Neonatal Fungemia by Non-Candida Rare Opportunistic Yeasts: A Systematic Review of Literature
Abstract
:1. Introduction
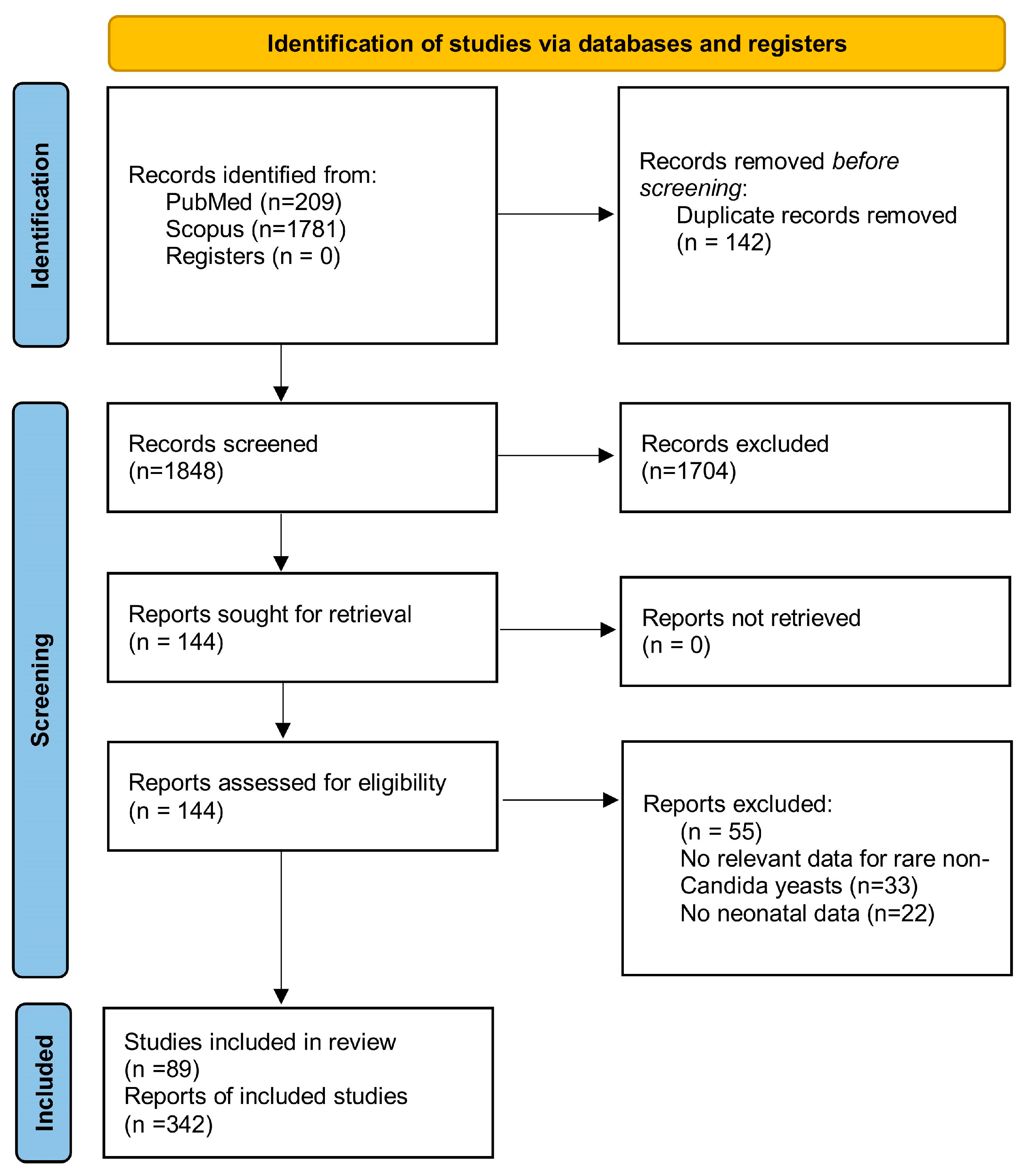
2. Methods
2.1. Formulation of the Research Question
- Population: Neonates
- Intervention: Rare opportunistic yeasts
- Comparison: Not required for this review
- Outcome: Cases of neonatal fungemia
2.2. Development of the Review Protocol
- Case reports and case series studies reporting on cases of neonatal fungemia from non-Candida rare opportunistic yeasts.
- Study designs focused on non-Candida rare opportunistic yeast infections, mainly defined as positive cultures from blood in neonates.
- Exclusion Criteria:
- Studies referring to other forms of fungal infections.
- Cases not involving neonates.
- Duplicate publications of the same cases.
- Review articles, systematic reviews, and meta-analyses. Conference proceedings will be excluded.
- Neonatal fungemia by Candida species
- Clinical presentation of non-Candida rare yeasts infections in neonates
- Risk factors and characteristics of the affected population
- Outcomes of the infections, including mortality
2.3. Search Strategy-Data Sources
2.4. Conflict Resolution
2.5. Data Synthesis and Presentation
3. Results
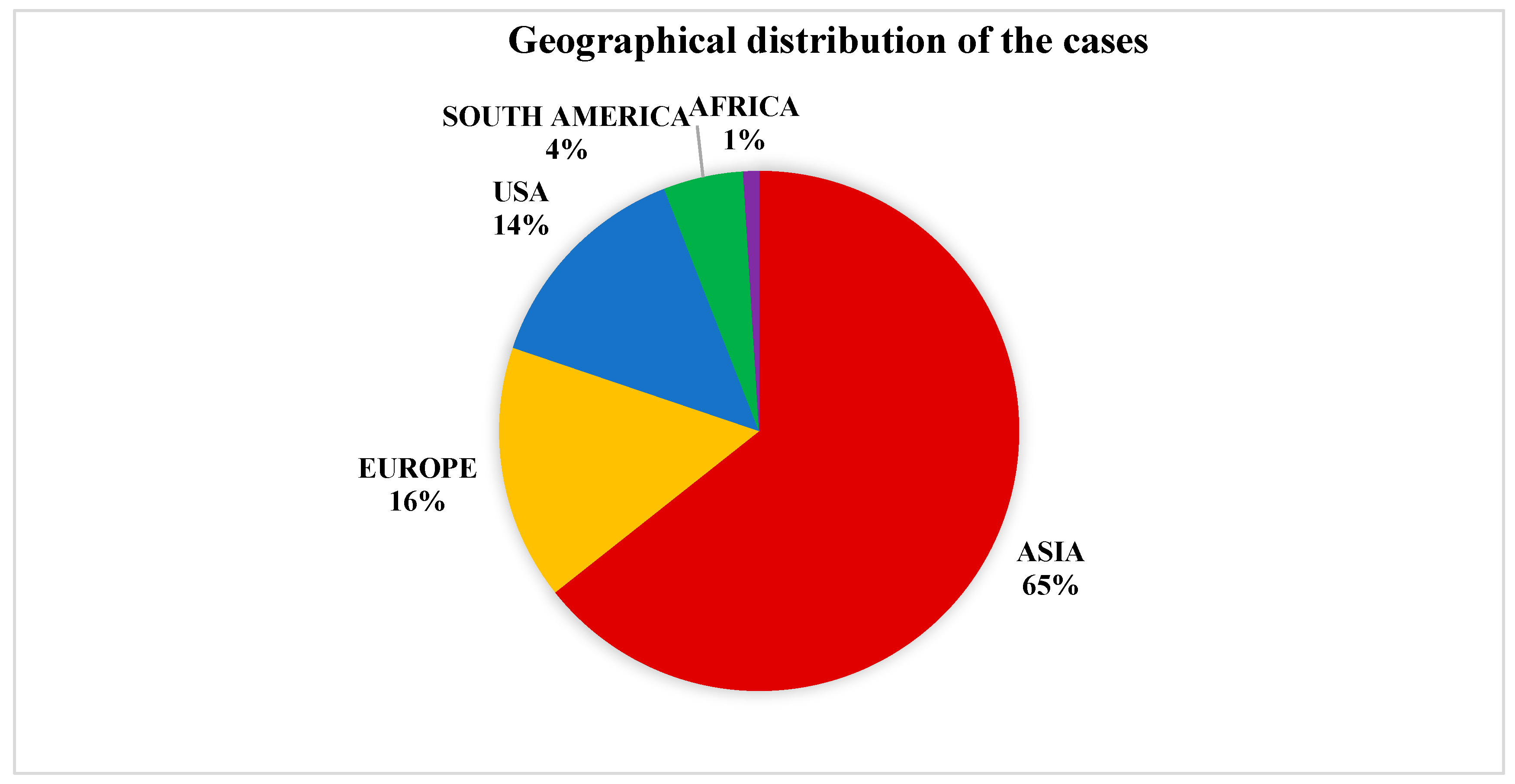
3.1. Species Taxonomy and Geographical Distribution
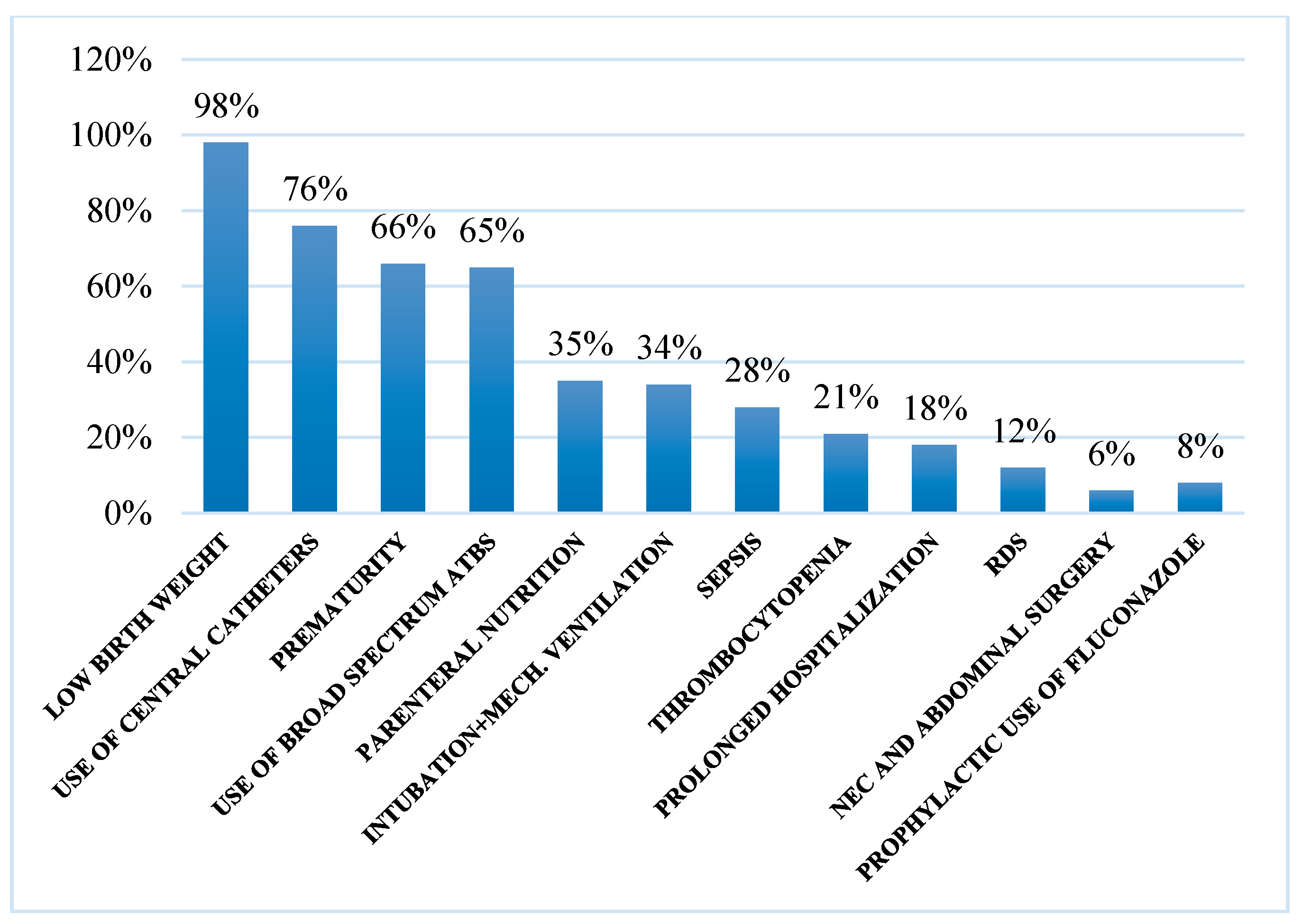
3.2. Risk Factors
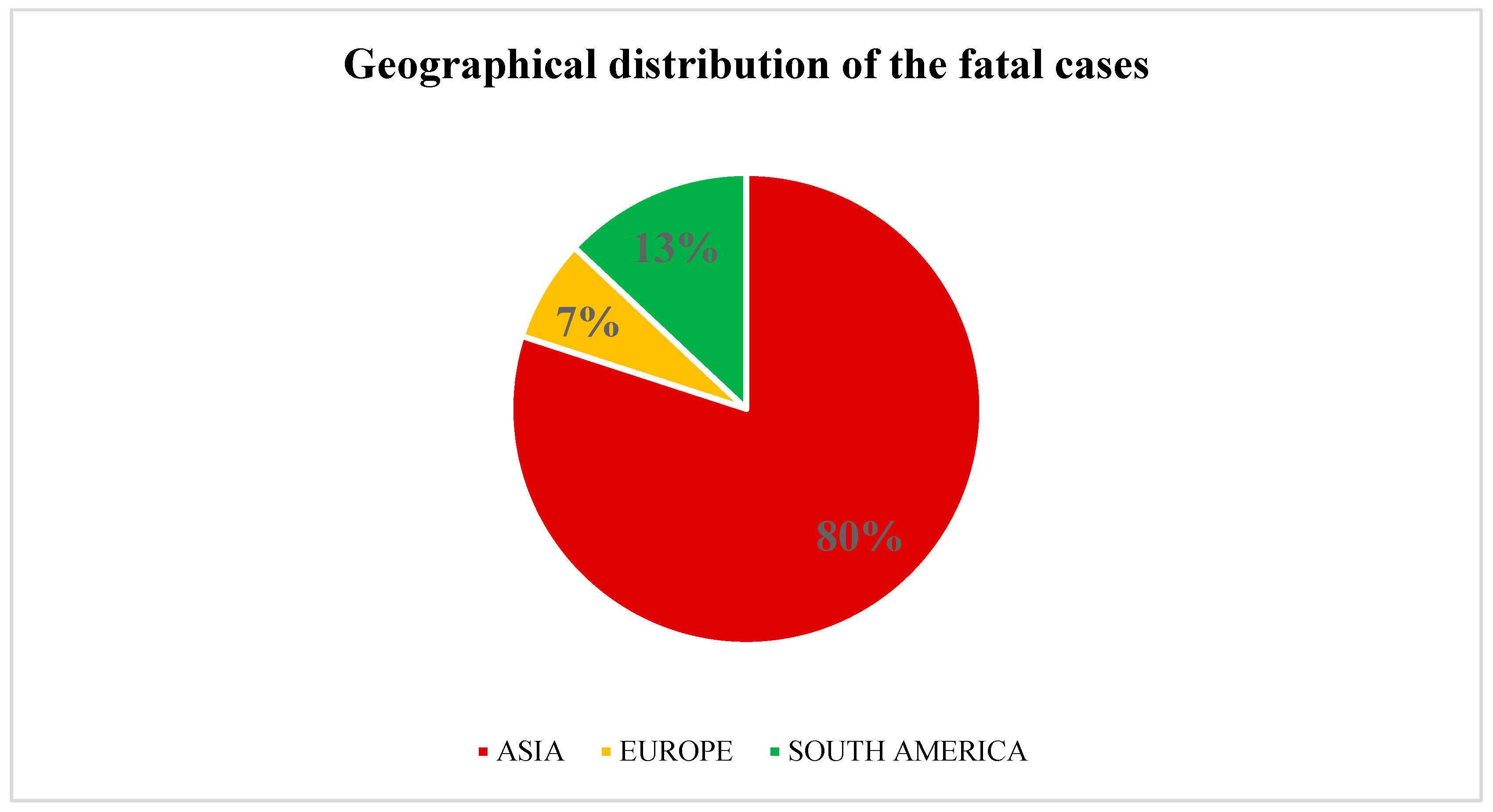
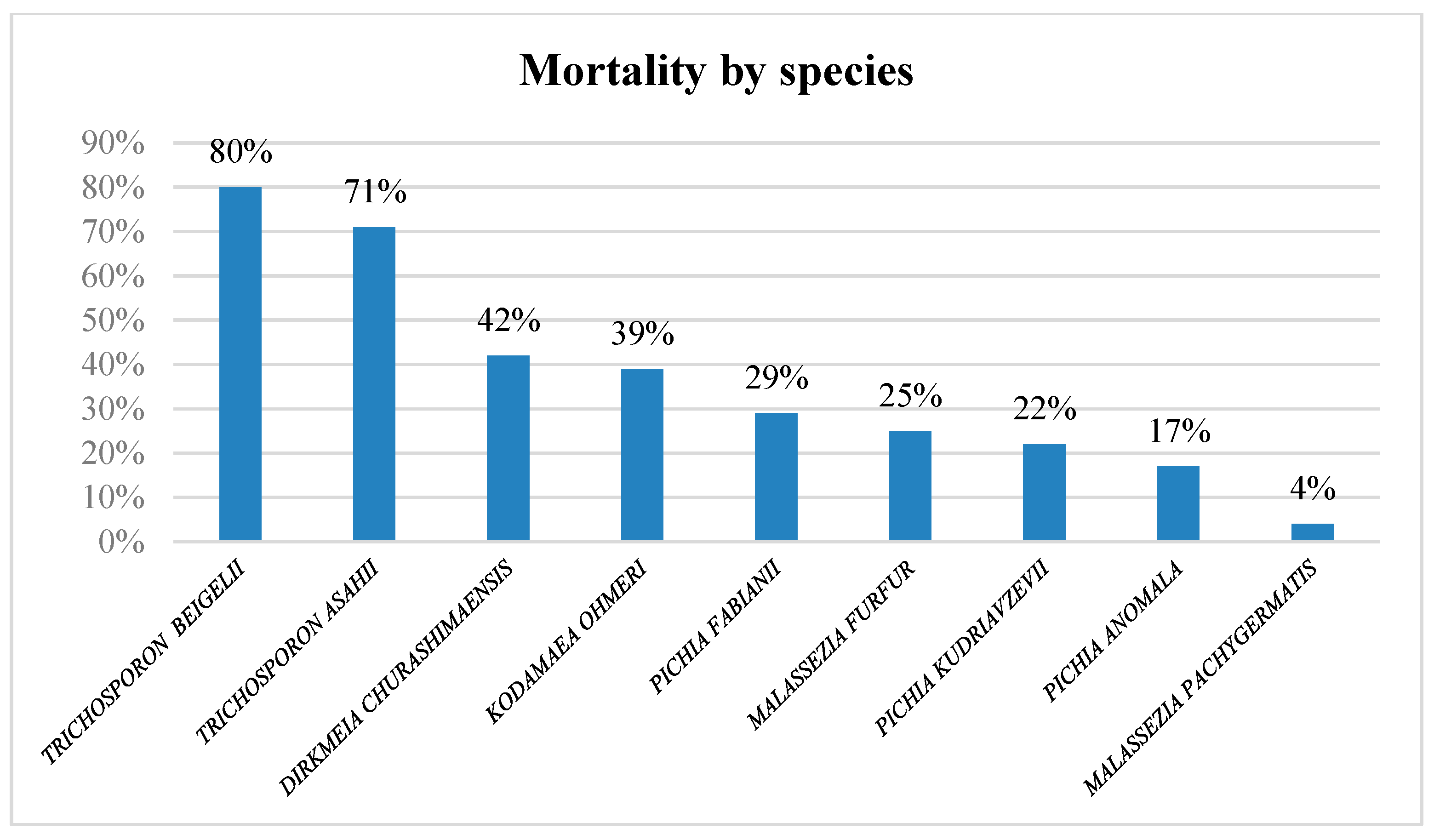
3.3. Outcome
3.4. Antifungal Susceptibility
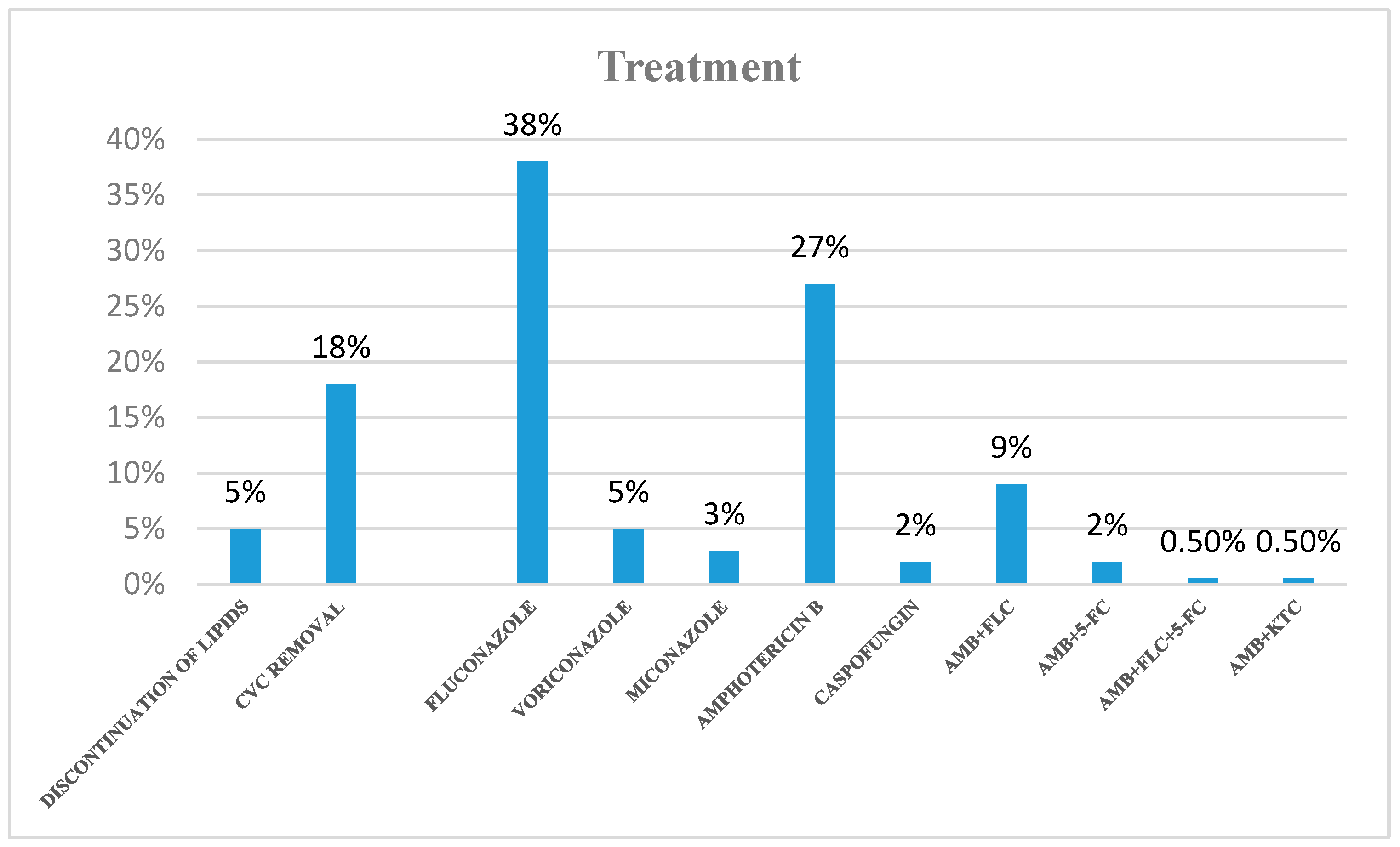
3.5. Treatment
