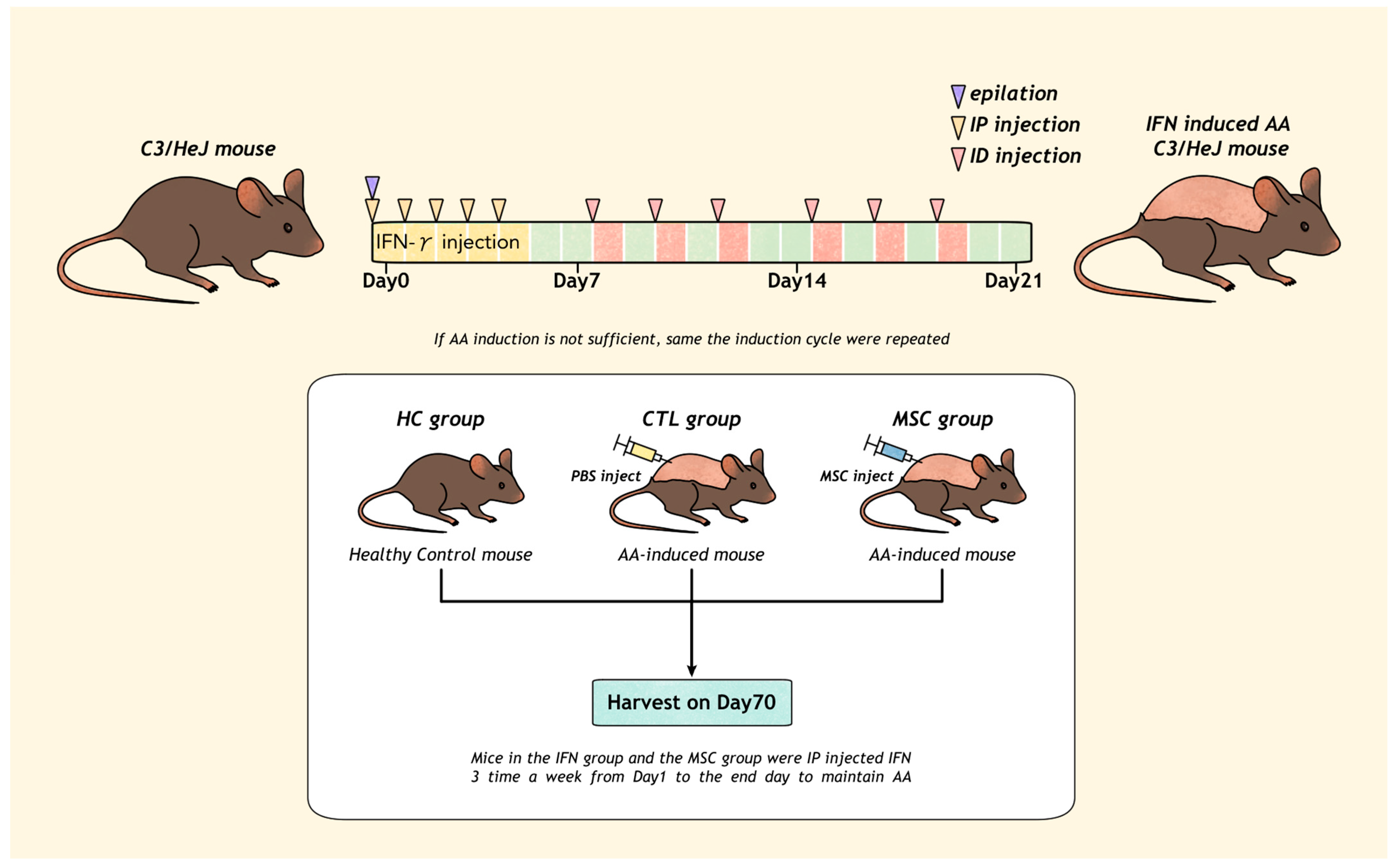
2.1. AA Induction
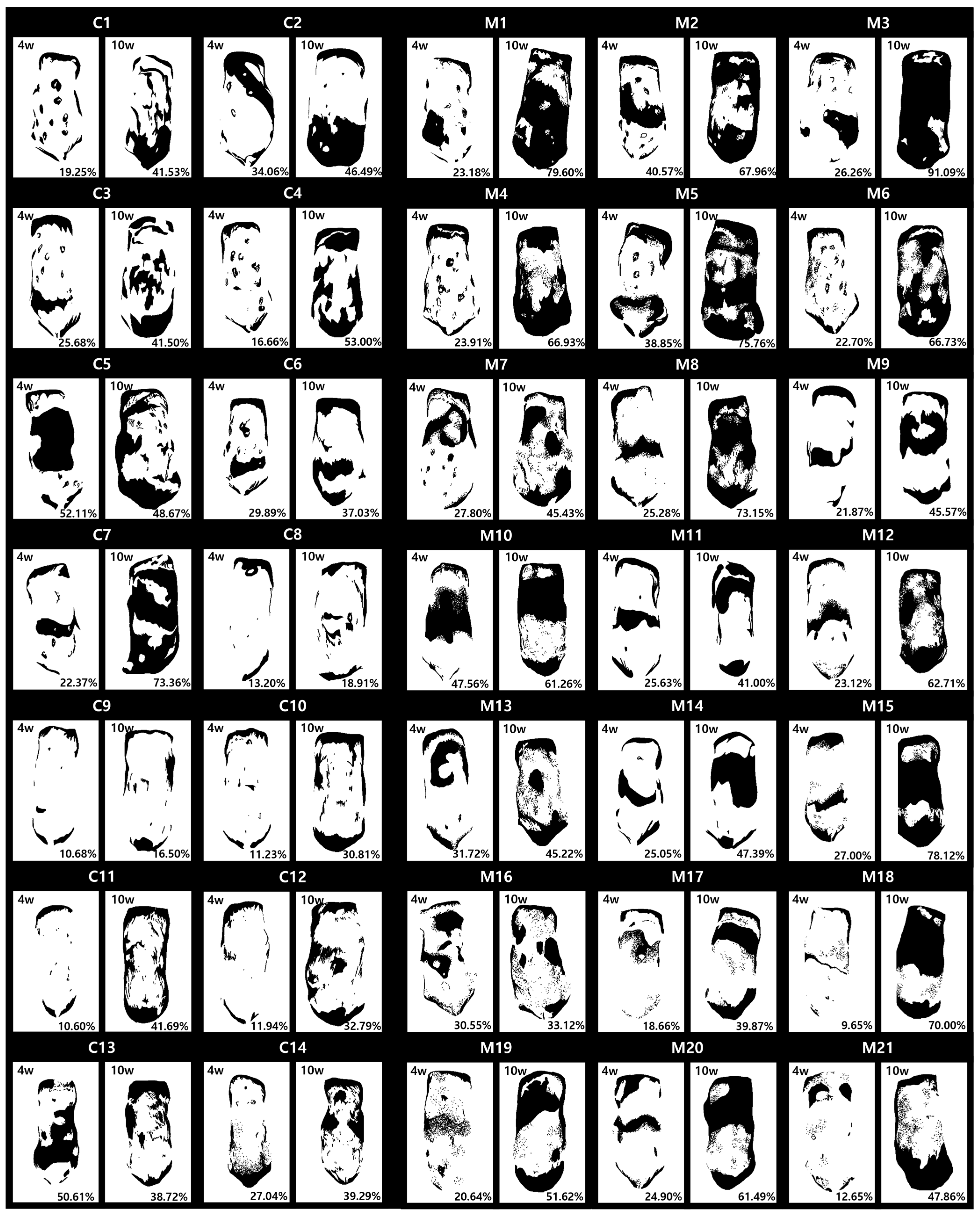
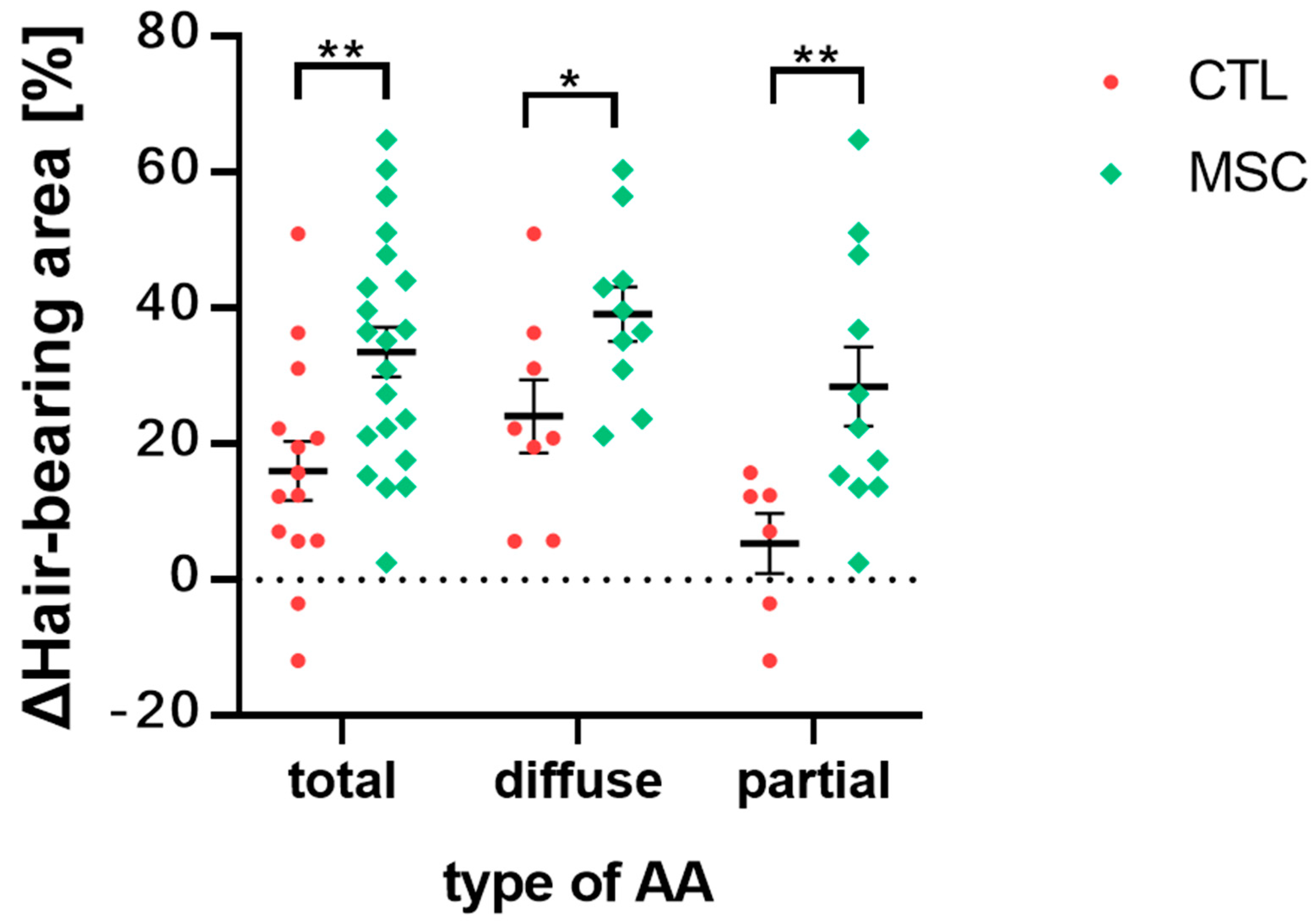
2.2. Global Assessment of Efficacy of MSCT in AA Mice
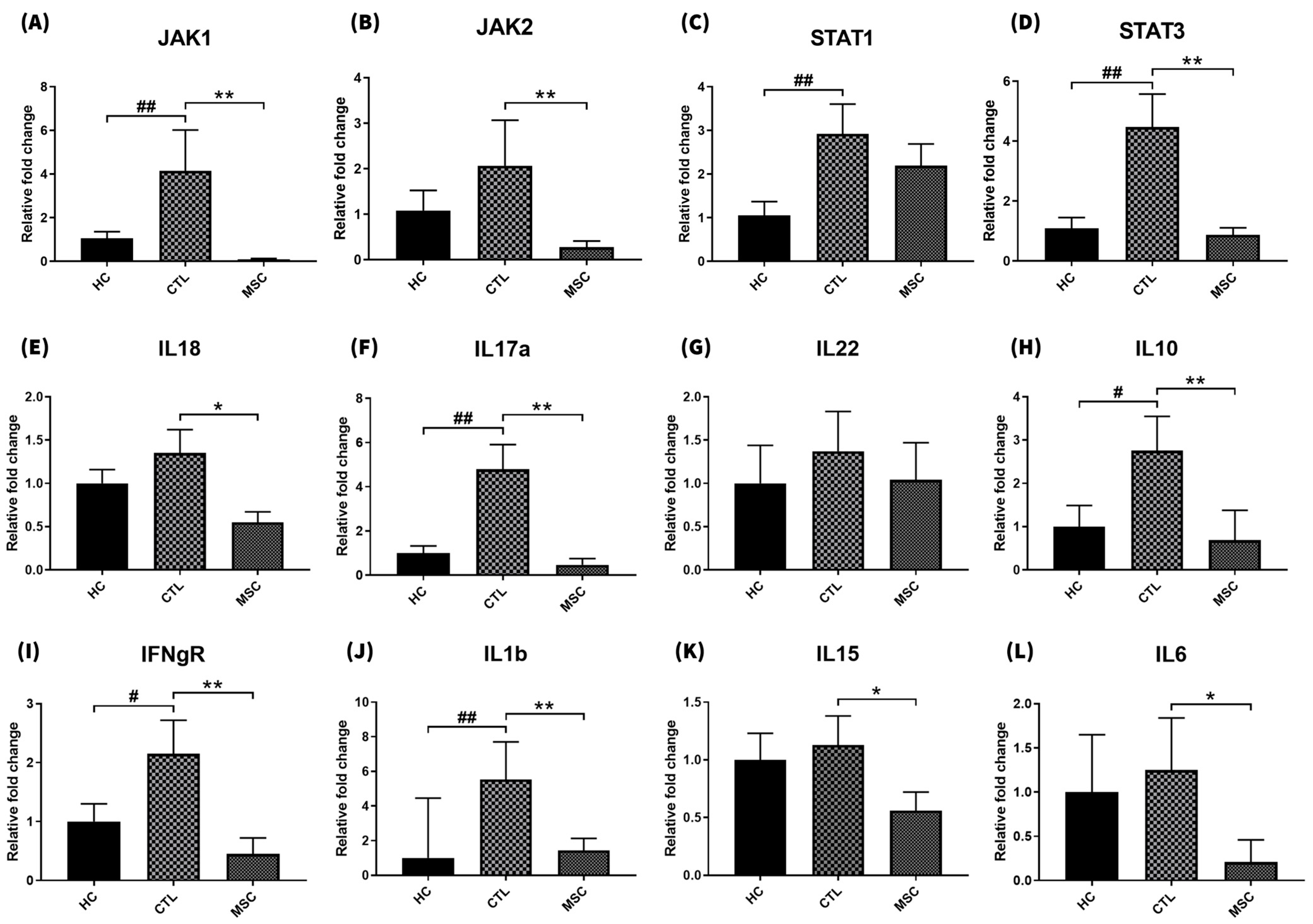
2.3. Effect of MSCT on Skin Inflammatory Factor Gene Expression
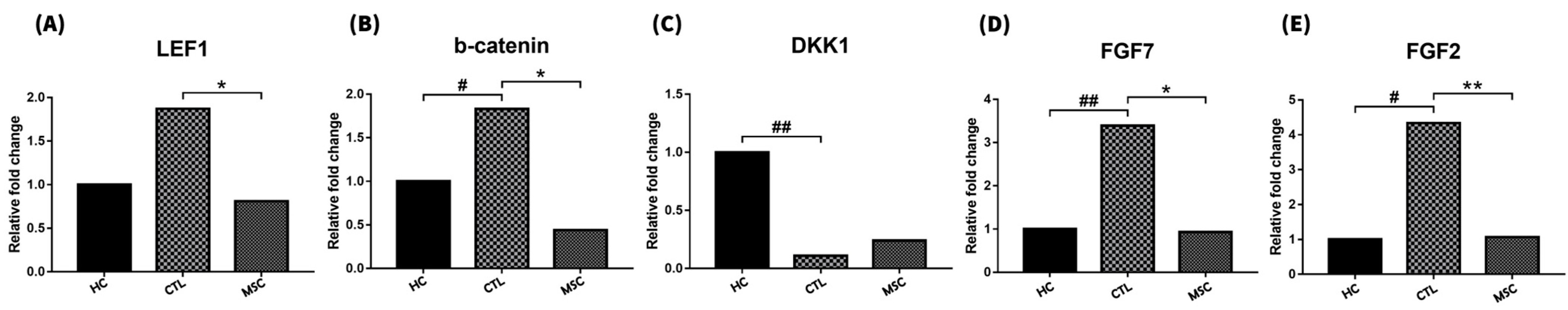
2.4. Effect of MSCT on Hair Cycle-Related Gene Expression
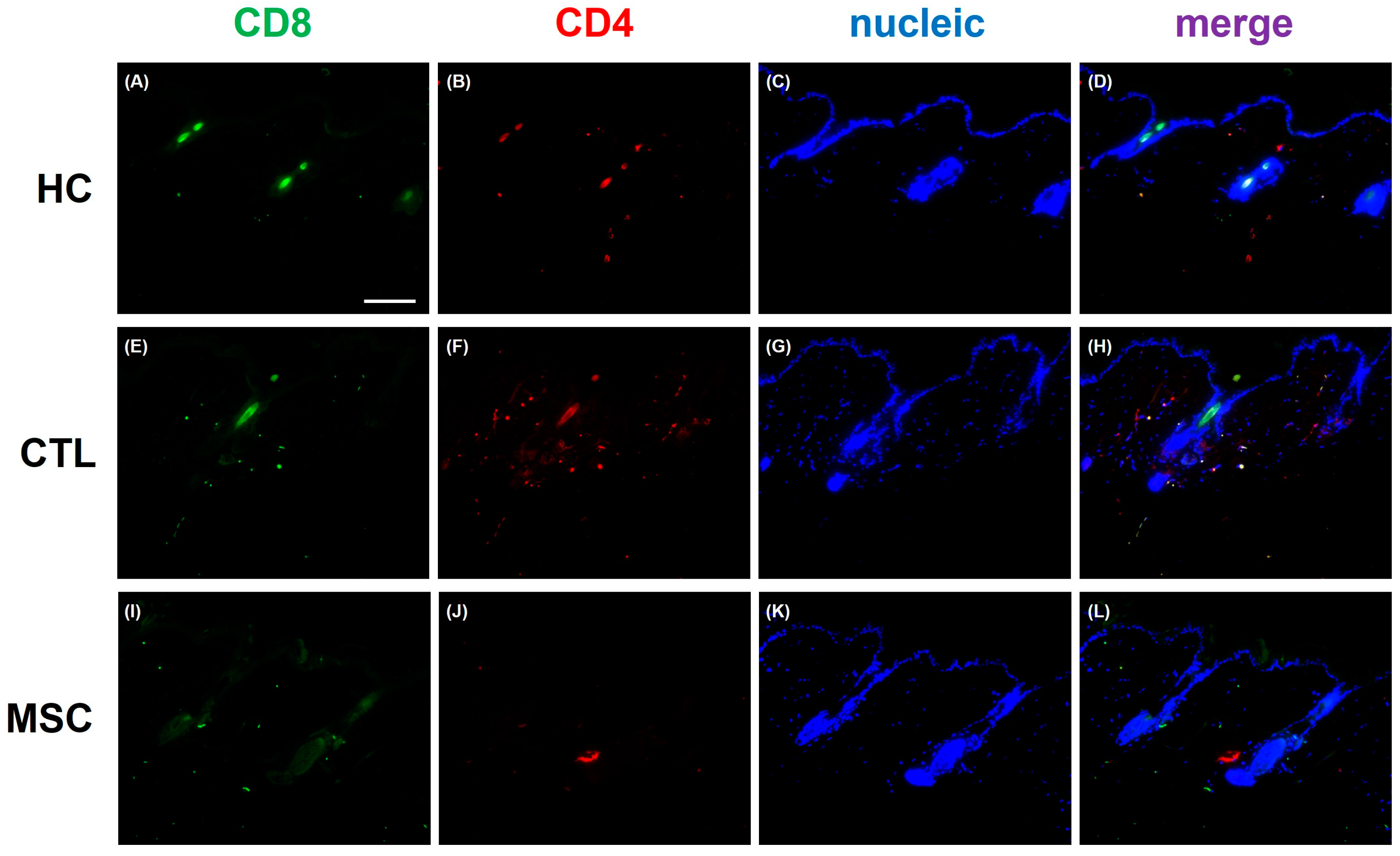
2.5. Histologic Changes via MSC Treatment in AA Mice
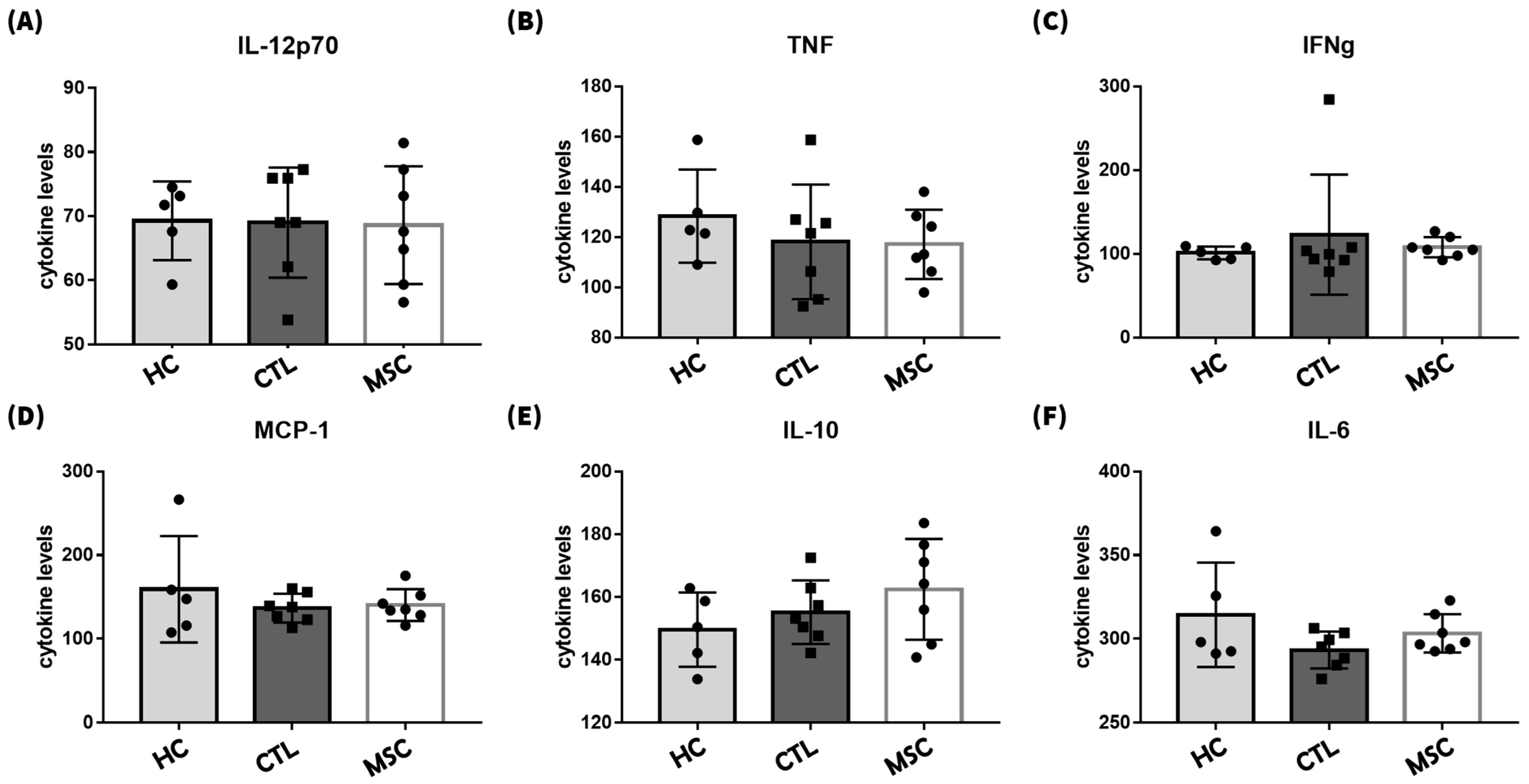
2.6. Effects of MSCT on Circulating Inflammatory Factor Regulation
3. Discussion
4. Materials and Methods
4.1. Animals and Human Bone Marrow-Derived Mesenchymal Stem Cells (hMSCs)
4.2. Induction of AA
4.3. Administration of hMSCs
4.4. Image Analysis
4.5. Mouse Tissue Sample Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirzoyev, S.A.; Schrum, A.G.; Davis, M.D.P.; Torgerson, R.R. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. J. Investig. Dermatol. 2014, 134, 1141–1142. [Google Scholar] [CrossRef] [PubMed]
- Safavi, K.H.; Muller, S.A.; Suman, V.J.; Moshell, A.N.; Melton, L.J., 3rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin. Proc. 1995, 70, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.; McHale, S. The psychological impact of alopecia. BMJ 2005, 331, 951–953. [Google Scholar] [CrossRef]
