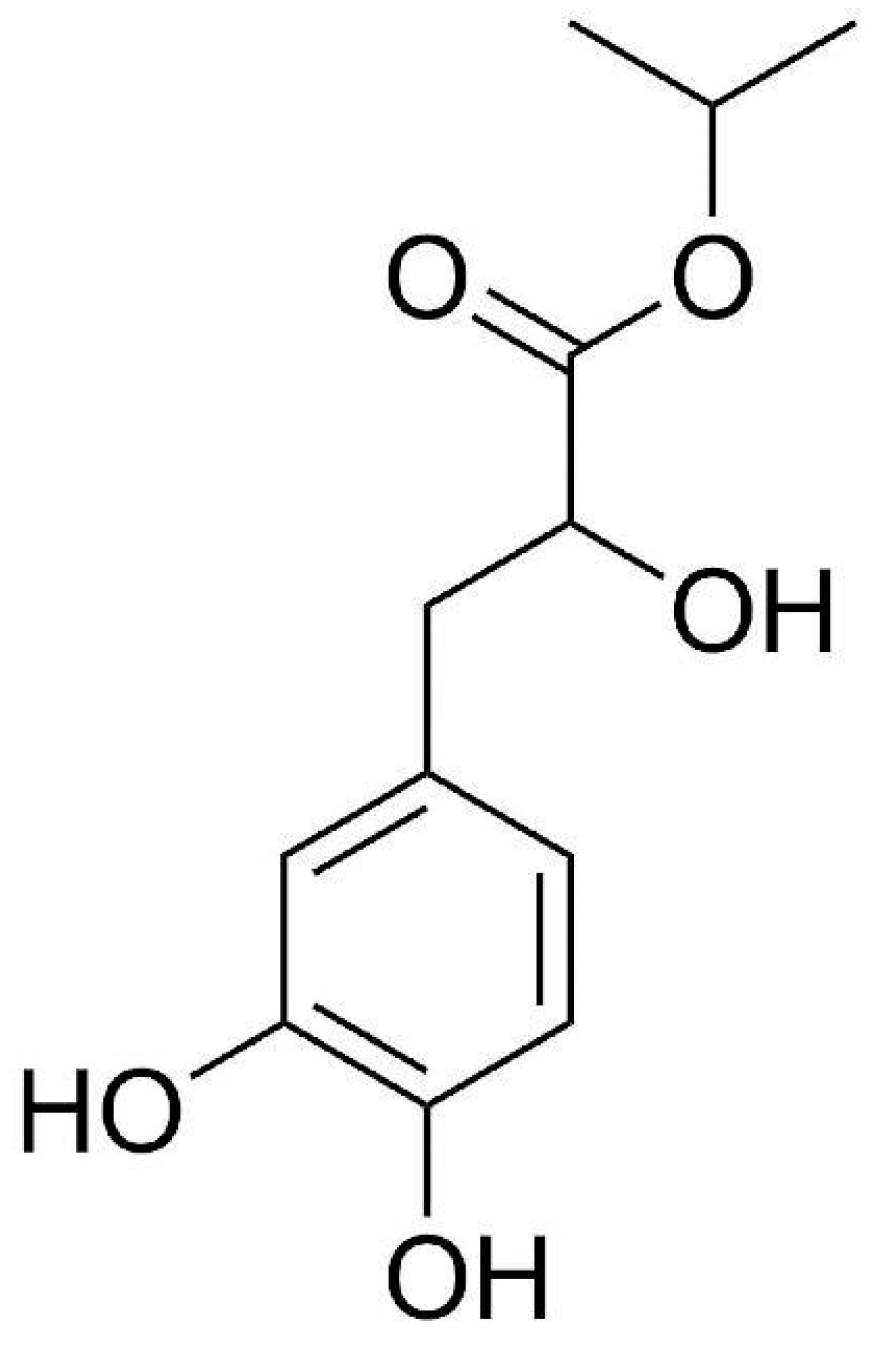
Isopropyl 3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoate Alleviates Palmitic Acid-Induced Vascular Aging in HUVEC Cells through ROS/Ferroptosis Pathway
Abstract
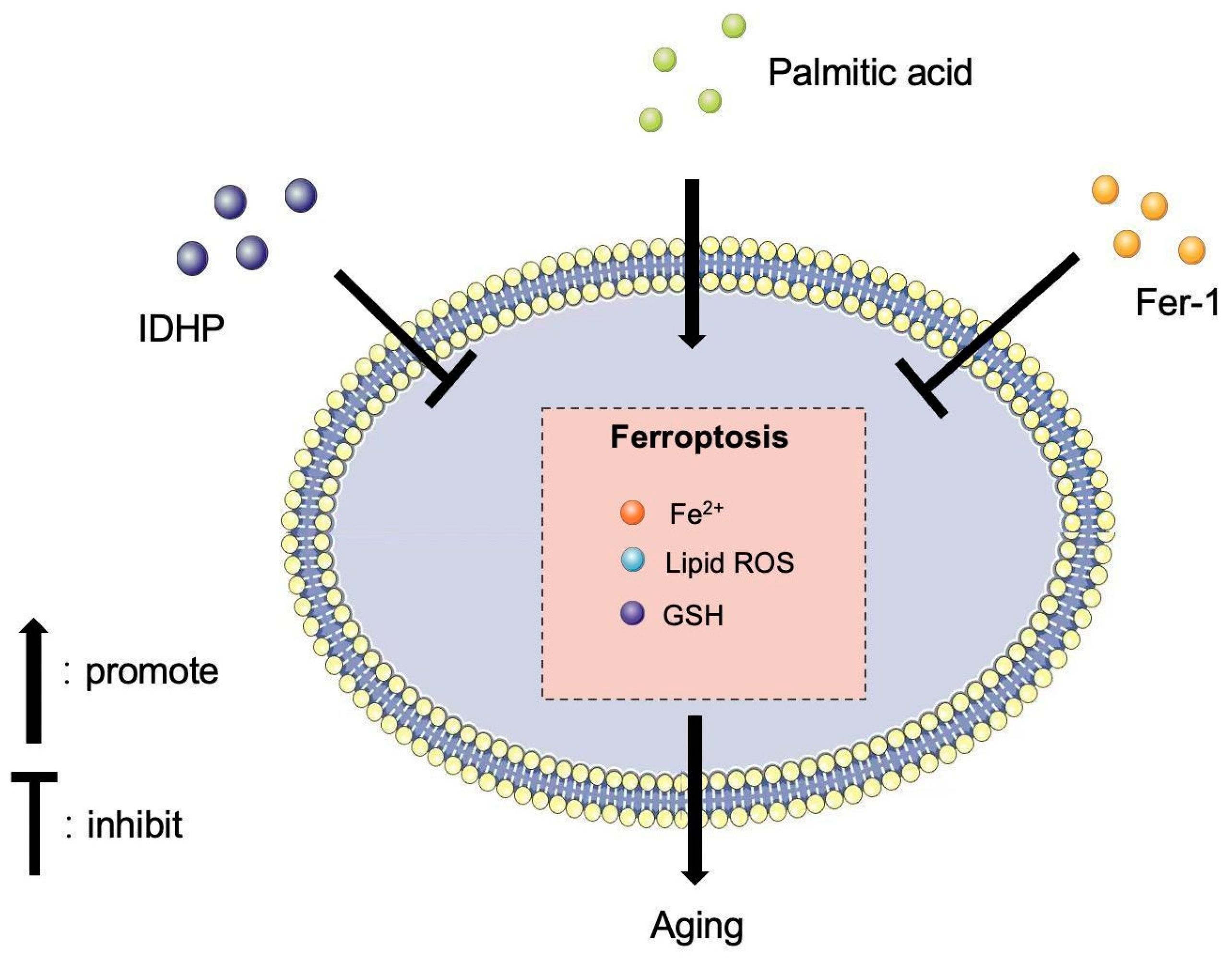
1. Introduction
2. Results
2.1. Experimental Results of MTT
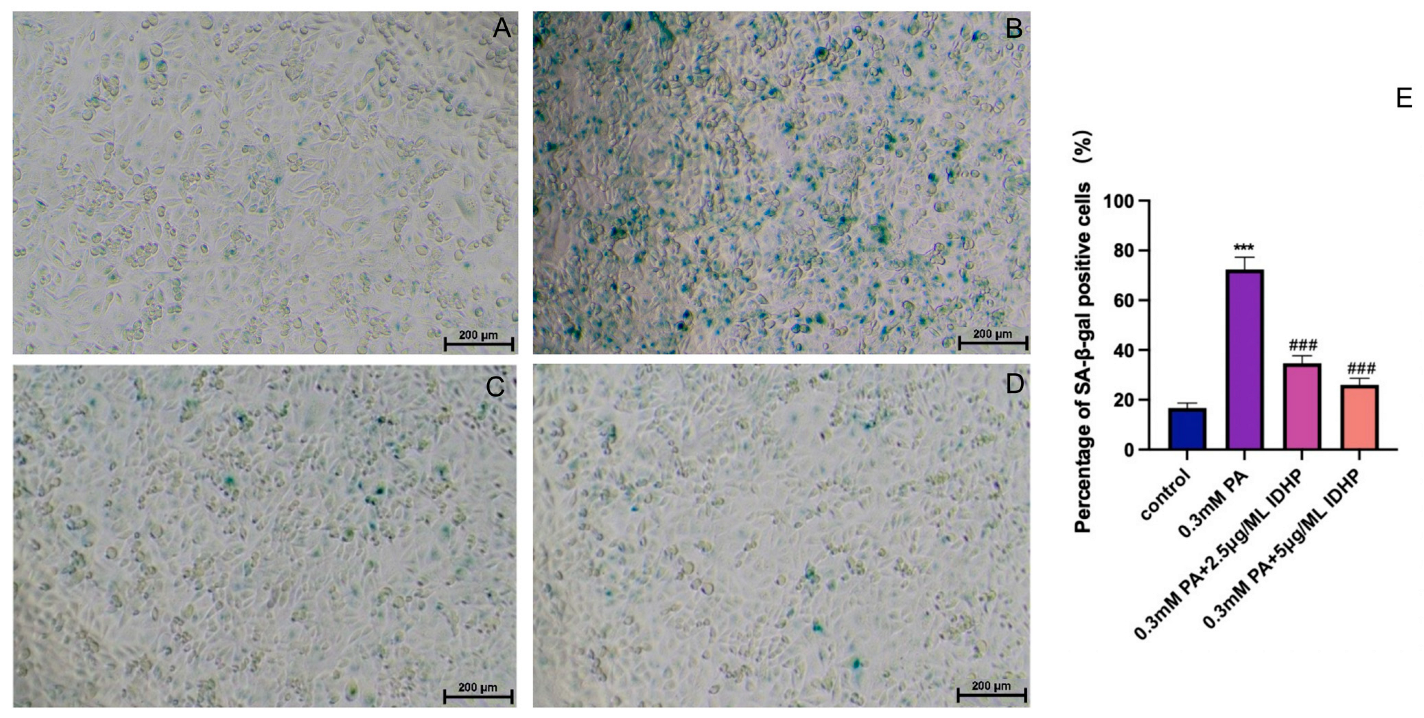
2.2. IDHP Delays PA-Induced HUVEC Cell Senescence
2.3. Cellular ROS Experimental Results
2.4. GSH Detection
2.5. Fe2+ Detection
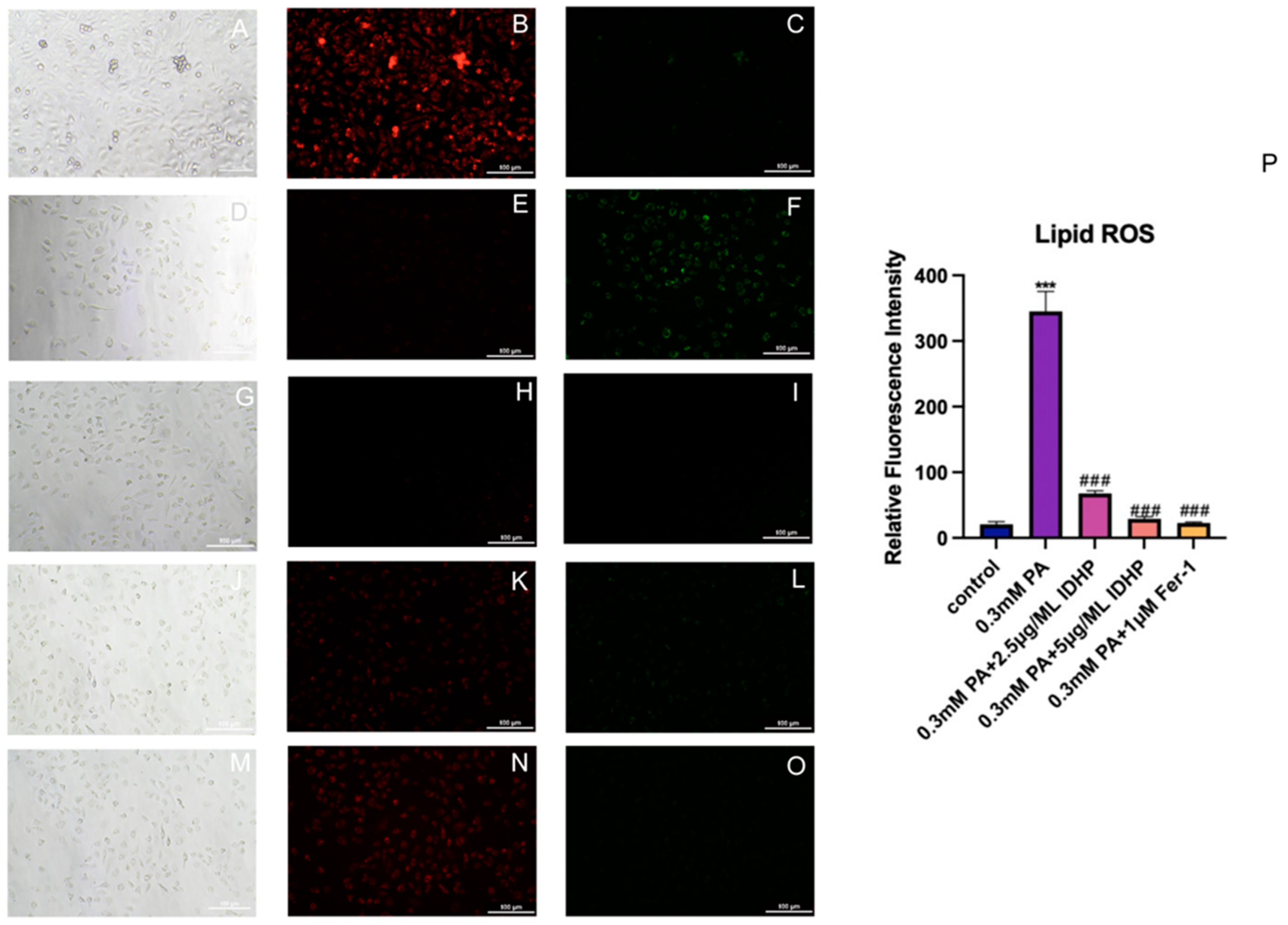
2.6. Detection of Lipid ROS
2.7. Flow Cytometry to Detect Cell Proliferation Rate
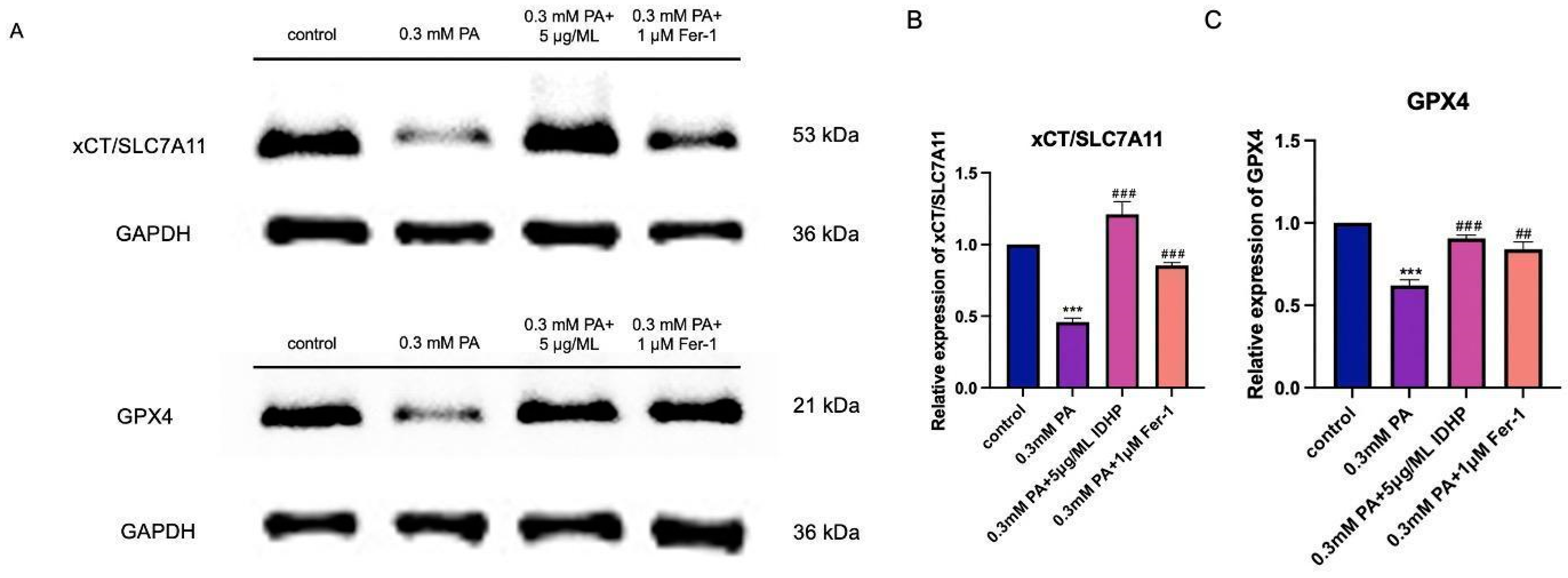
2.8. Western Blot
3. Discussion
4. Materials and Methods
4.1. Cell Line and Culture
4.2. Cell Viability—MTT Assay
4.3. Senescence-Associated β-Galactosidase Staining Assay
4.4. Cellular ROS Assay—DCFH-DA Probe
4.5. Lipid ROS Detection
4.6. Fe2+ Detection
4.7. GSH Detection
4.8. Flow Cytometry
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Ma, D.; He, L.; Yang, D. Senolystics from natural products for extending health and lifespan. Tradit. Med. Res. 2024, 9, 67. [Google Scholar] [CrossRef]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.S.; Björck, M.; Chandra, A.; Clouse, W.D.; Dalsing, M.C.; Oderich, G.S.; Smeds, M.R.; Murad, M.H. Chronic mesenteric ischemia: Clinical practice guidelines from the Society for Vascular Surgery. J. Vasc. Surg. 2021, 73, 87s–115s. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yang, W.Q.; Luo, W.W.; Zhang, L.L.; Mai, Y.Q.; Li, Z.Q.; Liu, S.T.; Jiang, L.J.; Liu, P.Q.; Li, Z.M. Disturbance of Fatty Acid Metabolism Promoted Vascular Endothelial Cell Senescence via Acetyl-CoA-Induced Protein Acetylation Modification. Oxid. Med. Cell Longev. 2022, 2022, 1198607. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, N.; Herman, A.B.; Gorospe, M.; Lee, J.S. Factors and Pathways Modulating Endothelial Cell Senescence in Vascular Aging. Int. J. Mol. Sci. 2022, 23, 10135. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- Robert, L.; Molinari, J.; Ravelojaona, V.; Andrès, E.; Robert, A.M. Age- and passage-dependent upregulation of fibroblast elastase-type endopeptidase activity. Role of advanced glycation endproducts, inhibition by fucose- and rhamnose-rich oligosaccharides. Arch. Gerontol. Geriatr. 2010, 50, 327–331. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes. Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Zheng, X.W.; Du, M.F.; Zhang, X.; Chu, C.; Wang, D.; Liao, Y.Y.; Ma, Q.; Jia, H.; et al. Early-Life Cardiovascular Risk Factor Trajectories and Vascular Aging in Midlife: A 30-Year Prospective Cohort Study. Hypertension 2023, 80, 1057–1066. [Google Scholar] [CrossRef]
