Oral Administration of Probiotic Bifidobacterium breve Ameliorates Tonic–Clonic Seizure in a Pentylenetetrazole-Induced Kindling Mouse Model via Integrin-Linked Kinase Signaling
Abstract
1. Introduction
2. Results
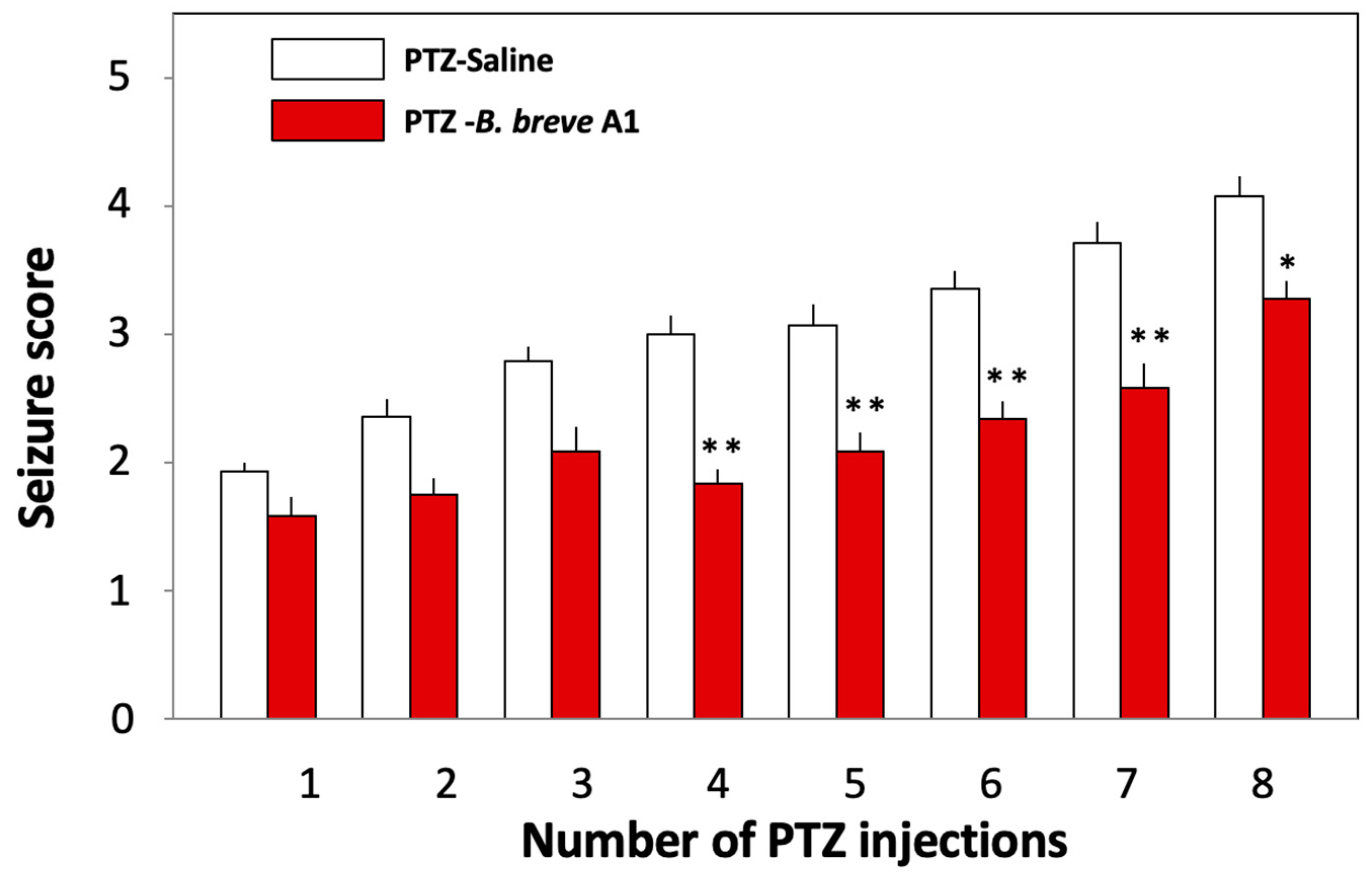
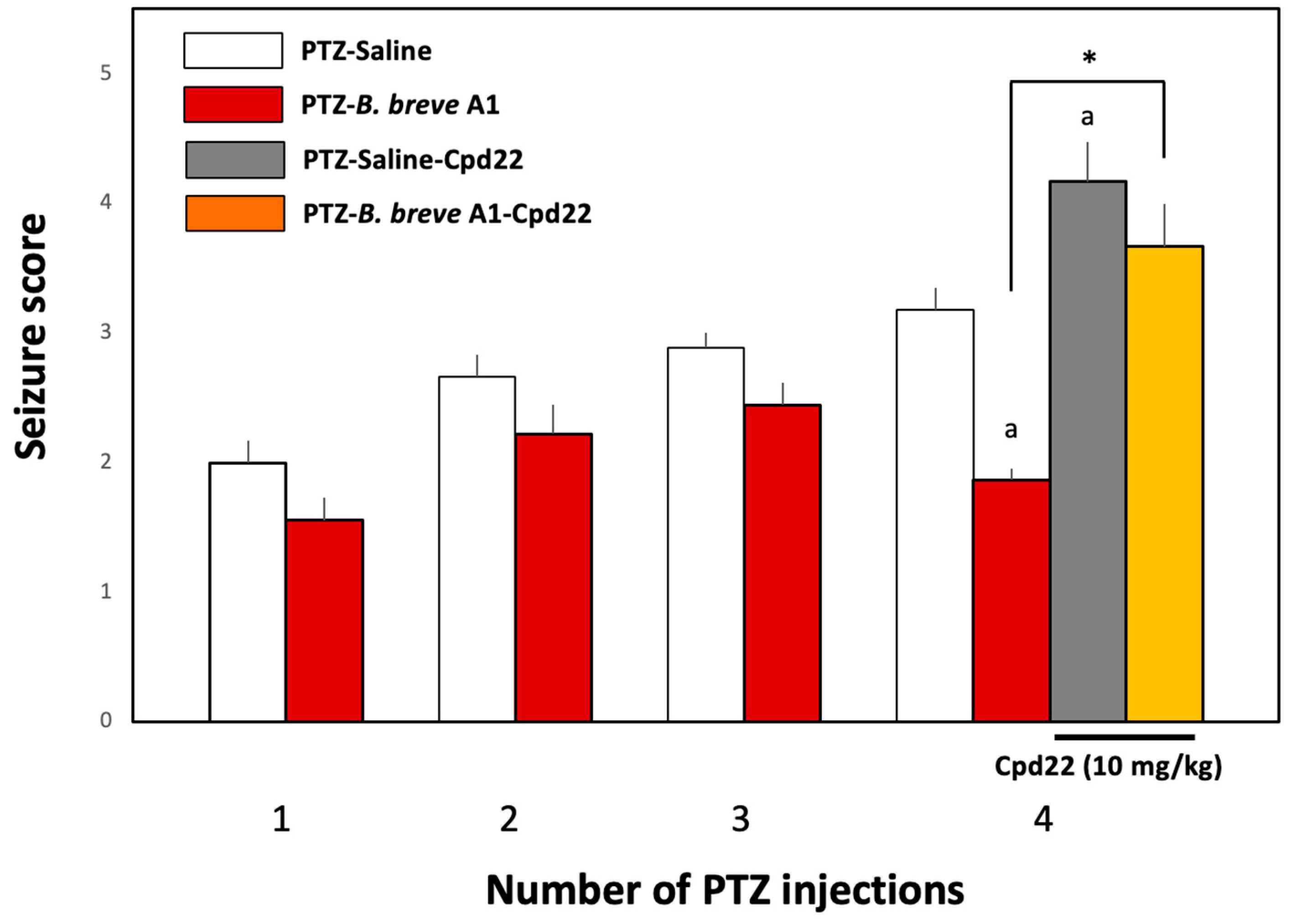
2.1. Administration of B. breve A1 to KD Mice Ameliorated PTZ-Induced Seizures
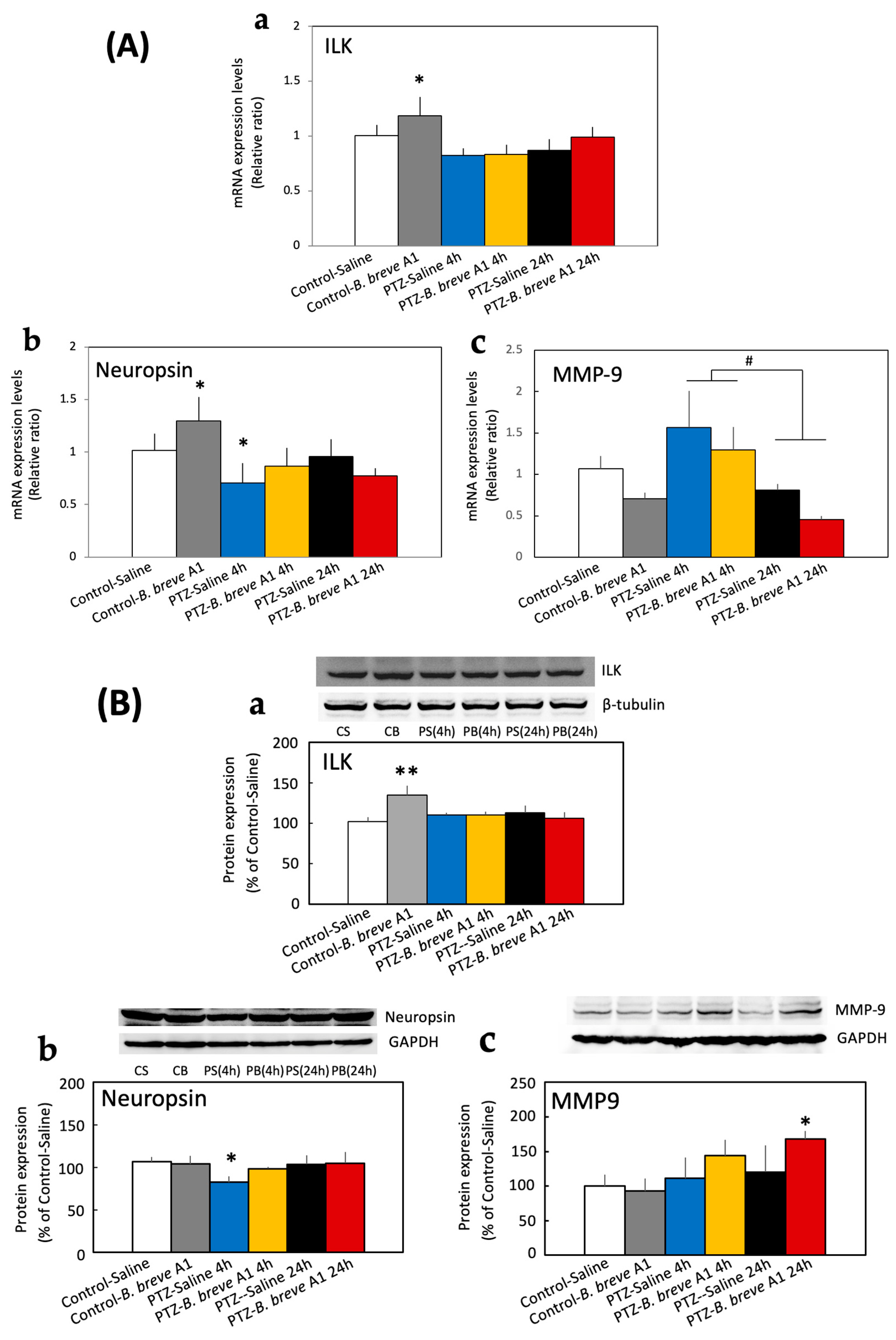
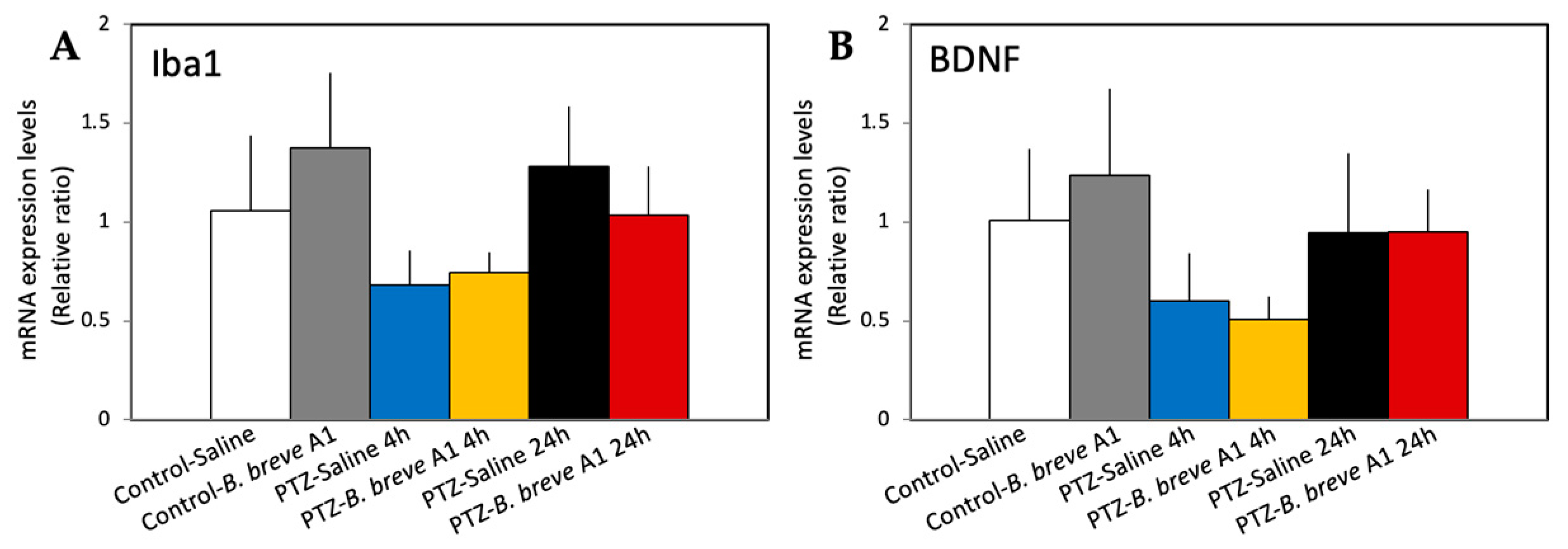
2.2. Effect of Administration of B. breve A1 on the Hippocampal mRNA Expression Levels of Neuropsin, MMP-9, Integrin-Linked Kinase (ILK), Brain-Derived Neurotrophic Factor (BDNF), and Ionized Calcium-Binding Adapter Molecule 1 (Iba1)
2.3. Effect of B. breve A1 on the Hippocampal Protein Expression Levels of ILK, Neuropsin, and MMP-9
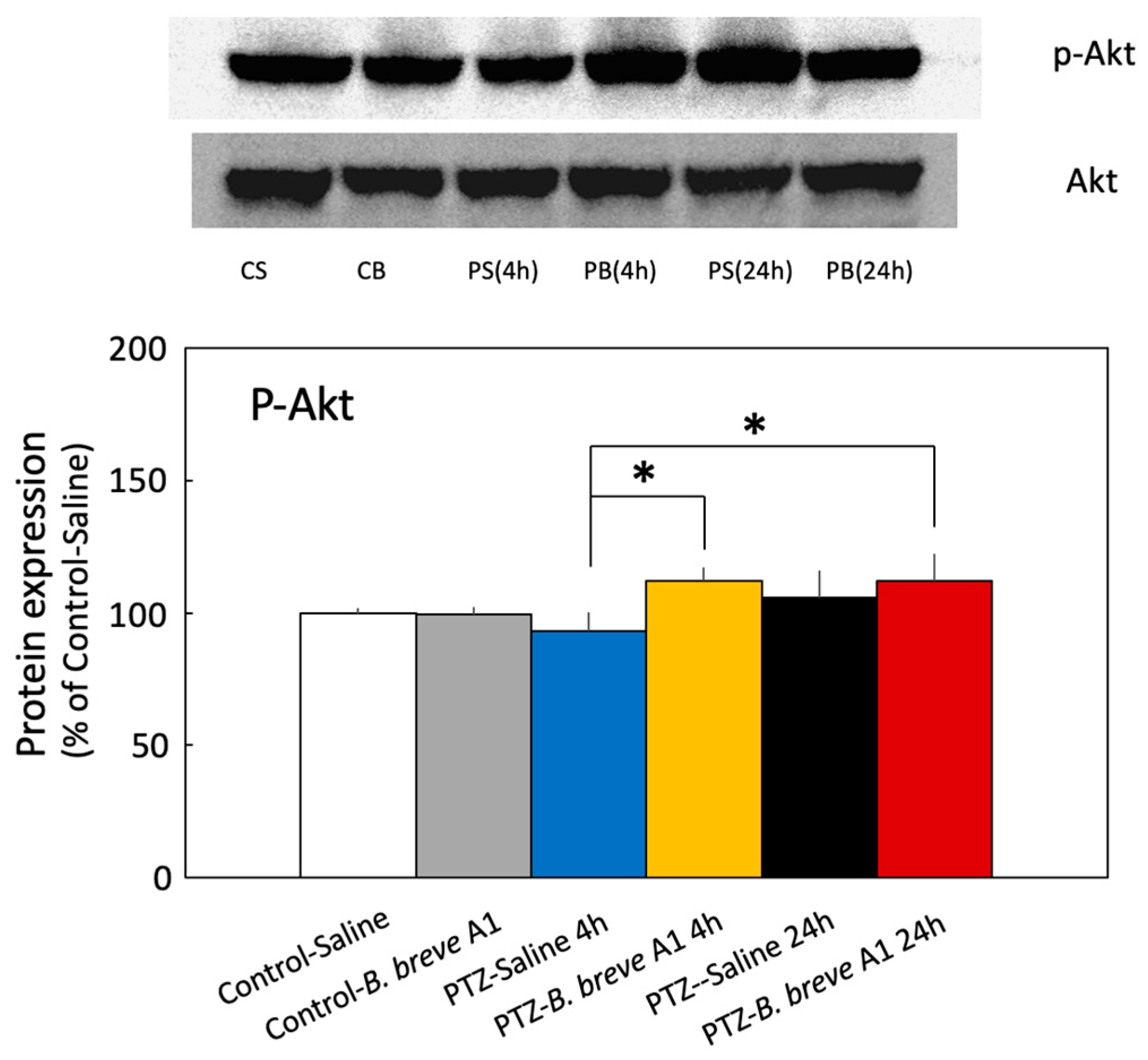
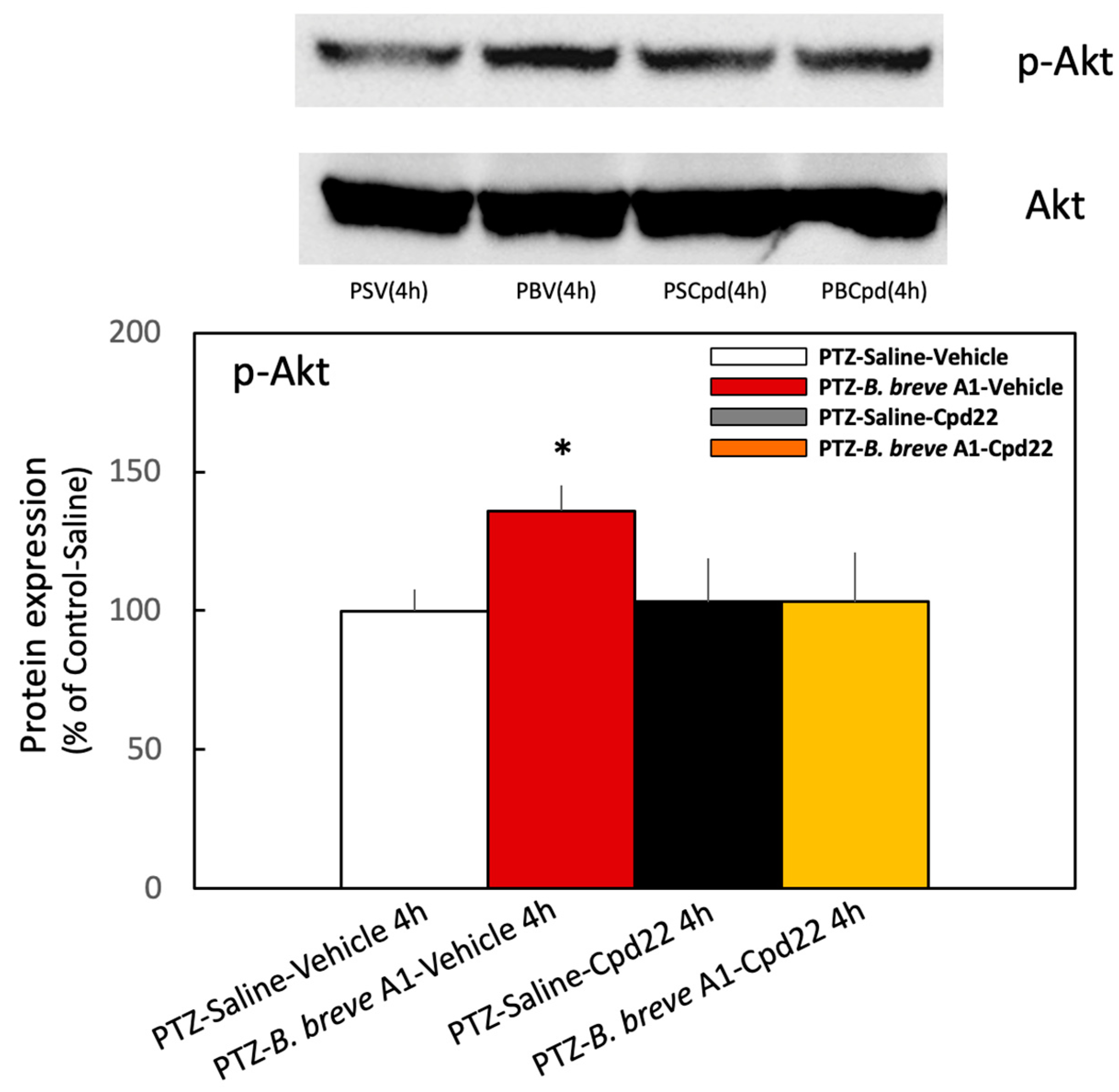
2.4. Effect of B. breve A1 on the Phosphorylation of Akt Ser473 (p-Akt) Expression in the Hippocampus
2.5. Effect of the ILK Inhibitor Cpd22 on the Antiepileptic Effect of B. breve A1
2.6. Effect of the ILK Inhibitor Cpd22 on p-Akt Expression Levels in KD Mice with or without the Administration of B. breve A1
3. Discussion
4. Materials and Methods
4.1. Animals and PTZ Treatment
4.2. RNA Extraction and RT–qPCR Assay
4.3. Western Blot Analysis
4.4. Administration of B. breve A1
4.5. Administration of Cpd22
