Elucidating the Role of SlBBX31 in Plant Growth and Heat-Stress Resistance in Tomato
Abstract
:1. Introduction
2. Results
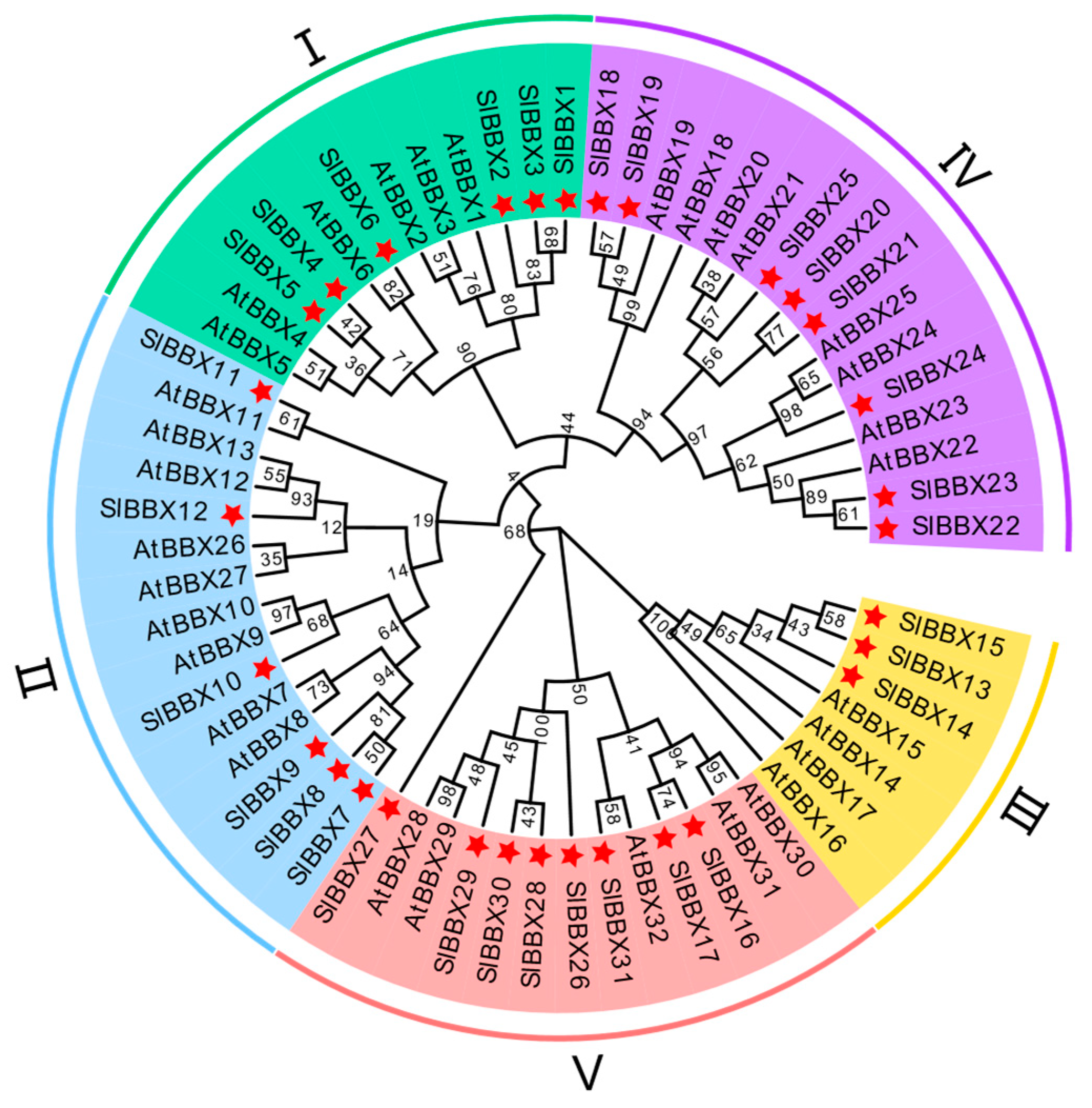
2.1. Sequence and Phylogenetic Analysis of SlBBX31
2.2. Expression Patterns of SlBBX31
2.3. SlBBX31 Expression Is Responsive to Heat Stress
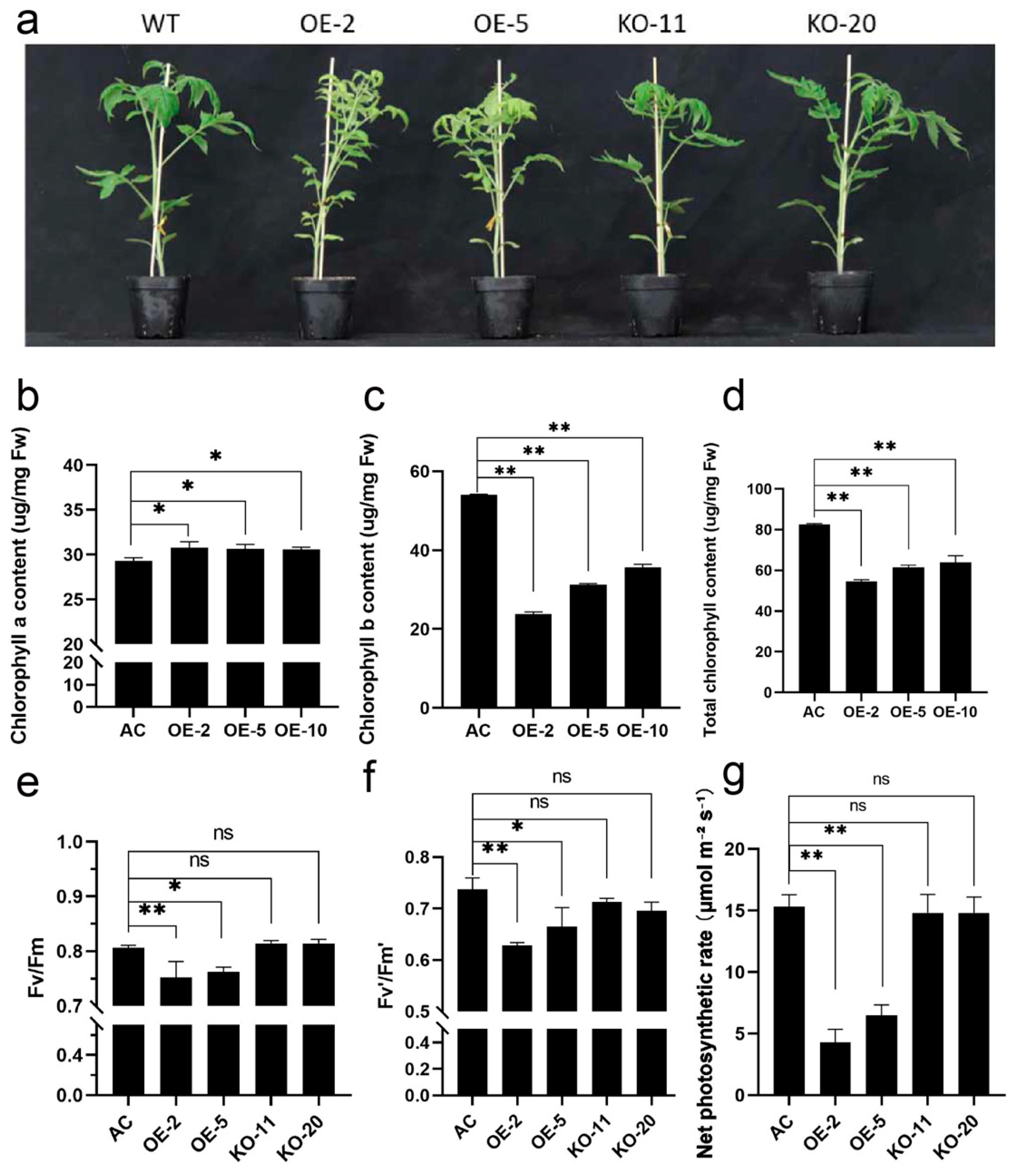
2.4. Overexpression of SlBBX31 Inhibits Chlorophyll Accumulation and Photosynthesis in Tomato
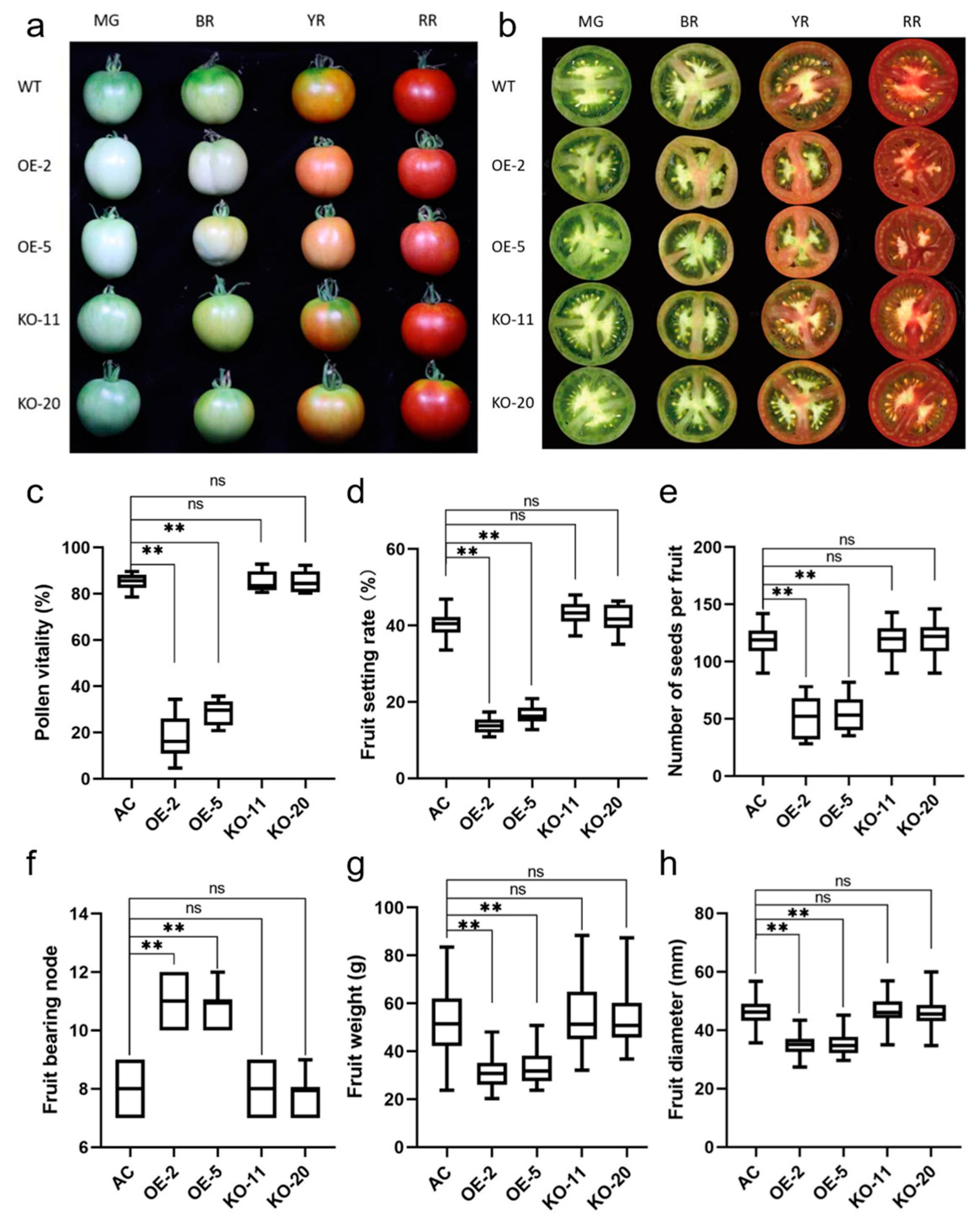
2.5. Overexpression of SlBBX31 Affects the Reproductive Growth Process in Tomato
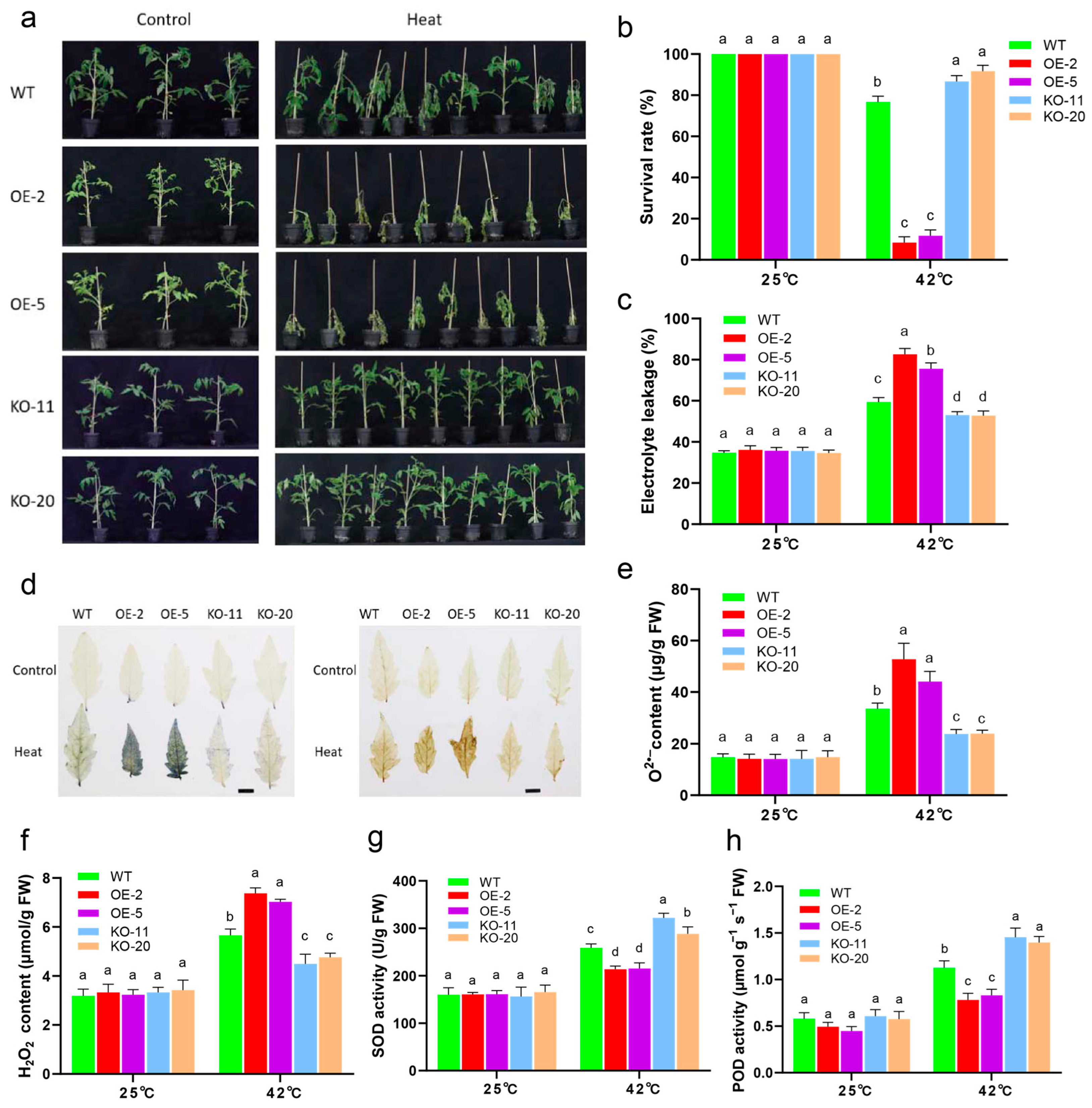
2.6. SlBBX31 Is Important for Heat Tolerance in Tomato
2.7. SlBBX31 Is a Negative Regulator for ROS Detoxification under Heat Stress
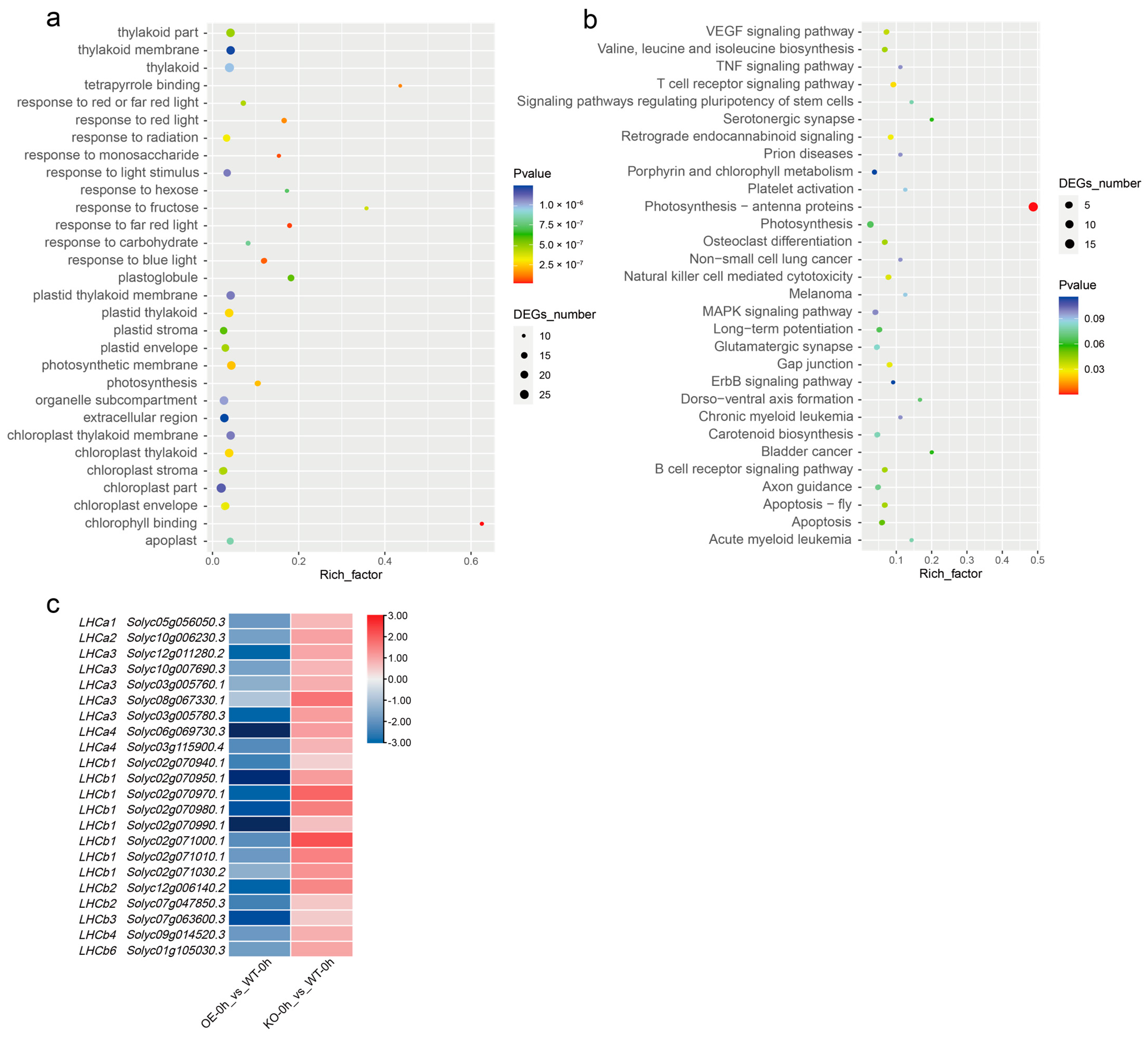
2.8. SlBBX31 Is Involved in the Heat-Stress Response Gene Regulatory Networks
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Tomato Transformation
4.2. Plant Growth Conditions and Stress Treatments
4.3. RNA Extraction and qRT-PCR Analysis
4.4. Protein Physicochemical Properties Prediction and Phylogenetic Analysis
4.5. RNA Sequencing and Data Analysis
4.6. Subcellular Localization
4.7. Measurement of Chlorophyll Content and Photosynthetic Correlation Indices
4.8. Measurement of Pollen Vigor and Yield-Related Indices
4.9. Analysis of the H2O2 and O2•–
4.10. Assessment of Antioxidant Enzyme Activities
4.11. Data Statistics and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osyczka, P.; Mysliwa-Kurdziel, B. Do the expected heatwaves pose a threat to lichens?: Linkage between a passive decline in water content in thalli and response to heat stress. Plant Cell Environ. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Habibi, P.; Razmjouei, J.; Moradi, A.; Mahdavi, F.; Fallah-Aliabadi, S.; Heydari, A. Climate change and heat stress resilient outdoor workers: Findings from systematic literature review. BMC Public. Health 2024, 24, 1711. [Google Scholar] [CrossRef] [PubMed]