- Ji, H.; Kwan, A.C.; Chen, M.T.; Ouyang, D.; Ebinger, J.E.; Bell, S.P.; Niiranen, T.J.; Bello, N.A.; Cheng, S. Sex Differences in Myocardial and Vascular Aging. Circ. Res. 2022, 130, 566–577. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Louka, A.M.; Sagris, D.; Ntaios, G. Immunity, Vascular Aging and Stroke. Curr. Med. Chem. 2022, 29, 5510–5521. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Zhang, M.; Beard, D.A.; Goldstein, D.R. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ. Res. 2020, 126, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Li, C.; Liu, R.; Xiong, Z.; Bao, X.; Liang, S.; Zeng, H.; Jin, W.; Gong, Q.; Liu, L.; Guo, J. Ferroptosis: A potential target for the treatment of atherosclerosis. Acta Biochim. Biophys. Sin. 2024, 56, 331–344. [Google Scholar] [CrossRef]
- Liu, C.; Shen, Y.; Cavdar, O.; Huang, J.; Fang, H. Angiotensin II-induced vascular endothelial cells ferroptosis via P53-ALOX12 signal axis. Clin. Exp. Hypertens. 2023, 45, 2180019. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, E.; Yang, H.; Chen, Y.; Tao, L.; Xu, Y.; Chen, T.; Shen, X. Gastrodin Ameliorates Cognitive Dysfunction in Vascular Dementia Rats by Suppressing Ferroptosis via the Regulation of the Nrf2/Keap1-GPx4 Signaling Pathway. Molecules 2022, 27, 6311. [Google Scholar] [CrossRef]
