Mitigating Cold Ischemic Injury: HTK, UW and IGL-2 Solution’s Role in Enhancing Antioxidant Defence and Reducing Inflammation in Steatotic Livers
Abstract
1. Introduction
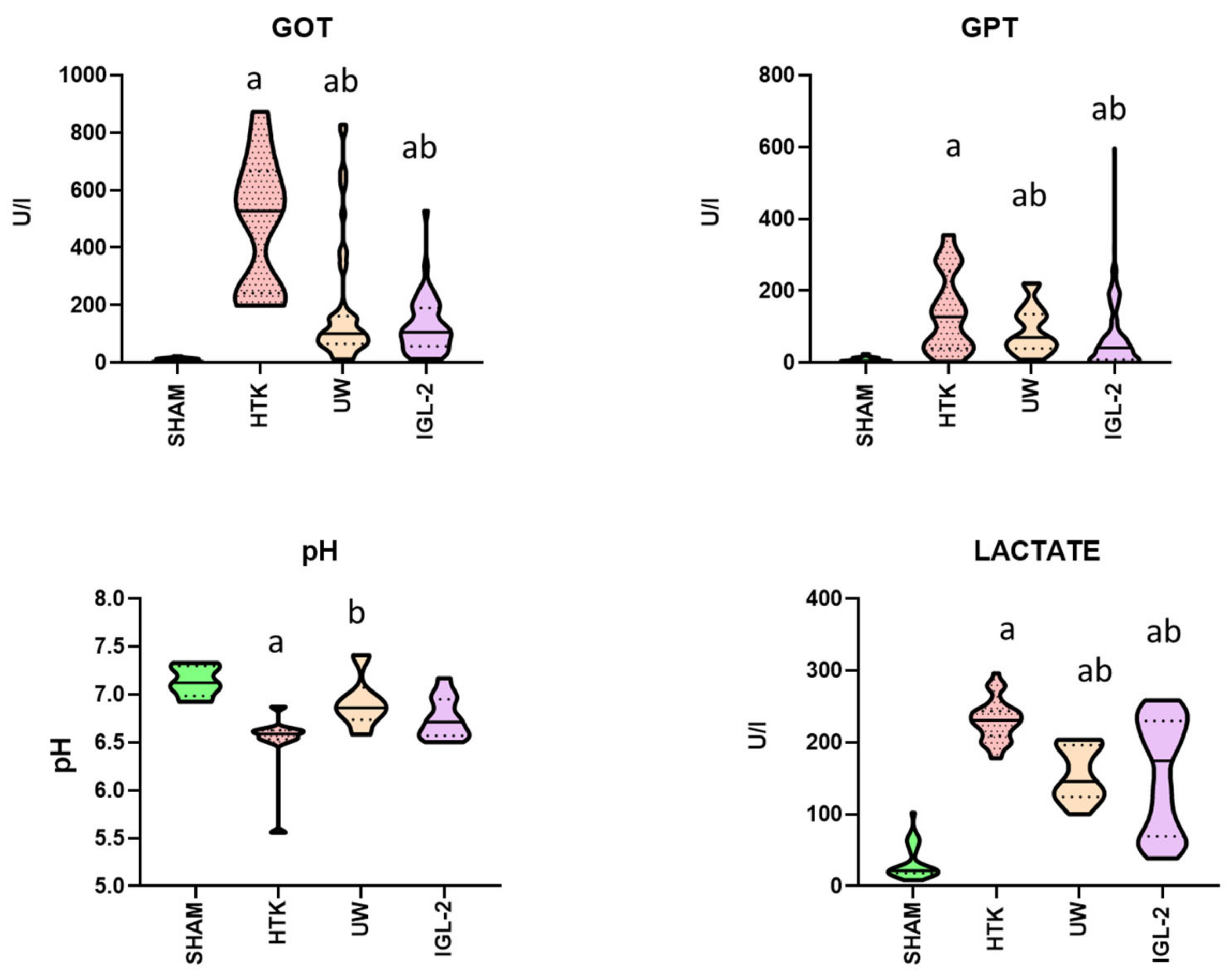
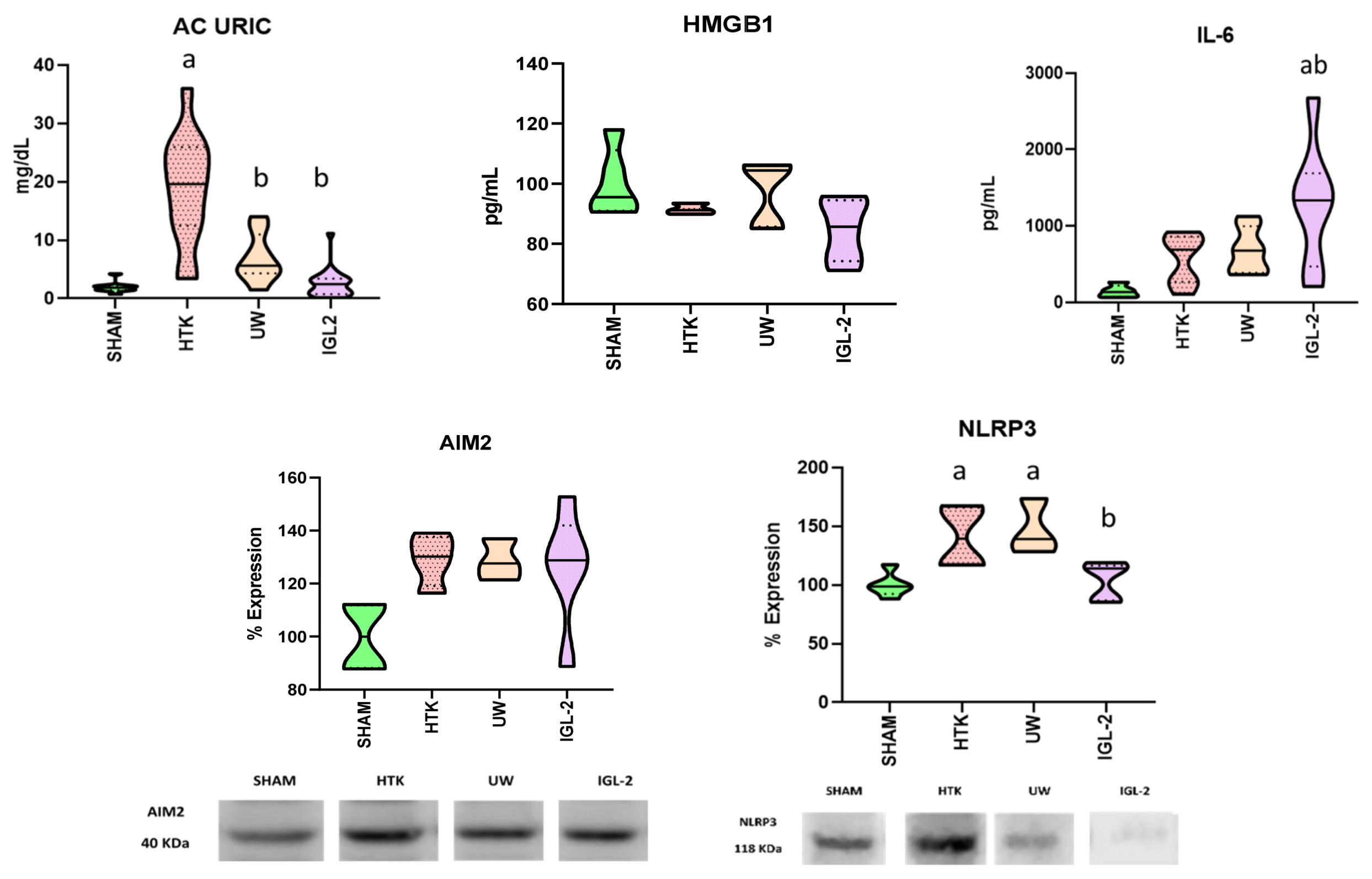
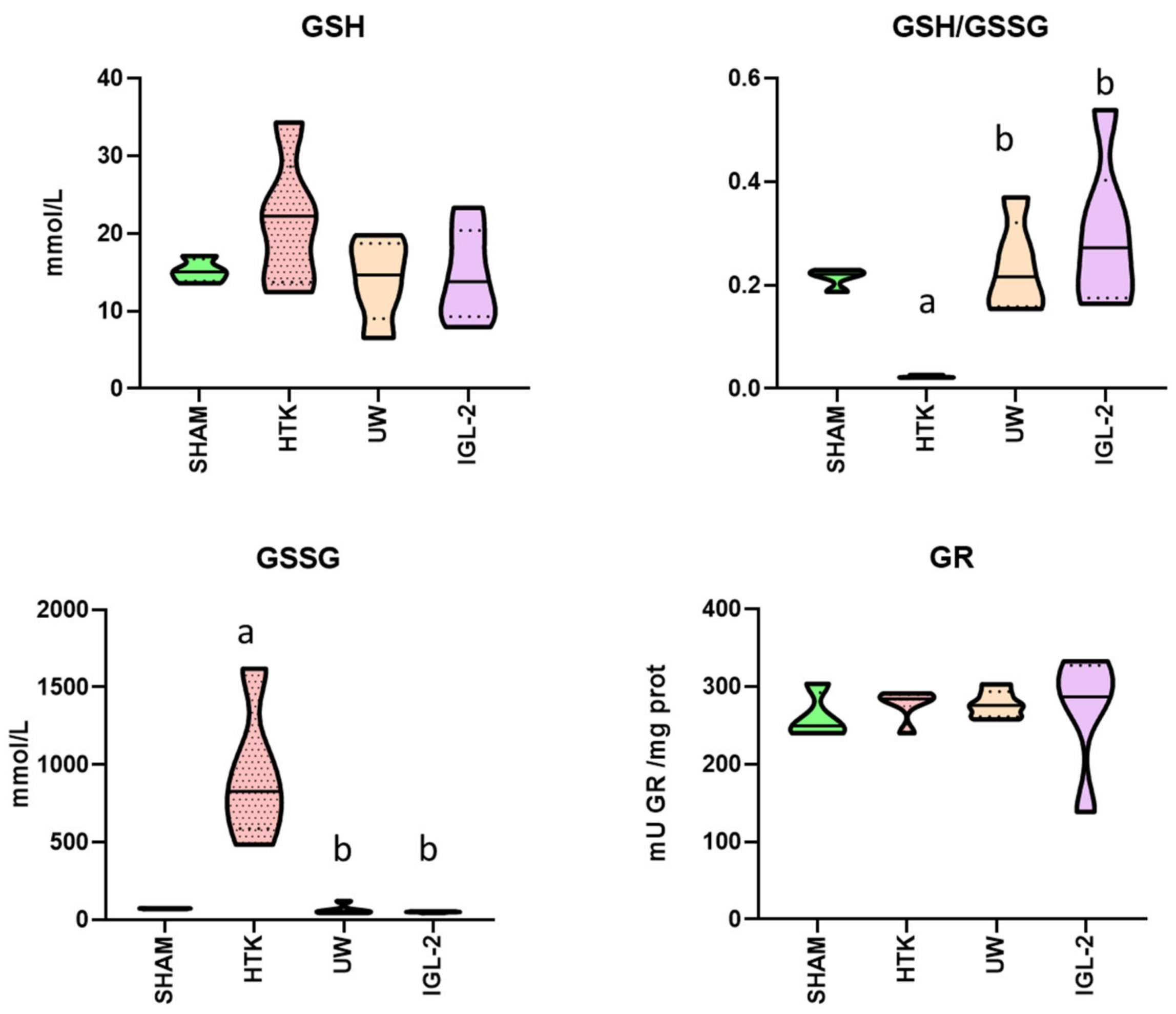
2. Results
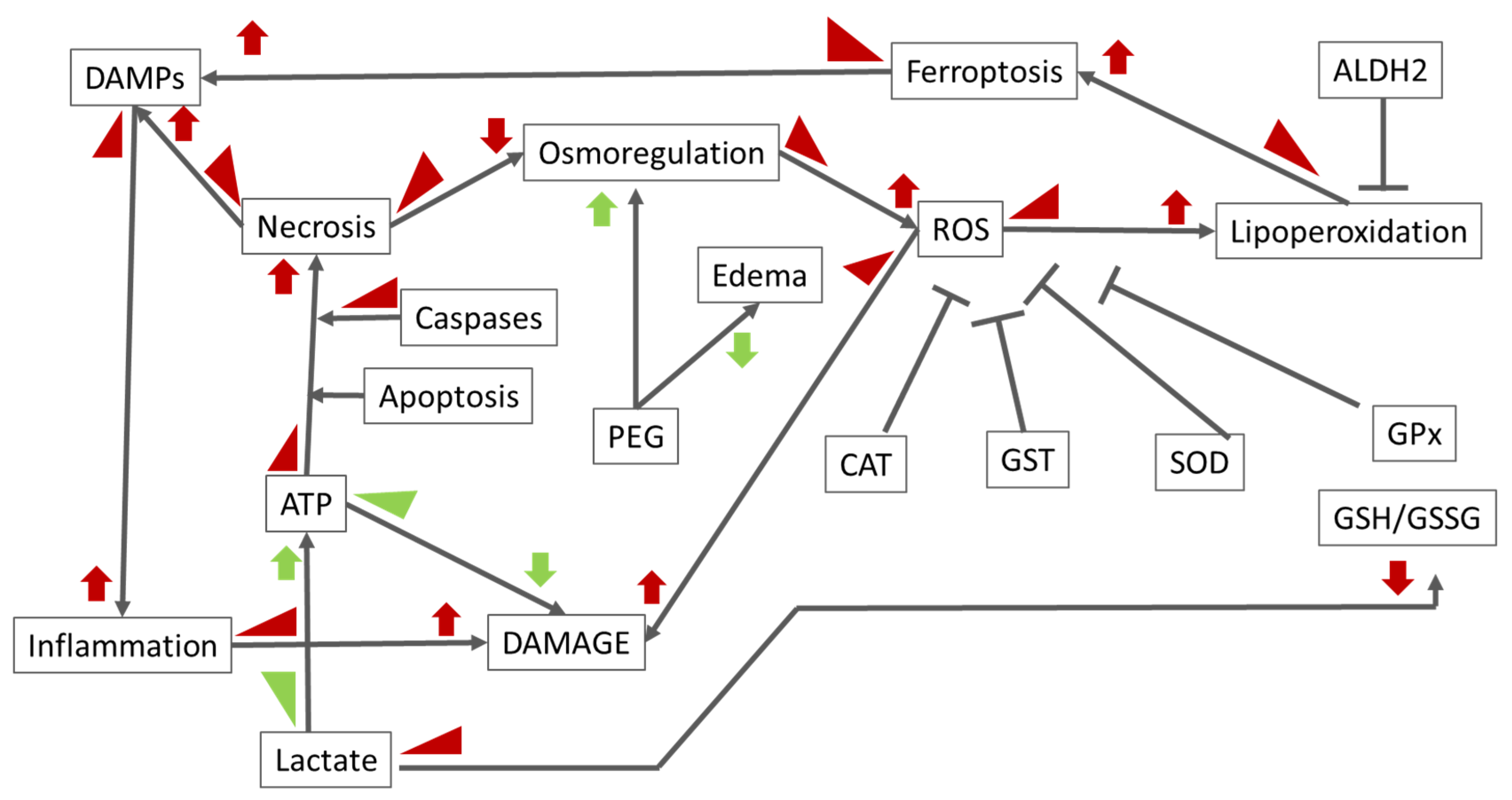
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Experimental Groups
4.3. Preservation Solutions
4.4. Biochemical Assays
4.5. Statistical Analysis
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De l’Hortet, A.C.; Takeishi, K.; Guzman-Lepe, J.; Handa, K.; Matsubara, K.; Fukumitsu, K.; Dorko, K.; Presnell, S.C.; Yagi, H.; Soto-Gutierrez, A. Liver-Regenerative Transplantation: Regrow and Reset. Am. J. Transplant. 2016, 16, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Zhou, K.; Chang, E.K.; Busuttil, R.W. De novo hepatocellular carcinoma after liver transplantation. J. Clin. Transl. Hepatol. 2015, 3, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Cholankeril, G.; Wong, R.J.; Hu, M.; Perumpail, R.B.; Yoo, E.R.; Puri, P.; Younossi, Z.M.; Harrison, S.A.; Ahmed, A. Liver Transplantation for Nonalcoholic Steatohepatitis in the US: Temporal Trends and Outcomes. Dig. Dis. Sci. 2017, 62, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- Ghinolfi, D.; Melandro, F.; Torri, F.; Martinelli, C.; Cappello, V.; Babboni, S.; Silvestrini, B.; De Simone, P.; Basta, G.; Del Turco, S. Extended criteria grafts and emerging therapeutics strategy in liver transplantation. The unstable balance between damage and repair. Transplant. Rev. 2021, 35, 100639. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.W.; Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transplant. 2003, 9, 651–663. [Google Scholar] [CrossRef]
- Teoh, N.C.; Farrell, G.C. Hepatic ischemia reperfusion injury: Pathogenic mechanisms and basis for hepatoprotection. J. Gastroenterol. Hepatol. 2003, 18, 891–902. [Google Scholar] [CrossRef]
- Tashiro, H.; Kuroda, S.; Mikuriya, Y.; Ohdan, H. Ischemia–reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg. Today 2014, 44, 1611–1625. [Google Scholar] [CrossRef]
