The Enhancement Origin of Antioxidant Property of Carboxylated Lignin Isolated from Herbaceous Biomass Using the Maleic Acid Hydrotropic Fractionation
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Physical Properties of AHL and MWL
2.2. Chemical Structures of AHL and MWL
2.3. Assessment of Radical Scavenging Ability of AHL and MWL
3. Materials and Methods
3.1. Materials
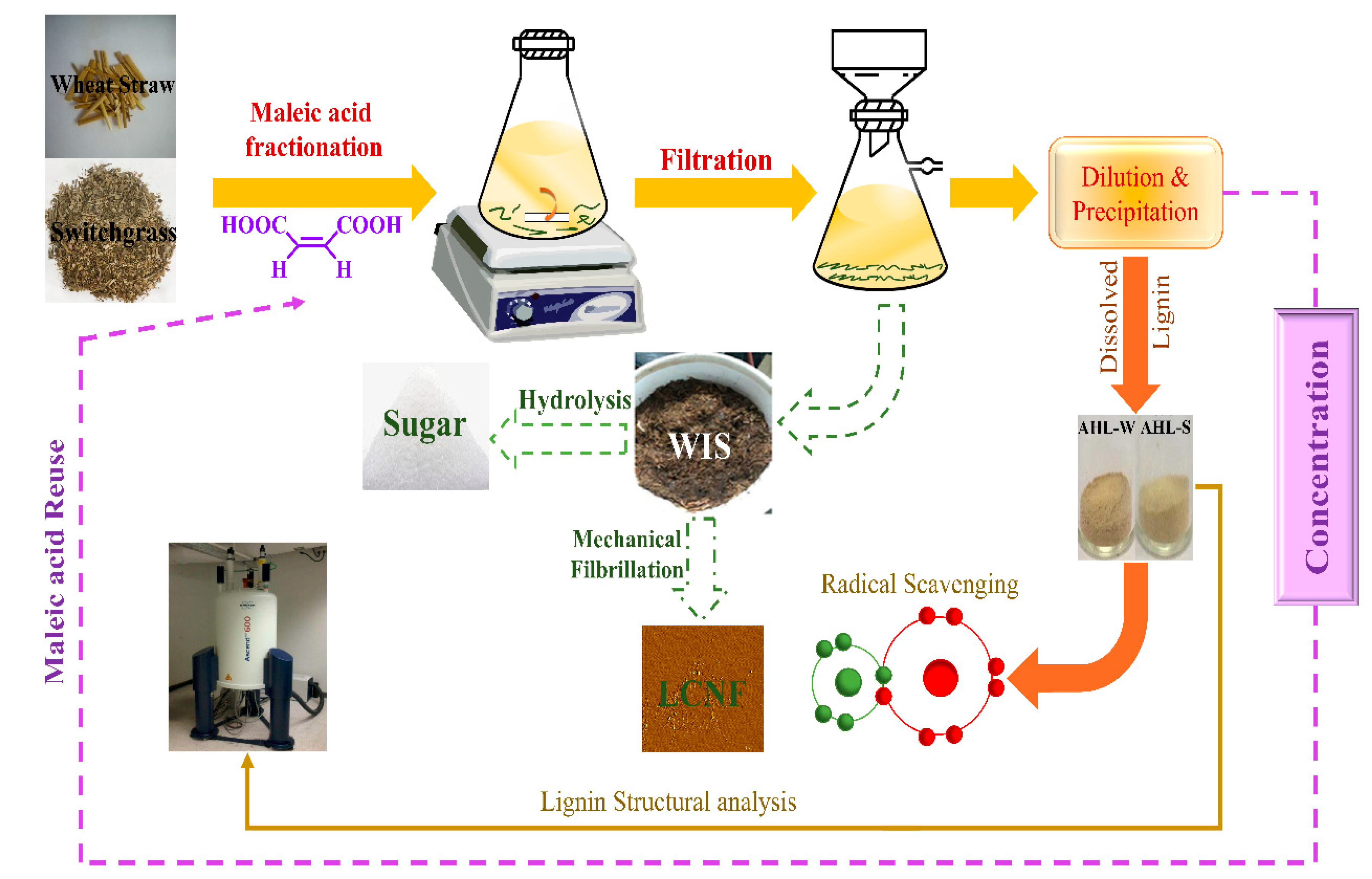
3.2. Separation of Acid Hydrotropic Lignin (AHL) from Gramineae Using Maleic Acid Hydrotropic Fractionation (MAHF) and Preparation of Milled Wood Lignin (MWL)
3.3. Characterization of AHL and MWL
3.4. DPPH and ABTS Radicals Scavenging Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Qu, Y.; Jiao, L.; Bian, H.; Wang, R.; Wu, W.; Fang, G.; Dai, H. Boosting the thermal conductivity of CNF-based composites by cross-linked lignin nanoparticle and BN-OH: Dual construction of 3D thermally conductive pathways. Compos. Sci. Technol. 2021, 204, 108641. [Google Scholar] [CrossRef]
- Su, C.; Wang, X.; Deng, Y.; Wu, T.; Jiao, J.; Huang, C.; Cao, Y.; Dai, H.; Fang, G. Revitalizing Multiple Bioactivities of the Lignin-Carbohydrate Complex through a Two-Step Strategy: Unraveling the Enhancement Origin from a Structural Variation Perspective. Sustain. Chem. Eng. 2024, 12, 10544. [Google Scholar] [CrossRef]
- Wang, X.; Ji, S.L.; Wang, X.Q.; Bian, H.Y.; Lin, L.R.; Dai, H.Q.; Xiao, H. Thermally conductive, super flexible and flame-retardant BN-OH/PVA composite film reinforced by lignin nanoparticles. J. Mater. Chem. C 2019, 7, 14159. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Li, J.; Mao, Z.; Zhang, S.; Zhao, S.; Li, M.; Hao, X.; Peng, F. Plant cell wall inspired lignin-based membrane with configurable radical scavenging activity. Chem. Eng. J. 2024, 495, 153317. [Google Scholar] [CrossRef]
- Patel, S.; Das, D.; Kim, S.; Cho, B.; Kalia, V.; Lee, J. Integrating strategies for the sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Rusănescu, C.; Ciobanu, M.; Rusănescu, M.; Dinculoiu, R. Pretreatments Applied to Wheat Straw to Obtain Bioethanol. Appl. Sci. 2024, 14, 1612. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Cárcamo-Martínez, Á.; Stewart, S.; Donnelly, R.; Larrañeta, E.; Borrega, M. Lignin for pharmaceutical and biomedical applications-Could this become a reality? Sustain. Chem. Pharm. 2020, 18, 100320. [Google Scholar] [CrossRef]
