Interferon-Induced Transmembrane Protein 1 (IFITM1) Is Downregulated in Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors
Abstract
:1. Introduction
2. Results
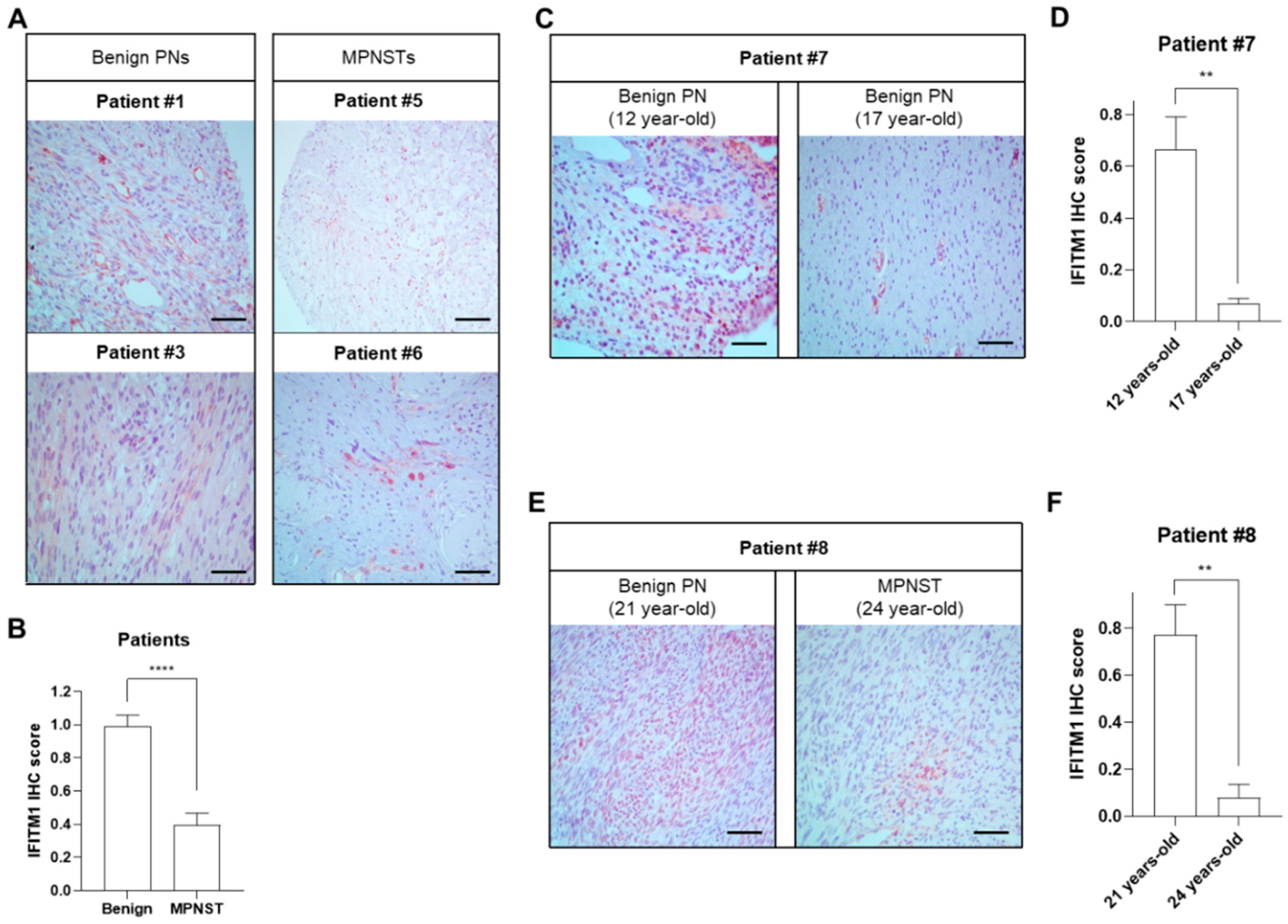
2.1. Downregulation of IFITM1 in MPNST Tissues of Patients with NF1
2.2. Close Association between IFITM1 Protein Levels and Ras/ERK Signaling in Normal and MPNST Schwann Cell Lines
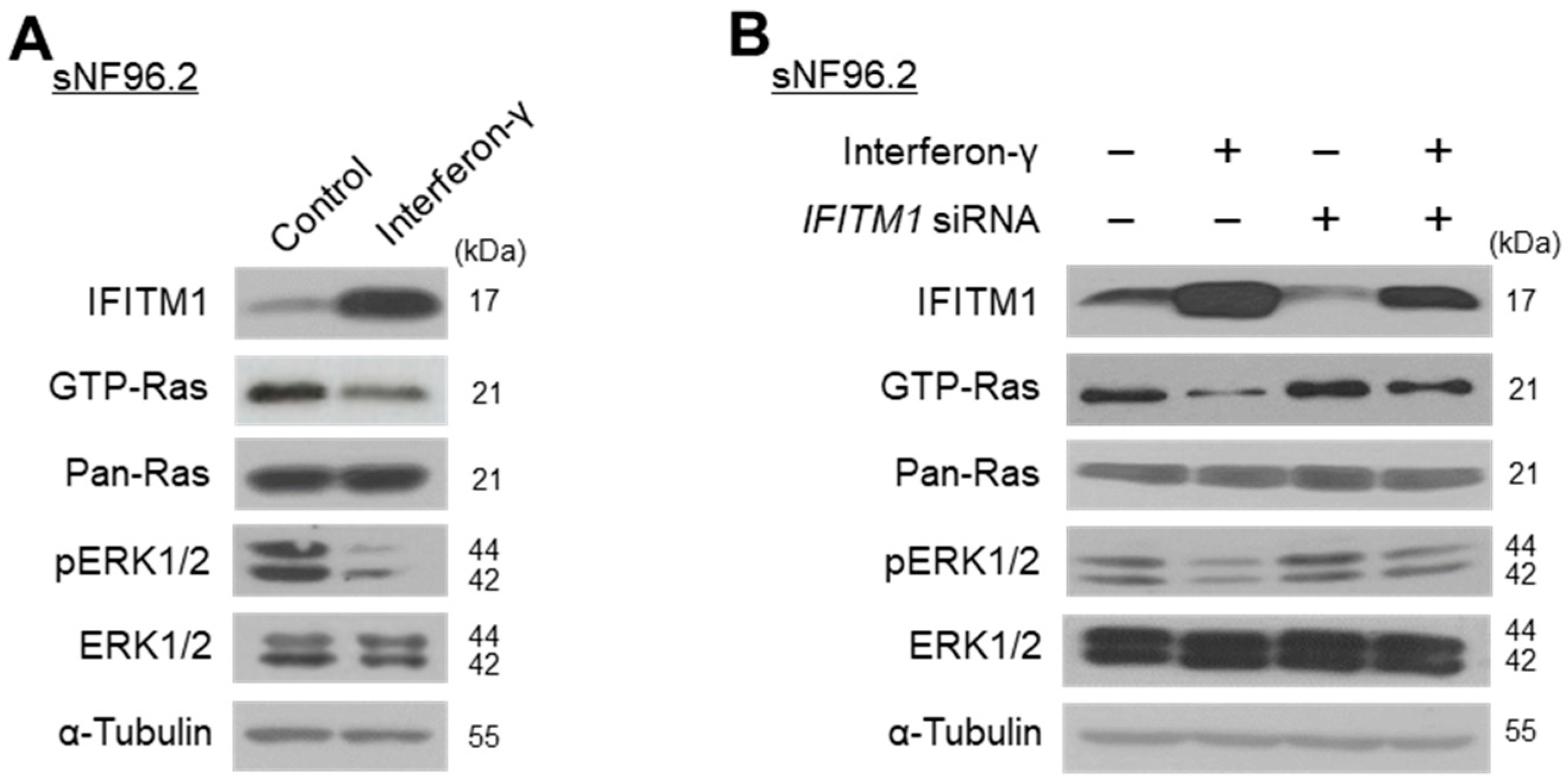
2.3. IFN-γ Upregulates IFITM1 and Inactivates Ras/ERK Signaling in MPNST Schwann Cells
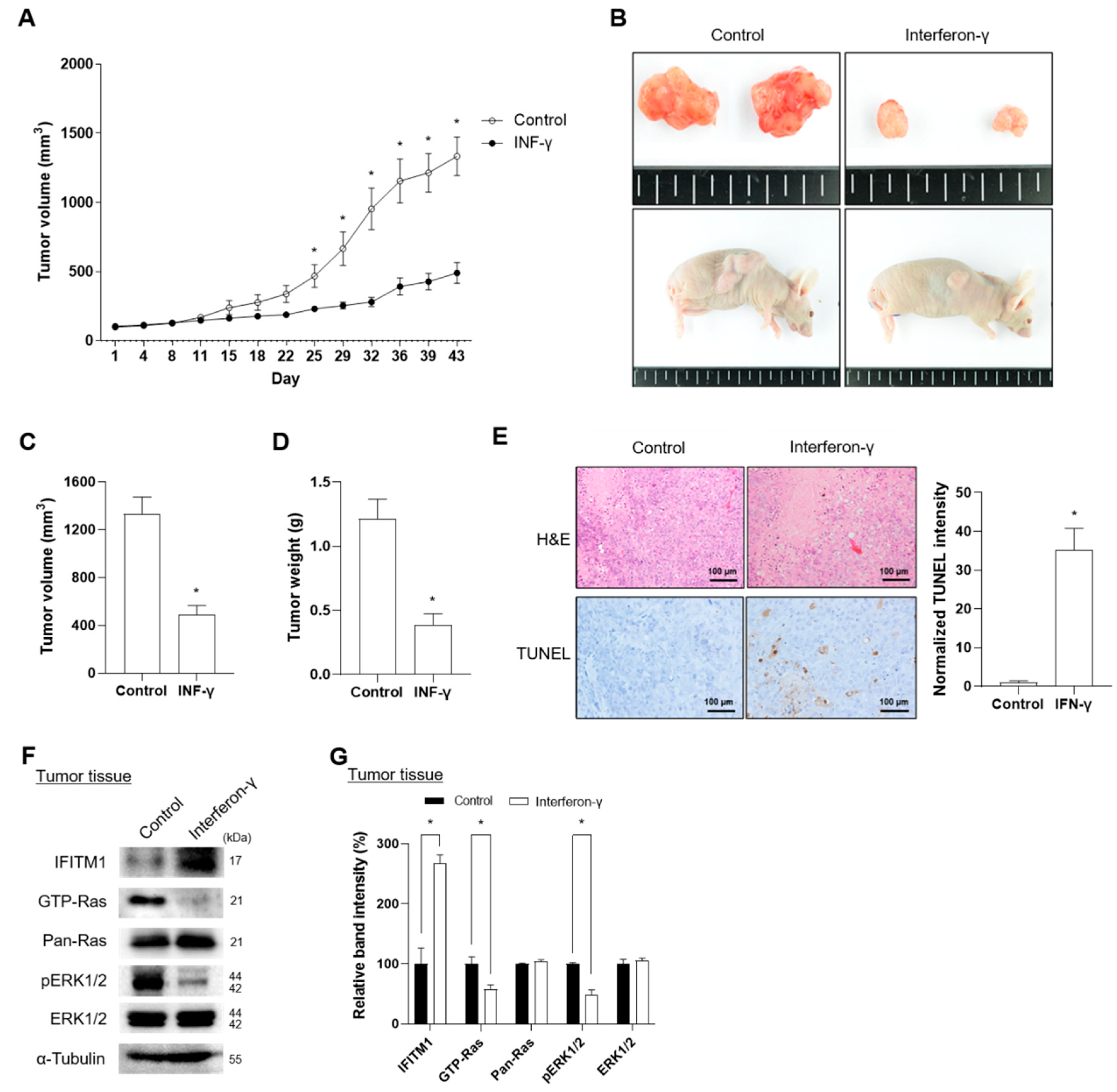
2.4. IFN-γ Suppresses Tumor Progression in Xenograft Mice Injected with NF1-Assocaited MPNST Cells
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Immunohistochemistry (IHC)
4.3. Reagents and Cell Lines
4.4. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.5. Short Interfering RNAs (siRNAs), Plasmids, and Transfection
4.6. Ras Activation Assay and Western Blot Analysis
4.7. Animal Study
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, K.P.; Korf, B.R.; Theos, A. Neurofibromatosis type 1. J. Am. Acad. Dermatol. 2009, 61, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McClatchey, A.I. Neurofibromatosis. Annu. Rev. Pathol. Mech. Dis. 2007, 2, 191–216. [Google Scholar] [CrossRef] [PubMed]
- Jett, K.; Friedman, J.M. Clinical and genetic aspects of neurofibromatosis 1. Genet. Med. 2010, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M.; Weiss, R.; Xu, G.; Viskochil, D.; Culver, M.; Stevens, J.; Robertson, M.; Dunn, D.; Gesteland, R.; O’Connell, P. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell 1990, 62, 193–201. [Google Scholar] [CrossRef]
- Brems, H.; Beert, E.; de Ravel, T.; Legius, E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009, 10, 508–515. [Google Scholar] [CrossRef]
- Spurlock, G.; Knight, S.J.; Thomas, N.; Kiehl, T.-R.; Guha, A.; Upadhyaya, M. Molecular evolution of a neurofibroma to malignant peripheral nerve sheath tumor (MPNST) in an NF1 patient: Correlation between histopathological, clinical and molecular findings. J. Cancer Res. Clin. Oncol. 2010, 136, 1869–1880. [Google Scholar] [CrossRef]
