Deciphering DNA Methylation in Gestational Diabetes Mellitus: Epigenetic Regulation and Potential Clinical Applications
Abstract
:1. Introduction
2. DNA Methylation in GDM
2.1. Global DNA Methylation in GDM
2.1.1. Placenta
2.1.2. Peripheral Blood
2.1.3. Cord Blood
2.2. Gene-Specific DNA Methylation in GDM
2.2.1. Energy Metabolism-Related Genes
2.2.2. IR-Related Genes
2.2.3. Inflammation and Immune-Related Genes
2.2.4. Imprinted Genes
2.3. Genome-Wide DNA Methylation in GDM
2.3.1. Pregnant Women
2.3.2. Offspring
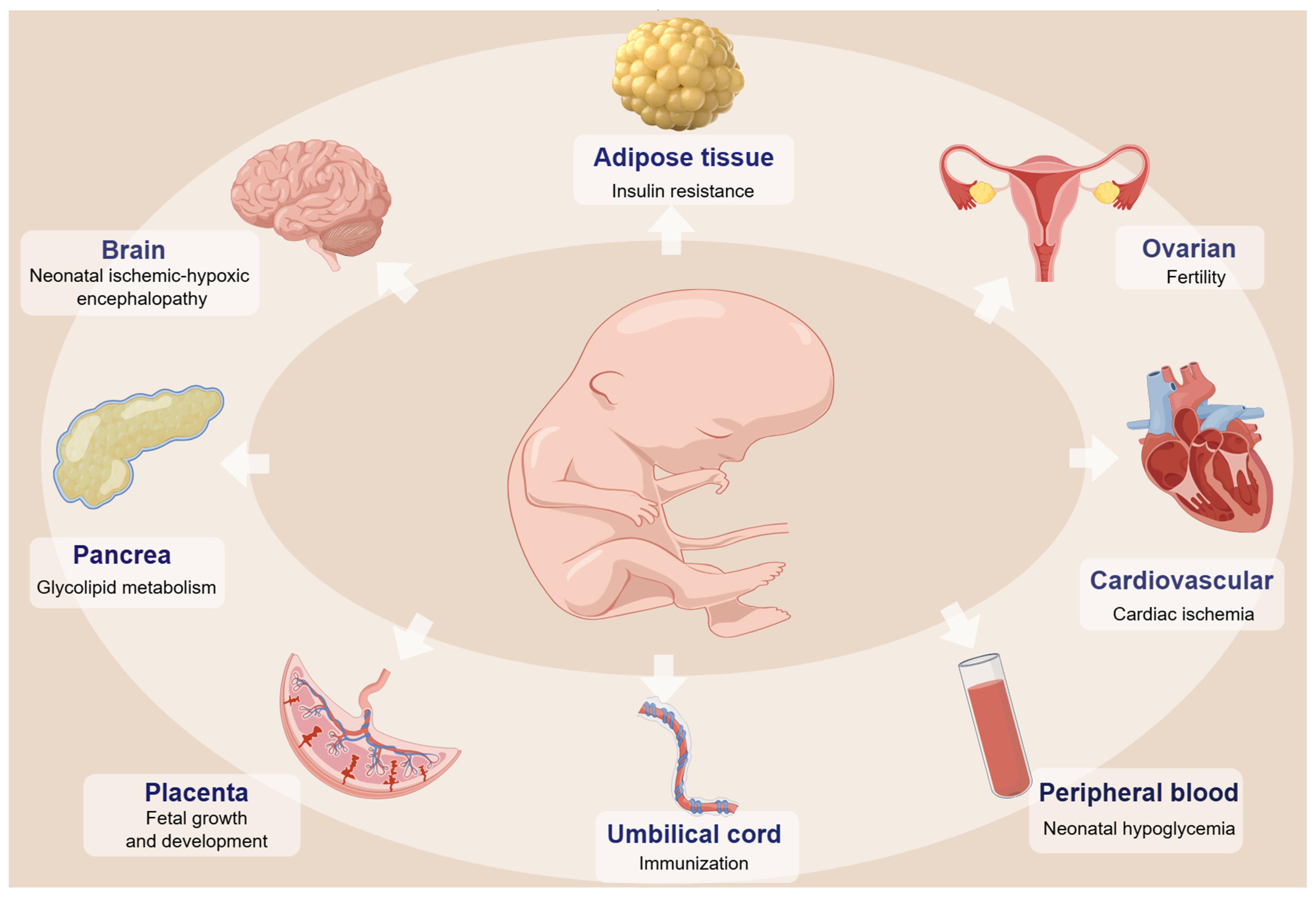
3. DNA Methylation Changes and the Health of Offspring with GDM
3.1. Neurodevelopmental Disorder
3.2. Cardiac Function
3.3. Metabolic Disorder
3.4. Obesity
3.5. Fertility
4. Environmental Factors Impact DNA Methylation in GDM
4.1. Nutritional Supplements
4.2. Pollutant Exposure
4.3. Gut Microecology
5. Diagnosis and Risk Prediction of GDM Based on DNA Methylation
5.1. Peripheral Blood
5.2. cfDNA
5.3. Epigenetic Clocks
6. Present Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDM | gestational diabetes mellitus |
| T2D | type 2 diabetes |
| IR | insulin resistance |
| CC | Carpenter and Coustan |
| NDDG | National Diabetes Data Group |
| IADPSG | International Association of the Diabetes and Pregnancy Study Groups |
| DNMTs | DNA methyltransferases |
| MBD-seq | methylated DNA-binding domain sequencing |
| EM-seq | enzymatic methyl sequencing |
| LUMA | luminometric methylation assays |
| LC-MS/MS | liquid chromatography–mass spectrometry/mass spectrometry |
| DMPs | differential methylation probes |
| PPARGC1A | peroxisome proliferator-activated receptor-gamma coactivator-1α |
| MC4R | melanocortin-4 receptor |
| G6PD | glucose-6-phosphate dehydrogenase |
| IGFBP | insulin-like growth factor-binding protein |
| IGFs | insulin-like growth factors |
| HIF3A | hypoxia-inducible factor 3 alpha |
| LEP | leptin |
| ADIPOQ | adiponectin |
| RETN | resistin |
| cfDNA | cell-free DNA |
| INS | insulin |
| IAPP | islet amyloid polypeptide |
| TNF-α | tumor necrosis factor-α |
| SOCS3 | suppressor of cytokine signaling 3 |
| URGCP | upregulator of cell proliferation |
| HbA1c | hemoglobin A1c |
| IL10 | interleukin 10 |
| SLC6A4 | solute carrier family 6, member 4 |
| SIRT1 | sirtuin 1 |
| SOX11 | SRY-related high-mobility-group box 11 |
| HDAC2 | histone deacetylase-2 |
| PDX1 | pancreatic and duodenal homeobox 1 |
| PGC-1α | proliferator-activated receptor gamma coactivator 1α |
| CDKN2A/B | cyclin-dependent kinase inhibitor 2A/B |
| FoxO1 | forkhead box protein O1 |
| DLK1 | delta-like 1 homologue |
| MEG3 | maternally expressed gene 3 |
| LPL | lipoprotein lipase |
| CART | cocaine- and amphetamine-regulated transcript |
| WGBS | whole-genome bisulfite sequencing |
| BMI | body mass index |
| ZNF696 | zinc finger protein 696 |
| ARHGEF11 | Rho Guanine Nucleotide Exchange Factor 11 |
| SAM | S-adenosylmethionine |
| CRH | corticotropin-releasing hormone |
| As | arsenic |
| COPS8 | COP9 signalosome complex subunit 8 |
| PIK3R5 | phosphoinositide-3-kinase regulatory subunit 5 |
| HAAO | 3-hydroxyanthranilate 3,4-dioxygenase |
| CCDC124 | coiled-coil domain containing 124 |
| C5orf34 | chromosome 5 open reading frame 34 |
| AUC | area under curve |
| HDL-C | high-density lipoprotein cholesterol |
References
- Diabetes Data Report 2000—2045; International Diabetes Federation: Amsterdam, The Netherlands, 2021.
- Shah, N.S.; Wang, M.C.; Freaney, P.M.; Perak, A.M.; Carnethon, M.R.; Kandula, N.R.; Gunderson, E.P.; Bullard, K.M.; Grobman, W.A.; O’Brien, M.J.; et al. Trends in Gestational Diabetes at First Live Birth by Race and Ethnicity in the US, 2011-2019. JAMA 2021, 326, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Leth-Møller, M.; Hulman, A.; Kampmann, U.; Hede, S.; Ovesen, P.G.; Knorr, S. Effect of gestational diabetes on fetal growth rate and later overweight in the offspring. J. Clin. Endocrinol. Metab. 2024. [CrossRef] [PubMed]
- Venkatesh, K.K.; Khan, S.S.; Powe, C.E. Gestational Diabetes and Long-Term Cardiometabolic Health. JAMA 2023, 330, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Mitsunami, M.; Manson, J.E.; Gaskins, A.J.; Rich-Edwards, J.W.; Wang, L.; Zhang, C.; Chavarro, J.E. Association of Gestational Diabetes with Subsequent Long-Term Risk of Mortality. JAMA Intern. Med. 2023, 183, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Ahmad, A.; Ashraf, H. Metabolic Profile of Offspring of Mothers with Gestational Diabetes Mellitus. Indian J. Endocrinol. Metab. 2024, 28, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.W.; Looker, H.C.; Kobes, S.; Touger, L.; Tataranni, P.A.; Hanson, R.L.; Knowler, W.C. