Surface Immune Checkpoints as Potential Biomarkers in Physiological Pregnancy and Recurrent Pregnancy Loss
Abstract
:1. Introduction
1.1. Programmed Cell Death Protein 1 (PD-1)
1.2. T Cell Immunoglobulin and Mucin Domain-Containing Protein 3 (TIM-3)
1.3. Lymphocyte Activation Gene-3 (LAG-3)
1.4. T Cell Immunoglobulin and ITIM Domain (TIGIT)
1.5. V-Domain Ig-Containing Suppressor of T Cell Activation (VISTA)
2. Results
3. Discussion
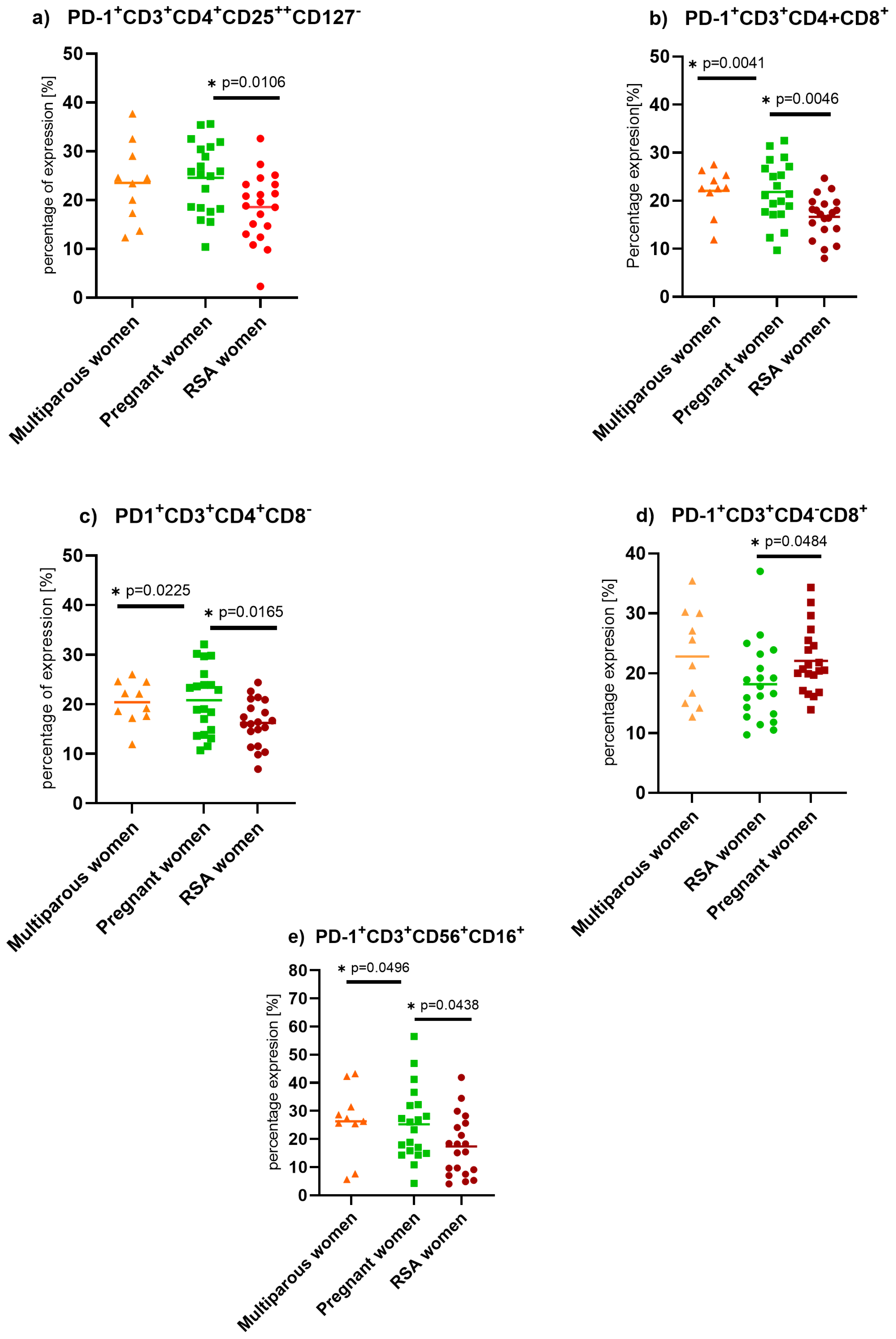
3.1. Programmed Death Protein (PD-1) Expression on Lymphocytes
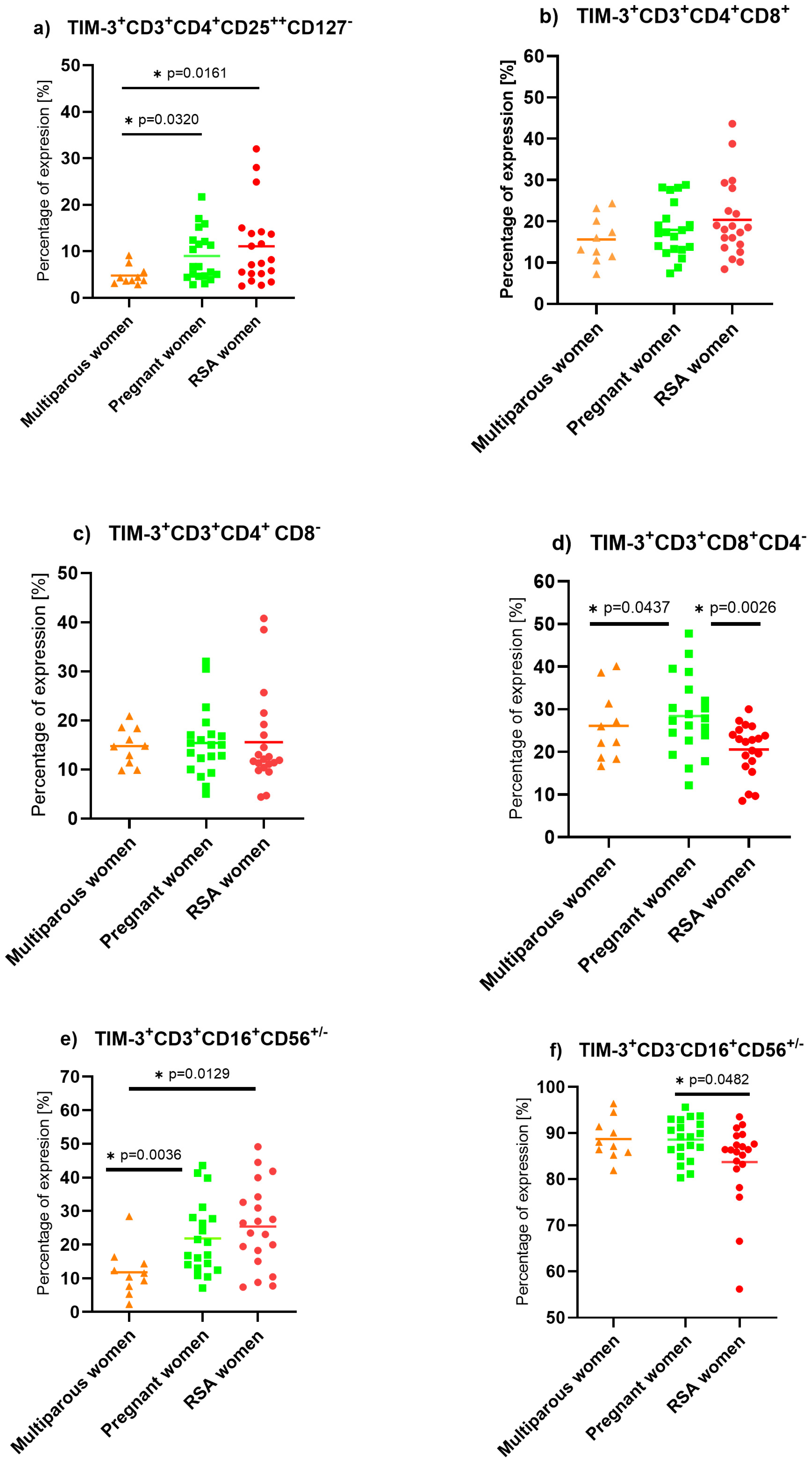
3.2. T Cell Immunoglobulin and Mucin Domain-Containing Protein (TIM-3) Expression on Lymphocytes
3.3. T Cell Immunoglobulin and ITIM Domain (TIGIT) Expression on Lymphocytes
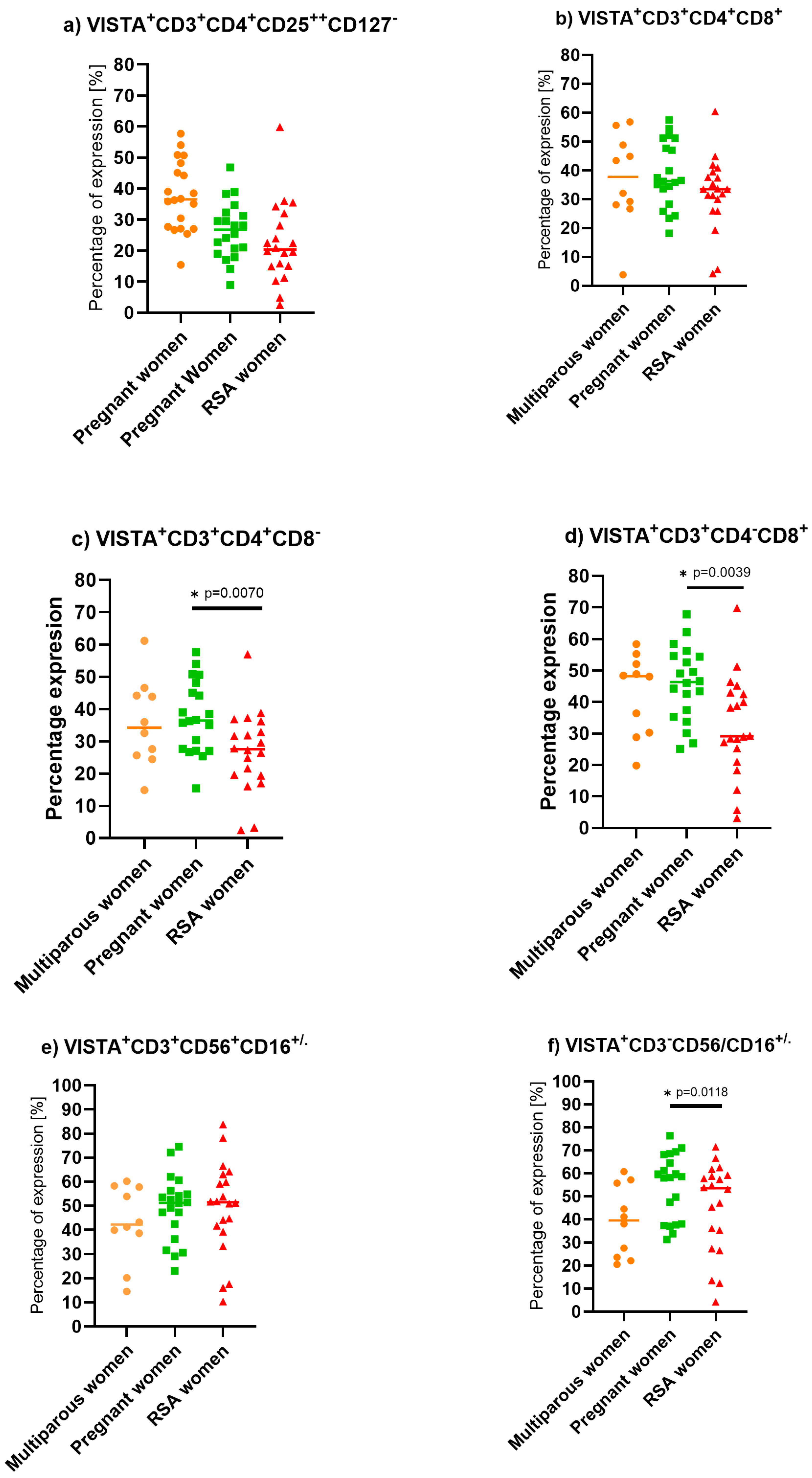
3.4. V-Domain Ig-Containing Suppressor of T Cell Activation (VISTA) Expression on Lymphocytes
3.5. Lymphocyte Activation Gene-3 (LAG-3) Expression on Lymphocytes
3.6. Potential Clinical Applications of the Findings
3.6.1. Diagnostic Tool for Immune Dysregulation in RPL
3.6.2. Personalized Immunomodulatory Therapies
3.6.3. Monitoring of Treatment Response
3.6.4. Predictive Biomarker for Pregnancy Outcomes
3.7. Strengths and Limitations of This Research
4. Materials and Methods
4.1. Materials
4.1.1. Non-Pregnant Multiparous Women
4.1.2. Pregnant Women