- Mariana Distefano, A.; Bauer, V.; Cascallares, M.; Lopez, G.A.; Fiol, D.F.; Zabaleta, E.; Pagnussat, G.C. Heat stress in plants: Sensing, signalling and ferroptosis. J. Exp. Bot. 2024, erae296. [Google Scholar] [CrossRef]
- Malinski, K.H.; Elizabeth Moore, M.; Kingsolver, J.G. Heat stress and host-parasitoid interactions: Lessons and opportunities in a changing climate. Curr. Opin. Insect Sci. 2024, 64, 101225. [Google Scholar] [CrossRef]
- Pratx, L.; Crawford, T.; Baurle, I. Mechanisms of heat stress-induced transcriptional memory. Curr. Opin. Plant Biol. 2024, 81, 102590. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yao, Q.; Mao, F.; Liu, M.; Wang, Y.; Wang, X.; Gao, Y.; Wang, Y.; Liao, S.; Wang, P.; et al. Heat stress and sexual reproduction in maize: Unveiling the most pivotal factors and the biggest opportunities. J. Exp. Bot. 2024, 75, 4219–4243. [Google Scholar] [CrossRef]
- Sedaghatmehr, M.; Balazadeh, S. Autophagy: A key player in the recovery of plants from heat stress. J. Exp. Bot. 2024, 75, 2246–2255. [Google Scholar] [CrossRef]
- Turck, F.; Fornara, F.; Coupland, G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 2008, 59, 573–594. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Li, D.; Yang, X.; Hu, T.; Fu, J. Comparative proteomics in tall fescue to reveal underlying mechanisms for improving Photosystem II thermotolerance during heat stress memory. BMC Genom. 2024, 25, 683. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Priya, M.; Dhanker, O.P.; Siddique, K.H.M.; HanumanthaRao, B.; Nair, R.M.; Pandey, S.; Singh, S.; Varshney, R.K.; Prasad, P.V.V.; Nayyar, H. Drought and heat stress-related proteins: An update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019, 132, 1607–1638. [Google Scholar] [CrossRef]
- Caseys, C. Regulatory Divergence in the Stress Response of Tomato. Plant Cell 2018, 30, 1380. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, G.K. A compact topic: How ethylene controls crown root development in compacted soil. Plant Cell 2024, 36, 2063–2064. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Liu, K.; Kim, E.Y.; Medina-Puche, L.; Dong, H.; Di, M.; Singh, R.M.; Li, M.; Qi, S.; Meng, Z.; et al. The m6A reader ECT1 drives mRNA sequestration to dampen salicylic acid-dependent stress responses in Arabidopsis. Plant Cell 2024, 36, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, G.; Ma, C.; Li, Y.; Jiang, C.; Wang, Y.; Zhang, B.; Wang, R.; Qiu, Y.; Ma, Y.; et al. The F-box protein RhSAF destabilizes the gibberellic acid receptor RhGID1 to mediate ethylene-induced petal senescence in rose. Plant Cell 2024, 36, 1736–1754. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ai, X.; Li, C.; Wang, S.; Zhang, N.; Ren, J.; Wang, J.; Zhong, C.; Zhao, X.; Zhang, H.; et al. A Genome-Wide Analysis of the Jasmonic Acid Biosynthesis Gene Families in Peanut Reveals Their Crucial Roles in Growth and Abiotic Stresses. Int. J. Mol. Sci. 2024, 25, 7054. [Google Scholar] [CrossRef]
- Zhan, X.; Qian, B.; Cao, F.; Wu, W.; Yang, L.; Guan, Q.; Gu, X.; Wang, P.; Okusolubo, T.A.; Dunn, S.L.; et al. An Arabidopsis PWI and RRM motif-containing protein is critical for pre-mRNA splicing and ABA responses. Nat. Commun. 2015, 6, 8139. [Google Scholar] [CrossRef]
- Gong, W.; Oubounyt, M.; Baumbach, J.; Dresselhaus, T. Heat-stress-induced ROS in maize silks cause late pollen tube growth arrest and sterility. iScience 2024, 27, 110081. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Wang, Q.; Dang, N.; Wang, L.; Liu, C.; Zhu, J.; Zhan, X. The tomato 2-oxoglutarate-dependent dioxygenase gene SlF3HL is critical for chilling stress tolerance. Hortic. Res. 2019, 6, 45. [Google Scholar] [CrossRef]
- Khanna, R.; Kronmiller, B.; Maszle, D.R.; Coupland, G.; Holm, M.; Mizuno, T.; Wu, S.H. The Arabidopsis B-box zinc finger family. Plant Cell 2009, 21, 3416–3420. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Heng, Y.; Wang, X.; Deng, X.W.; Xu, D. A Positive Feedback Loop of BBX11-BBX21-HY5 Promotes Photomorphogenic Development in Arabidopsis. Plant Commun. 2020, 1, 100045. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yan, T.; Liu, J.; Bian, Y.; Heng, Y.; Lin, F.; Jiang, Y.; Wang Deng, X.; Xu, D. BBX28/BBX29, HY5 and BBX30/31 form a feedback loop to fine-tune photomorphogenic development. Plant J. 2020, 104, 377–3909. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Luo, D.; Lin, A.; Zhang, C.; Shan, L.; He, P.; Li, B.; Zhang, Q.; Hua, B.; Yuan, Z.; et al. A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 2019, 221, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Holtan, H.E.; Bandong, S.; Marion, C.M.; Adam, L.; Tiwari, S.; Shen, Y.; Maloof, J.N.; Maszle, D.R.; Ohto, M.A.; Preuss, S.; et al. BBX32, an Arabidopsis B-Box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol. 2011, 156, 2109–2123. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Q.; Li, W.; Hu, T.; Wang, Q.; Yin, Y.; Liu, X.; He, S.; Zhang, M.; Liang, Y.; et al. Overexpression of SlBBX17 affects plant growth and enhances heat tolerance in tomato. Int. J. Biol. Macromol. 2022, 206, 799–811. [Google Scholar] [CrossRef]
- Chu, Z.; Wang, X.; Li, Y.; Yu, H.; Li, J.; Lu, Y.; Li, H.; Ouyang, B. Genomic Organization, Phylogenetic and Expression Analysis of the B-BOX Gene Family in Tomato. Front. Plant Sci. 2016, 7, 1552. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Dai, Y.; Chen, X.; Wang, X. Genome-wide identification and expression analysis of the B-box gene family in the Apple (Malus domestica Borkh.) genome. Mol. Genet. Genom. MGG 2018, 293, 303–315. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, D.; Han, Y.; Chen, T.; Jiao, C.; Chen, Y.; Jin, Q.; Cai, Y. Comparative analysis of B-BOX genes and their expression pattern analysis under various treatments in Dendrobium officinale. BMC Plant Biol. 2019, 19, 245. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, G.; Xu, R.; Jiao, Z.; Yang, J.; Lin, T.; Wang, Z.; Huang, S.; Chong, L.; Zhu, J.K. A natural promoter variation of SlBBX31 confers enhanced cold tolerance during tomato domestication. Plant Biotechnol. J. 2023, 21, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, T.; Tomczak, A.; Michelmore, R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol. J. 2005, 3, 259–273. [Google Scholar] [CrossRef]