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoll, B.J.; Hansen, N.; Fanaroff, A.A.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Lemons, J.A.; Donovan, E.F.; Stark, A.R.; Tyson, J.E.; et al. Late-Onset Sepsis in Very Low Birth Weight Neonates: The Experience of the NICHD Neonatal Research Network. Pediatrics 2002, 110, 285–291. [Google Scholar] [CrossRef]
- Weimer, K.E.D.; Smith, P.B.; Puia-Dumitrescu, M.; Aleem, S. Invasive fungal infections in neonates: A review. Pediatr. Res. 2021, 91, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Taliaka, P.K.; Tsantes, A.G.; Konstantinidi, A.; Houhoula, D.; Tsante, K.A.; Vaiopoulos, A.G.; Piovani, D.; Nikolopoulos, G.K.; Bonovas, S.; Iacovidou, N.; et al. Risk Factors, Diagnosis, and Treatment of Neonatal Fungal Liver Abscess: A Systematic Review of the Literature. Life 2023, 13, 167. [Google Scholar] [CrossRef]
- Nelson, R. Emergence of resistant Candida auris. Lancet Microbe 2023, 4, e396. [Google Scholar] [CrossRef]
- Sokou, R.; Palioura, A.E.; Kopanou Taliaka, P.; Konstantinidi, A.; Tsantes, A.G.; Piovani, D.; Tsante, K.A.; Gounari, E.A.; Iliodromiti, Z.; Boutsikou, T.; et al. Candida auris Infection, a Rapidly Emerging Threat in the Neonatal Intensive Care Units: A Systematic Review. J. Clin. Med. 2024, 13, 1586. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Redline, R.W.; Dahms, B.B. Malassezia Pulmonary Vasculitis in an Infant on Long-Term Intralipid Therapy. N. Engl. J. Med. 1981, 305, 1395–1398. [Google Scholar] [CrossRef]
- Powell, D.A.; Aungst, J.; Snedden, S.; Hansen, N.; Brady, M. Broviac catheter-related Malassezia furfur sepsis in five infants receiving intravenous fat emulsions. J. Pediatr. 1984, 105, 987–990. [Google Scholar] [CrossRef]