4.1.3. Recurrent Spontaneous Abortion (RSA) Women
4.2. Sample Isolation and Preparation
4.3. Flow Cytometry Staining
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Meggyes, M.; Miko, E.; Szigeti, B.; Farkas, N.; Szereday, L. The importance of the PD-1/PD-L1 pathway at the maternal-fetal interface. BMC Pregnancy Childbirth 2019, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.R.; Aslani, S.; Salmaninejad, A.; Javan, M.R.; Rezaei, N. PD-1/PD-L and autoimmunity: A growing relationship. Cell. Immunol. 2016, 310, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell. Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Asano, T.; Meguri, Y.; Yoshioka, T.; Kishi, Y.; Iwamoto, M.; Nakamura, M.; Sando, Y.; Yagita, H.; Koreth, J.; Kim, H.T.; et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood 2017, 129, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Meggyes, M.; Nagy, D.U.; Szereday, L. Investigation of the PD-1 and PD-L1 Immune Checkpoint Molecules Throughout Healthy Human Pregnancy and in Nonpregnant Women. J. Clin. Med. 2020, 9, 2536. [Google Scholar] [CrossRef] [PubMed]
- Ghiotto, M.; Gauthier, L.; Serriari, N.; Pastor, S.; Truneh, A.; Nunès, J.A.; Olive, D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int. Immunol. 