4.6. Data Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; French, J.; Bialer, M. Development of new antiepileptic drugs: Challenges, incentives, and recent advances. Lancet Neurol. 2007, 6, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Temkin, N.R. Preventing and treating posttraumatic seizures: The human experience. Epilepsia 2009, 50 (Suppl. S2), 10–13. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B. Effects of antiepileptic drugs on mood and behavior. Epilepsia 2006, 47, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Ushida, K. Health-benefical effects of probiotics: Its mode of action. Anim. Sci. J. 2009, 80, 361–371. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Review control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Peterson, C.T. Dysfunction of the microbiota-gut-brain axis in neurodegenerative disease: The promise of therapeutic modulation with prebiotics, medicinal herbs, probiotics, and synbiotics. J. Evid. Based. Integr. Med. 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef]
- Castelli, V.; d’Angelo, M.; Quintiliani, M.; Benedetti, E.; Cifone, M.G.; Cimini, A. The emerging role of probiotics in neurodegenerative diseases: New hope of Parkinson’s disease? Neural. Regen. Res. 2021, 16, 628–634. [Google Scholar]
- He, Z.; Cui, B.T.; Zhang, T.; Li, P.; Long, C.Y.; Ji, G.Z.; Zhang, F.M. Fecal microbiota transplantation cured epilepsy in a case with Grohn’s Disease: The first report. World J. Gastroenterol. 2017, 23, 3565–3568. [Google Scholar] [CrossRef]