- Redline, R.W.; Redline, S.S.; Boxerbaum, B.; Dahms, B.B. Systemic Malassezia furfur infections in patients receiving intralipid therapy. Hum. Pathol. 1985, 16, 815–822. [Google Scholar] [CrossRef]
- Murphy, N.; Damjanovic, V.; Hart, C.A.; Buchanan, C.R.; Whitaker, R.; Cooke, R.W.I. Infection and colonisation of neonates by hansenula anomala. Lancet 1986, 327, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Dankner, W.M.; Spector, S.A.; Fierer, J.; Davis, C.E. Malassezia fungemia in neonates and adults: Complication of hyperalimentation. Rev. Infect. Dis. 1987, 9, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Alpert, G.; Bell, L.M.; Campos, J.M. Malassezia furfur fungemia in infancy. Clin. Pediatr. 1987, 26, 528–531. [Google Scholar] [CrossRef]
- Shek, Y.H.; Tucker, M.C.; Viciana, A.L.; Manz, H.J.; Connor, D.H. Malassezia furfur--disseminated infection in premature infants. Am. J. Clin. Pathol. 1989, 92, 595–603. [Google Scholar] [CrossRef]
- Surmont, I.; Gavilanes, A.; Vandepitte, J.; Devlieger, H.; Eggermont, E. Malassezia furfur fungaemia in infants receiving intravenous lipid emulsions. A rarity or just underestimated? Eur. J. Pediatr. 1989, 148, 435–438. [Google Scholar] [CrossRef]
- Weiss Malassezia Furfur Fungemia Associated with Central Venous Catheter Lipid Emulsion Infusion—Aναζήτηση Google. Available online: https://www.google.com/search?q=weiss+Malassezia+furfur+fungemia+associated+with+central+venous+catheter+lipid+emulsion+infusion&sca_esv=578293274&ei=soJBZf2BAoyGxc8Pn--UgA0&udm=&ved=0ahUKEwj9ooTkraGCAxUMQ_EDHZ83BdAQ4dUDCBA&uact=5&oq=weiss+Malassezia+furf (accessed on 1 November 2023).