- Janßen, H.; Janßen, P.H.E.; Broelsch, C.E. Value of Energy Substrates in HTK and UW to Protect Human Liver Endothelial Cells against Ischemia and Reperfusion Injury. Eur. Surg. Res. 2004, 36, 26–32. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Liu, M.; Moriyama, M.; Ramakrishnan, R.; Forbush, B.; Reddy, P.V.B.; Norenberg, M.D. Na-K-Cl Cotransporter-1 in the Mechanism of Ammonia-induced Astrocyte Swelling. J. Biol. Chem. 2008, 283, 33874–33882. [Google Scholar] [CrossRef]
- Heijnen, B.H.M.; Elkhaloufi, Y.; Straatsburg, I.H.; van Gulik, T.M. Influence of acidosis and hypoxia on liver ischemia and reperfusion injury in an in vivo rat model. J. Appl. Physiol. 2002, 93, 319–323. [Google Scholar] [CrossRef]
- Landry, D.W.; Oliver, A.J. The ATP-sensitive K+ channel mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J. Clin. Investig. 1992, 89, 2071–2074. [Google Scholar] [CrossRef]
- Varela, A.T.; Rolo, A.P.; Palmeira, C.M. Fatty Liver and Ischemia/Reperfusion: Are there Drugs Able to Mitigate Injury? Curr. Med. Chem. 2011, 18, 4987–5002. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Robin, E.; Guzy, R.D.; Loor, G.; Iwase, H.; Waypa, G.B.; Marks, J.D.; Hoek, T.L.V.; Schumacker, P.T. Oxidant Stress during Simulated Ischemia Primes Cardiomyocytes for Cell Death during Reperfusion. J. Biol. Chem. 2007, 282, 19133–19143. [Google Scholar] [CrossRef]
- Erpicum, P.; Rowart, P.; Defraigne, J.-O.; Krzesinski, J.-M.; Jouret, F. What we need to know about lipid-associated injury in case of renalischemia-reperfusion. Am. J. Physiol. Physiol. 2018, 315, F1714–F1719. [Google Scholar] [CrossRef] [PubMed]
- Beneke, R.; Pollmann, C.; Bleif, I.; Leithäuser, R.M.; Hütler, H. How anaerobic is the wingate anaerobic test for humans? Eur. J. Appl. Physiol. 