- Evans, D.G.; Baser, M.E.; McGaughran, J.; Sharif, S.; Howard, E.; Moran, A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J. Med. Genet. 2002, 39, 311–314. [Google Scholar] [CrossRef]
- McCaughan, J.A.; Holloway, S.M.; Davidson, R.; Lam, W.W. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J. Med. Genet. 2007, 44, 463–466. [Google Scholar] [CrossRef]
- Tucker, T.; Wolkenstein, P.; Revuz, J.; Zeller, J.; Friedman, J. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 2005, 65, 205–211. [Google Scholar] [CrossRef]
- McQueen, M.; MacCollin, M.; Gusella, J.; Plotkin, S.R. Patient and physician attitudes regarding clinical trials in neurofibromatosis 1. J. Neurosci. Nurs. 2008, 40, 341–345. [Google Scholar] [CrossRef]
- Kluwe, L.; Friedrich, R.; Mautner, V.F. Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer 1999, 24, 283–285. [Google Scholar] [CrossRef]
- Zhu, Y.; Ghosh, P.; Charnay, P.; Burns, D.K.; Parada, L.F. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science 2002, 296, 920–922. [Google Scholar] [CrossRef]
- Kluwe, L.; Friedrich, R.E.; Mautner, V.F. Allelic loss of the NF1 gene in NF1-associated plexiform neurofibromas. Cancer Genet. Cytogenet. 1999, 113, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.P.; Stemmer-Rachamimov, A.O.; Ino, Y.; Møller, M.B.; Rosenberg, A.E.; Louis, D.N. Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am. J. Pathol. 1999, 155, 1879–1884. [Google Scholar] [CrossRef]
- Mawrin, C.; Kirches, E.; Boltze, C.; Dietzmann, K.; Roessner, A.; Schneider-Stock, R. Immunohistochemical and molecular analysis of p53, RB, and PTEN in malignant peripheral nerve sheath tumors. Virchows Arch. 2002, 440, 610–615. [Google Scholar] [CrossRef]
- Upadhyaya, M. Genetic basis of tumorigenesis in NF1 malignant peripheral nerve sheath tumors. Front. Biosci. 2011, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Chen, J.; Patel, A.; Zhang, L.; Chau, V.; Li, Y.; Cho, W.; Lim, K.; Xu, J.; Lazar, A.J. CXCR4/CXCL12 mediate autocrine cell-cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell 2013, 152, 1077–1090. [Google Scholar] [CrossRef]
- Legius, E.; Dierick, H.; Wu, R.; Hall, B.K.; Marynen, P.; Cassiman, J.J.; Glover, T.W. TP53 mutations are frequent in malignant NFI tumors. Genes. Chromosomes Cancer 1994, 10, 250–255. [Google Scholar] [CrossRef]