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 2006, 55, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, E.U.; Mamerto, T.P.; Chung, G.; Villavieja, A.; Gaus, N.L.; Morgan, E.; Pineda-Cortel, M.R.B. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int. J. Mol. Sci. 2020, 21, 5003. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Wang, X.; Yang, T.; Miao, J.; Liu, H.; Wu, K.; Guo, J.; Chen, J.; Li, T. Correlation Between Maternal and Fetal Insulin Resistance in Pregnant Women with Gestational Diabetes Mellitus. Clin. Lab. 2018, 64, 945–953. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H. Association between MTHFR gene C677T polymorphism and gestational diabetes mellitus in Chinese population: A meta-analysis. Front. Endocrinol. 2023, 14, 1273218. [Google Scholar] [CrossRef]
- She, L.; Li, W.; Guo, Y.; Zhou, J.; Liu, J.; Zheng, W.; Dai, A.; Chen, X.; Wang, P.; He, H.; et al. Association of glucokinase gene and glucokinase regulatory protein gene polymorphisms with gestational diabetes mellitus: A case-control study. Gene 2022, 824, 146378. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, K.; Evangelou, E.; Yiallouros, P.; Christophi, C.A.; Middleton, N.; Papatheodorou, E.; Papatheodorou, S.I. Risk factors for gestational diabetes: An umbrella review of meta-analyses of observational studies. PLoS ONE 2019, 14, e0215372. [Google Scholar] [CrossRef]
- Coustan, D.R.; Lowe, L.P.; Metzger, B.E.; Dyer, A.R. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: Paving the way for new diagnostic criteria for gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2010, 202, 654.e1–654.e6. [Google Scholar] [CrossRef]
- Bartha, J.L.; Martinez-Del-Fresno, P.; Comino-Delgado, R. Early diagnosis of gestational diabetes mellitus and prevention of diabetes-related complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 109, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Bartha, J.L.; Martinez-Del-Fresno, P.; Comino-Delgado, R. Gestational diabetes mellitus diagnosed during early pregnancy. Am. J. Obstet. Gynecol. 2000, 182, 346–350. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef]
- Dłuski, D.F.; Wolińska, E.; Skrzypczak, M. Epigenetic Changes in Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 7649. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Yan, R.; Cheng, X.; Gu, C.; Xu, Y.; Long, X.; Zhai, J.; Sun, F.; Qian, J.; Du, Y.; Wang, H.; et al. Dynamics of DNA hydroxymethylation and methylation during mouse embryonic and germline development. Nat. Genet. 2023, 55, 130–143. [Google Scholar] [CrossRef]
- Künstner, A.; Schwarting, J.; Witte, H.M.; Xing, P.; Bernard, V.; Stölting, S.; Lohneis, P.; Janke, F.; Salehi, M.; Chen, X.; et al. Genome-wide DNA methylation-analysis of blastic plasmacytoid dendritic cell neoplasm identifies distinct molecular features. Leukemia 2024, 38, 1086–1098. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Chen, X.; Rodgers, B.; Miura, F.; Bashtrykov, P.; Bonhomme, F.; Salinas-Luypaert, C.; Haxholli, D.; Gutekunst, N.; Aygenli, B.; et al. Non-canonical functions of UHRF1 maintain DNA methylation homeostasis in cancer cells. Nat. Commun. 2024, 15, 2960. [Google Scholar] [CrossRef]
- Shireby, G.; Dempster, E.L.; Policicchio, S.; Smith, R.G.; Pishva, E.; Chioza, B.; Davies, J.P.; Burrage, J.; Lunnon, K.; Seiler Vellame, D.; et al. DNA methylation signatures of Alzheimer’s disease neuropathology in the cortex are primarily driven by variation in non-neuronal cell-types. Nat. Commun. 2022, 13, 5620. [Google Scholar] [CrossRef] [PubMed]
- van Jaarsveld, R.H.; Reilly, J.; Cornips, M.C.; Hadders, M.A.; Agolini, E.; Ahimaz, P.; Anyane-Yeboa, K.; Bellanger, S.A.; van Binsbergen, E.; van den Boogaard, M.J.; et al. Delineation of a KDM2B-related neurodevelopmental disorder and its associated DNA methylation signature. Genet. Med. 2023, 25, 49–62. [Google Scholar] [CrossRef]
- Kubo, N.; Uehara, R.; Uemura, S.; Ohishi, H.; Shirane, K.; Sasaki, H. Combined and differential roles of ADD domains of DNMT3A and DNMT3L on DNA methylation landscapes in mouse germ cells. Nat. Commun. 2024, 15, 3266. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, J.; Yin, X.; Chang, Y.; Wang, C.; Yan, M.; Feng, L.; Cheng, Y.; Gao, Y.; Xu, B.; et al. Base editing-mediated one-step inactivation of the Dnmt gene family reveals critical roles of DNA methylation during mouse gastrulation. Nat. Commun. 2023, 14, 2922. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, J.; Zheng, Y.; Long, S.; Lin, H.; Zhang, N.; Tian, M.; Wu, X.; An, R.; Ma, S.; et al. Study on the relationship between DNA methylation of target CpG sites in peripheral blood and gestational diabetes during early pregnancy. Sci. Rep. 2021, 11, 20455. [Google Scholar] [CrossRef]