- Spiridon, I. Extraction of lignin and therapeutic applications of lignin-derived compounds. A review. Environ. Chem. Lett. 2020, 18, 771. [Google Scholar] [CrossRef]
- Torres, L.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.; Karp, S.; Lorenci, L.G.; Faulds, C.; Soccol, C. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Ullah, I.; Chen, Z.; Xie, Y.; Khan, S.; Singh, S.; Yu, C.; Cheng, G. Recent advances in biological activities of lignin and emerging biomedical applications: A short review. Int. J. Biol. Macromol. 2022, 208, 819. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV light blocker—A review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Xie, D.; Gan, T.; Su, C.; Han, Y.; Liu, Z.; Cao, Y. Structural characterization and antioxidant activity of water-soluble lignin-carbohydrate complexes (LCCs) isolated from wheat straw. Int. J. Biol. Macromol. 2020, 161, 315. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Gu, L.; Wu, W.; Zhao, H.; Jin, Y. Structural elucidation and antioxidant activity of lignin isolated from rice straw and alkalioxygen black liquor. Int. J. Biol. Macromol. 2018, 116, 513. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Huang, L.; Zhang, C.; Zhang, Y. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods 2015, 16, 460. [Google Scholar] [CrossRef]
- An, L.; Si, C.; Wang, G.; Sui, W.; Tao, Z. Enhancing the solubility and antioxidant activity of high-molecular-weight lignin by moderate depolymerization via in situ ethanol/acid catalysis. Ind. Crops Prod. 2019, 128, 177. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, W.; Gleisner, R.; Pan, X.; Zhu, J. Comparisons of SPORL and dilute acid pretreatments for sugar and ethanol productions from aspen. Biotechnol. Prog. 2011, 27, 419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Pan, X. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation. Bioresour. Technol. 2010, 101, 4992. [Google Scholar] [CrossRef] [PubMed]
- Ewanick, S.; Bura, R.; Saddler, J. Acid-catalyzed steam pretreatment of lodgepole pine and subsequent enzymatic hydrolysis and fermentation to ethanol. Biotechnol. Bioeng. 2007, 98, 737. [Google Scholar] [CrossRef]
- Pan, X.; Gilkes, N.; Kadla, J.; Pye, K.; Saka, S.; Gregg, D.; Ehara, K.; Xie, D.; Lam, D.; Saddler, J. Bioconversion of hybrid poplar to ethanol and co-products using an organosolv fractionation process: Optimization of process yields. Biotechnol. Bioeng. 2006, 94, 851. [Google Scholar] [CrossRef] [PubMed]