- Hruszkewycz, V.; Holtrop, P.C.; Batton, D.G.; Morden, R.S.; Gibson, P.; Band, J.D. Complications associated with central venous catheters inserted in critically ill neonates. Infect. Control Hosp. Epidemiol. 1991, 12, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Masure, O.; Leostic, C.; Abalain, M.; Chastel, C.; Yakoub-Agha, I.; Berthou, C.; Briere, J. Malassezia furfur septicaemia in a child with leukaemia. J. Infect. 1991, 23, 335–336. [Google Scholar] [CrossRef]
- Giacoia, G.P. Trichosporon beigelii: A Potential Cause of Sepsis in Premature Infants. South. Med. J. 1992, 85, 1247–1248. [Google Scholar] [CrossRef]
- Herbrecht, R.; Waller, J.; Dufour, P.; Koenig, H.; Lioure, B.; Marcellin, L.; Oberling, F. Rare opportunistic fungal diseases in patients with organ or bone marrow transplantation. Agressologie 1992, 33, 77–80. [Google Scholar]
- Fisher, D.J.; Christy, C.; Spafford, P.; Maniscalco, W.M.; Hardy, D.J.; Graman, P.S. Neonatal Trichosporon beigelii infection: Report of a cluster of cases in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 1993, 12, 149–155. [Google Scholar] [CrossRef]
- Welbel, S.F.; McNeil, M.M.; Pramanik, A.; Silberman, R.; Oberle, A.D.; Midgley, G.; Crow, S.; Jarvis, W.R. Nosocomial Malassezia pachydermatis bloodstream infections in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 1994, 13, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Yoss, B.S.; Sautter, R.L.; Brenker, H.J. Trichosporon beigelii, a new neonatal pathogen. Am. J. Perinatol. 1997, 14, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.B.; Bradley, S.F.; Kauffman, C.A. Fungaemia due to Cryptococcus laurentii and a review of non-neoformans cryptococcaemia. Mycoses 1998, 41, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Miller, H.L.; Watkins, N.; Arduino, M.J.; Ashford, D.A.; Midgley, G.; Aguero, S.M.; Pinto-Powell, R.; von Reyn, C.F.; Edwards, W.; et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N. Engl. J. Med. 1998, 338, 706–711. [Google Scholar] [CrossRef]
- Sweet, D.; Reid, M. Disseminated neonatal Trichosporon beigelii infection: Successful treatment with liposomal amphotericin B. J. Infect. 1998, 36, 120–121. [Google Scholar] [CrossRef]
- Singh, K.; Chakrabarti, A.; Narang, A.; Gopalan, S. Yeast colonisation & fungaemia in preterm neonates in a tertiary care centre. Indian J. Med. Res. 1999, 110, 169. [Google Scholar]
- Perapoch, J.; Planes, A.M.; Querol, A.; López, V.; Martínez-Bendayán, I.; Tormo, R.; Fernández, F.; Peguero, G.; Salcedo, S. Fungemia with Saccharomyces cerevisiae in two newborns, only one of whom had been treated with ultra-levura. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 468–470. [Google Scholar] [CrossRef]
- Ma, J.S.; Chen, P.Y.; Chen, C.H.; Chi, C.S. Neonatal fungemia caused by Hansenula anomala: A case report. J. Microbiol. Immunol. Infect. 2000, 33, 267–270. [Google Scholar]
- Wong, A.R.; Ibrahim, H.; Van Rostenberghe, H.; Ishak, Z.; Radzi, M.J. Hansenula anomala infection in a neonate. J. Paediatr. Child. Health 2000, 36, 609–610. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Singh, K.; Narang, A.; Singhi, S.; Batra, R.; Rao, K.L.; Ray, P.; Gopalan, S.; Das, S.; Gupta, V.; et al. Outbreak of Pichia anomala infection in the pediatric service of a tertiary-care center in Northern India. J. Clin. Microbiol. 2001, 39, 1702–1706. [Google Scholar] [CrossRef]
- Aragão, P.A.; Oshiro, I.C.V.; Manrique, E.I.; Gomes, C.C.; Matsuo, L.L.; Leone, C.; Moretti-Branchini, M.L.; Levin, A.S. Pichia anomala outbreak in a nursery: Exogenous source? Pediatr. Infect Dis. J. 2001, 20, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-F.; Chiou, C.C.; Liu, Y.-C.; Wang, H.-Z.; Hsieh, K.-S. Cryptococcus laurentii Fungemia in a Premature Neonate. J. Clin. Microbiol. 2001, 39, 1608–1611. [Google Scholar] [CrossRef]
- Chryssanthou, E.; Broberger, U.; Petrini, B. Malassezia pachydermatis fungaemia in a neonatal intensive care unit. Acta Paediatr. 2007, 90, 323–327. [Google Scholar] [CrossRef]
- Panagopoulou, P.; Evdoridou, J.; Bibashi, E.; Filioti, J.; Sofianou, D.; Kremenopoulos, G.; Roilides, E. Trichosporon asahii: An unusual cause of invasive infection in neonates. Pediatr. Infect. Dis. J. 2002, 21, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Yıldıran, A.; Kücüködük, Ş.; Saniç, A.; Belet, N.; Güvenli, A. Disseminated Trichosporon asahii Infection in a Preterm. Am. J. Perinatol. 2003, 20, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Gökahmetoğlu, S.; Nedret Koç, A.; Güneş, T.; Cetin, N. Case reports. Trichosporon mucoides infection in three premature newborns. Mycoses 2002, 45, 123–125. [Google Scholar] [CrossRef]
- Baron, E.; Anaissie, E.; Dumphy, F.; McCredie, K.; Fainstein, V. Hansenula anomala fungemia. Clin. Infect. Dis. 1988, 10, 1182–1186. [Google Scholar] [CrossRef]
- Bakır, M.; Çerikcioğlu, N.; Tırtır, A.; Berrak, S.; Özek, E.; Canpolat, C. Pichia anomala fungaemia in immunocompromised children. Mycoses 2004, 47, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Belet, N.; Dalgiç, N.; Oncel, S.; Ciftçi, E.; Ince, E.; Güriz, H.; Barlas, M.; Doğru, U. Catheter-related fungemia caused by Saccharomyces cerevisiae in a newborn. Pediatr. Infect. Dis. J. 2005, 24, 1125. [Google Scholar] [CrossRef]
- Taj-Aldeen, S.J.; Doiphode, S.H.; Han, X.Y. Kodamaea (Pichia) ohmeri fungaemia in a premature neonate. J. Med. Microbiol. 2006, 55, 237–239. [Google Scholar] [CrossRef]
- Bhally, H.S.; Jain, S.; Shields, C.; Halsey, N.; Cristofalo, E.; Merz, W.G. Infection in a neonate caused by Pichia fabianii: Importance of molecular identification. Med. Mycol. 2006, 44, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Paula, C.R.; Krebs, V.L.; Auler, M.E.; Ruiz, L.S.; Matsumoto, F.E.; Silva, E.H.; Diniz, E.M.; Vaz, F.A. Nosocomial infection in newborns by Pichia anomala in a Brazilian intensive care unit. Med. Mycol. 2006, 44, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Perniola, R.; Faneschi, M.L.; Manso, E.; Pizzolante, M.; Rizzo, A.; Damiani, A.S.; Longo, R. Rhodotorula mucilaginosa outbreak in neonatal intensive care unit: Microbiological features, clinical presentation, and analysis of related variables. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Vivas, R.; Beltran, C.; Munera, M.I.; Trujillo, M.; Restrepo, A.; Garcés, C. Fungemia due to Kodamaea ohmeri in a young infant and review of the literature. Med. Mycol. Case Rep. 2016, 13, 5–8. [Google Scholar] [CrossRef]
- Ngerncham, S.; Lapphra, K. Cryptococcus neoformans septicemia in an immunocompetent neonate: First case report in Thailand. Southeast Asian J. Trop. Med. Public Health 2008, 39, 697–700. [Google Scholar]
- Téllez-Castillo, C.J.; Gil-Fortuño, M.; Centelles-Sales, I.; Sabater-Vidal, S.; Serrano, F.P. Trichosporon asahii fatal infection in a preterm newborn. Rev. Chil. Infectología 2008, 25, 213–215. [Google Scholar]
- Poojary, A.; Sapre, G. Kodamaea ohmeri infection in a neonate. Indian Pediatr. 2009, 46, 629–631. [Google Scholar]
- Pereira, D.N.; Nader, S.S.; Nader, P.; Martins, P.G.; Furlan, S.P.; Hentges, C.R. Disseminated Trichosporon spp infection in preterm newborns: A case report. J. Pediatr. 2009, 85, 459–461. [Google Scholar]
- Noni, M.; Stathi, A.; Velegraki, A.; Malamati, M.; Kalampaliki, A.; Zachariadou, L.; Michos, A. Rare Invasive Yeast Infections in Greek Neonates and Children, a Retrospective 12-Year Study. J. Fungi 2020, 6, 194. [Google Scholar] [CrossRef]
- Grenouillet, F.; Millon, L.; Chamouine, A.; Thiriez, G.; Schulze, O.; Leroy, J. Pichia fabianii Fungemia in a Neonate. Pediatr. Infect. Dis. J. 2010, 29, 191. [Google Scholar] [CrossRef]
- Duggal, S.; Jain, H.; Tyagi, A.; Sharma, A.; Chugh, T.D. Rhodotorula fungemia: Two cases and a brief review. Med. Mycol. 2011, 49, 879–882. [Google Scholar] [PubMed]
- Oliveri, S.; Trovato, L.; Betta, P.; Romeo, M.G.; Nicoletti, G. Malassezia furfur fungaemia in a neonatal patient detected by lysis-centrifugation blood culture method: First case reported in Italy. Mycoses 2011, 54, 638. [Google Scholar] [CrossRef]
- Al-Sweih, N.; Khan, Z.U.; Ahmad, S.; Devarajan, L.; Khan, S.; Joseph, L.; Chandy, R. Kodamaea ohmeri as an emerging pathogen: A case report and review of the literature. Med. Mycol. 2011, 49, 766–770. [Google Scholar] [PubMed]
- Vashishtha, V.M.; Mittal, A.; Garg, A. A fatal outbreak of Trichosporon asahii Sepsis in a neonatal intensive care unit. Indian Pediatr. 2012, 49, 745–747. [Google Scholar] [CrossRef]
- Lin, H.-C.; Lin, H.-Y.; Su, B.-H.; Ho, M.-W.; Ho, C.-M.; Lee, C.-Y.; Lin, M.-H.; Hsieh, H.-Y.; Lin, H.-C.; Li, T.-C.; et al. Reporting an outbreak of Candida pelliculosa fungemia in a neonatal intensive care unit. J. Microbiol. Immunol. Infect. 2013, 46, 456–462. [Google Scholar] [CrossRef]
- da Silva, C.M.; de Carvalho Parahym, A.M.; Leão, M.P.; de Oliveira, N.T.; de Jesus Machado Amorim, R.; Neves, R.P. Fungemia by Candida pelliculosa (Pichia anomala) in a neonatal intensive care unit: A possible clonal origin. Mycopathologia 2013, 175, 175–179. [Google Scholar] [CrossRef]
- Iatta, R.; Cafarchia, C.; Cuna, T.; Montagna, O.; Laforgia, N.; Gentile, O.; Rizzo, A.; Boekhout, T.; Otranto, D.; Montagna, M.T. Bloodstream infections by Malassezia and Candida species in critical care patients. Med. Mycol. 2014, 52, 264–269. [Google Scholar] [CrossRef]
- Chioukh, F.Z.; Ben Hmida, H.; Ben Ameur, K.; Toumi, A.; Monastiri, K. Saccharomyces cerevisiae fungemia in a premature neonate treated receiving probiotics. Med. Mal. Infect. 2013, 43, 359–360. [Google Scholar] [CrossRef]
- Liu, C.X.; Yang, J.H.; Dong, L.; Mai, J.Y.; Zhang, L.; Zhu, J.H. Clinical features and homological analysis of Pichia ohmeri-caused hospital-acquired fungemia in premature infants. Zhonghua Yi Xue Za Zhi 2013, 93, 285–288. [Google Scholar]
- Wu, Y.; Wang, J.; Li, W.; Jia, H.; Che, J.; Lu, J.; Liu, L.; Cheng, Y. Pichia fabianii blood infection in a premature infant in China: Case report. BMC Res. Notes 2013, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Kodamaea Ohmeri as an Emerging Pathogen in Mainland China—Aναζήτηση Google. Available online: https://www.google.com/search?q=%0D%0AKodamaea+ohmeri+as+an+Emerging+Pathogen+in+Mainland+China&sca_esv=578502807&ei=aWtCZfutD86Rxc8PoKSAsAc&udm=&ved=0ahUKEwi7htvbi6OCAxXOSPEDHSASAHYQ4dUDCBA&uact=5&oq=%0D%0AKodamaea+ohmeri+as+an+Emerging+Pathogen+in+Mainland+China&gs_lp=Egxnd3Mtd2l6LXNlcnAiOgpLb2RhbWFlYSBvaG1lcmkgYXMgYW4gRW1lcmdpbmcgUGF0aG9nZW4gaW4gTWFpbmxhbmQgQ2hpbmFIwwtQnwhYnwhwAXgBkAEAmAEAoAEAqgEAuAEDyAEA-AEB-AECqAIA4gMEGAAgQYgGAQ&sclient=gws-wiz-serp (accessed on 1 November 2023).
