SlHB8 Is a Novel Factor in Enhancing Cold Resistance in Tomato Anthers by Modulating Tapetal Cell Death
Abstract
:1. Introduction
2. Results
2.1. SlHB8 Showed a Cold-Induced Expression Pattern within the Tapetal Cells of Tomato Anthers
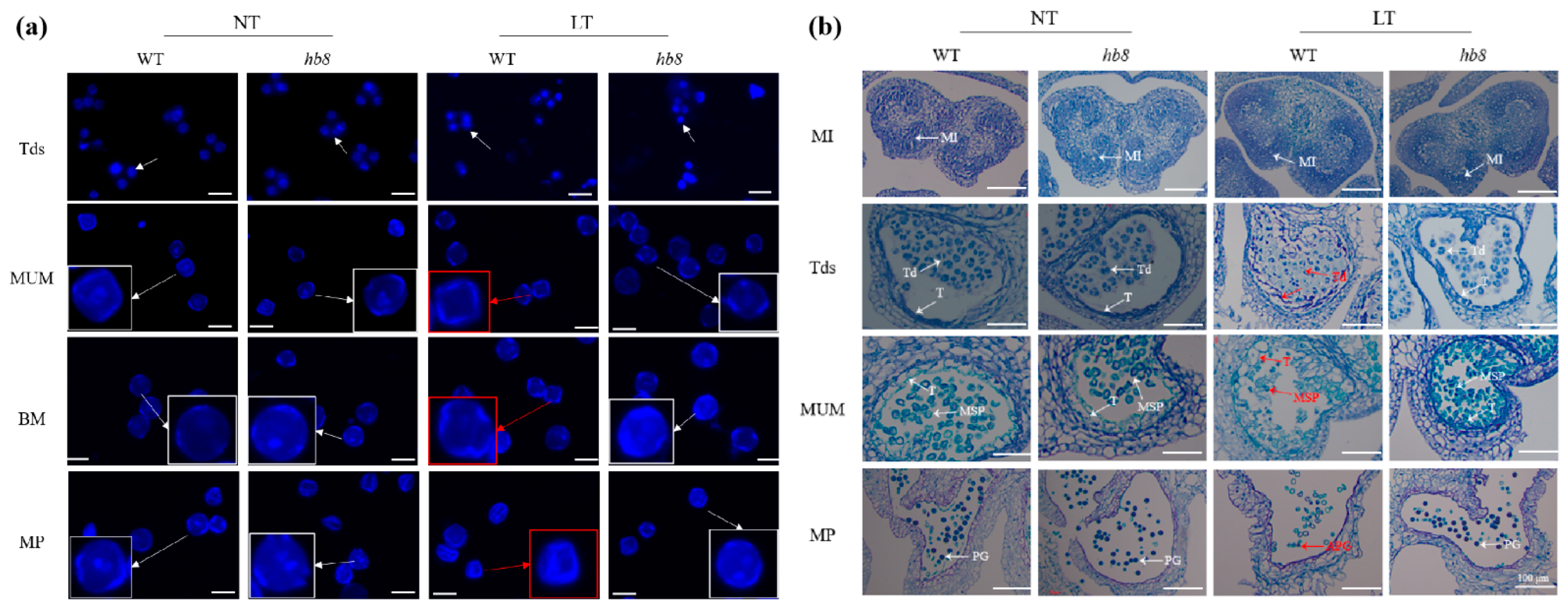
2.2. Knocking Out SlHB8 Enhances the Tolerance of Anthers to Low-Temperature Stress
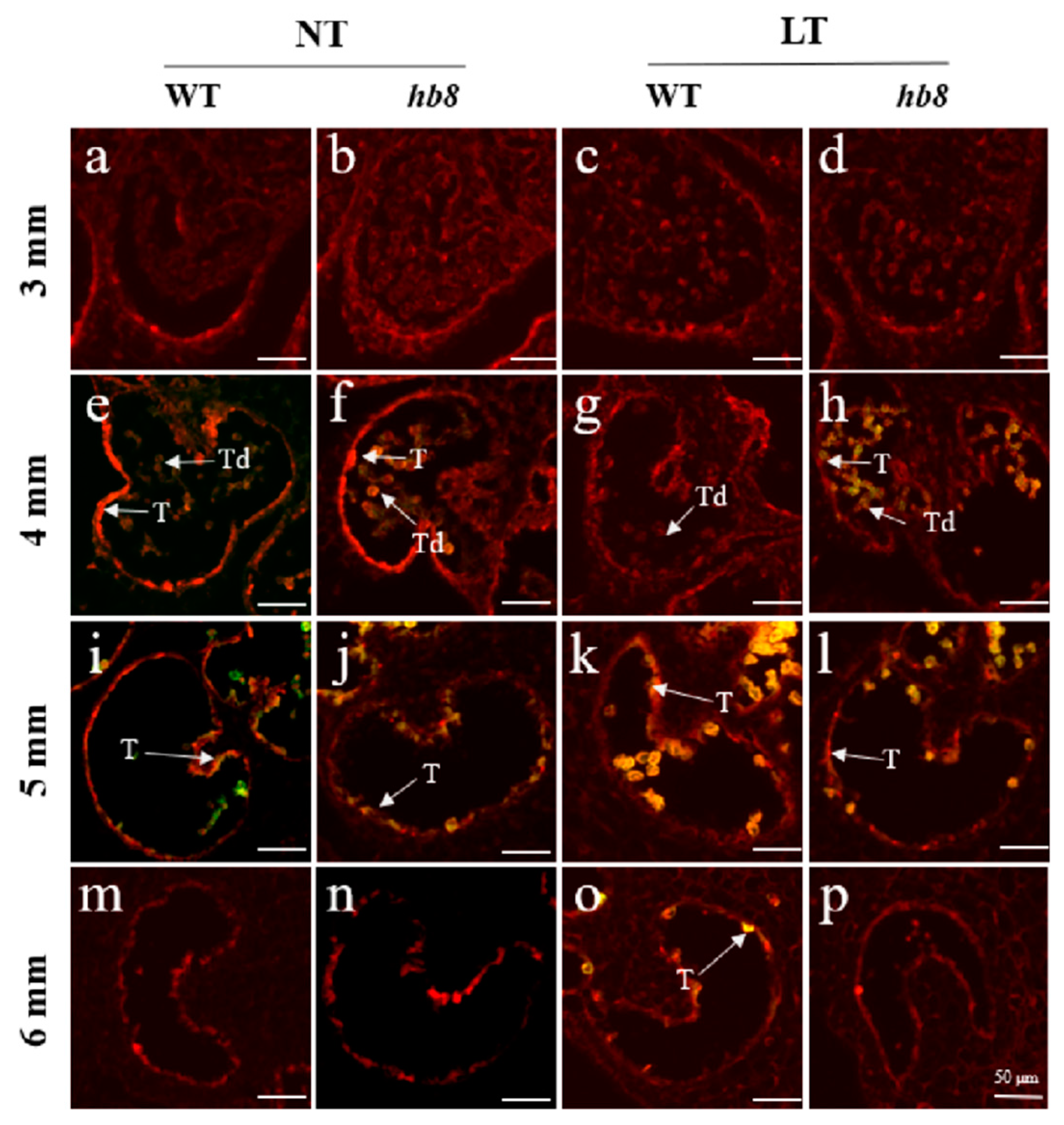
2.3. SlHB8 Negatively Regulates Low Temperature Tolerance of Tomato Anthers by Regulating Tapetal PCD
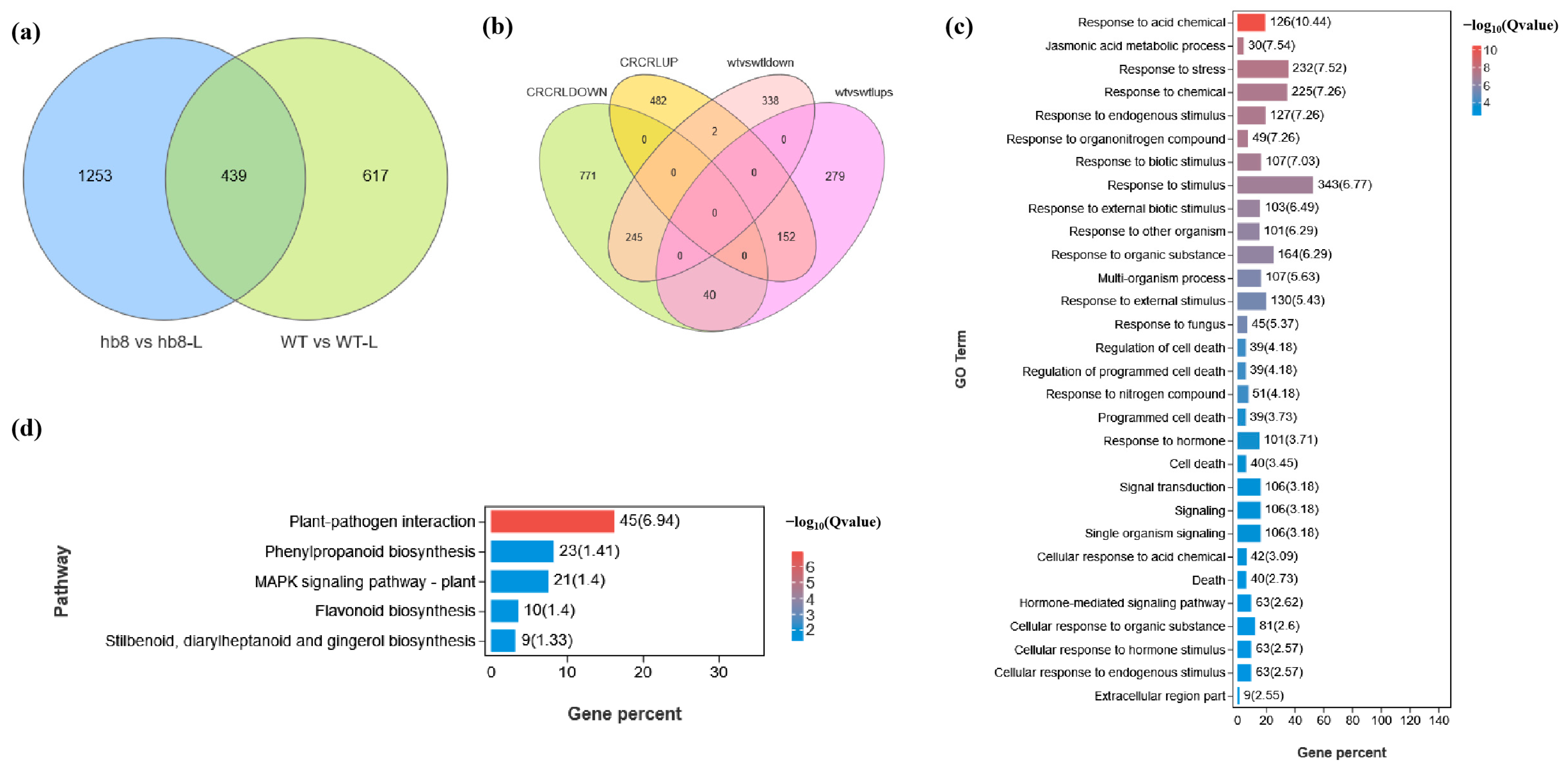
2.4. Transcriptome Analysis of DEGs in Anthers of the Wild Type and hb8 under Cold Treatment
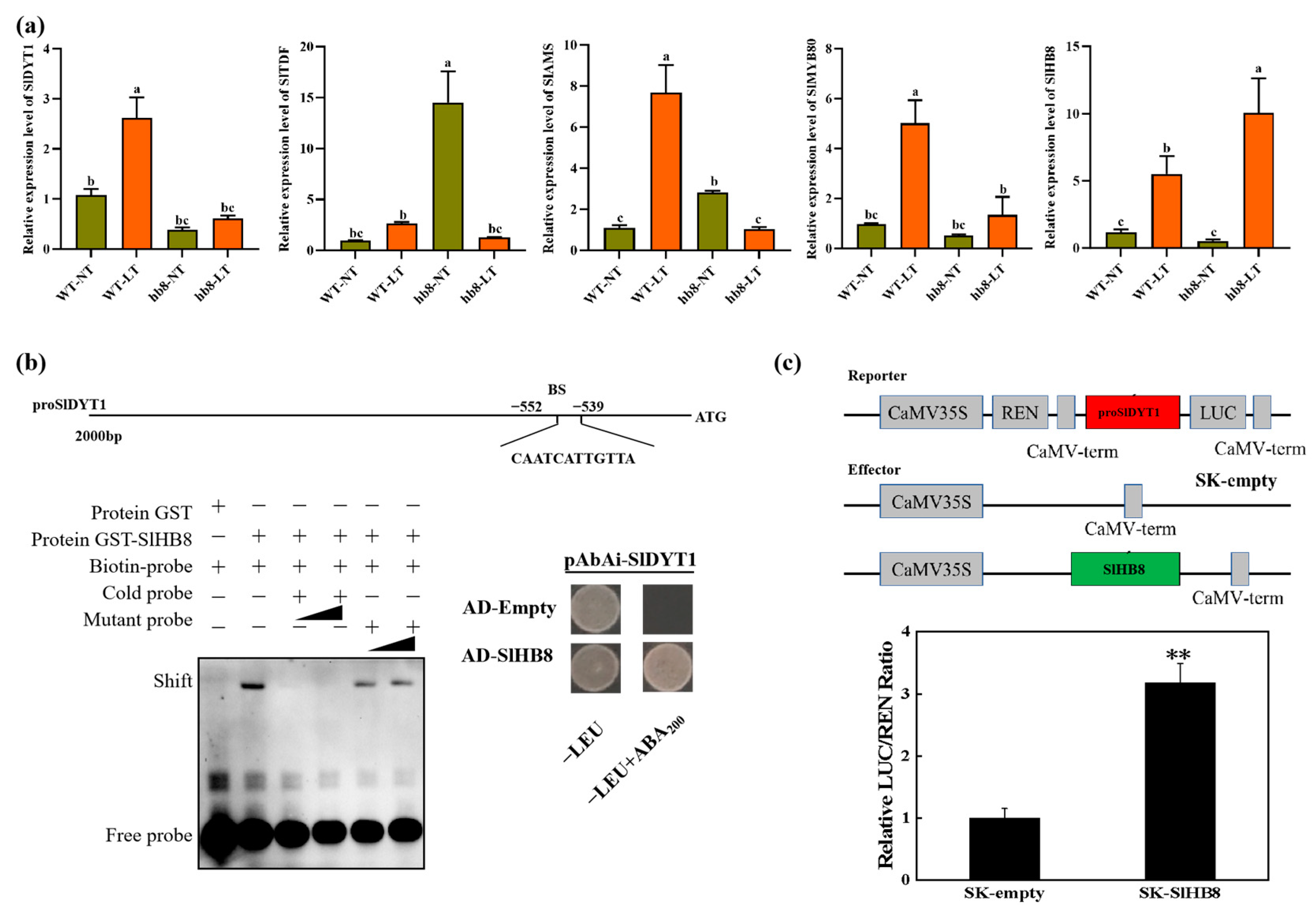
2.5. Knocking Out SlHB8 Blocks Cold-Induced Activation of DYT1-TDF1-AMS-MYB80
2.6. The SlHB8 Interact with SlTDF1-1 and SlMYB80
3. Discussion
3.1. SlHB8 Exhibited a Critical Influence on the Cold Resistance of Tomato Pollen, Primarily by Regulating the Delayed Degradation of the Tapetum under Low Temperature
3.2. SlHB8 Enhanced Cold Tolerance in Anthers through Interaction with the Tapetum Regulatory Module
4. Materials and Methods
4.1. Plant Material and Cold Treatment
4.2. Pollen Viability and Pollen Germination Assay
4.3. Fruit Set and Fruit Morphology Analysis
4.4. DAPI Staining
4.5. Cytological Characterization of Anthers
4.6. In Situ Hybridization Assay
4.7. RNA-seq and qRT-PCR Analysis
4.8. Yeast One-Hybrid Assay (Y1H)
4.9. Dual-Luciferase Transient Expression (DLR) Assay
4.10. Yeast Two-Hybrid (Y2H) System
4.11. Luciferase Complementation (LCI) Assay
4.12. Electrophoretic Mobility Shift Assay (EMSA)
