Disruption of Poly(ADP-ribosyl)ation Improves Plant Tolerance to Methyl Viologen-Mediated Oxidative Stress via Induction of ROS Scavenging Enzymes
Abstract
1. Introduction
2. Results
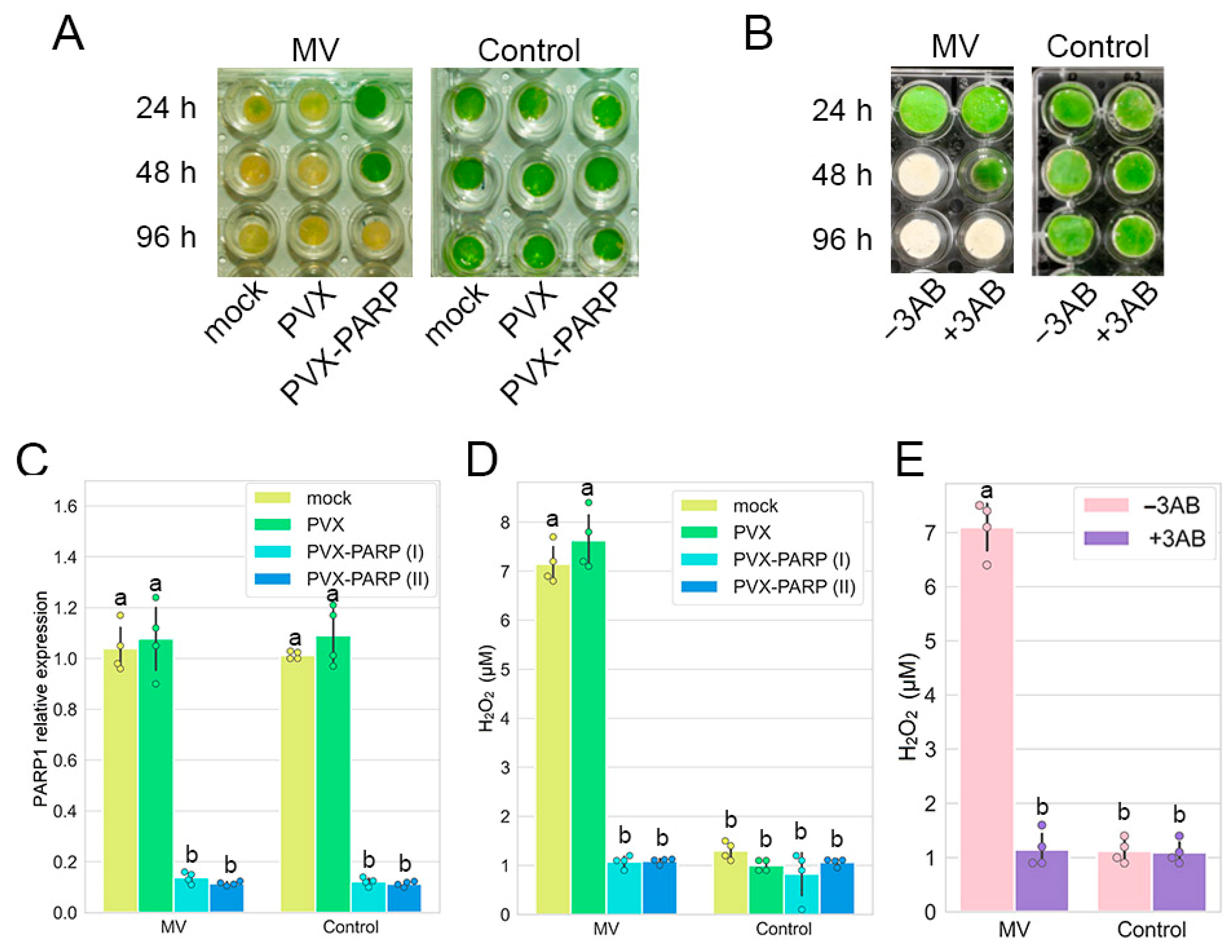
2.1. RNAi Silencing as Well as Pharmacological Inhibition of PARP1 Induces Tolerance to MV—Mediated Stress in Nicotiana Benthamiana
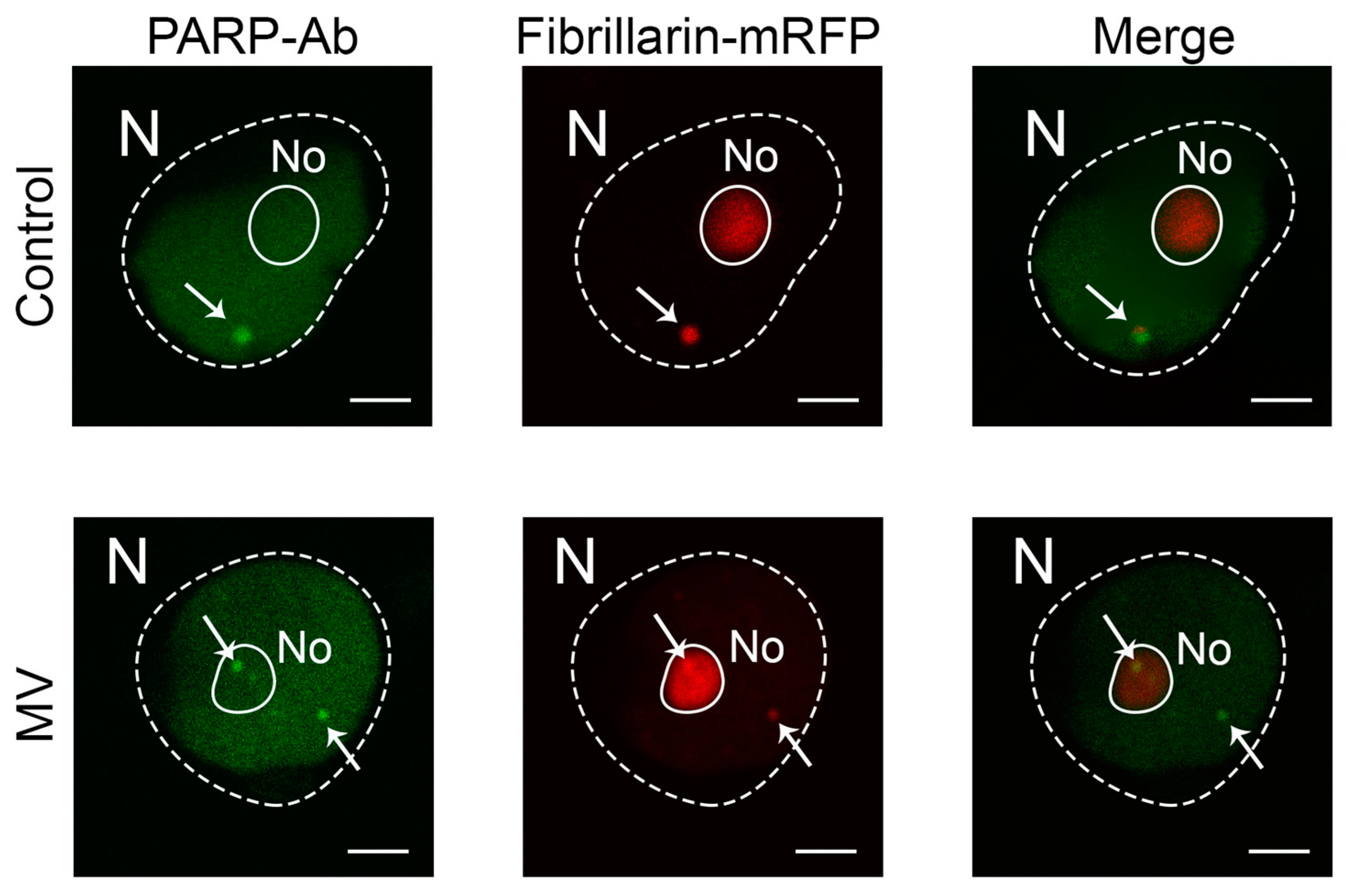
2.2. MV Does Not Affect Gene Expression and Subcellular Localization of PARP1
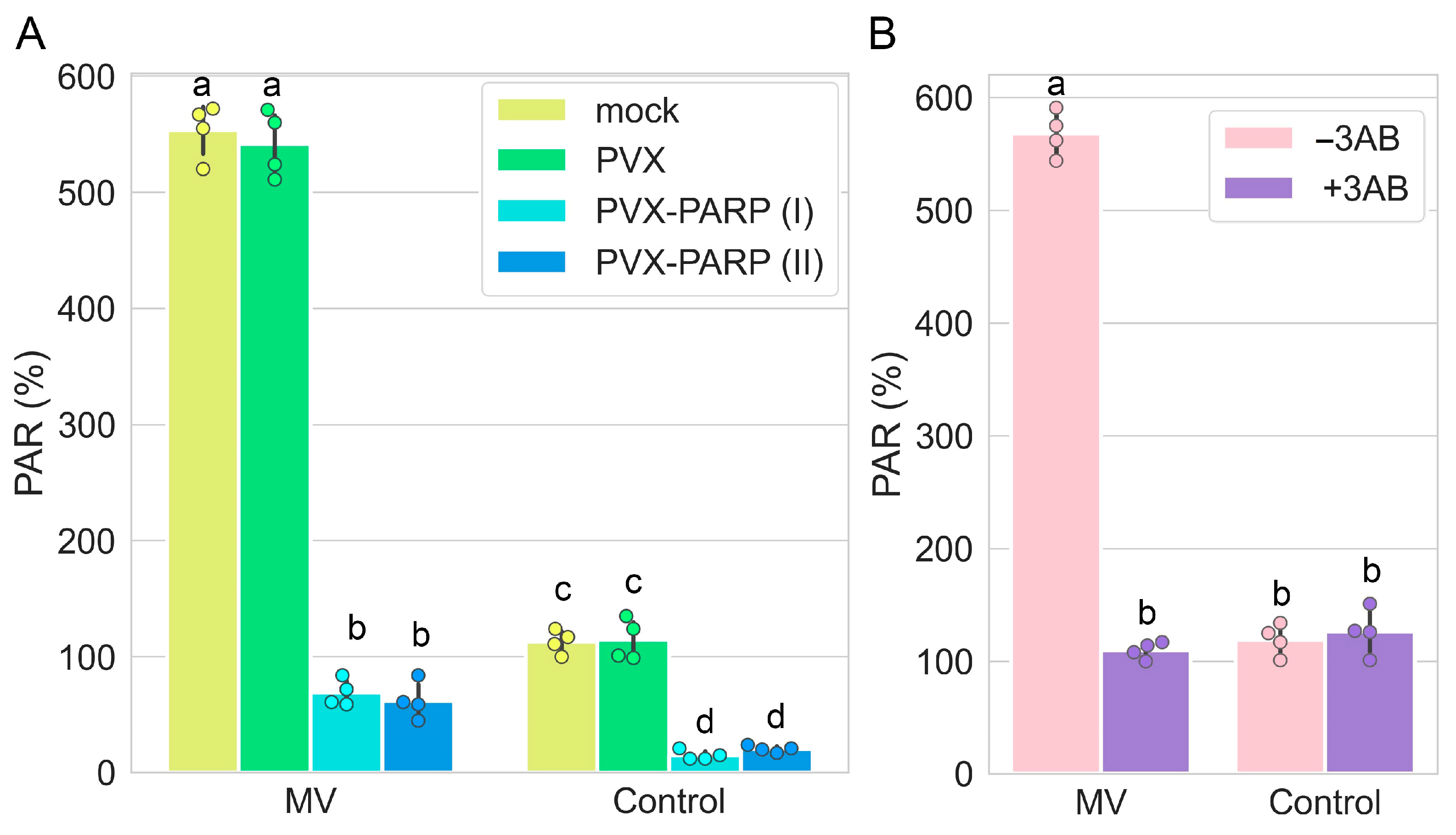
2.3. MV Activates Poly(ADP-ribosyl)ation
2.4. The Role of PARP1 in Accumulation of Plant Hormones
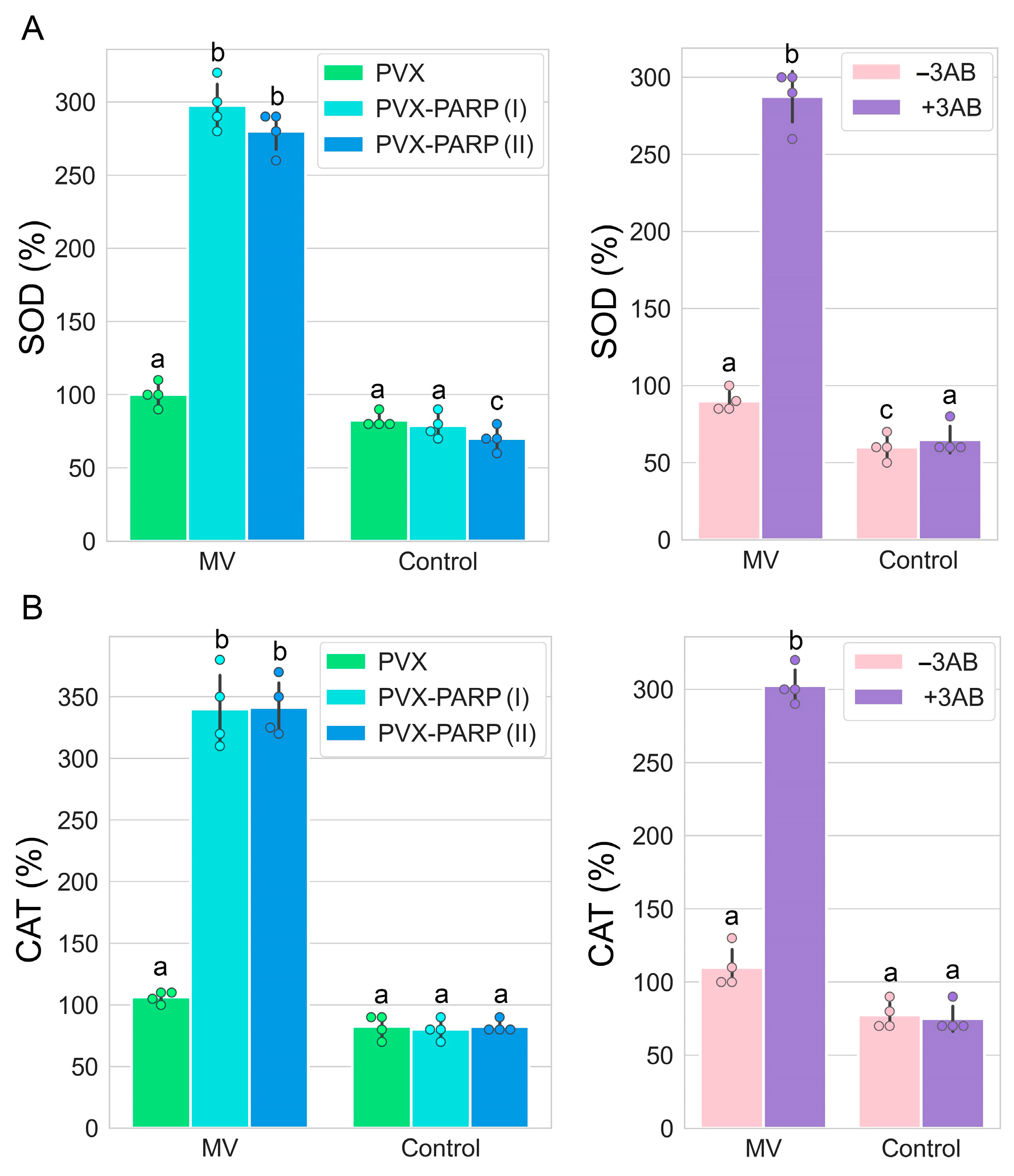
2.5. RNAi Silencing and Pharmacological Inhibition of PARP1 Activates Expression of Genes Encoding Major ROS Scavenging Antioxidant Enzymes
3. Discussion
4. Materials and Methods
4.1. Virus-Induced Silencing of PARP1 Expression
4.2. MV Stress and 3AB Treatments
4.3. Chemiluminescence Assay for H2O2
4.4. Immunolabelling and Confocal Imaging Analysis
4.5. Real Time Quantitative RT-PCR (RT-qPCR)
4.6. Immunological Detection of Poly ADP-Ribose (PAR)
4.7. Measurement of Endogenous SA
4.8. Quantification of SOD and CAT Activities
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rissel, D.; Peiter, E. Poly (ADP-Ribose) Polymerases in Plants and Their Human Counterparts: Parallels and Peculiarities. Int. J. Mol. Sci. 2019, 20, 1638. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.; Stokes, M.S.; Kraus, W.L. MARTs and MARylation in the Cytosol: Biological Functions, Mechanisms of Action, and Therapeutic Potential. Cells 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Maluchenko, N.V.; Koshkina, D.O.; Feofanov, A.V.; Studitsky, V.M.; Kirpichnikov, M.P. Poly (ADP-Ribosyl) Code Functions. Acta Naturae 2021, 13, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.G.; Bent, A.F. Poly (ADP-Ribosyl) Ation in Plants. Trends Plant Sci. 2011, 16, 372–380. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Shapiguzov, A.; Vaattovaara, A.; Kangasjärvi, J. Plant PARPs, PARGs and PARP-like Proteins. Curr. Protein Pept. Sci. 2016, 17, 713–723. [Google Scholar] [CrossRef]
- Gu, Z.; Pan, W.; Chen, W.; Lian, Q.; Wu, Q.; Lv, Z.; Cheng, X.; Ge, X. New Perspectives on the Plant PARP Family: Arabidopsis PARP3 Is Inactive, and PARP1 Exhibits Predominant Poly (ADP-Ribose) Polymerase Activity in Response to DNA Damage. BMC Plant Biol. 2019, 19, 364. [Google Scholar] [CrossRef]
- Yoshimura, K.; Shigeoka, S. Versatile Physiological Functions of the Nudix Hydrolase Family in Arabidopsis. Biosci. Biotechnol. Biochem. 2015, 79, 354–366. [Google Scholar] [CrossRef]
- Spechenkova, N.; Kalinina, N.O.; Zavriev, S.K.; Love, A.J.; Taliansky, M. ADP-Ribosylation and Antiviral Resistance in Plants. Viruses 2023, 15, 241. [Google Scholar] [CrossRef]
- Cui, F.; Brosché, M.; Shapiguzov, A.; He, X.-Q.; Vainonen, J.P.; Leppälä, J.; Trotta, A.; Kangasjärvi, S.; Salojärvi, J.; Kangasjärvi, J.; et al. Interaction of Methyl Viologen-Induced Chloroplast and Mitochondrial Signalling in Arabidopsis. Free Radic. Biol. Med. 2019, 134, 555–566. [Google Scholar] [CrossRef]
- Kanojia, A.; Dijkwel, P.P. Abiotic Stress Responses Are Governed by Reactive Oxygen Species and Age. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 295–326. ISBN 978-1-119-31299-4. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Xu, P.; Xiang, C.-B. Loss of AtPDR11, a Plasma Membrane-Localized ABC Transporter, Confers Paraquat Tolerance in Arabidopsis thaliana. Plant J. 2012, 69, 782–791. [Google Scholar] [CrossRef]