- Levy, P.; Ripoche, H.; Laurendeau, I.; Lazar, V.; Ortonne, N.; Parfait, B.a.; Leroy, K.; Wechsler, J.; Salmon, I.; Wolkenstein, P. Microarray-based identification of tenascin C and tenascin XB, genes possibly involved in tumorigenesis associated with neurofibromatosis type 1. Clin. Cancer Res. 2007, 13, 398–407. [Google Scholar] [CrossRef]
- Watanabe, T.; Oda, Y.; Tamiya, S.; Masuda, K.; Tsuneyoshi, M. Malignant peripheral nerve sheath tumour arising within neurofibroma. An immunohistochemical analysis in the comparison between benign and malignant components. J. Clin. Pathol. 2001, 54, 631. [Google Scholar] [CrossRef]
- Johannessen, C.M.; Reczek, E.E.; James, M.F.; Brems, H.; Legius, E.; Cichowski, K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc. Natl. Acad. Sci. USA 2005, 102, 8573–8578. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Lee, S.-J.; Sohn, Y.B.; Jin, H.-S.; Han, J.H.; Kim, Y.-B.; Yim, H.; Jeong, S.-Y. NF1 deficiency causes Bcl-xL upregulation in Schwann cells derived from neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Int. J. Oncol. 2013, 42, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.L.; Ratner, N. How does the Schwann cell lineage form tumors in NF1? Glia 2008, 56, 1590–1605. [Google Scholar] [CrossRef]
- Gregorian, C.; Nakashima, J.; Dry, S.M.; Nghiemphu, P.L.; Smith, K.B.; Ao, Y.; Dang, J.; Lawson, G.; Mellinghoff, I.K.; Mischel, P.S. PTEN dosage is essential for neurofibroma development and malignant transformation. Proc. Natl. Acad. Sci. USA 2009, 106, 19479–19484. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Deshmukh, H.; Payton, J.E.; Dunham, C.; Scheithauer, B.W.; Tihan, T.; Prayson, R.A.; Guha, A.; Bridge, J.A.; Ferner, R.E. Array-based comparative genomic hybridization identifies CDK4 and FOXM1 alterations as independent predictors of survival in malignant peripheral nerve sheath tumor. Clin. Cancer Res. 2011, 17, 1924–1934. [Google Scholar] [CrossRef]
- Stricker, T.P.; Henriksen, K.J.; Tonsgard, J.H.; Montag, A.G.; Krausz, T.N.; Pytel, P. Expression profiling of 519 kinase genes in matched malignant peripheral nerve sheath tumor/plexiform neurofibroma samples is discriminatory and identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed with transformation. Mod. Pathol. 2013, 26, 930–943. [Google Scholar] [CrossRef]