4.13. Pull-Down Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alenazi, M.M.; Shafiq, M.; Alsadon, A.A.; Alhelal, I.M.; Alhamdan, A.M.; Solieman, T.; Ibrahim, A.A.; Shady, M.R.; Al-Selwey, W.A. Improved functional and nutritional properties of tomato fruit during cold storage. Saudi J. Biol. Sci. 2020, 27, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Popko, J.; Hänsch, R.; Mendel, R.R.; Polle, A.; Teichmann, T. The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 2010, 12, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef]
- Peng, G.; Liu, M.; Zhu, L.; Luo, W.; Wang, Q.; Wang, M.; Chen, H.; Luo, Z.; Xiao, Y.; Zhang, Y.; et al. The E3 ubiquitin ligase CSIT1 regulates critical sterility-inducing temperature by ribosome-associated quality control to safeguard two-line hybrid breeding in rice. Mol. Plant 2023, 16, 1695–1709. [Google Scholar] [CrossRef]
- Mamun, E.A.; Alfred, S.; Cantrill, L.C.; Overall, R.L.; Sutton, B.G. Effects of chilling on male gametophyte development in rice. Cell Biol. Int. 2006, 30, 583–591. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, D.H. Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiol. 2003, 132, 517–529. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Wi, S.D.; Lee, E.S.; Park, J.H.; Chae, H.B.; Paeng, S.K.; Bae, S.B.; Phan, T.; Kim, W.Y.; Yun, D.J.; Lee, S.Y. Redox-mediated structural and functional switching of C-repeat binding factors enhances plant cold tolerance. New Phytol. 2022, 233, 1067–1073. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Dong, S.; Jiang, X.; Wang, L.; Yu, J.; Zhou, Y. Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato. Plant Biotechnol. J. 2020, 18, 1041–1055. [Google Scholar] [CrossRef]