- Li, J.; Mu, J.; Bai, J.; Fu, F.; Zou, T.; An, F.; Zhang, J.; Jing, H.; Wang, Q.; Li, Z.; et al. Paraquat Resistant1, a Golgi-Localized Putative Transporter Protein, Is Involved in Intracellular Transport of Paraquat. Plant Physiol. 2013, 162, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Shinozaki, K. Identification of Polyamine Transporters in Plants: Paraquat Transport Provides Crucial Clues. Plant Cell Physiol. 2014, 55, 855–861. [Google Scholar] [CrossRef]
- Sipari, N.; Lihavainen, J.; Shapiguzov, A.; Kangasjärvi, J.; Keinänen, M. Primary Metabolite Responses to Oxidative Stress in Early-Senescing and Paraquat Resistant Arabidopsis thaliana Rcd1 (Radical-Induced Cell Death1). Front. Plant Sci. 2020, 11, 194. [Google Scholar] [CrossRef]
- Chichkova, N.V.; Shaw, J.; Galiullina, R.A.; Drury, G.E.; Tuzhikov, A.I.; Kim, S.H.; Kalkum, M.; Hong, T.B.; Gorshkova, E.N.; Torrance, L.; et al. Phytaspase, a Relocalisable Cell Death Promoting Plant Protease with Caspase Specificity. EMBO J. 2010, 29, 1149–1161. [Google Scholar] [CrossRef]
- Block, M.D.; Verduyn, C.; Brouwer, D.D.; Cornelissen, M. Poly (ADP-Ribose) Polymerase in Plants Affects Energy Homeostasis, Cell Death and Stress Tolerance. Plant J. 2005, 41, 95–106. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ogawa, T.; Hirosue, E.; Nakayama, Y.; Harada, K.; Fukusaki, E.; Yoshimura, K.; Shigeoka, S. Modulation of the Poly (ADP-Ribosyl)Ation Reaction via the Arabidopsis ADP-Ribose/NADH Pyrophosphohydrolase, AtNUDX7, Is Involved in the Response to Oxidative Stress. Plant Physiol. 2009, 151, 741–754. [Google Scholar] [CrossRef]
- Ogawa, T.; Ishikawa, K.; Harada, K.; Fukusaki, E.; Yoshimura, K.; Shigeoka, S. Overexpression of an ADP-Ribose Pyrophosphatase, AtNUDX2, Confers Enhanced Tolerance to Oxidative Stress in Arabidopsis Plants. Plant J. 2009, 57, 289–301. [Google Scholar] [CrossRef]