- Chakrabarti, A.; Rudramurthy, S.M.; Kale, P.; Hariprasath, P.; Dhaliwal, M.; Singhi, S.; Rao, K.L.N. Epidemiological study of a large cluster of fungaemia cases due to Kodamaea ohmeri in an Indian tertiary care centre. Clin. Microbiol. Infect. 2014, 20, O83–O89. [Google Scholar] [CrossRef] [PubMed]
- Borade, A.; Kapdi, M.; Suryavanshi, K. Kodamaea Ohmeri—An Emerging Fungal Pathogen in Neonatal Intensive Care Unit. Pediatr. Oncall 2014, 11, 114–116. [Google Scholar] [CrossRef]
- Prakash, A.; Wankhede, S.; Singh, P.K.; Agarwal, K.; Kathuria, S.; Sengupta, S.; Barman, P.; Meis, J.F.; Chowdhary, A. First neonatal case of fungaemia due to Pseudozyma aphidis and a global literature review. Mycoses 2014, 57, 64–68. [Google Scholar] [CrossRef]
- Al-Sweih, N.; Ahmad, S.; Joseph, L.; Khan, S.; Khan, Z. Malassezia pachydermatis fungemia in a preterm neonate resistant to fluconazole and flucytosine. Med. Mycol. Case Rep. 2014, 5, 9–11. [Google Scholar] [CrossRef]
- Biswal, D.; Sahu, M.; Mahajan, A.; Advani, S.H.; Shah, S. Kodameae ohmeri—An Emerging Yeast: Two Cases and Literature Review. J. Clin. Diagn. Res. 2015, 9, DD01. [Google Scholar] [CrossRef]
- Mlinarić-Missoni, E.; Hatvani, L.; Kocsube, S.; Vágvölgyi, C.; Škarić, I.; Lukić-Grlić, A. Cyberlindnera fabianii in the neonatal and paediatric intensive care unit: Case reports. JMM Case Rep. 2015, 2, e000032. [Google Scholar] [CrossRef]
- Basu, S.; Tilak, R.; Kumar, A. Multidrug-resistant Trichosporon: An unusual fungal sepsis in preterm neonates. Ann. Trop. Med. Parasitol. 2015, 109, 202–206. [Google Scholar] [CrossRef]
- Okolo, O.M.; Van Diepeningen, A.D.; Toma, B.; Nnadi, N.E.; Ayanbimpe, M.G.; Onyedibe, I.K.; Sabitu, M.Z.; Banwat, B.E.; Groenewald, M.; Scordino, F.; et al. First report of neonatal sepsis due to Moesziomyces bullatus in a preterm low-birth-weight infant. JMM Case Rep. 2015, 2, e000011. [Google Scholar] [CrossRef]
- Socarras, J.A.; Rojas Torres, J.P.; Vargas Soler, J.A.; Guerrero, C. Kodamaea ohmeri infection in a newborn with a mediastinal mass. Arch. Argent. Pediatr. 2016, 114, e319–e322. [Google Scholar]
- Yılmaz-Semerci, S.; Demirel, G.; Taştekin, A. Wickerhamomyces anomalus blood stream infection in a term newborn with pneumonia. Turk. J. Pediatr. 2017, 59, 349–351. [Google Scholar] [CrossRef]
- Nagarathnamma, T.; Chunchanur, S.K.; Rudramurthy, S.M.; Vineetha, K.R.; Ramamurthy, K.; Joseph, J.; Ambica, R. Outbreak of Pichia kudriavzevii fungaemia in a neonatal intensive care unit. J. Med. Microbiol. 2017, 66, 1759–1764. [Google Scholar] [CrossRef]
- Kulkarni, V.; Manasa, B.M. Rhodotorula: An emerging pathogen in NICU. Perinatology 2017, 18, 77–80. [Google Scholar]
- Kumar, A.; Roy, P.; Rai, G.; Das, S.; Ansari, M.A. Cyberlindnera fabianii and Wickerhamomyces anomalous fungemia in newborns: An experience from a North Indian tertiary-care centre. Indian J. Med. Spéc. 2017, 8, 131–133. [Google Scholar] [CrossRef]
- Roy, U.; Jessani, L.G.; Rudramurthy, S.M.; Gopalakrishnan, R.; Dutta, S.; Chakravarty, C.; Jillwin, J.; Chakrabarti, A. Seven cases of Saccharomyces fungaemia related to use of probiotics. Mycoses 2017, 60, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mishra, A.K.; Singh, S.K. Cryptococcus laurentii fungemia in a low birth weight preterm neonate: India. J. Infect. Public. Health 2018, 11, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.F.; Lisboa, C.; Rodrigues, A.G. Malassezia infections with systemic involvement: Figures and facts. J. Dermatol. 2018, 45, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Iatta, R.; Battista, M.; Miragliotta, G.; Boekhout, T.; Otranto, D.; Cafarchia, C. Blood culture procedures and diagnosis of Malassezia furfur bloodstream infections: Strength and weakness. Med. Mycol. 2017, 56, 828–833. [Google Scholar] [CrossRef]
- Al-Sweih, N.; Ahmad, S.; Khan, S.; Joseph, L.; Asadzadeh, M.; Khan, Z. Cyberlindnera fabianii fungaemia outbreak in preterm neonates in Kuwait and literature review. Mycoses 2018, 62, 51–61. [Google Scholar] [CrossRef]
- Chen, I.T.; Chen, C.C.; Huang, H.C.; Kuo, K.C. Malassezia furfur Emergence and Candidemia Trends in a Neonatal Intensive Care Unit During 10 Years: The Experience of Fluconazole Prophylaxis in a Single Hospital. Adv. Neonatal Care 2020, 20, E3–E8. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Peng, C.-C.; Hsu, C.-H.; Chang, J.-H.; Chiu, N.-C.; Chi, H. Systemic Infection Caused by Malassezia pachydermatis in Infants: Case Series and Review of the Literature. Pediatr. Infect. Dis. J. 2020, 39, 444–448. [Google Scholar] [CrossRef]
- Chow, N.A.; Chinn, R.; Pong, A.; Schultz, K.; Kim, J.; Gade, L.; Jackson, B.R.; Beer, K.D.; Litvintseva, A.P. Use of whole-genome sequencing to detect an outbreak of Malassezia pachydermatis infection and colonization in a neonatal intensive care unit-California, 2015–2016. Infect. Control. Hosp. Epidemiol. 2020, 41, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharada, K.; Singh, P.K.; Bhagwani, D.K.; Kumar, N.; de Groot, T.; Meis, J.F. Outbreak of Dirkmeia churashimaensis Fungemia in a Neonatal Intensive Care Unit, India. Emerg. Infect. Dis. 2020, 26, 764. [Google Scholar] [CrossRef]
- Pandey, N.; Paul, P.; Kumar, D.; Tilak, R. Case report of a rare yeast Cyberlindnera fabianii fungemia in preterm twin neonates from North India: Diagnostic and therapeutic challenge. Indian J. Case Rep. 2020, 6, 563–565. [Google Scholar] [CrossRef]
- Al-Otaibi, H.; Asadzadeh, M.; Ahmad, S.; Al-Sweih, N.; Joseph, L. Papiliotrema laurentii fungemia in a premature, very low-birth-weight neonate in Kuwait successfully treated with liposomal amphotericin B. J. Med. Mycol. 2021, 31, 101123. [Google Scholar] [CrossRef]
- Galvis-Marín, J.C.; Giraldo-Ospina, B.; Martínez-Ríos, J.B.; Echeverri-Peláez, S. Fungemia by malassezia sympodialis in a neonatal intensive care unit in Colombia. Infectio 2021, 25, 130–134. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, W.; Cheng, Z. Candida pelliculosa sepsis in a neonate: A casereport. J. Int. Med. Res. 2021, 49, 0300060520982804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, Y.; Li, Y.; Chen, X.; Ding, C.; Liu, Y. Risk factors and biofilm formation analyses of hospital-acquired infection of Candida pelliculosa in a neonatal intensive care unit. BMC Infect. Dis. 2021, 21, 620. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, W.; Ding, L.; Yang, L.; Su, J.; Wu, B. Two different clones of Candida pelliculosa bloodstream infection in a tertiary neonatal intensive care unit. J. Infect. Dev. Ctries. 2021, 15, 870–876. [Google Scholar] [CrossRef]
- Mpakosi, A.; Siopi, M.; Demetriou, M.; Falaina, V.; Theodoraki, M.; Meletiadis, J. Fungemia due to Moesziomyces aphidis (Pseudozyma aphidis) in a premature neonate. Challenges in species identification and antifungal susceptibility testing of rare yeasts. J. Med. Mycol. 2022, 32, 101258. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Al-Sweih, N.; Ahmad, S.; Khan, S.; Alfouzan, W.; Joseph, L. Fatal Lodderomyces elongisporus Fungemia in a Premature, Extremely Low-Birth-Weight Neonate. J. Fungi. 2022, 8, 906. [Google Scholar] [CrossRef]