- Hahn, A.; Bublak, D.; Schleiff, E.; Scharf, K.D. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 2011, 23, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Li, X.; Li, Z.; Shen, L.; Li, X.; Yang, Y.; Wang, W.; Kuang, T.; Shen, J.R.; Han, G. Structure and distinct supramolecular organization of a PSII-ACPII dimer from a cryptophyte alga Chroomonas placoidea. Nat. Commun. 2024, 15, 4535. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ye, J.J.; Yang, H.Z.; Li, D.; Li, X.X.; Wang, Y.K.; Zheng, X.Q.; Ye, J.H.; Li, Q.S.; Liang, Y.R.; et al. Shading-Dependent Greening Process of the Leaves in the Light-Sensitive Albino Tea Plant ‘Huangjinya’: Possible Involvement of the Light-Harvesting Complex II Subunit of Photosystem II in the Phenotypic Characteristic. Int. J. Mol. Sci. 2023, 24, 10314. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Alvi, A.F.; Saify, S.; Iqbal, N.; Khan, N.A. The Ethylene Biosynthetic Enzymes, 1-Aminocyclopropane-1-Carboxylate (ACC) Synthase (ACS) and ACC Oxidase (ACO): The Less Explored Players in Abiotic Stress Tolerance. Biomolecules 2024, 14, 90. [Google Scholar] [CrossRef]
- Mead, A.J.; Ahluwalia, K.; Ebright, B.; Zhang, Z.; Dave, P.; Li, Z.; Zhou, E.; Naik, A.A.; Ngu, R.; Chester, C.; et al. Loss of 15-Lipoxygenase in Retinodegenerative RCS Rats. Int. J. Mol. Sci. 2024, 25, 2309. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, Y.; Li, Y.; Shi, H.; Yao, J.; Liu, X.; Wang, F.; Huang, S.; Zhu, G.; Zhu, J.K. Natural variations in SlSOS1 contribute to the loss of salt tolerance during tomato domestication. Plant Biotechnol. J. 2021, 19, 20–22. [Google Scholar] [CrossRef]
- Xu, D. COP1 and BBXs-HY5-mediated light signal transduction in plants. New Phytol. 2020, 228, 1748–1753. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, Y.; Li, J.; Lin, F.; Holm, M.; Deng, X.W. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA 2016, 113, 7655–7660. [Google Scholar] [CrossRef]