- Paus, R.; Bulfone-Paus, S.; Bertolini, M. Hair Follicle Immune Privilege Revisited: The Key to Alopecia Areata Management. J. Investig. Dermatol. Symp. Proc. 2018, 19, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Kalish, R.S. Alopecia areata: A tissue specific autoimmune disease of the hair follicle. Autoimmun. Rev. 2006, 5, 64–69. [Google Scholar] [CrossRef]
- Petukhova, L.; Duvic, M.; Hordinsky, M.; Norris, D.; Price, V.; Shimomura, Y.; Kim, H.; Singh, P.; Lee, A.; Chen, W.V.; et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010, 466, 113–117. [Google Scholar] [CrossRef]
- Chu, S.Y.; Chen, Y.J.; Tseng, W.C.; Lin, M.W.; Chen, T.J.; Hwang, C.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; et al. Comorbidity profiles among patients with alopecia areata: The importance of onset age, a nationwide population-based study. J. Am. Acad. Dermatol. 2011, 65, 949–956. [Google Scholar] [CrossRef]
- Kuin, R.A.; Spuls, P.I.; Limpens, J.; van Zuuren, E.J. Diphenylcyclopropenone in patients with alopecia areata. A critically appraised topic. Br. J. Dermatol. 2015, 173, 896–909. [Google Scholar] [CrossRef]
- Kennedy Crispin, M.; Ko, J.M.; Craiglow, B.G.; Li, S.; Shankar, G.; Urban, J.R.; Chen, J.C.; Cerise, J.E.; Jabbari, A.; Winge, M.C.; et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight 2016, 1, e89776. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Luger, D.; Epstein, S.E. Mesenchymal Stem Cell Therapy for the Treatment of Heart Failure Caused by Ischemic or Non-ischemic Cardiomyopathy: Immunosuppression and Its Implications. Handb. Exp. Pharmacol. 2017, 243, 329–353. [Google Scholar] [CrossRef]