- Jaspers, P.; Blomster, T.; Brosché, M.; Salojärvi, J.; Ahlfors, R.; Vainonen, J.P.; Reddy, R.A.; Immink, R.; Angenent, G.; Turck, F.; et al. Unequally Redundant RCD1 and SRO1 Mediate Stress and Developmental Responses and Interact with Transcription Factors. Plant J. 2009, 60, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Bassham, D.C. Plant Autophagy—More than a Starvation Response. Curr. Opin. Plant Biol. 2007, 10, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic Stress Generates ROS That Signal Expression of Anionic Glutamate Dehydrogenases to Form Glutamate for Proline Synthesis in Tobacco and Grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, S.; Farooq, M.A.; Zhao, T.; Wang, P.; Tabusam, J.; Wang, Y.; Xuan, S.; Zhao, J.; Chen, X.; Shen, S.; et al. Virus-Induced Gene Silencing (VIGS): A Powerful Tool for Crop Improvement and Its Advancement towards Epigenetics. Int. J. Mol. Sci. 2023, 24, 5608. [Google Scholar] [CrossRef]
- Spechenkova, N.; Samarskaya, V.O.; Kalinina, N.O.; Zavriev, S.K.; MacFarlane, S.; Love, A.J.; Taliansky, M. Plant Poly (ADP-Ribose) Polymerase 1 Is a Potential Mediator of Cross-Talk between the Cajal Body Protein Coilin and Salicylic Acid-Mediated Antiviral Defence. Viruses 2023, 15, 1282. [Google Scholar] [CrossRef]
- Love, A.J.; Yu, C.; Petukhova, N.V.; Kalinina, N.O.; Chen, J.; Taliansky, M.E. Cajal Bodies and Their Role in Plant Stress and Disease Responses. RNA Biol. 2017, 14, 779–790. [Google Scholar] [CrossRef]
- Kotova, E.; Jarnik, M.; Tulin, A.V. Poly (ADP-Ribose) Polymerase 1 Is Required for Protein Localization to Cajal Body. PLoS Genet. 2009, 5, e1000387. [Google Scholar] [CrossRef]