- Datta, S.; Johansson, H.; Hettiarachchi, C.; Irigoyen, M.L.; Desai, M.; Rubio, V.; Holm, M. LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell 2008, 20, 2324–2338. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Bakshi, S.; Yadukrishnan, P.; Lingwan, M.; Dolde, U.; Wenkel, S.; Masakapalli, S.K.; Datta, S. The B-Box-Containing MicroProtein miP1a/BBX31 Regulates Photomorphogenesis and UV-B Protection. Plant Physiol. 2019, 179, 1876–1892. [Google Scholar] [CrossRef]
- Heng, Y.; Lin, F.; Jiang, Y.; Ding, M.; Yan, T.; Lan, H.; Zhou, H.; Zhao, X.; Xu, D.; Deng, X.W. B-Box Containing Proteins BBX30 and BBX31, Acting Downstream of HY5, Negatively Regulate Photomorphogenesis in Arabidopsis. Plant Physiol. 2019, 180, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Li, W.; Shao, M.; Cui, Z.; Wang, H.; Huo, J.; Chen, L.; Chen, B.; Ma, Z. Shading Treatment Reduces Grape Sugar Content by Suppressing Photosynthesis-Antenna Protein Pathway Gene Expression in Grape Berries. Int. J. Mol. Sci. 2024, 25, 5029. [Google Scholar] [CrossRef]
- Garty, Y.; Bussi, Y.; Levin-Zaidman, S.; Shimoni, E.; Kirchhoff, H.; Charuvi, D.; Nevo, R.; Reich, Z. Thylakoid membrane stacking controls electron transport mode during the dark-to-light transition by adjusting the distances between PSI and PSII. Nat. Plants 2024, 10, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Guadagno, C.R.; Ewers, B.E.; Mackay, D.S. Combining PSII photochemistry and hydraulics improves predictions of photosynthesis and water use from mild to lethal drought. Plant Cell Environ. 2024, 47, 1255–1268. [Google Scholar] [CrossRef]
- Hassidim, M.; Harir, Y.; Yakir, E.; Kron, I.; Green, R.M. Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 2009, 230, 481–491. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Dai, Y.; Yuan, L.; Sun, Q.; Zhang, S.; Wang, X. A B-box zinc finger protein, MdBBX10, enhanced salt and drought stresses tolerance in Arabidopsis. Plant Mol. Biol. 2019, 99, 437–447. [Google Scholar] [CrossRef]