- da Silva, C.M.; Jucá, M.B.; Melo AS de, A.; Lima, S.L.; Galvão, P.V.M.; Macêdo, D.P.C.; Neves, R.P. Neonatal fungemia caused by Lodderomyces elongisporus: First case report in Latin America. Diagn. Microbiol. Infect. Dis. 2023, 107, 116077. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.A.; Osuagwu, C.S.; Aikhomu, V.; Oladele, R.O.O. Cryptococcus laurentii fungaemia in a Tertiary Hospital in Nigeria: Case reports. Microbes Infect. Dis. 2023, 4, 1428–1434. [Google Scholar] [CrossRef]
- Samaddar, A.; Sharma, A. First case of neonatal fungemia caused by Aureobasidium melanogenum. J. Med. Mycol. 2023, 33, 101334. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.A.; Karnaker, V.K.; Sreelatha, S.V.; Ramdas, S.; Nair, S.; Varma, S.R. A Rare Case of Fungaemia Due to Kodamaea ohmeri in a Neonate. Online J. Health Allied Sci. 2023, 22, 11. [Google Scholar]
- Kaur, H.; Chakrabarti, A. Strategies to Reduce Mortality in Adult and Neonatal Candidemia in Developing Countries. J. Fungi 2017, 3, 41. [Google Scholar] [CrossRef]
- McCrossan, B.; McHenry, E.; O‘Neill, F.; Ong, G.; Sweet, D.G. Selective fluconazole prophylaxis in high-risk babies to reduce invasive fungal infection. Arch. Dis. Child.-Fetal Neonatal Ed. 2007, 92, F454–F458. [Google Scholar] [CrossRef]
- Anaraki, M.R.; Nouri-Vaskeh, M.; Oskoei, S.A. Fluconazole prophylaxis against invasive candidiasis in very low and extremely low birth weight preterm neonates: A systematic review and meta-analysis. Clin. Exp. Pediatr. 2021, 64, 172–179. [Google Scholar] [CrossRef]
- Bretagne, S.; Renaudat, C.; Desnos-Ollivier, M.; Sitbon, K.; Lortholary, O.; Dromer, F.; the Cochrane Adverse Effects Methods Group. Predisposing factors and outcome of uncommon yeast species-related fungaemia based on an exhaustive surveillance programme (2002–2014). J. Antimicrob. Chemother. 2017, 72, 1784–1793. [Google Scholar] [CrossRef]
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. Fluconazole Resistance in Isolates of Uncommon Pathogenic Yeast Species from the United Kingdom. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-S.; Shin, S.H.; Choi, C.W.; Kim, E.-K.; Choi, E.H.; Kim, B.I.; Choi, J.-H. Efficacy and safety of fluconazole prophylaxis in extremely low birth weight infants: Multicenter pre-post cohort study. BMC Pediatr. 2016, 16, 67. [Google Scholar] [CrossRef]
- Iosifidis, E.; Papachristou, S.; Roilides, E. Advances in the Treatment of Mycoses in Pediatric Patients. J. Fungi. 2018, 4, 115. [Google Scholar] [CrossRef]
- Nunes, J.M.; Bizerra, F.C.; Carmona e Ferreira, R.; Colombo, A.L. Molecular Identification, Antifungal Susceptibility Profile, and Biofilm Formation of Clinical and Environmental Rhodotorula Species Isolates. Antimicrob. Agents Chemother. 2013, 57, 382–389. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans Biofilm Formation Depends on Surface Support and Carbon Source and Reduces Fungal Cell Susceptibility to Heat, Cold, and UV Light. Appl. Environ. Microbiol. 2007, 73, 4592–4601. [Google Scholar] [CrossRef]
- Iturrieta-González, I.A.; Padovan, A.C.B.; Bizerra, F.C.; Hahn, R.C.; Colombo, A.L. Multiple Species of Trichosporon Produce Biofilms Highly Resistant to Triazoles and Amphotericin B. PLoS ONE 2014, 9, e109553. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Merz, W.G. Infections caused by non-Candida, non-Cryptococcus yeasts. Clin. Mycol. 2009, 10, 251–270. [Google Scholar]
- Jindal, N.; Arora, S.; Dhuria, N.; Arora, D. Cyberlindnera (Pichia) fabianii infection in a neutropenic child: Importance of molecular identification. JMM Case Rep. 2015, 2, e000033. [Google Scholar] [CrossRef]
- Hamal, P.; Ostransky, J.; Dendis, M.; Horváth, R.; Ruzicka, F.; Buchta, V.; Vejsova, M.; Sauer, P.; Hejnar, P.; Raclavsky, V. A case of endocarditis caused by the yeast Pichia fabianii with biofilm production and developed in vitro resistance to azoles in the course of antifungal treatment. Sabouraudia 2008, 46, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Tortorano, A.M.; Di Francesco, L.F.; Cogliati, M.; Scalise, G.; Viviani, M.A. In-vitro activity of five antifungal agents against uncommon clinical isolates of Candida spp. J. Antimicrob. Chemother. 1999, 43, 295–299. [Google Scholar] [CrossRef]
- Chitasombat, M.N.; Kofteridis, D.P.; Jiang, Y.; Tarrand, J.; Lewis, R.E.; Kontoyiannis, D.P. Rare opportunistic (non-Candida, non-Cryptococcus) yeast bloodstream infections in patients with cancer. J. Infect. 2012, 64, 68–75. [Google Scholar] [CrossRef]
- Kaur, H.; Shankarnarayana, S.A.; Hallur, V.; Muralidharan, J.; Biswal, M.; Ghosh, A.K.; Ray, P.; Chakrabarti, A.; Rudramurthy, S.M. Prolonged Outbreak of Candida krusei Candidemia in Paediatric Ward of Tertiary Care Hospital. Mycopathologia 2020, 185, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Pannanusorn, S.; Fernandez, V.; Römling, U. Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses 2012, 56, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Rojas, F.D.; Sosa, M.d.L.A.; Fernández, M.S.; Cattana, M.E.; Córdoba, S.B.; Giusiano, G.E. Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Med. Mycol. 2014, 52, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Danesi, P.; James, T.Y.; Al-Hatmi, A.M.S.; Najafzadeh, M.J.; Dolatabadi, S.; Ming, C.; Liou, G.; Kang, Y.; de Hoog, S. Comparative pathogenicity of opportunistic black yeasts in Aureobasidium. Mycoses 2019, 62, 803–811. [Google Scholar] [CrossRef]
- Smith, D.F.Q.; Casadevall, A. Disaster Microbiology—A New Field of Study. mBio 2022, 13, e0168022. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J. Lodderomyces elongisporus: An emerging human fungal pathogen. PLoS Pathog. 2023, 19, e1011613. [Google Scholar] [CrossRef]
- Al-Obaid, K.; Ahmad, S.; Joseph, L.; Khan, Z. Lodderomyces elongisporus: A bloodstream pathogen of greater clinical significance. New Microbes New Infect. 2018, 26, 20–24. [Google Scholar] [CrossRef]
- Heath, J.A.; Zerr, D.M. Infections Acquired in the Nursery: Epidemiology and Control. Infect. Dis. Fetus Newborn Infant. 2006, 2006, 1179–1205. [Google Scholar]
- Hobi, S.; Cafarchia, C.; Romano, V.; Barrs, V.R. Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals. J. Fungi. 2022, 8, 708. [Google Scholar] [CrossRef]
- Schei, K.; Avershina, E.; Øien, T.; Rudi, K.; Follestad, T.; Salamati, S.; Ødegård, R.A. Early gut mycobiota and mother-offspring transfer. Microbiome. BioMed 2017, 5, 107. [Google Scholar] [CrossRef]
- Golubkova, A.; Hunter, C.J. Development of the Neonatal Intestinal Barrier, Microbiome, and Susceptibility to NEC. Microorganisms 2023, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, L.; Zhai, B. The mycobiome: Interactions with host and implications in diseases. Curr. Opin. Microbiol. 2023, 75, 102361. [Google Scholar] [CrossRef]
- O‘Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Summary of Recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin. Infect. Dis. 2011, 52, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Ding, Y.; Jiang, Y.; Mo, S.; Xu, S.; Qin, P. Persistent candidemia in very low birth weight neonates: Risk factors and clinical significance. BMC Infect. Dis. 2018, 18, 558. [Google Scholar] [CrossRef] [PubMed]