- Kang, J.; Lee, C.N.; Li, H.Y.; Hsu, K.H.; Lin, S.Y. Genome-wide DNA methylation variation in maternal and cord blood of gestational diabetes population. Diabetes Res. Clin. Pract. 2017, 132, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.T.; Workman, R.E.; Zuzarte, P.C.; David, M.; Dursi, L.J.; Timp, W. Detecting DNA cytosine methylation using nanopore sequencing. Nat. Methods 2017, 14, 407–410. [Google Scholar] [CrossRef]
- Aberg, K.A.; Chan, R.F.; van den Oord, E. MBD-seq—Realities of a misunderstood method for high-quality methylome-wide association studies. Epigenetics 2020, 15, 431–438. [Google Scholar] [CrossRef]
- Vaisvila, R.; Ponnaluri, V.K.C.; Sun, Z.; Langhorst, B.W.; Saleh, L.; Guan, S.; Dai, N.; Campbell, M.A.; Sexton, B.S.; Marks, K.; et al. Enzymatic methyl sequencing detects DNA methylation at single-base resolution from picograms of DNA. Genome Res. 2021, 31, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Gu, H.; Wang, X. Technologies and applications of single-cell DNA methylation sequencing. Theranostics 2023, 13, 2439–2454. [Google Scholar] [CrossRef]
- Illsley, N.P.; Baumann, M.U. Human placental glucose transport in fetoplacental growth and metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165359. [Google Scholar] [CrossRef]
- Awamleh, Z.; Butcher, D.T.; Hanley, A.; Retnakaran, R.; Haertle, L.; Haaf, T.; Hamilton, J.; Weksberg, R. Exposure to Gestational Diabetes Mellitus (GDM) alters DNA methylation in placenta and fetal cord blood. Diabetes Res. Clin. Pract. 2021, 174, 108690. [Google Scholar] [CrossRef]
- Nomura, Y.; Lambertini, L.; Rialdi, A.; Lee, M.; Mystal, E.Y.; Grabie, M.; Manaster, I.; Huynh, N.; Finik, J.; Davey, M.; et al. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod. Sci. 2014, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Reichetzeder, C.; Dwi Putra, S.E.; Pfab, T.; Slowinski, T.; Neuber, C.; Kleuser, B.; Hocher, B. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin. Epigenetics 2016, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Linares-Pineda, T.M.; Peña-Montero, N.; Gutiérrez-Repiso, C.; Lima-Rubio, F.; Sánchez-Pozo, A.; Tinahones, F.J.; Molina-Vega, M.; Picón-César, M.J.; Morcillo, S. Epigenome wide association study in peripheral blood of pregnant women identifies potential metabolic pathways related to gestational diabetes. Epigenetics 2023, 18, 2211369. [Google Scholar] [CrossRef]
- Dias, S.; Adam, S.; Van Wyk, N.; Rheeder, P.; Louw, J.; Pheiffer, C. Global DNA methylation profiling in peripheral blood cells of South African women with gestational diabetes mellitus. Biomarkers 2019, 24, 225–231. [Google Scholar] [CrossRef]
- Zheng, S.C.; Breeze, C.E.; Beck, S.; Teschendorff, A.E. Identification of differentially methylated cell types in epigenome-wide association studies. Nat. Methods 2018, 15, 1059–1066. [Google Scholar] [CrossRef]
- Lu, T.; Cardenas, A.; Perron, P.; Hivert, M.F.; Bouchard, L.; Greenwood, C.M.T. Detecting cord blood cell type-specific epigenetic associations with gestational diabetes mellitus and early childhood growth. Clin. Epigenetics 2021, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Gao, H.; Zeng, W.; Chen, S.; Feng, L.; Deng, D.; Qiao, F.Y.; Liao, L.; McCormick, K.; Ning, Q.; et al. Placental DNA methylation of peroxisome-proliferator-activated receptor-γ co-activator-1α promoter is associated with maternal gestational glucose level. Clin. Sci. 2015, 129, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Porreca, A.; D’Ardes, M.; Di Nicola, M.; Di Tizio, L.; Liberati, M.; Stuppia, L.; Vitacolonna, E. The Obesogenic Environment: Epigenetic Modifications in Placental Melanocortin 4 Receptor Gene Connected to Gestational Diabetes and Smoking. Front. Nutr. 2022, 9, 879526. [Google Scholar] [CrossRef] [PubMed]
- Steyn, A.; Crowther, N.J.; Norris, S.A.; Rabionet, R.; Estivill, X.; Ramsay, M. Epigenetic modification of the pentose phosphate pathway and the IGF-axis in women with gestational diabetes mellitus. Epigenomics 2019, 11, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Hivert, M.F.; White, F.; Allard, C.; James, K.; Majid, S.; Aguet, F.; Ardlie, K.G.; Florez, J.C.; Edlow, A.G.; Bouchard, L.; et al. Placental IGFBP1 levels during early pregnancy and the risk of insulin resistance and gestational diabetes. Nat. Med. 2024, 30, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, W.; Lv, S.; Qu, H.; He, Y. Berberine improves insulin resistance in adipocyte models by regulating the methylation of hypoxia-inducible factor-3α. Biosci. Rep. 2019, 39, BSR20192059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Qu, H.; Wang, Y. Methylation of HIF3A promoter CpG islands contributes to insulin resistance in gestational diabetes mellitus. Mol. Genet. Genom. Med. 2019, 7, e00583. [Google Scholar] [CrossRef] [PubMed]