- Riva, A.; Sahin, E.; Volpedo, G.; Petretto, A.; Lavarello, C.; Sapia, R.D.; Barbarossa, D.; Zaniani, N.R.; Craparotta, I.; Barbera, M.C.; et al. Identification of an epilepsy-linked gut microbiota signature in a pediatric rat model of acquired epilepsy. Neurobiol. Dis. 2024, 194, 106469. [Google Scholar] [CrossRef]
- Ishii, T.; Furuoka, H.; Kaya, M.; Kuhara, T. Oral administration of probiotic Bifidobacterium breve improves facilitation of hippocampal memory extinction via restoration of aberrant higher induction of neuropsin in an MPTP-induced mouse model of Parkinson’s disease. Biomedicines 2021, 9, 167. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Xiao, J.; Katsumata, N.; Bernier, F.; Ohno, K.; Yamauchi, Y.; Odamaki, T.; Yoshikawa, K.; Ito, K.; Kaneko, T. Probiotic Bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. J. Alzheimers Dis. 2020, 77, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Helmstaedter, C.; Witt, J.A. Epilepsy and cognition—A bidirectional relationship? Seizure 2017, 49, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yamagata, K. Pentylenetetrazole-induced kindling mouse model. J. Vis. Exp. 2018, 136, 56573. [Google Scholar]
- Schmidt, J. Changes in seizure susceptibility in rats following chronic administration of pentylenetetrazol. Biomed. Biochim. Acta 1987, 46, 267–270. [Google Scholar]
- Angelatou, F.; Pagonopoulou, O.; Kostopoulog, G. Changes in seizure latency correlate with alterations in A1 adenosine receptor binding during daily repeated pentylenetetrazol-induced convulsions in different mouse brain area. Neurosci. Lett. 1991, 132, 203–206. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Nakade, J.; Tachibana, M.; Ibi, D.; Someya, E.; Koike, H.; Kamei, H.; Nabeshima, T.; Itohara, S.; Takuma, K.; et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetrtrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J. Neurosci. 2011, 31, 12963–12971. [Google Scholar] [CrossRef]
- Yong, W.V.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology, pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagi, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, J.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006, 15, 1923–1934. [Google Scholar] [CrossRef]
- Kim, G.W.; Kim, H.J.; Cho, K.J.; Kim, H.W.; Cho, Y.J.; Lee, B.I. The role of MMP-9 in integrin-mediated hippocampal cell death after pilocarpine-induced status epilepticus. Neurobiol. Dis. 2009, 36, 169–180. [Google Scholar] [CrossRef]
- Jourquin, J.; Tremblay, E.; Decanis, N.; Charton, G.; Hanessian, S.; Chollet, A.M.; Diguardher, T.L.; Khrestchatisky, M.; Rivera, S. Neuronal activity-dependent increase of net matrix metalloproteinase activity is associated with MMP-9 neurotoxicity after kainite. Eur. J. Neurosci. 2003, 18, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Jareo-Basulto, J.; Gasca-Martínez, Y.; Rivera-Cervantes, M.C.; Ureña-Guerrero, M.E.; Ferina-Velasco, A.I.; Beas-Zarate, C. Interactions between epilepsy and plasticity. Pharmaceuticals 2018, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E. Epilepsy as an example of neural plasticity. Neuroscientist 2002, 8, 154–173. [Google Scholar] [CrossRef]