- Wang, Q.; Tu, X.; Zhang, J.; Chen, X.; Rao, L. Heat stress-induced BBX18 negatively regulates the thermotolerance in Arabidopsis. Mol. Biol. Rep. 2013, 40, 2679–2688. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Zhang, X.W.; You, C.X.; Hao, Y.J. Apple B-box protein BBX37 regulates jasmonic acid mediated cold tolerance through the JAZ-BBX37-ICE1-CBF pathway and undergoes MIEL1-mediated ubiquitination and degradation. New Phytol. 2021, 229, 2707–2729. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Yan, J.; Zhang, Y.; Zhou, S.; Sun, X.; Yang, Y.; Ahammed, G.J.; Liu, Y.; Qi, M.; et al. Genome-Wide Characterization of B-Box Gene Family and Its Roles in Responses to Light Quality and Cold Stress in Tomato. Front. Plant Sci. 2021, 12, 698525. [Google Scholar] [CrossRef]
- Dong, H.; Hu, C.; Liu, C.; Wang, J.; Zhou, Y.; Yu, J. ELONGATED HYPOCOTYL 5 mediates blue light-induced starch degradation in tomato. J. Exp. Bot. 2021, 72, 2627–2641. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, J.; Wang, Y.; Hu, C.; Shi, K.; Zhou, J.; Xia, X.; Zhou, Y.; Foyer, C.H.; Yu, J. The phyB-dependent induction of HY5 promotes iron uptake by systemically activating FER expression. EMBO Rep. 2021, 22, e51944. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, C.; Dong, H.; Liu, X.; Guo, H.; Tong, B.; Fang, F.; Zhao, Y.; Yu, Y.; Liu, Y.; et al. Activation and negative feedback regulation of SlHY5 transcription by the SlBBX20/21-SlHY5 transcription factor module in UV-B signaling. Plant Cell 2022, 34, 2038–2055. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, F.; Li, B.; Guo, C.; Hu, T.; Zhang, M.; Liang, Y.; Zhu, J.; Zhan, X. A bHLH transcription factor, SlbHLH96, promotes drought tolerance in tomato. Hortic. Res. 2022, 9, uhac198. [Google Scholar] [CrossRef] [PubMed]