2002, 87, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Darwin, D.; Cord-Ruwisch, R.; Charles, W. Ethanol and lactic acid production from sugar and starch wastes by anaerobic acidification. Eng. Life Sci. 2018, 18, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Bae, H.C.; Lee, H.; Jang, Y.; Park, Y.; Ryu, W.; Choi, B.H.; Kim, J.H.; Jeong, S.H.; Son, S.W. Intracellular ROS levels determine the apoptotic potential of keratinocyte by Quantum Dot via blockade of AKT Phosphorylation. Exp. Dermatol. 2017, 26, 1046–1052. [Google Scholar] [CrossRef]
- Hao, S.; Yu, J.; He, W.; Huang, Q.; Zhao, Y.; Liang, B.; Zhang, S.; Wen, Z.; Dong, S.; Rao, J.; et al. Cysteine Dioxygenase 1 Mediates Erastin-Induced Ferroptosis in Human Gastric Cancer Cells. Neoplasia 2017, 19, 1022–1032. [Google Scholar] [CrossRef]
- Tai, C.; Chang, S.; Chien, L.; Leung, P.C.; Tzeng, C. Adenosine Triphosphate Induces Activation of Caspase-3 in Apoptosis of Human Granulosa-luteal Cells. Endocr. J. 2005, 52, 327–335. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Y.; Lu, J.; Duan, W.; He, X.; Lin, L.; Ding, J.; Qin, Y. Caspase-8 preferentially senses the apoptosis-inducing action of NG-18, A gambogic acid derivative, in human leukemia HL-60 cells. Cancer Biol. Ther. 2007, 6, 691–696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López, E.; Figueroa, S.; Oset-Gasque, M.J.; González, M.P. Apoptosis and necrosis: Two distinct events induced by cadmium in cortical neurons in culture. Br. J. Pharmacol. 2003, 138, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Single, B.; Castoldi, A.F.; Kühnle, S.; Nicotera, P. Intracellular Adenosine Triphosphate (ATP) Concentration: A Switch in the Decision Between Apoptosis and Necrosis. J. Exp. Med. 1997, 185, 1481–1486. [Google Scholar] [CrossRef]
- Tonnus, W.; Gembardt, F.; Latk, M.; Parmentier, S.; Hugo, C.; Bornstein, S.R.; Linkermann, A. The clinical relevance of necroinflammation—Highlighting the importance of acute kidney injury and the adrenal glands. Cell Death Differ. 2019, 26, 68–82. [Google Scholar] [CrossRef]
- Marshall, K.D.; Edwards, M.A.; Krenz, M.; Davis, J.W.; Baines, C.P. Proteomic mapping of proteins released during necrosis and apoptosis from cultured neonatal cardiac myocytes. Am. J. Physiol.-Cell Physiol. 2014, 306, C639–C647. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. ESCRT-III-mediated membrane repair in cell death and tumor resistance. Cancer Gene Ther. 2021, 28, 1–4. [Google Scholar] [CrossRef]