- Holtkamp, N.; Okuducu, A.F.; Mucha, J.; Afanasieva, A.; Hartmann, C.; Atallah, I.; Estevez-Schwarz, L.; Mawrin, C.; Friedrich, R.E.; Mautner, V.-F. Mutation and expression of PDGFRA and KIT in malignant peripheral nerve sheath tumors, and its implications for imatinib sensitivity. Carcinogenesis 2006, 27, 664–671. [Google Scholar] [CrossRef]
- Holtkamp, N.; Malzer, E.; Zietsch, J.; Okuducu, A.F.; Mucha, J.; Mawrin, C.; Mautner, V.-F.; Schildhaus, H.-U.; Von Deimling, A. EGFR and erbB2 in malignant peripheral nerve sheath tumors and implications for targeted therapy. Neuro-Oncology 2008, 10, 946–957. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Han, J.-H.; Park, Y.-Y.; Kim, H.-J. Identification of differentially expressed genes related to NF1-associated malignant transformation from a patient with neurofibromatosis type 1. Genes Genom. 2008, 30, 407–418. [Google Scholar]
- Akyerli, C.B.; Beksac, M.; Holko, M.; Frevel, M.; Dalva, K.; Özbek, U.; Soydan, E.; Özcan, M.; Özet, G.; Ilhan, O. Expression of IFITM1 in chronic myeloid leukemia patients. Leuk. Res. 2005, 29, 283–286. [Google Scholar] [CrossRef]
- Yang, G.; Xu, Y.; Chen, X.; Hu, G. IFITM1 plays an essential role in the antiproliferative action of interferon-γ. Oncogene 2007, 26, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Z.; Yi, X.; Zuo, Q.; Li, H.; Guo, X.; Li, D.; He, H.; Pan, Z.; Fan, P. Down-regulation of IFITM1 and its growth inhibitory role in cervical squamous cell carcinoma. Cancer Cell Int. 2017, 17, 88. [Google Scholar] [CrossRef]
- Katz, D.; Lazar, A.; Lev, D. Malignant peripheral nerve sheath tumour (MPNST): The clinical implications of cellular signalling pathways. Expert. Rev. Mol. Med. 2009, 11, e30. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, V.W.; Smalley, K.S. Change or die: Targeting adaptive signaling to kinase inhibition in cancer cells. Biochem. Pharmacol. 2014, 91, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Parada, L. Tumor microenvironment and neurofibromatosis type I: Connecting the GAPs. Oncogene 2007, 26, 4609–4616. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Stalnecker, C.A.; Der, C.J. RAS, wanted dead or alive: Advances in targeting RAS mutant cancers. Sci. Signal. 2020, 13, eaay6013. [Google Scholar] [CrossRef]