- Houshmand-Oeregaard, A.; Hansen, N.S.; Hjort, L.; Kelstrup, L.; Broholm, C.; Mathiesen, E.R.; Clausen, T.D.; Damm, P.; Vaag, A. Differential adipokine DNA methylation and gene expression in subcutaneous adipose tissue from adult offspring of women with diabetes in pregnancy. Clin. Epigenetics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Baecker, A.; Song, Y.; Kiely, M.; Liu, S.; Zhang, C. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: A systematic review. Metabolism 2015, 64, 756–764. [Google Scholar] [CrossRef]
- Ott, R.; Melchior, K.; Stupin, J.H.; Ziska, T.; Schellong, K.; Henrich, W.; Rancourt, R.C.; Plagemann, A. Reduced Insulin Receptor Expression and Altered DNA Methylation in Fat Tissues and Blood of Women with GDM and Offspring. J. Clin. Endocrinol. Metab. 2019, 104, 137–149. [Google Scholar] [CrossRef]
- Lappas, M.; Georgiou, H.M.; Willcox, J.C.; Permezel, M.; Shub, A.; Maynard, C.L.; Joglekar, M.V.; Hardikar, A.A. Postpartum Circulating Cell-Free Insulin DNA Levels Are Higher in Women with Previous Gestational Diabetes Mellitus Who Develop Type 2 Diabetes in Later Life. J. Diabetes Res. 2019, 2019, 3264184. [Google Scholar] [CrossRef] [PubMed]
- Linares-Pineda, T.M.; Gutiérrez-Repiso, C.; Peña-Montero, N.; Molina-Vega, M.; Rubio, F.L.; Arana, M.S.; Tinahones, F.J.; Picón-César, M.J.; Morcillo, S. Higher β cell death in pregnant women, measured by DNA methylation patterns of cell-free DNA, compared to new-onset type 1 and type 2 diabetes subjects: A cross-sectional study. Diabetol. Metab. Syndr. 2023, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Kenna, L.A.; Olsen, J.A.; Spelios, M.G.; Radin, M.S.; Akirav, E.M. β-Cell death is decreased in women with gestational diabetes mellitus. Diabetol. Metab. Syndr. 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Soubrier, F. TET2: A Bridge Between DNA Methylation and Vascular Inflammation. Circulation 2020, 141, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, J.; Cui, Z.; Ma, K.; Wu, D.; Luo, J.; Li, F.; Xiong, W.; Rao, S.; Xiang, Q.; et al. Lachnospiraceae-derived butyrate mediates protection of high fermentable fiber against placental inflammation in gestational diabetes mellitus. Sci. Adv. 2023, 9, eadi7337. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Jin, X.; Wang, J.; Hu, Q.; Dai, B. Placenta inflammation is closely associated with gestational diabetes mellitus. Am. J. Transl. Res. 2021, 13, 4068–4079. [Google Scholar] [PubMed]
- Coughlan, M.T.; Oliva, K.; Georgiou, H.M.; Permezel, J.M.; Rice, G.E. Glucose-induced release of tumour necrosis factor-alpha from human placental and adipose tissues in gestational diabetes mellitus. Diabet. Med. 2001, 18, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Rancourt, R.C.; Ott, R.; Ziska, T.; Schellong, K.; Melchior, K.; Henrich, W.; Plagemann, A. Visceral Adipose Tissue Inflammatory Factors (TNF-Alpha, SOCS3) in Gestational Diabetes (GDM): Epigenetics as a Clue in GDM Pathophysiology. Int. J. Mol. Sci. 2020, 21, 479. [Google Scholar] [CrossRef] [PubMed]
- Rancourt, R.C.; Ott, R.; Schellong, K.; Ziska, T.; Melchior, K.; Henrich, W.; Plagemann, A. Altered SOCS3 DNA methylation within exon 2 is associated with increased mRNA expression in visceral adipose tissue in gestational diabetes. Epigenetics 2021, 16, 488–494. [Google Scholar] [CrossRef]
- He, Y.; Hariharan, M.; Gorkin, D.U.; Dickel, D.E.; Luo, C.; Castanon, R.G.; Nery, J.R.; Lee, A.Y.; Zhao, Y.; Huang, H.; et al. Spatiotemporal DNA methylome dynamics of the developing mouse fetus. Nature 2020, 583, 752–759. [Google Scholar] [CrossRef]
- Juvinao-Quintero, D.L.; Starling, A.P.; Cardenas, A.; Powe, C.E.; Perron, P.; Bouchard, L.; Dabelea, D.; Hivert, M.F. Epigenome-wide association study of maternal hemoglobin A1c in pregnancy and cord blood DNA methylation. Epigenomics 2021, 13, 203–218. [Google Scholar] [CrossRef]
- Kang, J.; Lee, C.N.; Li, H.Y.; Hsu, K.H.; Wang, S.H.; Lin, S.Y. Association of Interleukin-10 Methylation Levels with Gestational Diabetes in a Taiwanese Population. Front. Genet. 2018, 9, 222. [Google Scholar] [CrossRef]
- Tucci, V.; Isles, A.R.; Kelsey, G.; Ferguson-Smith, A.C. Genomic Imprinting and Physiological Processes in Mammals. Cell 2019, 176, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, B.A.; Bartolomei, M.S. DNA methylation reprogramming of genomic imprints in the mammalian germline: A TET-centric view. Andrology 2023, 11, 884–890. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Shen, L.; Liu, Y.; Sendler, D.; Zhang, Y. Role of Tet1 in erasure of genomic imprinting. Nature 2013, 504, 460–464. [Google Scholar] [CrossRef]