- Luque-Campos, N.; Contreras-López, R.A.; Jose Paredes-Martínez, M.; Torres, M.J.; Bahraoui, S.; Wei, M.; Espinoza, F.; Djouad, F.; Elizondo-Vega, R.J.; Luz-Crawford, P. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front. Immunol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 2021, 12, 631291. [Google Scholar] [CrossRef]
- Kadri, N.; Amu, S.; Iacobaeus, E.; Boberg, E.; Le Blanc, K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell. Mol. Immunol. 2023, 20, 613–625. [Google Scholar] [CrossRef]
- Byun, J.W.; Kim, H.J.; Na, K.; Ko, H.S.; Song, H.J.; Song, S.U.; Jeon, M.S.; Choi, G.S. Bone marrow-derived mesenchymal stem cells prevent alopecia areata development through the inhibition of NKG2D expression: A pilot study. Exp. Dermatol. 2017, 26, 532–535. [Google Scholar] [CrossRef]
- Li, Y.; Yan, B.; Wang, H.; Li, H.; Li, Q.; Zhao, D.; Chen, Y.; Zhang, Y.; Li, W.; Zhang, J.; et al. Hair regrowth in alopecia areata patients following Stem Cell Educator therapy. BMC Med. 2015, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Oh, J.H.; Woo, Y.J.; Jung, J.H.; Jeong, K.H.; Kang, H. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models. Exp. Dermatol. 2020, 29, 265–272. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, S.H.; Park, H.R.; Lee, Y.; Kang, H.; Kim, J.E. Mesenchymal Stem Cells Antagonize IFN-Induced Proinflammatory Changes and Growth Inhibition Effects via Wnt/β-Catenin and JAK/STAT Pathway in Human Outer Root Sheath Cells and Hair Follicles. Int. J. Mol. Sci. 2021, 22, 4581. [Google Scholar] [CrossRef] [PubMed]