- Williams, K.B.; Largaespada, D.A. New model systems and the development of targeted therapies for the treatment of neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Genes 2020, 11, 477. [Google Scholar] [CrossRef]
- Lee, S.-J.; Park, H.-J.; Kim, Y.-H.; Kim, B.-Y.; Jin, H.-S.; Kim, H.J.; Han, J.-H.; Yim, H.; Jeong, S.-Y. Inhibition of Bcl-xL by ABT-737 enhances chemotherapy sensitivity in neurofibromatosis type 1-associated malignant peripheral nerve sheath tumor cells. Int. J. Mol. Med. 2012, 30, 443–450. [Google Scholar] [CrossRef]
- Lau, S.L.Y.; Yuen, M.L.; Kou, C.Y.C.; Au, K.W.; Zhou, J.; Tsui, S.K.W. Interferons induce the expression of IFITM1 and IFITM3 and suppress the proliferation of rat neonatal cardiomyocytes. J. Cell. Biochem. 2012, 113, 841–847. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I-and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Romerio, F.; Riva, A.; Zella, D. Interferon-α 2b reduces phosphorylation and activity of MEK and ERK through a Ras/Raf-independent mechanism. Br. J. Cancer 2000, 83, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Spyra, M.; Kluwe, L.; Hagel, C.; Nguyen, R.; Panse, J.; Kurtz, A.; Mautner, V.F.; Rabkin, S.D.; Demestre, M. Cancer stem cell-like cells derived from malignant peripheral nerve sheath tumors. PLoS ONE 2011, 6, e21099. [Google Scholar] [CrossRef] [PubMed]
- Meehan, S.; Wu, A.J.; Kang, E.C.; Sakai, T.; Ambudkar, I.S. Interferon-γ induces a decrease in the intracellular calcium pump in a human salivary gland cell line. Am. J. Physiol.-Cell Physiol. 1997, 273, C2030–C2036. [Google Scholar] [CrossRef]
- Balbous, A.; Cortes, U.; Guilloteau, K.; Villalva, C.; Flamant, S.; Gaillard, A.; Milin, S.; Wager, M.; Sorel, N.; Guilhot, J. A mesenchymal glioma stem cell profile is related to clinical outcome. Oncogenesis 2014, 3, e91. [Google Scholar] [CrossRef]