- Galkin, M.; Samec, J. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery. ChemSusChem 2016, 9, 1544. [Google Scholar]
- Qin, H.; Hu, X.; Wang, J.; Cheng, H.; Chen, L.; Qi, Z. Overview of acidic deep eutectic solvents on synthesis, properties and applications. Green Energy Environ. 2020, 5, 8. [Google Scholar] [CrossRef]
- Ovejero-Perez, A.; Rigual, V.; Dominguez, J.; Alonso, M.; Oliet, M.; Rodriguez, F. Acidic depolymerization vs ionic liquid solubilization in lignin extraction from eucalyptus wood using the protic ionic liquid 1-methylimidazolium chloride. Int. J. Biol. Macromol. 2020, 157, 461. [Google Scholar] [CrossRef]
- Su, C.; Hirth, K.; Liu, Z.; Cao, Y.; Zhu, J. Acid hydrotropic fractionation of switchgrass at atmospheric pressure using maleic acid in comparison with p-TsOH: Advantages of lignin esterification. Ind. Crops Prod. 2021, 159, 113017. [Google Scholar] [CrossRef]
- Cai, C.; Hirth, K.; Gleisner, R.; Lou, H.; Qiu, X.; Zhu, J. Maleic acid as a dicarboxylic acid hydrotrope for sustainable fractionation of wood at atmospheric pressure and ≤100 °C: Mode and utility of lignin esterification. Green Chem. 2020, 22, 1605. [Google Scholar] [CrossRef]
- Su, C.; Hirth, K.; Liu, Z.; Cao, Y.; Zhu, J. Maleic acid hydrotropic fractionation of wheat straw to facilitate value-added multi-product biorefinery at atmospheric pressure. GCB Bioenergy 2021, 13, 1407. [Google Scholar] [CrossRef]
- Cai, C.; Li, J.; Hirth, K.; Huber, G.; Lou, H.; Zhu, J. Comparison of Two Acid Hydrotropes for Sustainable Fractionation of Birch Wood. ChemSusChem 2020, 13, 4649. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, S.; Hirth, K.; Cheng, J.; Wen, J.; Li, N.; Fang, Y.; Pan, X.; Zhu, J. Preserving both lignin and cellulose chemical structures: Flow-through acid hydrotropic fractionation at atmospheric pressure for complete wood valorization. Sustain. Chem. Eng. 2019, 7, 10808. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, C.; Zhan, Y.; Han, S.; Wang, J.; Meng, X.; Yoo, C.G.; Fang, G.; Ragauskas, A.J. Effective biomass fractionation and lignin stabilization using a diol DES system. Chem. Eng. J. 2022, 443, 136395. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, L.; Wang, R.; Yang, R.; Zhu, J.Y. Direct production of lignin nanoparticles (LNPs) from wood using p-toluenesulfonic acid in an aqueous system at 80 °C: Characterization of LNP morphology, size, and surface charge. Holzforschung 2018, 72, 933. [Google Scholar] [CrossRef]