- Zaaroura, H.; Gilding, A.J.; Sibbald, C. Biomarkers in alopecia Areata: A systematic review and meta-analysis. Autoimmun. Rev. 2023, 22, 103339. [Google Scholar] [CrossRef]
- Ito, T.; Kageyama, R.; Nakazawa, S.; Honda, T. Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp. Dermatol. 2020, 29, 726–732. [Google Scholar] [CrossRef]
- Waśkiel-Burnat, A.; Osińska, M.; Salińska, A.; Blicharz, L.; Goldust, M.; Olszewska, M.; Rudnicka, L. The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications. Cells 2021, 10, 3397. [Google Scholar] [CrossRef]
- Koelman, E.M.R.; Yeste-Vázquez, A.; Grossmann, T.N. Targeting the interaction of β-catenin and TCF/LEF transcription factors to inhibit oncogenic Wnt signaling. Bioorg. Med. Chem. 2022, 70, 116920. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, O.; Sivrikaya, A.; Unlu, A.; Altinyazar, H.C. Serum cytokine and chemokine profiles in patients with alopecia areata. J. Dermatol. Treat. 2016, 27, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska-Zdrojowy, M.; Jankowska-Konsur, A.; Nowicka-Suszko, D.; Szepietowski, J.C.; Hryncewicz-Gwóźdź, A. Comparison of serum concentrations of interleukins 10, 12, 17 and 35 between patients with alopecia areata and controls. Postep. Dermatol. I Alergol. 2021, 38, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, K.; Kozłowska, M.; Kaszuba, A.; Lesiak, A.; Narbutt, J.; Zalewska-Janowska, A. Increased Serum Levels of IFN-γ, IL-1β, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxidative Med. Cell. Longev. 