2010, 22, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, T.; Schust, D.J.; Sugimoto, J.; Barrier, B.F. Human decidual stromal cells suppress cytokine secretion by allogenic CD4+ T cells via PD-1 ligand interactions. Hum. Reprod. 2009, 24, 3160–3171. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- van de Weyer, P.S.; Muehlfeit, M.; Klose, C.; Bonventre, J.V.; Walz, G.; Kuehn, E.W. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem. Biophys. Res. Commun. 2006, 351, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.; Schraven, B.; Veillette, A. PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes. Mol. Cell. Biol. 2007, 27, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-H.; Tang, M.-X.; Mor, G.; Liao, A.-H. Tim-3: Expression on immune cells and roles at the maternal-fetal interface. J. Reprod. Immunol. 2016, 118, 92–99. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kabir-Salmani, M.; Azadbakht, M.; Sugihara, K.; Sakai, K.; Iwashita, M. Expression and localization of galectin-9 in the human uterodome. Endocr. J. 2008, 55, 879–887. [Google Scholar] [CrossRef]
- Miko, E.; Meggyes, M.; Doba, K.; Barakonyi, A.; Szereday, L. Immune Checkpoint Molecules in Reproductive Immunology. Front. Immunol. 2019, 10, 846. [Google Scholar] [CrossRef]