- Shao, S.; Liu, Y.; Hong, W.; Mo, Y.; Shu, F.; Jiang, L.; Tan, N. Influence of FOSL1 Inhibition on Vascular Calcification and ROS Generation through Ferroptosis via P53-SLC7A11 Axis. Biomedicines 2023, 11, 635. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef]
- Wu, S.; Mao, C.; Kondiparthi, L.; Poyurovsky, M.V.; Olszewski, K.; Gan, B. A ferroptosis defense mechanism mediated by glycerol-3-phosphate dehydrogenase 2 in mitochondria. Proc. Natl. Acad. Sci. USA 2022, 119, e2121987119. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; He, S.; Li, X.; He, J.; Luo, L. Mitochondria-mediated Ferroptosis in Diseases Therapy: From Molecular Mechanisms to Implications. Aging Dis. 2024, 15, 714–738. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Wang, C.; Wang, L.; Wu, H.; Song, X.; Wang, W.; Xu, H.; Dong, X. Potent nanoreactor-mediated ferroptosis-based strategy for the reversal of cancer chemoresistance to Sorafenib. Acta Biomater. 2023, 159, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Wang, M.; Chen, J.; Liu, Y.J.; Yu, Y.J.; Liu, L.M.; Zheng, X.H.; Xiao, Y.C.; Zhang, J.M.; Zhu, M.X.; et al. Isopropyl 3-(3, 4-dihydroxyphenyl)-2-hydroxypropanoate protects lipopolysaccharide-induced acute lung injury in mice by attenuating pyroptosis. Eur. J. Pharmacol. 