2020, 2020, 5693572. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.P.; Han, D.M.; Zhao, L.; Guo, Z.K.; Xiao, F.J.; Zhang, Y.K.; Zhang, X.Y.; Wang, L.S.; Wang, H.X.; Wang, H. Hepatocyte growth factor enhances the inflammation-alleviating effect of umbilical cord-derived mesenchymal stromal cells in a bronchiolitis obliterans model. Cytotherapy 2016, 18, 402–412. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Y.; Chen, H.; Yang, N.; Zhang, Y.; Liu, Q.; Jiang, Y.; Jin, S. From hair to pancreas: Transplanted hair follicle mesenchymal stem cells express pancreatic progenitor cell markers in a rat model of acute pancreatitis. Am. J. Transl. Res. 2021, 13, 1389–1399. [Google Scholar]
- Shi, B.; Qi, J.; Yao, G.; Feng, R.; Zhang, Z.; Wang, D.; Chen, C.; Tang, X.; Lu, L.; Chen, W.; et al. Mesenchymal stem cell transplantation ameliorates Sjögren’s syndrome via suppressing IL-12 production by dendritic cells. Stem Cell Res. Ther. 2018, 9, 308. [Google Scholar] [CrossRef]
- Phan, K.; Sebaratnam, D.F. JAK inhibitors for alopecia areata: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Ungar, B.; Noda, S.; Shroff, A.; Mansouri, Y.; Fuentes-Duculan, J.; Czernik, A.; Zheng, X.; Estrada, Y.D.; Xu, H.; et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J. Allergy Clin. Immunol. 2015, 136, 1277–1287. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Büscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef]