- Yu, C.Y.; Xu, X.F.; Ge, J.; Guo, Y.F.; Dong, J.G.; Dong, Z.S. Premature breakdown of tapetum associated with reverse thermo-sensitive genic male-sterile line Huiyou50S in rapeseed (Brassica napus). Acta Physiol. Plant. 2016, 38, 54. [Google Scholar] [CrossRef]
- Ma, H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu. Rev. Plant Biol. 2005, 56, 393–434. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Li, H.; Gao, J.F.; Jiang, H.; Wang, C.; Guan, Y.F.; Yang, Z.N. Defective in Tapetal Development and Function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J. 2008, 55, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Franchi, G.G.; Hesse, M. The tapetum: Its form, function, and possible phylogeny in Embryophyta. Plant Syst. Evol. 1985, 149, 155–185. [Google Scholar] [CrossRef]
- Luo, D.; Xu, H.; Liu, Z.; Guo, J.; Li, H.; Chen, L.; Fang, C.; Zhang, Q.; Bai, M.; Yao, N.; et al. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 2013, 45, 573–577. [Google Scholar] [CrossRef]
- Kawanabe, T.; Ariizumi, T.; Kawai-Yamada, M.; Uchimiya, H.; Toriyama, K. Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol. 2006, 47, 784–787. [Google Scholar] [CrossRef]
- Zheng, S.; Dong, J.; Lu, J.; Li, J.; Jiang, D.; Yu, H.; Ye, S.; Bu, W.; Liu, Z.; Zhou, H.; et al. A cytosolic pentatricopeptide repeat protein is essential for tapetal plastid development by regulating OsGLK1 transcript levels in rice. New Phytol. 2022, 234, 1678–1695. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.; Yoon, H.; Suh, H.S.; Chung, Y.Y. Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 2003, 217, 559–565. [Google Scholar] [CrossRef]