2023, 942, 175545. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, H.; Jing, H.; Wang, S.; Kang, L.; Gao, X.; Hu, L.; Zheng, X. Anti-inflammatory effects of isopropyl 3-(3, 4-dihydroxyphenyl)-2-hydroxypropanoate, a novel metabolite from danshen, on activated microglia. Chin. J. Physiol. 2012, 55, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Xu, X.; Li, N.; Zhang, Y.; Tang, R.; Li, X.; Tang, J.; Wu, X.; Lu, C.; Bai, Y.; et al. Isopropyl 3-(3,4-dihydroxyphenyl) 2-hydroxypropanoate protects septic myocardial injury via regulating GAS6/Axl-AMPK signaling pathway. Biochem. Pharmacol. 2024, 221, 116035. [Google Scholar] [CrossRef]
- Baranov, V.S.; Baranova, E.V. Aging and Ambiguous ROS. System Genetics Analysis. Curr. Aging Sci. 2017, 10, 6–11. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure With Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef]
- Faleeva, M.; Ahmad, S.; Theofilatos, K.; Lynham, S.; Watson, G.; Whitehead, M.; Marhuenda, E.; Iskratsch, T.; Cox, S.; Shanahan, C.M. Sox9 Accelerates Vascular Aging by Regulating Extracellular Matrix Composition and Stiffness. Circ. Res. 2024, 134, 307–324. [Google Scholar] [CrossRef]