- Abad, F.R.M.; Hajizadeh, M.R.; Mahmoodi, M.; Jalali, Z.; Kazeruni, F.N.; Swann, J.; Hosseiniara, R.; Karimabad, M.N. Evaluation of H19, Mest, Meg3, and Peg3 genes affecting growth and metabolism in umbilical cord blood cells of infants born to mothers with gestational diabetes and healthy mothers in Rafsanjan City, Iran. J. Dev. Orig. Health Dis. 2023, 14, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.M.; Wei, J.; Xu, L.L.; Yan, Y.S.; Chen, Y.; Lv, M.; Jiang, Y.; Luo, Q. Altered expression of long noncoding RNA MEG3 in the offspring of gestational diabetes mellitus induces impaired glucose tolerance in adulthood. Acta Diabetol. 2024, 61, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Wang, C.; Feng, H.; Lin, L.; Liu, X.; Wei, Y.; Yang, H. Alteration in Expression and Methylation of IGF2/H19 in Placenta and Umbilical Cord Blood Are Associated with Macrosomia Exposed to Intrauterine Hyperglycemia. PLoS ONE 2016, 11, e0148399. [Google Scholar] [CrossRef] [PubMed]
- Petry, C.J.; Ong, K.K.; Hughes, I.A.; Acerini, C.L.; Dunger, D.B. The influence of maternal pregnancy glucose concentrations on associations between a fetal imprinted gene allele score and offspring size at birth. BMC Res. Notes 2018, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, H.; Chen, Z.; Yu, Y.C.; Guo, X.H.; Chen, Y.; Yang, M.M.; Chen, B.W.; Sagnelli, M.; Xu, D.; et al. Hepatic IGF2/H19 Epigenetic Alteration Induced Glucose Intolerance in Gestational Diabetes Mellitus Offspring via FoxO1 Mediation. Front. Endocrinol. 2022, 13, 844707. [Google Scholar] [CrossRef]
- Ding, G.L.; Wang, F.F.; Shu, J.; Tian, S.; Jiang, Y.; Zhang, D.; Wang, N.; Luo, Q.; Zhang, Y.; Jin, F.; et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 2012, 61, 1133–1142. [Google Scholar] [CrossRef]
- Song, J.Y.; Lee, K.E.; Byeon, E.J.; Choi, J.; Kim, S.J.; Shin, J.E. Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta. Life 2022, 12, 1869. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; Zhou, L.; Wu, Y.; Lu, H.; Luo, J. Altered expression of PGC-1α and PDX1 and their methylation status are associated with fetal glucose metabolism in gestational diabetes mellitus. Biochem. Biophys. Res. Commun. 2018, 501, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.H.; Jiang, Y.; Zhu, H.; Xi, F.F.; Chen, Y.; Xu, Y.T.; Liu, F.; Wang, Y.Y.; Hu, W.S.; Lv, W.G.; et al. Placental Delta-Like 1 Gene DNA Methylation Levels Are Related to Mothers’ Blood Glucose Concentration. J. Diabetes Res. 2019, 2019, 9521510. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Y.; Yan, T.; Chen, Y.; Yang, M.; Lv, M.; Xi, F.; Lu, J.; Zhao, B.; Luo, Q. Placental maternally expressed gene 3 differentially methylated region methylation profile is associated with maternal glucose concentration and newborn birthweight. J. Diabetes Investig. 2021, 12, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Gagné-Ouellet, V.; Houde, A.A.; Guay, S.P.; Perron, P.; Gaudet, D.; Guérin, R.; Jean-Patrice, B.; Hivert, M.F.; Brisson, D.; Bouchard, L. Placental lipoprotein lipase DNA methylation alterations are associated with gestational diabetes and body composition at 5 years of age. Epigenetics 2017, 12, 616–625. [Google Scholar] [CrossRef]
- Kasuga, Y.; Kawai, T.; Miyakoshi, K.; Saisho, Y.; Tamagawa, M.; Hasegawa, K.; Ikenoue, S.; Ochiai, D.; Hida, M.; Tanaka, M.; et al. Epigenetic Changes in Neonates Born to Mothers with Gestational Diabetes Mellitus May Be Associated with Neonatal Hypoglycaemia. Front. Endocrinol. 2021, 12, 690648. [Google Scholar] [CrossRef]
- Yan, J.; Su, R.; Zhang, W.; Wei, Y.; Wang, C.; Lin, L.; Feng, H.; Yang, H. Epigenetic alteration of Rho guanine nucleotide exchange Factor 11 (ARHGEF11) in cord blood samples in macrosomia exposed to intrauterine hyperglycemia. J. Matern.-Fetal Neonatal Med. 2021, 34, 422–431. [Google Scholar] [CrossRef]
- Bečeheli, I.; Horvatiček, M.; Perić, M.; Nikolić, B.; Holuka, C.; Klasić, M.; Ivanišević, M.; Starčević, M.; Desoye, G.; Hranilović, D.; et al. Methylation of serotonin regulating genes in cord blood cells: Association with maternal metabolic parameters and correlation with methylation in peripheral blood cells during childhood and adolescence. Clin. Epigenetics 2024, 16, 4. [Google Scholar] [CrossRef]
- Nazari, Z.; Shahryari, A.; Ghafari, S.; Nabiuni, M.; Golalipour, M.J. In Utero Exposure to Gestational Diabetes Alters DNA Methylation and Gene Expression of CDKN2A/B in Langerhans Islets of Rat Offspring. Cell J. 2020, 22, 203–211. [Google Scholar] [PubMed]