- Marques, P.E.; Vandendriessche, S.; de Oliveira, T.H.; Crijns, H.; Lopes, M.E.; Blanter, M.; Schuermans, S.; Yu, K.; Poosti, F.; Vanheule, V.; et al. Inhibition of Drug-Induced Liver Injury in Mice Using a Positively Charged Peptide That Binds DNA. Hepatol. Commun. 2021, 5, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, M.; Wang, C.; Feng, X.; Zhao, Z.; Yang, Y.; Sahoo, N.; Gu, M.; Yang, Y.; Xiao, S.; et al. LRRC8 family proteins within lysosomes regulate cellular osmoregulation and enhance cell survival to multiple physiological stresses. Proc. Natl. Acad. Sci. USA 2020, 117, 29155–29165. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Li, H.; Zhang, O.; Hui, C.; Huang, Y.; Shao, H.; Song, M.; Gao, L.; Jin, S.; Ding, C.; Xu, L. Deuterium-Reinforced Polyunsaturated Fatty Acids Prevent Diet-Induced Nonalcoholic Steatohepatitis by Reducing Oxidative Stress. Medicina 2022, 58, 790. [Google Scholar] [CrossRef]
- Bardallo, R.G.; da Silva, R.T.; Carbonell, T.; Folch-Puy, E.; Palmeira, C.; Roselló-Catafau, J.; Pirenne, J.; Adam, R.; Panisello-Roselló, A. Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach. Int. J. Mol. Sci. 2021, 22, 5332. [Google Scholar] [CrossRef]
- Panisello-Roselló, A.; Alva, N.; Flores, M.; Lopez, A.; Benítez, C.C.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Adam, R.; Carbonell, T.; et al. Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2479. [Google Scholar] [CrossRef]
- Panisello-Roselló, A.; Lopez, A.; Folch-Puy, E.; Carbonell, T.; Rolo, A.; Palmeira, C.; Adam, R.; Net, M.; Roselló-Catafau, J. Role of aldehyde dehydrogenase 2 in ischemia reperfusion injury: An update. World J. Gastroenterol. 2018, 24, 2984–2994. [Google Scholar] [CrossRef]