- Pianta, S.; Bonassi Signoroni, P.; Muradore, I.; Rodrigues, M.F.; Rossi, D.; Silini, A.; Parolini, O. Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets. Stem Cell Rev. Rep. 2015, 11, 394–407. [Google Scholar] [CrossRef]
- Xiao, C.Y.; Pan, Y.F.; Guo, X.H.; Wu, Y.Q.; Gu, J.R.; Cai, D.Z. Expression of β-catenin in rheumatoid arthritis fibroblast-like synoviocytes. Scand. J. Rheumatol. 2011, 40, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.; Hwang, J.; Kim, D.Y.; Kim, J.; Song, S.Y.; Sung, J.H. Involvement of DKK1 secreted from adipose-derived stem cells in alopecia areata. Cell Prolif. 2024, 57, e13562. [Google Scholar] [CrossRef]
- Yan, H.; Gao, Y.; Ding, Q.; Liu, J.; Li, Y.; Jin, M.; Xu, H.; Ma, S.; Wang, X.; Zeng, W.; et al. Exosomal Micro RNAs Derived from Dermal Papilla Cells Mediate Hair Follicle Stem Cell Proliferation and Differentiation. Int. J. Biol. Sci. 2019, 15, 1368–1382. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.C.; Cheng, K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.H.; Roh, K.H.; Jun, H.J.; Kang, K.S.; Kim, T.Y. Clinical Trial of Human Umbilical Cord Blood-Derived Stem Cells for the Treatment of Moderate-to-Severe Atopic Dermatitis: Phase I/IIa Studies. Stem Cells 2017, 35, 248–255. [Google Scholar] [CrossRef]
- Yan, D.; Fan, H.; Chen, M.; Xia, L.; Wang, S.; Dong, W.; Wang, Q.; Niu, S.; Rao, H.; Chen, L.; et al. The efficacy and safety of JAK inhibitors for alopecia areata: A systematic review and meta-analysis of prospective studies. Front. Pharmacol. 2022, 13, 950450. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]