- Hao, W.; Shan, W.; Wan, F.; Luo, J.; Niu, Y.; Zhou, J.; Zhang, Y.; Xu, N.; Xie, W. Canagliflozin Delays Aging of HUVECs Induced by Palmitic Acid via the ROS/p38/JNK Pathway. Antioxidants 2023, 12, 838. [Google Scholar] [CrossRef]
- Wan, F.; He, X.; Xie, W. Canagliflozin Inhibits Palmitic Acid-Induced Vascular Cell Aging In Vitro through ROS/ERK and Ferroptosis Pathways. Antioxidants 2024, 13, 831. [Google Scholar] [CrossRef]
- Kuang, H.; Sun, X.; Liu, Y.; Tang, M.; Wei, Y.; Shi, Y.; Li, R.; Xiao, G.; Kang, J.; Wang, F.; et al. Palmitic acid-induced ferroptosis via CD36 activates ER stress to break calcium-iron balance in colon cancer cells. FEBS J. 2023, 290, 3664–3687. [Google Scholar] [CrossRef]
- Tan, X.H.; Gu, Y.Y.; Song, W.P.; Nan, T.G.; Song, W.D.; Fang, D.; Yuan, Y.M.; Xin, Z.C.; Li, X.S.; Guan, R.L. Transcriptome analysis highlights the role of ferroptosis in palmitic acid-induced endothelial dysfunction. Sex. Med. 2023, 11, qfac008. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Ma, J.; Ikeda, M.; Ide, T.; Griffin, C.T.; Ding, X.Q.; Wang, S. Comparative mechanistic study of RPE cell death induced by different oxidative stresses. Redox Biol. 2023, 65, 102840. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, L.; Zhong, Y.; Zhang, Y.; Yin, Q.; Li, S.; Zhang, X.; Han, H.; Yao, K. Ferrostatin-1-loaded liposome for treatment of corneal alkali burn via targeting ferroptosis. Bioeng. Transl. Med. 2022, 7, e10276. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Zheng, X.; Xie, W. Isopropyl 3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoate Alleviates Palmitic Acid-Induced Vascular Aging in HUVEC Cells through ROS/Ferroptosis Pathway. Int. J. Mol. Sci. 2024, 25, 9278. https://doi.org/10.3390/ijms25179278
He X, Zheng X, Xie W. Isopropyl 3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoate Alleviates Palmitic Acid-Induced Vascular Aging in HUVEC Cells through ROS/Ferroptosis Pathway. International Journal of Molecular Sciences. 2024; 25(17):9278. https://doi.org/10.3390/ijms25179278
Chicago/Turabian StyleHe, Xin, Xiaohui Zheng, and Weidong Xie. 2024. "Isopropyl 3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoate Alleviates Palmitic Acid-Induced Vascular Aging in HUVEC Cells through ROS/Ferroptosis Pathway" International Journal of Molecular Sciences 25, no. 17: 9278. https://doi.org/10.3390/ijms25179278
APA StyleHe, X., Zheng, X., & Xie, W. (2024). Isopropyl 3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoate Alleviates Palmitic Acid-Induced Vascular Aging in HUVEC Cells through ROS/Ferroptosis Pathway. International Journal of Molecular Sciences, 25(17), 9278. https://doi.org/10.3390/ijms25179278