- Saiman, L.; Ludington, E.; Pfaller, M.; Rangel-Frausto, S.; Wiblin, R.T.; Dawson, J.; Blumberg, H.M.; Patterson, J.E.; Rinaldi, M.; Edwards, J.E. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr. Infect. Dis. J. 2000, 19, 319–324. [Google Scholar] [CrossRef] [PubMed]
- De Rose, D.U.; Santisi, A.; Ronchetti, M.P.; Martini, L.; Serafini, L.; Betta, P.; Maino, M.; Cavigioli, F.; Cocchi, I.; Pugni, L.; et al. Invasive Candida Infections in Neonates after Major Surgery: Current Evidence and New Directions. Pathogens 2021, 10, 319. [Google Scholar] [CrossRef]
- Auriti, C.; De Rose, D.; Santisi, A.; Martini, L.; Ronchetti, M.; Ravà, L.; Antenucci, V.; Bernaschi, P.; Serafini, L.; Catarzi, S.; et al. Incidence and risk factors of bacterial sepsis and invasive fungal infection in neonates and infants requiring major surgery: An Italian multicentre prospective study. J. Hosp. Infect. 2022, 130, 122–130. [Google Scholar] [CrossRef]
- McPherson, C.; Wambach, J.A. Prevention and Treatment of Respiratory Distress Syndrome in Preterm Neonates. Neonatal Netw. 2018, 37, 169–177. [Google Scholar] [CrossRef]
- Cosio, T.; Pica, F.; Fontana, C.; Pistoia, E.S.; Favaro, M.; Valsecchi, I.; Zarabian, N.; Campione, E.; Botterel, F.; Gaziano, R. Stephanoascus ciferrii Complex: The Current State of Infections and Drug Resistance in Humans. J. Fungi 2024, 10, 294. [Google Scholar] [CrossRef]
- Garvey, M.; Rowan, N.J. Pathogenic Drug Resistant Fungi: A Review of Mitigation Strategies. Int. J. Mol. Sci. 2023, 24, 1584. [Google Scholar] [CrossRef]
- Brion, L.P.; Uko, S.E.; Goldman, D.L. Risk of resistance associated with fluconazole prophylaxis: Systematic review. J. Infect. 2007, 54, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.K.; Price, D.; Herxheimer, A.; the Cochrane Adverse Effects Methods Group. Systematic reviews of adverse effects: Framework for a structured approach. BMC Med. Res. Methodol. 2007, 7, 32. [Google Scholar] [CrossRef]
- Nambiema, A.; Sembajwe, G.; Lam, J.; Woodruff, T.; Mandrioli, D.; Chartres, N.; Fadel, M.; Le Guillou, A.; Valter, R.; Deguigne, M.; et al. A Protocol for the Use of Case Reports/Studies and Case Series in Systematic Reviews for Clinical Toxicology. Front. Med. 2021, 8, 708380. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chakrabarti, A. Candidiasis and Other Emerging Yeasts. Curr. Fungal Infect. Rep. 2023, 17, 15–24. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]



| Location | Year | Cases | Yeast Species | Identification Method | Reference |
|---|---|---|---|---|---|
| USA | 1981 | 1 case | Malassezia furfur | NA | [8] |
| USA | 1984 | 4 cases | Malassezia furfur | NA | [9] |
| USA | 1985 | 3 cases | Malassezia furfur | NA | [10] |
| UK | 1986 | 7 cases | Hansenula anomala | API 20C | [11] |
| USA | 1987 | 5 cases | Malassezia furfur | NA | [12] |
| USA | 1987 | 6 cases | Malassezia furfur | NA | [13] |
| USA | 1989 | 3 cases | Malassezia furfur | NA | [14] |
| BELGIUM | 1989 | 6 cases | Malassezia furfur | NA | [15] |
| NY | 1991 | 1 case | Malassezia furfur | NA | [16] |
| USA | 1991 | 2 cases | Malassezia furfur | NA | [17] |
| FRANCE | 1991 | 1 case | Malassezia furfur | NA | [18] |
| USA | 1992 | 1 case | Trichosporon beigelii | NA | [19] |
| EUROPE | 1992 | 1 case | Trichosporon asteroides | NA | [20] |
| NY | 1993 | 2 cases | Trichosporon beigelii | BacT/Alert | [21] |
| ATLANTA | 1994 | 5 cases | Malassezia pachydermatis | NA | [22] |
| USA | 1997 | 2 cases | Trichosporon beigelii | NA | [23] |
| USA | 1997 | 1 case | Cryptococcus laurentii | NA | [24] |
| USA | 1998 | 8 cases | Malassezia pachydermatis | Molecular | [25] |
| UK | 1998 | 1 case | Trichosporon beigelii | API 32 ATB | [26] |
| CHANDIGARH | 1999 | 10 cases | Pichia anomala | NA | [27] |
| SPAIN | 2000 | 2 case | Saccharomyces cerevisiae | Vitek 2 API 20C Molecular | [28] |
| TAIWAN | 2000 | 1 case | Hansenula anomala | NA | [29] |
| MALAYSIA | 2000 | 1 case | Hansenula anomala | NA | [30] |
| INDIA | 2001 | 33 cases | Pichia anomala | Morphology Biochemical tests | [31] |
| BRAZIL | 2001 | 4 cases | Pichia anomala | NA | [32] |
| TAIWAN | 2001 | 1 case | Cryptococcus laurentii | Morphology India ink Vitek 2 | [33] |
| SWEDEN | 2001 | 8 cases | Malassezia pachydermatis | NA | [34] |
| EUROPE | 2002 | 1 case | Trichosporon asahii | API ID 32C | [35] |
| TURKEY | 2003 | 1 case | Trichosporon asahii | NA | [36] |
| TURKEY | 2003 | 3 cases | Trichosporon mucoides | API AUX C | [37] |
| ARGENTINA | 2003 | 1 case | Hansenula anomala | API ID 32C | [38] |
| TURKEY | 2004 | 1 case | Pichia anomala | API ID 32C | [39] |
| TURKEY | 2005 | 1 case | Saccharomyces cerevisiae | NA | [40] |
| QATAR | 2006 | 1 case | Kodamaea ohmeri | Vitek 2 API ID 32C Molecular | [41] |
| USA | 2006 | 1 case | Pichia fabianii | Molecular | [42] |
| BRAZIL | 2006 | 2 cases | Pichia anomala | Vitek 2 | [43] |
| ITALY | 2006 | 4 cases | Rhodotorula mucilaginosa | Vitek 2 API ID 32C | [44] |
| SOUTH KOREA | 2007 | 1 case | Kodamaea ohmeri | Vitek 2 API20C | [45] |
| THAILAND | 2008 | 1 case | Cryptococcus neoformans | NA | [46] |
| EUROPE | 2008 | 1 case | Trichosporon asahii | Vitek 2 | [47] |
| INDIA | 2009 | 1 case | Kodamaea ohmeri | BacT/Alert 3D API ID 32C | [48] |
| BRAZIL | 2009 | 1 case | Trichosporon spp. | Morphology | [49] |
| GREECE | 2009 | 1 case | Cryptococcus terreus | Morphology MALDI-TOF MS Molecular | [50] |
| FRANCE | 2010 | 1 case | Pichia fabianii | Molecular | [51] |
| INDIA | 2011 | 1 case | Rhodotorula mucilaginosa | Morphology API 20C | [52] |
| ITALY | 2011 | 1 case | Malassezia furfur | Morphology Molecular | [53] |
| KUWAIT | 2011 | 1 case | Kodamaea ohmeri | Vitek 2 API 20C AUX Molecular | [54] |
| INDIA | 2012 | 8 cases | Trichosporon asahii | Vitek 2 | [55] |
| TAIWAN | 2013 | 6 cases | P. anomala (C. pelliculosa) | API-32C Mini API Molecular | [56] |
| SOUTH AMERICA | 2013 | 4 cases | P. anomala (C. pelliculosa) | Morphology Molecular | [57] |
| GREECE | 2013 | 1 case | Malassezia furfur | Morphology MALDI-TOF MS Molecular | [50] |
| ITALY | 2013 | 6 cases | Malassezia furfur | Vitek2 MALDI-TOF MS Molecular | [58] |
| TUNISIA | 2013 | 1 case | Saccharomyces cerevisiae | NA | [59] |
| CHINA | 2013 | 6 cases | Pichia ohmeri | Molecular | [60] |
| CHINA | 2013 | 1 case | Pichia fabianii | API 20C AUX Molecular | [61] |
| CHINA | 2013 | 1 case | Kodamaea ohmeri | API 20C AUX Vitek 2 Molecular | [62] |
| INDIA | 2013 | 38 cases | Kodamaea ohmeri | Molecular | [63] |
| INDIA | 2014 | 1 case | Kodamaea ohmeri | BacT/ALERT Vitek2 | [64] |
| INDIA | 2014 | 1 case | Pseudozyma aphidis | API ID 32C Vitek 2 Molecular | [65] |
| KUWAIT | 2014 | 1 case | Malassezia pachydermatis | Vitek 2 MALDI–TOF MS Molecular | [66] |
| INDIA | 2015 | 2 cases | Kodamaea ohmeri | Vitek 2 Molecular | [67] |
| CROATIA | 2015 | 2 cases | Cyberlindnera fabianii | Molecular | [68] |