- Ueno, H.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Takahashi, Y.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Alteration of extracellular matrix molecules and perineuronal nets in the hippocampus of pentylenetetrazol-kindled mice. Neural Plast. 2019, 2019, 8924634. [Google Scholar] [CrossRef]
- Shiosaka, S.; Ishikawa, Y. Neuropsin-A possible modulator of synaptic plasticity. J. Chem. Neuroanat. 2011, 42, 24–29. [Google Scholar] [CrossRef]
- Gary, D.S.; Mihavet, O.; Camandola, S.; Mattson, M.P. Essential role for integrin linked kinase in Akt-mediated integrin survival signaling in hippocampal neurons. J. Neurochem. 2003, 84, 878–890. [Google Scholar] [CrossRef]
- Matrisian, L.M. The matrix-degrading metalloproteinases. Bioessays 1992, 14, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Delcommenne, M.; Tan, C.; Gray, V.; Rue, L.; Woodgett, J.; Dedhar, S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 11211–11216. [Google Scholar] [CrossRef]
- Persad, S.; Attwell, S.; Gray, V.; Nawji, M.; Deng, J.T.; Leung, D.; Yan, J.; Sanghera, J.; Walsh, M.P.; Dedhar, S. Regulation of protein/Akt-serine 473 phosphorylation by integrin-linked kinase. J. Biol. Chem. 2001, 276, 27462–27469. [Google Scholar] [CrossRef]
- Lee, S.L.; Hsu, E.C.; Chou, C.C.; Chuang, H.C.; Bai, L.Y.; Kulp, S.K.; Chen, C.S. Identification and characterization of a novel integrin-linked kinase inhibitor. J. Med. Chem. 2011, 54, 6364–6374. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-brain-gut axis and neurodegenerative diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Wilczynski, G.M.; Knopoacki, F.A.; Wilczek, E.; Lasiecka, Z.; Gorlewicz, A.; Michaluk, P.; Wawrzyniak, M.; Malinowska, M.; Okulski, P.; Kolodziej, L.R.; et al. Important role of matorix metalloproteinase-9 in epileptogenesis. J. Cell Biol. 2008, 10180, 1021–1035. [Google Scholar] [CrossRef]
- Kazmi, Z.; Zeeshan, S.; Khan, A.; Malik, S.; Shehzad, A.; Seo, E.K.; Khan, S. Anti-epileptic activity of daidzin in PTZ-induced mice model by targeting oxidative stress and BDNF/VEGF signaling. Neurotoxicology 2020, 79, 150–163. [Google Scholar] [CrossRef]
- Kaur, H.; Patro, I.; Tikoo, K.; Sandhir, R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 2015, 89, 40–50. [Google Scholar] [CrossRef]
- Guo, X.Q.; Cao, Y.L.; Hao, F.; Yan, Z.R.; Wang, M.L.; Liu, X.W. Tangeretin alters neuronal apoptosis and ameliorates the severity of seizures in experimental epilepsy-induced rats by modulating apoptotic protein expressions, regulating matrix metalloproteinases, and activating the PI3K/Akt cell survival pathway. Adv. Med. Sci. 2017, 62, 246–253. [Google Scholar] [CrossRef]
- Dedhar, S.; Williams, B.; Hannigan, G. Integrin-linked kinase (ILK): A regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999, 9, 319–323. [Google Scholar] [CrossRef]
- Hannigan, G.; Troussard, A.A.; Dedhar, S. Integrin-linked kinase: A cancer therapeutic target unique among its ILK. Nat. Rev. Cancer 2005, 5, 51–63. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, X.; Yang, F.; Wang, H. CB2R induces a protective response for epileptic seizure via the PI3K 100α-AKT signaling pathway. Exp. Ther. Med. 2018, 16, 4784–4790. [Google Scholar]