- Dhalla, N. Status of myocardial antioxidants in ischemia–reperfusion injury. Cardiovasc. Res. 2000, 47, 446–456. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Bacanli, M.; Başaran, N.; Başaran, A.A. Lycopene: Is it Beneficial to Human Health as an Antioxidant? Turk. J. Pharm. Sci. 2017, 14, 311–318. [Google Scholar] [CrossRef]
- Bontor, K.; Gabryel, B. Sulodexide Increases Glutathione Synthesis and Causes Pro-Reducing Shift in Glutathione-Redox State in HUVECs Exposed to Oxygen–Glucose Deprivation: Implication for Protection of Endothelium against Ischemic Injury. Molecules 2022, 27, 5465. [Google Scholar] [CrossRef]
- Rodgers, C.; Sanborn, C.; Taylor, O.; Gundy, P.; Pasvogel, A.; Moore, I.M.; Hockenberry, M.J. Fatigue and Oxidative Stress in Children Undergoing Leukemia Treatment. Biol. Res. Nurs. 2016, 18, 515–520. [Google Scholar] [CrossRef]
- Khurana, S.; Corbally, M.T.; Manning, F.; Armenise, T.; Kierce, B.; Kilty, C. Glutathione S-transferase: A potential new marker of intestinal ischemia. J. Pediatr. Surg. 2002, 37, 1543–1548. [Google Scholar] [CrossRef]
- Burra, P.; Zanetto, A.; Russo, F.; Germani, G. Organ Preservation in Liver Transplantation. Semin. Liver Dis. 2018, 38, 260–269. [Google Scholar] [CrossRef]
- Bodzin, A.S.; Baker, T.B. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transplant. 2018, 24, 1470–1475. [Google Scholar] [CrossRef]
- Bejaoui, M.; Pantazi, E.; Folch-Puy, E.; Baptista, P.M.; García-Gil, A.; Adam, R.; Roselló-Catafau, J. Emerging concepts in liver graft preservation. World J. Gastroenterol. 2015, 21, 396. [Google Scholar] [CrossRef]
- Adam, R.; Karam, V.; Cailliez, V.; Grady, J.G.O.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Da Silva, R.T.; Bardallo, R.G.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.M.; Fondevila, C.; Adam, R.; Roselló-Catafau, J.; Panisello-Roselló, A. IGL-2 as a Unique Solution for Cold Static Preservation and Machine Perfusion in Liver and Mitochondrial Protection. Transplant. Proc. 2022, 54, 73–76. [Google Scholar] [CrossRef]
- Belzer, S.; Southard, J.H.; Belzer, F.O. Organ Preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar]