- Kim, S.H.; MacFarlane, S.; Kalinina, N.O.; Rakitina, D.V.; Ryabov, E.V.; Gillespie, T.; Haupt, S.; Brown, J.W.S.; Taliansky, M. Interaction of a Plant Virus-Encoded Protein with the Major Nucleolar Protein Fibrillarin Is Required for Systemic Virus Infection. Proc. Natl. Acad. Sci. USA 2007, 104, 11115–11120. [Google Scholar] [CrossRef]
- Hurtado-Bagès, S.; Knobloch, G.; Ladurner, A.G.; Buschbeck, M. The Taming of PARP1 and Its Impact on NAD+ Metabolism. Mol. Metab. 2020, 38, 100950. [Google Scholar] [CrossRef]
- Kim, D.-S.; Camacho, C.V.; Nagari, A.; Malladi, V.S.; Challa, S.; Kraus, W.L. Activation of PARP-1 by snoRNAs Controls Ribosome Biogenesis and Cell Growth via the RNA Helicase DDX21. Mol. Cell 2019, 75, 1270–1285.e14. [Google Scholar] [CrossRef]
- Love, A.J.; Geri, C.; Laird, J.; Carr, C.; Yun, B.-W.; Loake, G.J.; Tada, Y.; Sadanandom, A.; Milner, J.J. Cauliflower Mosaic Virus Protein P6 Inhibits Signaling Responses to Salicylic Acid and Regulates Innate Immunity. PLoS ONE 2012, 7, e47535. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Schumaker, K.; Zhu, J. Molecular Genetic Perspectives on Cross-talk and Specificity in Abiotic Stress Signalling in Plants. J. Exp. Bot. 2004, 55, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ding, H.; Chen, Q.; Ouyang, L.; Li, S.; Zhang, J. Enhanced Tolerance to Methyl Viologen-Mediated Oxidative Stress via AtGR2 Expression From Chloroplast Genome. Front. Plant Sci. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Genomics in Plant Abiotic Stress Responses and Tolerance: From Gene Discovery to Complex Regulatory Networks and Their Application in Breeding. Proc. Jpn. Acad. Ser. B 2022, 98, 470–492. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Oxidative Stress in Plants. Antioxidants 2020, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.V.; Orsi, R.O.; Maraschin, M.; Lima, G.P.P. Chapter 27-Oxidative Stress in Plants and the Biochemical Response Mechanisms. In Plant Stress Mitigators; Ghorbanpour, M., Adnan Shahid, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 455–468. ISBN 978-0-323-89871-3. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. Defensive Strategies of ROS in Plant–Pathogen Interactions. In Plant Pathogen Interaction; Verma, P.K., Mishra, S., Srivastava, V., Mehrotra, S., Eds.; Springer: Singapore, 2023; pp. 163–183. ISBN 978-981-9948-90-1. [Google Scholar]
- Gomez-Cadenas, A.; Vives, V.; Zandalinas, S.I.; Manzi, M.; Sanchez-Perez, A.M.; Perez-Clemente, R.M.; Arbona, V. Abscisic Acid: A Versatile Phytohormone in Plant Signaling and Beyond. Curr. Protein Pept. Sci. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Li, G.; Nasar, V.; Yang, Y.; Li, W.; Liu, B.; Sun, L.; Li, D.; Song, F. Arabidopsis Poly (ADP-Ribose) Glycohydrolase 1 Is Required for Drought, Osmotic and Oxidative Stress Responses. Plant Sci. 2011, 180, 283–291. [Google Scholar] [CrossRef]
- Song, J.; Keppler, B.D.; Wise, R.R.; Bent, A.F. PARP2 Is the Predominant Poly (ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses. PLoS Genet. 2015, 11, e1005200. [Google Scholar] [CrossRef] [PubMed]
- Samarskaya, V.O.; Spechenkova, N.; Markin, N.; Suprunova, T.P.; Zavriev, S.K.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Impact of Exogenous Application of Potato Virus Y-Specific dsRNA on RNA Interference, Pattern-Triggered Immunity and Poly (ADP-Ribose) Metabolism. Int. J. Mol. Sci. 2022, 23, 7915. [Google Scholar] [CrossRef] [PubMed]