| Gene | Primer | Sequence (5′ to 3′) | Length |
|---|---|---|---|
| JAK1 | Forward | TAT TGG GTG GCC AGA AGC AG | 20 |
| Reverse | GTG GTT CAT GAG GTC TCG CA | 20 | |
| JAK2 | Forward | GAG GTG GTC GCT GTG AAG AA | 20 |
| Reverse | GCA CTG TAG CAC ACT CCC TT | 20 | |
| STAT1 | Forward | TTC AGC AGC TGG ACT CCA AG | 20 |
| Reverse | ACG AGA CAT CAT AGG CAG CG | 20 | |
| STAT3 | Forward | AAC GAC CTG CAG CAA TAC CA | 20 |
| Reverse | TCC ATG TCA AAC GTG AGC GA | 20 | |
| IFNγR | Forward | GCA ATG ACC CAA GAC CAG TG | 20 |
| Reverse | ACT GTG AAT GGG TGT GGG AA | 20 | |
| IL-10 | Forward | CGG GAA GAC AAT AAC TGC ACC C | 22 |
| Reverse | CGG TTA GCA GTA TGT TGT CCA GC | 23 | |
| IL-15 | Forward | CAT TTT GGG CTG TGT CAG TG | 20 |
| Reverse | GCA ATT CCA GGA GAA AGC AG | 20 | |
| IL-17α | Forward | CAG ACT ACC TCA ACC GTT CCA C | 22 |
| Reverse | TCC AGC TTT CCC TCC GCA TTG A | 22 | |
| IL-18 | Forward | CAG GCC TGA CAT CTT CTC CAA | 21 |
| Reverse | CTG ACA TGG CAG CCA TTG T | 19 | |
| IL-1β | Forward | TGG ACC TTC CAG GAT GAG GAC A | 22 |
| Reverse | GTT CAT CTC GGA GCC TGT AGT G | 22 | |
| IL-22 | Forward | GCT TGA GGT GTC CAA CTT CCA G | 22 |
| Reverse | ACT CCT CGG AAC AGT TTC TCC C | 22 | |
| IL-6 | Forward | TAC CAC TTC ACA AGT CGG AGG C | 22 |
| Reverse | CTG CAA GTG CAT CAT CGT TGT TC | 23 | |
| DKK1 | Forward | ATA TGC ATG CCC TCT GAC CA | 20 |
| Reverse | CGG AGC CTT CTT GTC CTT TG | 20 | |