- Nakayama, J.; Tanaka, T.; Arakawa, F.; Terao, H.; Shimura, H.; Ikeda, S.; Kuroki, M. Gamma interferon gene transfection efficiently inhibits proliferation of neurofibroma cell lines in vitro. J. Dermatol. 2003, 30, 181–188. [Google Scholar] [CrossRef]
- Nakayama, J.; Tanaka, T.; Furumura, M.; Takahashi, A.; Yamaguchi, T.; Shimura, H.; Ikeda, S.; Kuroki, M. Inhibition of the proliferation of a malignant peripheral nerve sheath tumor cell line by gamma interferon gene transfection. J. Dermatol. 2003, 30, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Terao, H. Gamma interferon directly inhibits the growth of neurofibroma cells in vitro. J. Dermatol. 2002, 29, 556–561. [Google Scholar] [CrossRef]
- He, J.; Li, J.; Feng, W.; Chen, L.; Yang, K. Prognostic significance of INF-induced transmembrane protein 1 in colorectal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 16007. [Google Scholar]
- Sari, N.I.; Yang, Y.-G.; Phi, L.T.H.; Kim, H.; Baek, M.J.; Jeong, D.; Kwon, H.Y. Interferon-induced transmembrane protein 1 (IFITM1) is required for the progression of colorectal cancer. Oncotarget 2016, 7, 86039. [Google Scholar] [CrossRef]
- Borg, D.; Hedner, C.; Gaber, A.; Nodin, B.; Fristedt, R.; Jirström, K. Expression of IFITM1 as a prognostic biomarker in resected gastric and esophageal adenocarcinoma. Biomark. Res. 2016, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.L. Molecular mechanisms promoting the pathogenesis of Schwann cell neoplasms. Acta Neuropathol. 2012, 123, 321–348. [Google Scholar] [CrossRef]
- Somatilaka, B.N.; Sadek, A.; McKay, R.M.; Le, L.Q. Malignant peripheral nerve sheath tumor: Models, biology, and translation. Oncogene 2022, 41, 2405–2421. [Google Scholar] [CrossRef]
- Pellerino, A.; Verdijk, R.M.; Nichelli, L.; Andratschke, N.H.; Idbaih, A.; Goldbrunner, R. Diagnosis and Treatment of Peripheral and Cranial Nerve Tumors with Expert Recommendations: An EUropean Network for RAre CANcers (EURACAN) Initiative. Cancers 2023, 15, 1930. [Google Scholar] [CrossRef] [PubMed]