- Coquelle, N.; Glover, J.N.M. PARP Pairs up to PARsylate. Nat. Struct. Mol. Biol. 2012, 19, 660–661. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, C.; Zhang, J.; Zhong, X.; Wang, R.; Zeng, X.; Ba, X. The Role of PARPs in Inflammation—And Metabolic—Related Diseases: Molecular Mechanisms and Beyond. Cells 2019, 8, 1047. [Google Scholar] [CrossRef]
- Jones, L.; Hamilton, A.J.; Voinnet, O.; Thomas, C.L.; Maule, A.J.; Baulcombe, D.C. RNA-DNA Interactions and DNA Methylation in Post-Transcriptional Gene Silencing. Plant Cell 1999, 11, 2291–2301. [Google Scholar]
- Adams-Phillips, L.; Briggs, A.G.; Bent, A.F. Disruption of Poly (ADP-Ribosyl)Ation Mechanisms Alters Responses of Arabidopsis to Biotic Stress. Plant Physiol. 2010, 152, 267–280. [Google Scholar] [CrossRef]
- Gómez-Gómez, L.; Felix, G.; Boller, T. A Single Locus Determines Sensitivity to Bacterial Flagellin in Arabidopsis thaliana. Plant J. 1999, 18, 277–284. [Google Scholar] [CrossRef]
- Kim, S.H.; Ryabov, E.V.; Kalinina, N.O.; Rakitina, D.V.; Gillespie, T.; MacFarlane, S.; Haupt, S.; Brown, J.W.; Taliansky, M. Cajal Bodies and the Nucleolus Are Required for a Plant Virus Systemic Infection. EMBO J. 2007, 26, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Allasia, V.; Industri, B.; Ponchet, M.; Quentin, M.; Favery, B.; Keller, H. Quantification of Salicylic Acid (SA) and SA-Glucosides in Arabidopsis thaliana. Bio-Protoc. 2018, 8, e2844. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhu, P.-X.; Xu, F.; Che, Y.-P.; Ma, Y.-M.; Ji, Z.-L. Alpha-momorcharin enhances Nicotiana benthamianaresistance to tobacco mosaic virus infection throughmodulation of reactive oxygen species. Mol. Plant Pathol. 2020, 21, 1212–1226. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Z.; Zhang, H.; Yang, Y.; Yang, X.; Zhao, X.; Guo, H.; Nagalakshmi, U.; Li, D.; Dinesh-Kumar, S.P.; et al. The MAPK-Alfin-like 7 module negatively regulates ROS scavenging genes to promote NLR-mediated immunity. Proc. Natl. Acad. Sci. USA 2023, 120, e2214750120. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, N.O.; Spechenkova, N.; Ilina, I.; Samarskaya, V.O.; Bagdasarova, P.; Zavriev, S.K.; Love, A.J.; Taliansky, M. Disruption of Poly(ADP-ribosyl)ation Improves Plant Tolerance to Methyl Viologen-Mediated Oxidative Stress via Induction of ROS Scavenging Enzymes. Int. J. Mol. Sci. 2024, 25, 9367. https://doi.org/10.3390/ijms25179367
Kalinina NO, Spechenkova N, Ilina I, Samarskaya VO, Bagdasarova P, Zavriev SK, Love AJ, Taliansky M. Disruption of Poly(ADP-ribosyl)ation Improves Plant Tolerance to Methyl Viologen-Mediated Oxidative Stress via Induction of ROS Scavenging Enzymes. International Journal of Molecular Sciences. 2024; 25(17):9367. https://doi.org/10.3390/ijms25179367
Chicago/Turabian StyleKalinina, Natalia O., Nadezhda Spechenkova, Irina Ilina, Viktoriya O. Samarskaya, Polina Bagdasarova, Sergey K. Zavriev, Andrew J. Love, and Michael Taliansky. 2024. "Disruption of Poly(ADP-ribosyl)ation Improves Plant Tolerance to Methyl Viologen-Mediated Oxidative Stress via Induction of ROS Scavenging Enzymes" International Journal of Molecular Sciences 25, no. 17: 9367. https://doi.org/10.3390/ijms25179367
APA StyleKalinina, N. O., Spechenkova, N., Ilina, I., Samarskaya, V. O., Bagdasarova, P., Zavriev, S. K., Love, A. J., & Taliansky, M. (2024). Disruption of Poly(ADP-ribosyl)ation Improves Plant Tolerance to Methyl Viologen-Mediated Oxidative Stress via Induction of ROS Scavenging Enzymes. International Journal of Molecular Sciences, 25(17), 9367. https://doi.org/10.3390/ijms25179367