- Sinha, N.; Biswas, A.; Nave, O.; Seger, C.; Sen, A. Gestational Diabetes Epigenetically Reprograms the Cart Promoter in Fetal Ovary, Causing Subfertility in Adult Life. Endocrinology 2019, 160, 1684–1700. [Google Scholar] [CrossRef] [PubMed]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Hivert, M.F.; Cardenas, A.; Allard, C.; Doyon, M.; Powe, C.E.; Catalano, P.M.; Perron, P.; Bouchard, L. Interplay of Placental DNA Methylation and Maternal Insulin Sensitivity in Pregnancy. Diabetes 2020, 69, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Drong, A.; Lehne, B.; Loh, M.; Scott, W.R.; Kunze, S.; Tsai, P.C.; Ried, J.S.; Zhang, W.; Yang, Y.; et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 2017, 541, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Opsahl, J.O.; Fragoso-Bargas, N.; Lee, Y.; Carlsen, E.; Lekanova, N.; Qvigstad, E.; Sletner, L.; Jenum, A.K.; Lee-Ødegård, S.; Prasad, R.B.; et al. Epigenome-wide association study of DNA methylation in maternal blood leukocytes with BMI in pregnancy and gestational weight gain. Int. J. Obes. 2024, 48, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, M.L.; Jaddoe, V.W.V.; Gaillard, R.; Felix, J.F. Associations of maternal early-pregnancy blood glucose and insulin concentrations with DNA methylation in newborns. Clin. Epigenetics 2020, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Liu, F.; Zhang, H.; Kan, M.; Wang, T.; Dong, M.; Liu, Y. Genome-wide DNA methylation profiling in infants born to gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 142, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, X.; Xiao, Y.; Wen, J.; Chen, J.; Wang, K.; Chen, G. Gestational diabetes mellitus alters DNA methylation profiles in pancreas of the offspring mice. J. Diabetes Complicat. 2019, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pinney, S.E.; Joshi, A.; Yin, V.; Min, S.W.; Rashid, C.; Condon, D.E.; Wang, P.Z. Exposure to Gestational Diabetes Enriches Immune-Related Pathways in the Transcriptome and Methylome of Human Amniocytes. J. Clin. Endocrinol. Metab. 2020, 105, 3250–3264. [Google Scholar] [CrossRef] [PubMed]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Jiang, S.; Tian, J.; Li, Y.; Yu, W.; Zhang, L.; Xiao, D. STZ-induced gestational diabetes exposure alters PTEN/AKT/mTOR-mediated autophagy signaling pathway leading to increase the risk of neonatal hypoxic-ischemic encephalopathy. Reprod. Toxicol. 2024, 123, 108494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gong, L.; Zhang, P.; Li, Y.; Liu, B.; Zhang, L.; Zhuang, J.; Xiao, D. Epigenetic Down-Regulation of Sirt 1 via DNA Methylation and Oxidative Stress Signaling Contributes to the Gestational Diabetes Mellitus-Induced Fetal Programming of Heart Ischemia-Sensitive Phenotype in Late Life. Int. J. Biol. Sci. 2019, 15, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Gao, Q.; Guan, L.; Sun, P.; Li, Q.; Shi, C.; Ma, X. Downregulation of SOX11 in fetal heart tissue, under hyperglycemic environment, mediates cardiomyocytes apoptosis. J. Biochem. Mol. Toxicol. 2021, 35, e22629. [Google Scholar] [CrossRef]
- Dalfrà, M.G.; Burlina, S.; Del Vescovo, G.G.; Lapolla, A. Genetics and Epigenetics: New Insight on Gestational Diabetes Mellitus. Front. Endocrinol. 2020, 11, 602477. [Google Scholar] [CrossRef]
- Sultan, S.; AlMalki, S. Analysis of global DNA methylation and epigenetic modifiers (DNMTs and HDACs) in human foetal endothelium exposed to gestational and type 2 diabetes. Epigenetics 2023, 18, 2201714. [Google Scholar] [CrossRef]
- Ren, J.; Cheng, Y.; Ming, Z.H.; Dong, X.Y.; Zhou, Y.Z.; Ding, G.L.; Pang, H.Y.; Rahman, T.U.; Akbar, R.; Huang, H.F.; et al. Intrauterine hyperglycemia exposure results in intergenerational inheritance via DNA methylation reprogramming on F1 PGCs. Epigenetics Chromatin 2018, 11, 20. [Google Scholar] [CrossRef]
- Alba-Linares, J.J.; Pérez, R.F.; Tejedor, J.R.; Bastante-Rodríguez, D.; Ponce, F.; Carbonell, N.G.; Zafra, R.G.; Fernández, A.F.; Fraga, M.F.; Lurbe, E. Maternal obesity and gestational diabetes reprogram the methylome of offspring beyond birth by inducing epigenetic signatures in metabolic and developmental pathways. Cardiovasc. Diabetol. 2023, 22, 44. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Chen, H.; Jiang, Y.; Wang, Y.; Wang, D.; Li, M.; Dou, Y.; Sun, X.; Huang, G.; et al. Association of Maternal Folate and Vitamin B12 in Early Pregnancy with Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2021, 44, 217–223. [Google Scholar] [CrossRef]
- Kadam, I.; Dalloul, M.; Hausser, J.; Huntley, M.; Hoepner, L.; Fordjour, L.; Hittelman, J.; Saxena, A.; Liu, J.; Futterman, I.D.; et al. Associations between nutrients in one-carbon metabolism and fetal DNA methylation in pregnancies with or without gestational diabetes mellitus. Clin. Epigenetics 2023, 15, 137. [Google Scholar] [CrossRef]