| ASIA | 2015 | 3 cases | Trichosporon asahii | NA | [69] |
| NIGERIA | 2015 | 1 case | Moesziomyces bullatus | Molecular | [70] |
| COLOMBIA | 2016 | 1 case | Kodamaea ohmeri | Vitek 2 | [71] |
| TURKEY | 2017 | 1 case | Wickerhamomyces anomalus | Vitek 2 | [72] |
| INDIA | 2017 | 9 cases | Pichia kudriavzevii | Molecular | [73] |
| INDIA | 2017 | 4 cases | Rhodotorula glutinis | NA | [74] |
| INDIA | 2017 | 8 cases | Cyberlindnera fabianii | Molecular | [75] |
| INDIA | 2017 | 1 case | Wickerhamomyces anomalus | Molecular | [75] |
| INDIA | 2017 | 2 cases | Saccharomyces cerevisiae | Molecular | [76] |
| INDIA | 2018 | 1 case | Cryptococcus laurentii | Vitek 2 | [77] |
| PORTUGAL | 2018 | 1 case | Malassezia furfur | MALDI–TOF MS | [78] |
| ITALY | 2018 | 9 cases | Malassezia furfur | BacT/Alert | [79] |
| KUWAIT | 2019 | 10 cases | Cyberlindnera fabianii | Molecular | [80] |
| TAIWAN | 2019 | 1 case | Malassezia furfur | BacT/Alert | [81] |
| TAIWAN | 2020 | 4 cases | Malassezia pachydermatis | BD BACTEC FX | [82] |
| CALIFORNIA | 2020 | 3 cases | Malassezia pachydermatis | Molecular | [83] |
| INDIA | 2020 | 12 cases | Dirkmeia churashimaensis | Molecular | [84] |
| INDIA | 2020 | 2 cases | Cyberlindnera fabianii | MALDI-TOF-MS | [85] |
| KUWAIT | 2021 | 1 case | Papiliotrema laurentii | Vitek 2 Molecular | [86] |
| COLOMBIA | 2021 | 1 case | Malassezia sympodialis | Molecular | [87] |
| CHINA | 2021 | 1 case | P. anomala (C. pelliculosa) | Molecular | [88] |
| CHINA | 2021 | 21 cases | P. anomala (C. pelliculosa) | Vitek MS | [89] |
| CHINA | 2021 | 14 cases | P. anomala (C. pelliculosa) | Molecular | [90] |
| GREECE | 2022 | 1 case | Moesziomyces aphidis | Vitek 2 Molecular | [91] |
| KUWAIT | 2022 | 1 case | Lodderomyces elongisporus | Vitek 2 Molecular | [92] |
| BRAZIL | 2023 | 1 case | Lodderomyces elongisporus | Molecular | [93] |
| NIGERIA | 2023 | 1 case | Cryptococcus laurentii | Vitek 2 | [94] |
| INDIA | 2023 | 1 case | Aureobasidium melanogenum | Molecular | [95] |
| INDIA | 2023 | 1 case | Kodamaea ohmeri | Vitek 2 MALDI–TOF | [96] |
| Years | Cases |
|---|---|
| 1981–1990 | 35 |
| 1991–2000 | 39 |
| 2001–2010 | 69 |
| 2011–2023 | 199 |
| Risk Factors | |||
|---|---|---|---|
| Total Cases n = 342 NA Outcome n = 41 | Survival n = 235/301 (78 %) | Exitus n = 66/301 (22%) | |
| Low birth weight | 335/342 (98%) | 181/235 (77%) | 55/66 (84%) |
| Central catheters | 260/342 (76%) | 178/235 (76%) | 49/66 (74%) |
| Prematurity | 226/342 (66%) | 141/235 (60%) | 44/66 (67%) |
| Broad spectrum antibiotics | 222/342 (65%) | 153/235 (65%) | 46/66 (70%) |
| Parenteral nutrition | 120/342 (35%) | 89/235 (38%) | 14/66 (21%) |
| Intubation/mechanical ventilation | 116/342 (34%) | 56/235 (24%) | 43/66 (65%) |
| Sepsis | 96/342 (28%) | 63/235 (27%) | 28/66 (43%) |
| Thrombocytopenia | 72/342 (21%) | 61/235 (26%) | 14/66 (21%) |
| Prolonged hospitalization | 61/342 (18%) | 61/235 (26%) | 28/66 (43%) |
| Respiratory distress syndrome | 41/342 (12%) | 28/235 (12%) | 12/66 (19%) |
| Use of fluconazole | 27/342 (8%) | 9/235 (4%) | 3/66 (5%) |
| Yeast | Number of Isolates | AMB | FLC | VRC | POS | ITC | ISA | 5-FC | AFG | MFG | CAS | Method Used | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kodamaea ohmeri | 1 | 0.064 | 32 | NA | NA | 0.25 | NA | <0.002 | NA | NA | NA | Etest | [41] |
| 1 | 0.023 | 4 | 0.047 | 0.012 | 0.125 | 0.032 | 0.064 | 0.125 | 0.25 | Etest | [54] | ||
| 38 | 0.25–1 | 0.5–64 | 0.03–8 | 0.06–4 | 0.06–4 | NA | NA | NA | NA | 0.12–1 | CLSI | [63] | |
| 2 | 0.25 | 4 | 0.25 | NA | NA | NA | 1 | NA | NA | 0.25 | VITEK2 | [67] | |
| 1 | <0.5 | NA | NA | NA | NA | NA | <1 | NA | NA | <0.25 | VITEK2 | [64] | |
| Cyberlindnera/Pichia fabianii | 1 | 0.03 | 8 | NA | NA | NA | NA | 0.125 | NA | NA | NA | NCCLS | [42] |
| 1 | 0.5 | 2 | NA | NA | NA | NA | 0.25 | NA | NA | NA | Etest | [51] | |
| 8 | 0.75–12 | 0.5–16 | NA | NA | NA | NA | NA | NA | NA | NA | Etest | [75] | |
| 1 | 1 | ≤1 | 0.125 | NA | 2 | NA | ≤4 | NA | NA | NA | CLSI | [61] | |
| 2 | NA | NA | NA | NA | NA | NA | NA | 0.016–0.064 | 1–4 | 0.125–0.19 | EUCAST | [68] | |
| Pichia anomala (Candida pelliculosa) | 2 | 0.023–0.032 | 3–4 | 0.064–0.125 | NA | NA | NA | NA | NA | NA | NA | Etest | [43] |
| 33 | NA | Only one isolate >64 | NA | NA | NA | NA | NA | NA | NA | NA | CLSI | [31] | |
| 6 | 1–2 | 0.125 | NA | 2 | NA | NA | NA | NA | 0.25–0.5 | 2 | CLSI | [56] | |
| 21 | <0.5 | 2–4 | 0.125–0.25 | NA | 0.125–0.25 | NA | <4 | NA | NA | NA | CLSI | [89] | |
| Pichia kudriavzevii | 9 | 1 | 2–16 | 0.25 | 0.25–1 | 0.25–0.5 | NA | NA | 0.25–0.5 | 0.25–0.5 | 0.25–0.5 | CLSI | [73] |
| Moesziomyces (Pseudozyma) aphidis | 1 | 2 | <0.125 | 8 | 0.03 | 0.03 | ≤0.016 | >64 | >8 | >8 | >8 | EUCAST | [91] |
| Moesziomyces (Pseudozyma) bullatus | 1 | 1 | 128 | 0.03 | 0.03 | 0.12 | NA | 64 | 8 | 8 | 8 | Sensititre YeastOne Y010 microdilution method | [70] |
| Dirkmeia (Pseudozyma) churashimaensis | 12 | 0.198 | 1 to 4 | 0.03–0.125 | 0.03–0.25 | 0.03–0.25 | 0.03–0.125 | 0.157 | >8 | >8 | >8 | CLSI | [84] |
| Rhodotoroula mucilaginosa | 4 | 0.25 | >256 | 2 | NA | 2 | NA | 0.125 | NA | NA | NA | NA | [44] |
| 1 | 1.5 | >256 | 0.38 | NA | NA | NA | NA | NA | NA | >16 | Etest | [52] | |
| Cryptococcus laurentii | 1 | 0.25 | 4 | NA | NA | NA | NA | NA | NA | NA | NA | NCCLS | [33] |
| 1 | 0.25 | 2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | [77] | |
| Cryptococcus terreus | 1 | 0.125–1.5 | 128–256 | 0.125–0.5 | 0.25–0.5 | NA | 1 | 8–32 | 8–32 | 8–32 | 8–32 | broth microdilution method Micronaut-AM and E-test | [50] |
| Malassezia furfur | 1 | 0.19 | 0.25 | 0.094 | 0.094 | 0.125 | 0.5 | 16 | 32 | 32 | 32 | MIC method on modified RPMI 1640 agar | [50] |
| Malassezia pachydermatis | 1 | 0.19 | >256 | 0.012 | 0.016 | NA | NA | >32 | NA | NA | >32 | Etest | [66] |
| Treatment | Survival | Exitus |
|---|---|---|
| CVC removal | 21% | 3% |
| Fluconazole (FLC) | 30% | 46% |
| Voriconazole (VOR) | 5% | 3% |
| Miconazole (MIC) | 3% | 2% |
| Caspofungin (CAS) | 2% | 0% or NA |
| Amphotericin B (AmB) | 21% | 33% |
| AmB + FLC | 8% | 10% |
| AmB + 5-FC (5-flucytosine) | 4% | 3% |
| AmB + FLC + 5-FC | 4% | 2% |
| AmB + KTC (ketoconazole) | 1% | 0% or NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpakosi, A.; Cholevas, V.; Meletiadis, J.; Theodoraki, M.; Sokou, R. Neonatal Fungemia by Non-Candida Rare Opportunistic Yeasts: A Systematic Review of Literature. Int. J. Mol. Sci. 2024, 25, 9266. https://doi.org/10.3390/ijms25179266
Mpakosi A, Cholevas V, Meletiadis J, Theodoraki M, Sokou R. Neonatal Fungemia by Non-Candida Rare Opportunistic Yeasts: A Systematic Review of Literature. International Journal of Molecular Sciences. 2024; 25(17):9266. https://doi.org/10.3390/ijms25179266
Chicago/Turabian StyleMpakosi, Alexandra, Vasileios Cholevas, Joseph Meletiadis, Martha Theodoraki, and Rozeta Sokou. 2024. "Neonatal Fungemia by Non-Candida Rare Opportunistic Yeasts: A Systematic Review of Literature" International Journal of Molecular Sciences 25, no. 17: 9266. https://doi.org/10.3390/ijms25179266