- Camargos, C.; Rezende, C. Antisolvent versus ultrasonication: Bottom-up and top-down approaches to produce lignin nanoparticles (LNPs) with tailored properties. Int. J. Biol. Macromol. 2021, 193, 647. [Google Scholar] [CrossRef]
- Zhai, Q.; Han, S.; Hse, C.; Jiang, J.; Xu, J. 5-Sulfosalicylic acid as an acid hydrotrope for the rapid and green fractionation of woody biomass. Ind. Crops Prod. 2022, 177, 114435. [Google Scholar] [CrossRef]
- Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Ranzi, E. Detailed kinetic modeling of the thermal degradation of lignins. Biomass Bioenergy 2010, 34, 290. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Jones, G. Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym. Degrad. Stab. 2000, 68, 111. [Google Scholar] [CrossRef]
- Karmanov, A.; Derkacheva, O. Application of fourier transform infrared spectroscopy for the study of lignins of herbaceous plants. Russ. J. Bioorg. Chem. 2013, 39, 677. [Google Scholar]
- Joffres, B.; Lorentz, C.; Vidalie, M.; Laurenti, D.; Quoineaud, A.; Charon, N.; Daudin, A.; Quignard, A.; Geantet, C. Catalytic hydroconversion of a wheat straw soda lignin: Characterization of the products and the lignin residue. Appl. Catal. B 2014, 145, 167. [Google Scholar] [CrossRef]
- Holtman, K.; Chang, H.M.; Jameel, H.; Kadla, J. Quantitative 13C NMR Characterization of Milled Wood Lignins Isolated by Different Milling Techniques. J. Wood Chem. Technol. 2006, 26, 21. [Google Scholar] [CrossRef]
- Capanema, E.; Balakshin, M.; Katahira, R.; Chang, H.; Jameel, H. How Well Do MWL and CEL Preparations Represent the Whole Hardwood Lignin? J. Wood Chem. Technol. 2014, 35, 17. [Google Scholar] [CrossRef]
- Su, C.; Gan, T.; Liu, Z.; Chen, Y.; Zhou, Q.; Xia, J.; Cao, Y. Enhancement of the antioxidant abilities of lignin and lignin-carbohydrate complex from wheat straw by moderate depolymerization via LiCl/DMSO solvent catalysis. Int. J. Biol. Macromol. 2021, 184, 369. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Zhou, Q.; Su, C.; Xia, J.; Xie, D.; Liu, Z.; Cao, Y. Efficient isolation of organosolv lignin-carbohydrate complexes (LCC) with high antioxidative activity via introducing LiCl/DMSO dissolving. Int. J. Biol. Macromol. 