| FGF2 | Forward | CGA CCC ACA CGT CAA ACT ACA | 21 |
| Reverse | GTA ACA CAC TTA GAA GCC AGC A | 22 | |
| FKF7 | Forward | ATA GAA ACA GGT CGT GAC AAG G | 22 |
| Reverse | CAG ACA GCA GAC ACG GAA C | 19 | |
| LEF1 | Forward | GTC GAC TTC AGG TGG TAA GAG A | 22 |
| Reverse | TGC TGT CAG TGT TCC TTG GG | 20 | |
| β-catenin | Forward | GGC AGC GGC AGG ATA CAC GG | 20 |
| Reverse | CAG GAC ACG AGC TGA CGC GG | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Song, S.-W.; Lee, Y.-J.; Kang, H.; Kim, J.-E. Mesenchymal Stem Cell Therapy in Alopecia Areata: Visual and Molecular Evidence from a Mouse Model. Int. J. Mol. Sci. 2024, 25, 9236. https://doi.org/10.3390/ijms25179236
Park S-H, Song S-W, Lee Y-J, Kang H, Kim J-E. Mesenchymal Stem Cell Therapy in Alopecia Areata: Visual and Molecular Evidence from a Mouse Model. International Journal of Molecular Sciences. 2024; 25(17):9236. https://doi.org/10.3390/ijms25179236
Chicago/Turabian StylePark, Song-Hee, Seo-Won Song, Yu-Jin Lee, Hoon Kang, and Jung-Eun Kim. 2024. "Mesenchymal Stem Cell Therapy in Alopecia Areata: Visual and Molecular Evidence from a Mouse Model" International Journal of Molecular Sciences 25, no. 17: 9236. https://doi.org/10.3390/ijms25179236
APA StylePark, S.-H., Song, S.-W., Lee, Y.-J., Kang, H., & Kim, J.-E. (2024). Mesenchymal Stem Cell Therapy in Alopecia Areata: Visual and Molecular Evidence from a Mouse Model. International Journal of Molecular Sciences, 25(17), 9236. https://doi.org/10.3390/ijms25179236