- Wilson, C.H.; Brook, N.R.; Talbot, D. Preservation Solutions for Solid Organ Transplantation. Mini-Rev. Med. Chem. 2006, 6, 1081–1090. [Google Scholar] [CrossRef]
- Baicu, S.C.; Taylor, M.J.; Brockbank, K.G.M. The role of preservation solution on acid-base regulation during machine perfusion of kidneys. Clin. Transplant. 2006, 20, 113–121. [Google Scholar] [CrossRef]
- Lucas-Ruiz, F.; Mateo, S.V.; Jover-Aguilar, M.; Alconchel, F.; Martínez-Alarcón, L.; de Torre-Minguela, C.; Vidal-Correoso, D.; Villalba-López, F.; López-López, V.; Ríos-Zambudio, A.; et al. Danger signals released during cold ischemia storage activate NLRP3 inflammasome in myeloid cells and influence early allograft function in liver transplantation. eBioMedicine 2023, 87, 104419. [Google Scholar] [CrossRef]
- Camargo, C.A.; Madden, J.F.; Gao, W.; Selvan, R.S.; Clavien, P. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology 1997, 26, 1513–1520. [Google Scholar] [CrossRef]
- Xu, S.; Yu, C.; Ma, X.; Li, Y.; Shen, Y.; Chen, Y.; Huang, S.; Zhang, T.; Deng, W.; Wang, Y. IL-6 promotes nuclear translocation of HIF-1α to aggravate chemoresistance of ovarian cancer cells. Eur. J. Pharmacol. 2021, 894, 173817. [Google Scholar] [CrossRef]
- Kim, Y.-W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, N.; Shukla, S.; Kanwal, R.; Srivastava, J.K.; Gupta, S. Induction of heme oxygenase-1 by chamomile protects murine macrophages against oxidative stress. Life Sci. 2012, 90, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Verde, E.; Lopez, A.; Flores, M.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Hotter, G.; Carbonell, T.; Adam, R.; et al. Cytoprotective Mechanisms in Fatty Liver Preservation against Cold Ischemia Injury: A Comparison between IGL-1 and HTK. Int. J. Mol. Sci. 2018, 19, 348. [Google Scholar] [CrossRef]
- Garcia, A.A.; Koperniku, A.; Ferreira, J.C.B.; Mochly-Rosen, D. Treatment strategies for glucose-6-phosphate dehydrogenase deficiency: Past and future perspectives. Trends Pharmacol. Sci. 2021, 42, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.S.; Palsson, B.O. Effect of medium osmolarity on hybridoma growth, metabolism, and antibody production. Biotechnol. Bioeng. 1991, 37, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, B.; Ni, H.; Sheng, X.; Jin, N. Prediction of pulmonary edema by plasma protein levels in patients with sepsis. J. Crit. Care 2012, 27, 623–629. [Google Scholar] [CrossRef]