2021, 181, 752. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Sun, S.; Yuan, T.; Xu, F.; Sun, R. Fractionation of bamboo culms by autohydrolysis, organosolv delignification and extended delignification: Understanding the fundamental chemistry of the lignin during the integrated process. Bioresour. Technol. 2013, 150, 278. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Q.; Xu, W.; Qin, M.; Fu, Y.; Wang, Z.; Willför, S.; Xu, C. Revealing the structure of bamboo lignin obtained by formic acid delignification at different pressure levels. Ind. Crops Prod. 2017, 108, 864. [Google Scholar] [CrossRef]
- del Rio, J.; Rencoret, J.; Prinsen, P.; Martinez, A.; Ralph, J.; Gutierrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, J.; Prinsen, P.; Cadena, E.; Martinez, A.; Gutierrez, A.; Rencoret, J. Lignin-carbohydrate complexes from sisal (Agave sisalana) and abaca (Musa textilis): Chemical composition and structural modifications during the isolation process. Planta 2016, 243, 1143. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.; Chang, H. MWL fraction with a high concentration of lignin-carbohydrate linkages: Isolation and 2D NMR spectroscopic analysis. Holzforschung 2007, 61, 1. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Gellerstedt, G.; Rencoret, J.; Del Rio, J.; Martinez, A.; Gutierrez, A. Understanding pulp delignification by laccase-mediator systems through isolation and characterization of lignin-carbohydrate complexes. Biomacromolecules 2013, 14, 3073. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Perez-Boada, M.; Fernandez, C.; Rencoret, J.; del Rio, J.; Jimenez-Barbero, J.; Li, J.; Gutierrez, A.; Martinez, A. Analysis of lignin-carbohydrate and lignin-lignin linkages after hydrolase treatment of xylan-lignin, glucomannan-lignin and glucan-lignin complexes from spruce wood. Planta 2014, 239, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.; Gracz, H.; Chang, H.M.; Jameel, H. Quantification of lignin-carbohydrate linkages with high-resolution NMR spectroscopy. Planta 2011, 233, 1097. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, X.; Liang, C.; Jiang, X.; Yang, G.; Xu, J.; Yong, Q. A sustainable process for procuring biologically active fractions of high-purity xylooligosaccharides and water-soluble lignin from Moso bamboo prehydrolyzate. Biotechnol. Biofuels 2019, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Ye, D.; Chen, X.; Wu, Y.; Yang, Z.; Mai, Y.; Liao, B.; Chen, J. Lignin-derived polyphenols with enhanced antioxidant activities by chemical demethylation and their structure-activity relationship. Int. J. Biol. Macromol. 2023, 237, 124030. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Song, D.; Mu, H.; Zhang, W.; Sun, F.; Duan, J. Investigation of three lignin complexes with antioxidant and immunological capacities from Inonotus obliquus. Int. J. Biol. Macromol. 2016, 86, 587. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Volperts, A.; Dobele, G.; Telysheva, G. Analytical pyrolysis—A tool for revealing of lignin structure-antioxidant activity relationship. J. Anal. Appl. Pyrolysis 2015, 113, 360. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins-natural antioxidants. Bioresour. Technol. 2004, 95, 309. [Google Scholar] [CrossRef]
- Pan, X.; Kadla, J.; Ehara, K.; Gilkes, N.; Saddler, J. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Li, N.; Liu, H.; Zhang, J.; Zhu, J.Y.; Wang, F. Extracting high β-O-4 content lignin and by-producing substrate susceptible to enzymatic hydrolysis by a green flow through process. Chem. Eng. J. 2023, 453, 139730. [Google Scholar] [CrossRef]
- Chen, L.; Dou, J.; Ma, Q.; Li, N.; Wu, R.; Bian, H.; Yelle, D.; Vuorinen, T.; Fu, S.; Pan, X.; et al. Rapid and near-complete dissolution of wood lignin at ≤80 °C by a recyclable acid hydrotrope. Sci. Adv. 2017, 3, e1701735. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tang, S.; Zhang, W.; Tao, Y.; Lai, C.; Li, X.; Yong, Q. Unveiling the Structural Properties of Lignin-Carbohydrate Complexes in Bamboo Residues and Its Functionality as Antioxidants and Immunostimulants. Sustain. Chem. Eng. 2018, 6, 12522. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Du, L.; Min, D.; Yong, Q. Structural Characterization of the Lignins from the Green and Yellow Bamboo of Bamboo Culm (Phyllostachys pubescens). J. Wood Chem. Technol. 2015, 36, 157. [Google Scholar] [CrossRef]
- Zheng, L.; Lu, G.; Pei, W.; Yan, W.; Li, Y.; Zhang, L.; Huang, C.; Jiang, Q. Understanding the relationship between the structural properties of lignin and their biological activities. Int. J. Biol. Macromol. 2021, 190, 291. [Google Scholar] [CrossRef]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crops Prod. 2011, 34, 1629. [Google Scholar] [CrossRef]