- Sun, W.X.; Shu, Y.P.; Yang, X.Y.; Huang, W.; Chen, J.; Yu, N.N.; Zhao, M. Effects of folic acid supplementation in pregnant mice on glucose metabolism disorders in male offspring induced by lipopolysaccharide exposure during pregnancy. Sci. Rep. 2023, 13, 7984. [Google Scholar] [CrossRef] [PubMed]
- Dilli, D.; Doğan, N.N.; İpek, M.; Çavuş, Y.; Ceylaner, S.; Doğan, H.; Dursun, A.; Küçüközkan, T.; Zenciroğlu, A. MaFOS-GDM trial: Maternal fish oil supplementation in women with gestational diabetes and cord blood DNA methylation at insulin like growth factor-1 (IGF-1) gene. Clin. Nutr. ESPEN 2018, 23, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Monk, D.; Mackay, D.J.G.; Eggermann, T.; Maher, E.R.; Riccio, A. Genomic imprinting disorders: Lessons on how genome, epigenome and environment interact. Nat. Rev. Genet. 2019, 20, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Gossai, A.; Chen, Y.; Chasan-Taber, L.; Baker, E.; Karagas, M. Maternal arsenic exposure and gestational diabetes and glucose intolerance in the New Hampshire birth cohort study. Environ. Health 2016, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, H.; Fu, G.; Feng, Y.; Wu, W.; Yang, H.; Zhang, Y.; Wang, S. DNA methylation analysis reveals the effect of arsenic on gestational diabetes mellitus. Genomics 2023, 115, 110674. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.S.; Lu, J.H.; Li, S.H.; Li, J.H.; Yuan, M.Y.; He, J.R.; Chen, N.N.; Xiao, W.Q.; Shen, S.Y.; Qiu, L.; et al. Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Comparative Studies of the Gut Microbiota in the Offspring of Mothers with and without Gestational Diabetes. Front. Cell. Infect. Microbiol. 2020, 10, 536282. [Google Scholar] [CrossRef]
- Mora-Janiszewska, O.; Faryniak-Zuzak, A.; Darmochwał-Kolarz, D. Epigenetic Links between Microbiota and Gestational Diabetes. Int. J. Mol. Sci. 2022, 23, 1831. [Google Scholar] [CrossRef]
- Wu, P.; Farrell, W.E.; Haworth, K.E.; Emes, R.D.; Kitchen, M.O.; Glossop, J.R.; Hanna, F.W.; Fryer, A.A. Maternal genome-wide DNA methylation profiling in gestational diabetes shows distinctive disease-associated changes relative to matched healthy pregnancies. Epigenetics 2018, 13, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Geng, H.; Duan, B.; Yang, X.; Ma, A.; Ding, X. Identification of Diagnostic CpG Signatures in Patients with Gestational Diabetes Mellitus via Epigenome-Wide Association Study Integrated with Machine Learning. Biomed. Res. Int. 2021, 2021, 1984690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Zhao, L. Identification of diagnostic cytosine-phosphate-guanine biomarkers in patients with gestational diabetes mellitus via epigenome-wide association study and machine learning. Gynecol. Endocrinol. 2021, 37, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Linares-Pineda, T.; Peña-Montero, N.; Fragoso-Bargas, N.; Gutiérrez-Repiso, C.; Lima-Rubio, F.; Suarez-Arana, M.; Sánchez-Pozo, A.; Tinahones, F.J.; Molina-Vega, M.; Picón-César, M.J.; et al. Epigenetic marks associated with gestational diabetes mellitus across two time points during pregnancy. Clin. Epigenetics 2023, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, M.; Gil-Lluís, P.; Ejarque, M.; Diaz-Perdigones, C.; Martinez-Guasch, L.; Fernández-Veledo, S.; Vendrell, J.; Megía, A. DNA Methylation in Gestational Diabetes and its Predictive Value for Postpartum Glucose Disturbances. J. Clin. Endocrinol. Metab. 2022, 107, 2748–2757. [Google Scholar] [CrossRef] [PubMed]
- Linares-Pineda, T.M.; Fragoso-Bargas, N.; Picón, M.J.; Molina-Vega, M.; Jenum, A.K.; Sletner, L.; Lee-Ødegård, S.; Opsahl, J.O.; Moen, G.H.; Qvigstad, E.; et al. DNA methylation risk score for type 2 diabetes is associated with gestational diabetes. Cardiovasc. Diabetol. 2024, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, G.; Li, Q.; Li, W.; Thamotharan, S.; Tosevska, A.; Morselli, M.; Sung, K.; Janzen, C.; Zhou, X.; Pellegrini, M.; et al. Cell-free DNA Methylation and Transcriptomic Signature Prediction of Pregnancies with Adverse Outcomes. Epigenetics 2021, 16, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Hayano, M.; Griffin, P.T.; Amorim, J.A.; Bonkowski, M.S.; Apostolides, J.K.; Salfati, E.L.; Blanchette, M.; Munding, E.M.; Bhakta, M.; et al. Loss of epigenetic information as a cause of mammalian aging. Cell 2023, 186, 305–326.e27. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic clock: A promising biomarker and practical tool in aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef]
- Kanney, N.; Patki, A.; Chandler-Laney, P.; Garvey, W.T.; Hidalgo, B.A. Epigenetic Age Acceleration in Mothers and Offspring 4-10 Years after a Pregnancy Complicated by Gestational Diabetes and Obesity. Metabolites 2022, 12, 1226. [Google Scholar] [CrossRef]