- Baron, K.N.; Schroeder, D.F.; Stasolla, C. Transcriptional response of abscisic acid (ABA) metabolism and transport to cold and heat stress applied at the reproductive stage of development in Arabidopsis thaliana. Plant Sci. 2012, 188–189, 48–59. [Google Scholar] [CrossRef]
- Omidi, M.; Siahpoosh, M.R.; Mamghani, R.; Modarresi, M. The Influence of Terminal Heat Stress on Meiosis Abnormalities in Pollen Mother Cells of Wheat. Cytologia 2014, 79, 49–58. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Li, R.; Peng, G.; Chen, W.; Rautengarten, C.; Liu, M.; Zhu, L.; Xiao, Y.; Song, F.; et al. BOTRYOID POLLEN 1 regulates ROS-triggered PCD and pollen wall development by controlling UDP-sugar homeostasis in rice. Plant Cell 2023, 35, 3522–3543. [Google Scholar] [CrossRef] [PubMed]
- De Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ. 2014, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Rieu, I. Acclimation to high temperature during pollen development. Plant Reprod. 2016, 29, 107–118. [Google Scholar] [CrossRef]
- Liu, B.; Mo, W.J.; Zhang, D.; De Storme, N.; Geelen, D. Cold Influences Male Reproductive Development in Plants: A Hazard to Fertility, but a Window for Evolution. Plant Cell Physiol. 2019, 60, 7–18. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Wang, Y.; Zhang, L.; Yao, S. A point mutation in LTT1 enhances cold tolerance at the booting stage in rice. Plant Cell Environ. 2020, 43, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Gothandam, K.M.; Kim, E.S.; Chung, Y.Y. Ultrastructural study of rice tapetum under low-temperature stress. J. Plant Biol. 2007, 50, 396–402. [Google Scholar] [CrossRef]