- Chan, D.L.; Freeman, L.M.; Rozanski, E.A.; Rush, J.E. Colloid osmotic pressure of parenteral nutrition components and intravenous fluids. J. Vet. Emerg. Crit. Care 2001, 11, 269–273. [Google Scholar] [CrossRef]
- Panisello-Rosello, A.; Castro-Benítez, C.; Lopez, A.; Balloji, S.; Folch-Puy, E.; Adam, R.; Roselló-Catafau, J. Graft Protection Against Cold Ischemia Preservation: An Institute George Lopez 1 and Histidine-tryptophan-ketoglutarate Solution Appraisal. Transplant. Proc. 2018, 50, 714–718. [Google Scholar] [CrossRef]
- Bardallo, R.G.; Company-Marin, I.; Folch-Puy, E.; Roselló-Catafau, J.; Panisello-Rosello, A.; Carbonell, T. PEG35 and Glutathione Improve Mitochondrial Function and Reduce Oxidative Stress in Cold Fatty Liver Graft Preservation. Antioxidants 2022, 11, 158. [Google Scholar] [CrossRef]
- Errante, P.R. Extravasation injuries in the intravenous therapy with drugs with properties vesicants and irritants in the veterinary medicine of small animals. J. Dairy Vet. Anim. Res. 2023, 12, 19–22. [Google Scholar] [CrossRef]
- Ramos, P.; Williams, P.; Salinas, J.; Vengohechea, J.B.; Lodge, J.P.A.; Fondevila, C.; Hessheimer, A.J. Abdominal Organ Preservation Solutions in the Age of Machine Perfusion. Transplantation 2023, 107, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Kand’ár, R.; Žáková, P.; Lotková, H.; Kučera, O.; Červinková, Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J. Pharm. Biomed. Anal. 2007, 43, 1382–1387. [Google Scholar] [CrossRef] [PubMed]


| HTK | UW | IGL-2 | |
|---|---|---|---|
| pH | 7.02–7.2 | 7.4 | 7.4 |
| Osmolarity, mOsm/L | 310 | 320 | 360 |
| Viscosity, cP | 1.8 | 5.70 | 1.7 |
| Colloids | – | 50 g/L HES | 5 g/L PEG |
| Impermeants | |||
| Lactobionate mmol/L: | – | 100 | 100 |
| Mannitol mmol/L: | 30 | – | 60 |