- Yu, X.; Guan, Q.; Wang, Y.; Shen, H.; Zhai, L.; Lu, X.; Jin, Y. Anticonvulsant and anti-apoptosis effects of salvianolic acid B on pentylenetetrazole-kindled rats via AKT/CREB/BDNF signaling. Epilepsy Res. 2019, 154, 90–96. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Dedhar, S. New perspectives on the role of integrin-linked kinase (ILK) signaling in cancer metastasis. Cancers 2022, 14, 3209. [Google Scholar] [CrossRef]
- Yong, V.W.; Krekoski, C.A.; Forsyth, P.A.; Bell, R.; Edwards, D.R. Matrix metalloproteinases and disease of the CNS. Trends Neurosci. 1998, 21, 75–80. [Google Scholar] [CrossRef]
- Yong, V.W. Metalloproteinase mediators of pathology and regeneration in CNS. Nat. Neurosci. 2005, 6, 931–944. [Google Scholar] [CrossRef]
- Rivera, S.; Khrestchatisky, M.; Kaczmark, L.; Rosenberg, G.A.; Jaworski, D.M. Metzincin proteases and their inhibitors, foes or friends in nervous system physiology. J. Neurosci. 2010, 30, 15337–15357. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR aand the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Transcript | Primers | |
|---|---|---|
| β-Actin | Sense | ATTGCTGACAGGATGCAGAAG |
| Antisense | TAGAAGCACTTGCGGTGCACG | |
| Neuropsin | Sense | CTCAACTGTGCGGAAGTGAA |
| Antisense | ACTCCAGGTTTCTCGGGTTT | |
| ILK | Sense | AAGGTGCTGAAGGTTCGAGA |
| Antisense | CAGTGTGTGATGAGGGTTGG | |
| BDNF | Sense | GCGGCAGATAAAAAGACTGC |