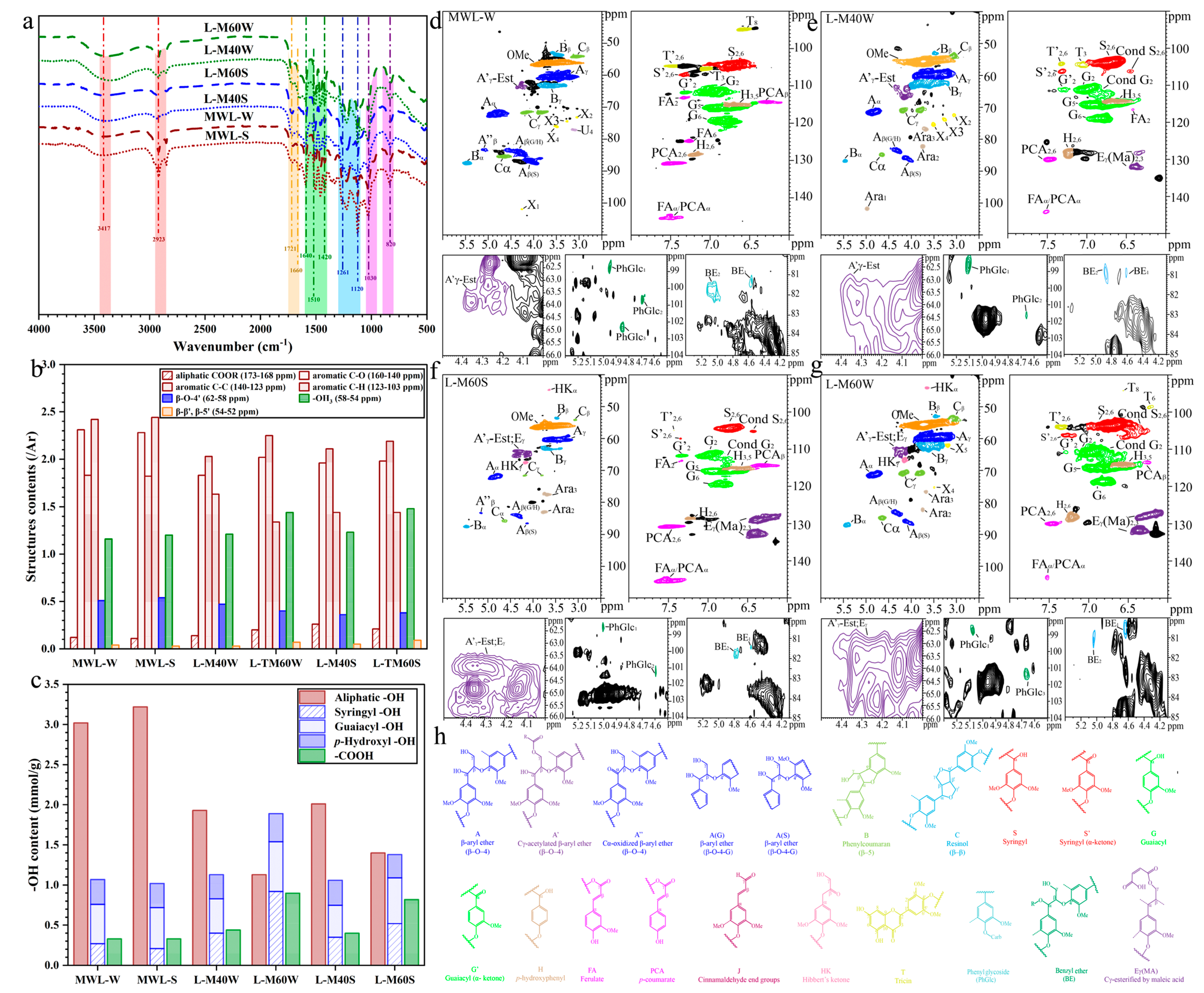

| Lignin Samples | MWL-W | MWL-S | L-M40W | L-M60W | L-M40S | L-M60S |
|---|---|---|---|---|---|---|
| Mw | 12,817 | 13,659 | 8798 | 4664 | 7915 | 4592 |
| Mn | 4589 | 4971 | 4176 | 3174 | 4067 | 3207 |
| Mw/Mn | 2.8 | 2.7 | 2.1 | 1.5 | 1.9 | 1.4 |
| Interunit linkages | ||||||
| β−O−4′ | 55.2 | 48.1 | 51.8 | 26.1 | 43.7 | 28.7 |
| β−5′ | 6.7 | 5.5 | 4.4 | 5.3 | 3.1 | 4.4 |
| β−β′ | 6.0 | 6.3 | 5.3 | 8.7 | 1.8 | 2.5 |
| Condensed degree | 18.7 | 19.7 | 18.6 | 34.9 | 10.1 | 19.3 |
| γ-esterification | 11.5 | 12.3 | 16.0 | 38.5 | 18.2 | 46.0 |
| HKα | - | - | - | 8.2 | 2.4 | 4.2 |
| Aromatic units | ||||||
| S | 39 | 33 | 49 | 58 | 34 | 40 |
| Cond S | - | - | 2 | 9 | 1 | 8 |
| G | 58 | 63 | 48 | 39 | 63 | 57 |
| Cond G | - | - | 3 | 21 | 3 | 17 |
| H | 3 | 4 | 3 | 3 | 3 | 3 |
| S/G ratio | 0.7 | 0.5 | 1.0 | 1.5 | 0.5 | 0.70 |
| LCC linkages | ||||||
| PhGlc | 6.2 | 4.9 | 3.9 | 1.2 | 1.7 | 1.6 |
| BE | 7.1 | 2.2 | 2.8 | 1.5 | 1.8 | 1.1 |
| Total | 13.3 | 7.1 | 6.7 | 2.7 | 3.5 | 2.7 |
| Raw Material | Wheat Straw | Switchgrass | ||||
|---|---|---|---|---|---|---|
| Fractionation | — | M40T80 t100 | M60T110 t60 | M40T80 t100 | M60T110 t60 | |
| Lignin samples | MWL-W | L-M40W | L-M60W | MWL-S | L-M40S | L-M60S |
| Delignification | - | 35.1 | 58.2 | 33.8 | 57.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Wang, X.; Deng, Y.; Min, D.; Fang, G.; Huang, C. The Enhancement Origin of Antioxidant Property of Carboxylated Lignin Isolated from Herbaceous Biomass Using the Maleic Acid Hydrotropic Fractionation. Int. J. Mol. Sci. 2024, 25, 9257. https://doi.org/10.3390/ijms25179257
Su C, Wang X, Deng Y, Min D, Fang G, Huang C. The Enhancement Origin of Antioxidant Property of Carboxylated Lignin Isolated from Herbaceous Biomass Using the Maleic Acid Hydrotropic Fractionation. International Journal of Molecular Sciences. 2024; 25(17):9257. https://doi.org/10.3390/ijms25179257
Chicago/Turabian StyleSu, Chen, Xiu Wang, Yongjun Deng, Douyong Min, Guigan Fang, and Chen Huang. 2024. "The Enhancement Origin of Antioxidant Property of Carboxylated Lignin Isolated from Herbaceous Biomass Using the Maleic Acid Hydrotropic Fractionation" International Journal of Molecular Sciences 25, no. 17: 9257. https://doi.org/10.3390/ijms25179257