- Jung, K.H.; Han, M.J.; Lee, Y.S.; Kim, Y.W.; Hwang, I.; Kim, M.J.; Kim, Y.K.; Nahm, B.H.; An, G. Rice Undeveloped Tapetum1 is a major regulator of early tapetum development. Plant Cell 2005, 17, 2705–2722. [Google Scholar] [CrossRef]
- Li, N.; Zhang, D.S.; Liu, H.S.; Yin, C.S.; Li, X.X.; Liang, W.Q.; Yuan, Z.; Xu, B.; Chu, H.W.; Wang, J.; et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 2006, 18, 2999–3014. [Google Scholar] [CrossRef]
- Li, D.D.; Xue, J.S.; Zhu, J.; Yang, Z.N. Gene Regulatory Network for Tapetum Development in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1559. [Google Scholar] [CrossRef]
- Phan, H.A.; Iacuone, S.; Li, S.F.; Parish, R.W. The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell 2011, 23, 2209–2224. [Google Scholar] [CrossRef]
- Pan, C.; Yang, D.; Zhao, X.; Liu, Y.; Li, M.; Ye, L.; Ali, M.; Yu, F.; Lamin-Samu, A.T.; Fei, Z.; et al. PIF4 negatively modulates cold tolerance in tomato anthers via temperature-dependent regulation of tapetal cell death. Plant Cell 2021, 33, 2320–2339. [Google Scholar] [CrossRef] [PubMed]
- Clepet, C.; Devani, R.S.; Boumlik, R.; Hao, Y.; Morin, H.; Marcel, F.; Verdenaud, M.; Mania, B.; Brisou, G.; Citerne, S.; et al. The miR166-SlHB15A regulatory module controls ovule development and parthenocarpic fruit set under adverse temperatures in tomato. Mol. Plant 2021, 14, 1185–1198. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Y.; Su, D.; Yu, C.; Xian, Z.; Pan, Z.; Guan, H.; Hu, G.; Chen, D.; Li, Z.; et al. The SlHB8 acts as a negative regulator in tapetum development and pollen wall formation in Tomato. Hortic. Res. 2022, 9, c185. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, Y.; Wu, C.; Yang, Y.; Su, D.; Xian, Z.; Zhu, Y.; Yu, C.; Hu, G.; Deng, W.; et al. The SlARF4-SlHB8 regulatory module mediates leaf rolling in tomato. Plant Sci. 2023, 335, 111790. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, C.; Su, D.; Yang, Y.; Xian, Z.; Yu, C.; Li, Z.; Hao, Y.; Chen, R. The SlHB8 Acts as a Negative Regulator in Stem Development and Lignin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 13343. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Liu, J.; Yan, J.; Zhu, K.; Sun, X.; Bu, X.; Wang, X.; Ahammed, G.J.; Liu, Y.; et al. Tetratricopeptide repeat protein SlREC2 positively regulates cold tolerance in tomato. Plant Physiol. 2023, 192, 648–665. [Google Scholar] [CrossRef]
- Kiran, A.; Kumar, S.; Nayyar, H.; Sharma, K.D. Low temperature-induced aberrations in male and female reproductive organ development cause flower abortion in chickpea. Plant Cell Environ. 2019, 42, 2075–2089. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.; Prasad, P.; Siddique, K. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Ni, E.; Lin, J.; Peng, G.; Huang, J.; Zhu, L.; Deng, L.; Yang, F.; Luo, Q.; et al. HMS1 interacts with HMS1I to regulate very-long-chain fatty acid biosynthesis and the humidity-sensitive genic male sterility in rice (Oryza sativa). New Phytol. 2020, 225, 2077–2093. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Guan, H.; Huang, B.; Chen, M.; Wang, X.; Song, S.; Liu, H.; Chen, R.; Hao, Y. Genome-wide identification, phylogeny analysis, expression profiling, and determination of protein-protein interactions of the LEUNIG gene family members in tomato. Gene 2018, 679, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bi, G.; Zhou, J.M. Luciferase