| Antisense | CTTATGAATCGCCAGCCAAT | |
| Iba-1 | Sense | GAAGCGAATGCTGGAGAAAC |
| Antisense | GACCAGTTGGCCTCTTGTGT | |
| MMP-9 | Sense | CGTGTCTGGAGATTCGACTTGA |
| Antisense | TGGAAGATGTCGTGTGAGTTCC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, T.; Kaya, M.; Muroi, Y. Oral Administration of Probiotic Bifidobacterium breve Ameliorates Tonic–Clonic Seizure in a Pentylenetetrazole-Induced Kindling Mouse Model via Integrin-Linked Kinase Signaling. Int. J. Mol. Sci. 2024, 25, 9259. https://doi.org/10.3390/ijms25179259
Ishii T, Kaya M, Muroi Y. Oral Administration of Probiotic Bifidobacterium breve Ameliorates Tonic–Clonic Seizure in a Pentylenetetrazole-Induced Kindling Mouse Model via Integrin-Linked Kinase Signaling. International Journal of Molecular Sciences. 2024; 25(17):9259. https://doi.org/10.3390/ijms25179259
Chicago/Turabian StyleIshii, Toshiaki, Motohiro Kaya, and Yoshikage Muroi. 2024. "Oral Administration of Probiotic Bifidobacterium breve Ameliorates Tonic–Clonic Seizure in a Pentylenetetrazole-Induced Kindling Mouse Model via Integrin-Linked Kinase Signaling" International Journal of Molecular Sciences 25, no. 17: 9259. https://doi.org/10.3390/ijms25179259
APA StyleIshii, T., Kaya, M., & Muroi, Y. (2024). Oral Administration of Probiotic Bifidobacterium breve Ameliorates Tonic–Clonic Seizure in a Pentylenetetrazole-Induced Kindling Mouse Model via Integrin-Linked Kinase Signaling. International Journal of Molecular Sciences, 25(17), 9259. https://doi.org/10.3390/ijms25179259