- Sahraeian, S.M.E.; Mohiyuddin, M.; Sebra, R.; Tilgner, H.; Afshar, P.T.; Au, K.F.; Bani Asadi, N.; Gerstein, M.B.; Wong, W.H.; Snyder, M.P.; et al. Gaining comprehensive biological insight into the transcriptome by performing a broad-spectrum RNA-seq analysis. Nat. Commun. 2017, 8, 59. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Ghosh, S.; Chan, C.K. Analysis of RNA-Seq Data Using TopHat and Cufflinks. Methods Mol. Biol. 2016, 1374, 339–361. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hu, E.; Cai, Y.; Xie, Z.; Luo, X.; Zhan, L.; Tang, W.; Wang, Q.; Liu, B.; Wang, R.; et al. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 2024, 1–29. [Google Scholar] [CrossRef]
- Pavlicevic, M.; Pagano, L.; Villani, M.; Zappettini, A.; Paesano, L.; Bonas, U.; Marmiroli, N.; Marmiroli, M. Comparison of effect of CdS QD and ZnS QD and their corresponding salts on growth, chlorophyll content and antioxidative capacity of tomato. Int. J. Phytoremediation 2024, 26, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Tang, Y.; Yang, W.; Hu, Z.; Huang, R.; Ding, J.; Yi, X.; Niu, J.; Chen, Z.; Wang, T.; et al. The vesicle trafficking gene, OsRab7, is critical for pollen development and male fertility in cytoplasmic male-sterility rice. Gene 2024, 915, 148423. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Guan, L.; Yin, Y.; Wang, Y.; Shi, H.; Li, W.; Zhang, D.; Song, R.; Hu, T.; Zhan, X. Genome-Wide Analysis of MBF1 Family Genes in Five Solanaceous Plants and Functional Analysis of SlER24 in Salt Stress. Int. J. Mol. Sci. 2023, 24, 13965. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhan, X. Elucidating the Role of SlBBX31 in Plant Growth and Heat-Stress Resistance in Tomato. Int. J. Mol. Sci. 2024, 25, 9289. https://doi.org/10.3390/ijms25179289
Wang Q, Zhan X. Elucidating the Role of SlBBX31 in Plant Growth and Heat-Stress Resistance in Tomato. International Journal of Molecular Sciences. 2024; 25(17):9289. https://doi.org/10.3390/ijms25179289
Chicago/Turabian StyleWang, Qiqi, and Xiangqiang Zhan. 2024. "Elucidating the Role of SlBBX31 in Plant Growth and Heat-Stress Resistance in Tomato" International Journal of Molecular Sciences 25, no. 17: 9289. https://doi.org/10.3390/ijms25179289