- Jakacki, R.I.; Dombi, E.; Steinberg, S.M.; Goldman, S.; Kieran, M.W.; Ullrich, N.J.; Pollack, I.F.; Goodwin, A.; Manley, P.E.; Fangusaro, J.; et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2017, 19, 289–297. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Zibelman, M.; MacFarlane, A.W.T.; Costello, K.; McGowan, T.; O’Neill, J.; Kokate, R.; Borghaei, H.; Denlinger, C.S.; Dotan, E.; Geynisman, D.M.; et al. A phase 1 study of nivolumab in combination with interferon-gamma for patients with advanced solid tumors. Nat. Commun. 2023, 14, 4513. [Google Scholar] [CrossRef]

| Patients | Histological Findings | Clinical Features | Genotype | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gender | Age at Diagnosis | H&E | S100 | IFITM1 | Café-Au-Lait Spots | Neuro-Fibromas | Freckling | Optic Glioma | Lisch Nodule | Skeletal Dysplasia | Family History | NF1 Gene Mutation |
| P1 | Male | 59 | Benign | + | ++ | Y | Y | Y | N | N | N | Y | N/A |
| P3 | Female | 5 | Benign | + | ++ | Y | Y | Y | N | N | N | Y | N/A |
| P5 | Male | 32 | Malignant | + | + | Y | Y | N | N | N | N | N | c.4861_4862GT>AG |
| P6 | Female | 41 | Malignant | + | + | Y | Y | Y | N | N | N | N | N/A |
| P7 | Male | 12 | Benign | + | ++ | Y | Y | Y | N | Y | Y | N | c.4537C>T |
| 17 | Benign | + | + | Y | Y | Y | N | Y | Y | N | c.4537C>T | ||
| P8 | Male | 21 | Benign | + | ++ | Y | Y | Y | N | Y | N | N | c.6792C>A |
| 24 | Malignant | + | + | Y | Y | Y | N | Y | N | N | c.6792C>A | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.-H.; Park, E.; Lee, S.-J.; Lim, K.; Kim, J.; Park, J.E.; Jeong, S.-Y. Interferon-Induced Transmembrane Protein 1 (IFITM1) Is Downregulated in Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors. Int. J. Mol. Sci. 2024, 25, 9265. https://doi.org/10.3390/ijms25179265
Park G-H, Park E, Lee S-J, Lim K, Kim J, Park JE, Jeong S-Y. Interferon-Induced Transmembrane Protein 1 (IFITM1) Is Downregulated in Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors. International Journal of Molecular Sciences. 2024; 25(17):9265. https://doi.org/10.3390/ijms25179265
Chicago/Turabian StylePark, Gun-Hoo, Eunkuk Park, Su-Jin Lee, Kyubin Lim, Jeonghyun Kim, Jun Eun Park, and Seon-Yong Jeong. 2024. "Interferon-Induced Transmembrane Protein 1 (IFITM1) Is Downregulated in Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors" International Journal of Molecular Sciences 25, no. 17: 9265. https://doi.org/10.3390/ijms25179265