Complementation Assay for Protein-Protein Interactions in Plants. Curr. Protoc. Plant Biol. 2018, 3, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, X.; Peng, G.; Liu, M.; Zhang, S.; Chen, M.; Liao, S.; Wei, X.; Xu, P.; Tan, X.; et al. Methylesterification of cell-wall pectin controls the diurnal flower-opening times in rice. Mol. Plant 2022, 15, 956–972. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, H.; Yu, C.; Zeng, Z.; Hu, H.; Lin, Y.; Wu, C.; Yao, Y.; Xia, R.; Li, Z.; Ma, C.; et al. SlHB8 Is a Novel Factor in Enhancing Cold Resistance in Tomato Anthers by Modulating Tapetal Cell Death. Int. J. Mol. Sci. 2024, 25, 9336. https://doi.org/10.3390/ijms25179336
Guan H, Yu C, Zeng Z, Hu H, Lin Y, Wu C, Yao Y, Xia R, Li Z, Ma C, et al. SlHB8 Is a Novel Factor in Enhancing Cold Resistance in Tomato Anthers by Modulating Tapetal Cell Death. International Journal of Molecular Sciences. 2024; 25(17):9336. https://doi.org/10.3390/ijms25179336
Chicago/Turabian StyleGuan, Hongling, Canye Yu, Zaohai Zeng, Huimin Hu, Yuxiang Lin, Caiyu Wu, Yiwen Yao, Rui Xia, Zhengguo Li, Chongjian Ma, and et al. 2024. "SlHB8 Is a Novel Factor in Enhancing Cold Resistance in Tomato Anthers by Modulating Tapetal Cell Death" International Journal of Molecular Sciences 25, no. 17: 9336. https://doi.org/10.3390/ijms25179336
APA StyleGuan, H., Yu, C., Zeng, Z., Hu, H., Lin, Y., Wu, C., Yao, Y., Xia, R., Li, Z., Ma, C., Chen, R., Huang, B., & Hao, Y. (2024). SlHB8 Is a Novel Factor in Enhancing Cold Resistance in Tomato Anthers by Modulating Tapetal Cell Death. International Journal of Molecular Sciences, 25(17), 9336. https://doi.org/10.3390/ijms25179336