- Shiau, S.; Wang, L.; Liu, H.; Zheng, Y.; Drong, A.; Joyce, B.T.; Wang, J.; Li, W.; Leng, J.; Shen, Y.; et al. Prenatal gestational diabetes mellitus exposure and accelerated offspring DNA methylation age in early childhood. Epigenetics 2021, 16, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ladd-Acosta, C.; Vang, E.; Barrett, E.S.; Bulka, C.M.; Bush, N.R.; Cardenas, A.; Dabelea, D.; Dunlop, A.L.; Fry, R.C.; Gao, X.; et al. Analysis of Pregnancy Complications and Epigenetic Gestational Age of Newborns. JAMA Netw. Open 2023, 6, e230672. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choufani, S.; Weksberg, R.; Wilson, S.L.; Yuan, V.; Burt, A.; Marsit, C.; Lu, A.T.; Ritz, B.; Bohlin, J.; et al. Placental epigenetic clocks: Estimating gestational age using placental DNA methylation levels. Aging 2019, 11, 4238–4253. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.D.; Han, D.S.C.; Jiang, P.; Chiu, R.W.K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 2021, 372, eaaw3616. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, F.; Zhang, J.; Zhang, Z.; Li, K.; Tian, Q.; Hou, H.; Xu, C.; Lu, Q.; Ren, Z.; et al. Whole-Genome Promoter Profiling of Plasma DNA Exhibits Diagnostic Value for Placenta-Origin Pregnancy Complications. Adv. Sci. 2020, 7, 1901819. [Google Scholar] [CrossRef]



| Diagnostic Criteria | Methods | Glucose Intake (g) | Blood Glucose Level (mmol/L) | |||
|---|---|---|---|---|---|---|
| Fasting | 1 h | 2 h | 3 h | |||
| IADPSG | One step | 75 | 5.1~6.9 | 10.0 | 8.5~11.0 | Null |
| CC | Two step | 100 | 5.3 | 10.0 | 8.6 | 7.8 |
| NDDG | Two step | 100 | 5.8 | 10.6 | 9.2 | 8.0 |
| Samples | Methods | Genes | DNAme | Significance | Reference |
|---|---|---|---|---|---|
| Placenta tissue | Pyrosequencing | PPARGC1A | Up | Maternal blood glucose ↑ | [43] |
| Pyrosequencing | MC4R | Down | Maternal blood glucose ↑ | [44] | |
| MS-PCR | G6PD | Up | Maternal blood glucose ↑ | [45] | |
| MS-PCR | IGFBP1 | Up | Maternal IR ↑ | [46] | |
| Pyrosequencing | SLC6A4 | Up | Neurodevelopmental disorders | [73] | |
| BS-seq | PGC-1α | Up | Offspring GMD | [74] | |
| MethylTarget | DLK1 | Up | Maternal blood glucose ↑ | [75] | |
| MethylTarget | MEG3 | Up | Maternal blood glucose ↑ | [76] | |
| Pyrosequencing | LPL | Up | Offspring obesity | [77] | |
| Umbilical cord blood | 850k BeadChip | URGCP | Down | Maternal blood glucose ↑ | [62] |
| MassArray | IGF2 | Down | Fetal macrosomia | [69] | |
| MassArray | H19 | Up | Fetal macrosomia | [69] | |
| 850k BeadChip | ZNF696 | Up | Offspring blood glucose ↑ | [78] | |
| 450k BeadChip | ARHGEF11 | Down | Fetal macrosomia | [79] | |
| Pyrosequencing | SLC6A4 | Up | Neurodevelopmental disorders | [80] | |
| Peripheral blood | MS-PCR | G6PD | Up | Maternal blood glucose ↑ | [45] |
| MethyLight | IL-10 | Down | Maternal inflammation ↑ | [63] | |
| Adipose tissue | BS-seq | HIF3A | Up | Maternal IR ↑ | [48] |
| Pyrosequencing | Insulin receptor | Up | Maternal IR ↑ | [51] | |
| Pyrosequencing | TNF-α | Down | Maternal inflammation ↑ | [59] | |
| Pyrosequencing | SOCS3 | Down | Maternal inflammation ↑ | [60] | |
| cfDNA | MS-PCR | INS/IAPP | Up | Maternal IR ↑ | [52] |
| Adipose tissue * | Pyrosequencing | ADIPOQ | Up | Offspring IR ↑ | [49,50] |
| Islet tissue * | MS-PCR | IGF2 | Up | Offspring impaired glucose tolerance | [72] |
| MS-PCR | H19 | Up | Offspring impaired glucose tolerance | [72] | |
| BS-seq | CDKN2A/B | Down | Offspring IR ↑ | [81] | |
| Liver tissue * | Pyrosequencing | MEG3 | Up | Offspring impaired glucose tolerance | [68] |
| Pyrosequencing | IGF2 | Up | Offspring impaired glucose tolerance | [71] | |
| Pyrosequencing | H19 | Up | Offspring impaired glucose tolerance | [71] | |
| Ovarian tissue * | MeDIP qPCR | CART | Down | Offspring ovarian dysfunction | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Liu, H.-Y.; Liu, S.-M. Deciphering DNA Methylation in Gestational Diabetes Mellitus: Epigenetic Regulation and Potential Clinical Applications. Int. J. Mol. Sci. 2024, 25, 9361. https://doi.org/10.3390/ijms25179361
Li N, Liu H-Y, Liu S-M. Deciphering DNA Methylation in Gestational Diabetes Mellitus: Epigenetic Regulation and Potential Clinical Applications. International Journal of Molecular Sciences. 2024; 25(17):9361. https://doi.org/10.3390/ijms25179361
Chicago/Turabian StyleLi, Nan, Huan-Yu Liu, and Song-Mei Liu. 2024. "Deciphering DNA Methylation in Gestational Diabetes Mellitus: Epigenetic Regulation and Potential Clinical Applications" International Journal of Molecular Sciences 25, no. 17: 9361. https://doi.org/10.3390/ijms25179361