| Raffinose mmol/L: | 30 | – | |
| Buffers | Histidine | Phosphate, Sulfate | HEPES, Histidine, Phosphate, Sulfate |
| Electrolytes | |||
| Calcium mmol/L: | 0.015 | – | – |
| Chloride mmol/L: | 50 | – | – |
| Magnesium mmol/L: | 4 | 5 | 5 |
| Potassium mmol/L: | 10 | 120 | 25 |
| Sodium mmol/L: | 15 | 25 | 125 |
| Zinc mmol/L: | – | – | 0.091 |
| Metabolic Precursors | α-ketoglutarate | Adenosine | Adenosine, sodium nitrite |
| Antioxidants and Free-Radical Scavengers | Tryptophan | Allopurinol, glutathione | Glutathione |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardallo, R.G.; Chullo, G.; Alva, N.; Rosello-Catafau, J.; Fundora-Suárez, Y.; Carbonell, T.; Panisello-Rosello, A. Mitigating Cold Ischemic Injury: HTK, UW and IGL-2 Solution’s Role in Enhancing Antioxidant Defence and Reducing Inflammation in Steatotic Livers. Int. J. Mol. Sci. 2024, 25, 9318. https://doi.org/10.3390/ijms25179318
Bardallo RG, Chullo G, Alva N, Rosello-Catafau J, Fundora-Suárez Y, Carbonell T, Panisello-Rosello A. Mitigating Cold Ischemic Injury: HTK, UW and IGL-2 Solution’s Role in Enhancing Antioxidant Defence and Reducing Inflammation in Steatotic Livers. International Journal of Molecular Sciences. 2024; 25(17):9318. https://doi.org/10.3390/ijms25179318
Chicago/Turabian StyleBardallo, Raquel G., Gabriela Chullo, Norma Alva, Joan Rosello-Catafau, Yiliam Fundora-Suárez, Teresa Carbonell, and Arnau Panisello-Rosello. 2024. "Mitigating Cold Ischemic Injury: HTK, UW and IGL-2 Solution’s Role in Enhancing Antioxidant Defence and Reducing Inflammation in Steatotic Livers" International Journal of Molecular Sciences 25, no. 17: 9318. https://doi.org/10.3390/ijms25179318
APA StyleBardallo, R. G., Chullo, G., Alva, N., Rosello-Catafau, J., Fundora-Suárez, Y., Carbonell, T., & Panisello-Rosello, A. (2024). Mitigating Cold Ischemic Injury: HTK, UW and IGL-2 Solution’s Role in Enhancing Antioxidant Defence and Reducing Inflammation in Steatotic Livers. International Journal of Molecular Sciences, 25(17), 9318. https://doi.org/10.3390/ijms25179318