- Freeman, G.J.; Casasnovas, J.M.; Umetsu, D.T.; DeKruyff, R.H. TIM genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 2010, 235, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Akiba, H.; Takeda, K.; Kojima, Y.; Hashiguchi, M.; Azuma, M.; Yagita, H.; Okumura, K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 2009, 113, 3821–3830. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Zhang, J.-P.; Liang, J.; Li, L.; Zheng, L. Tim-3 expression defines regulatory T cells in human tumors. PLoS ONE 2013, 8, e58006. [Google Scholar] [CrossRef] [PubMed]
- Sakuishi, K.; Ngiow, S.F.; Sullivan, J.M.; Teng, M.W.L.; Kuchroo, V.K.; Smyth, M.J.; Anderson, A.C. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2013, 2, e23849. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shu, Q.; Gao, L.; Hou, N.; Zhao, D.; Liu, X.; Zhang, X.; Xu, L.; Yue, X.; Zhu, F.; et al. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin. Immunol. 2010, 137, 288–295. [Google Scholar] [CrossRef]
- Yang, L.; Anderson, D.E.; Kuchroo, J.; Hafler, D.A. Lack of TIM-3 immunoregulation in multiple sclerosis. J. Immunol. 2008, 180, 4409–4414. [Google Scholar] [CrossRef]
- Koguchi, K.; Anderson, D.E.; Yang, L.; O’Connor, K.C.; Kuchroo, V.K.; Hafler, D.A. Dysregulated T cell expression of TIM3 in multiple sclerosis. J. Exp. Med. 2006, 203, 1413–1418. [Google Scholar] [CrossRef]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.-M.; Okazaki, T. LAG-3: From molecular functions to clinical applications. J. Immunother. Cancer 2020, 8, e001014. [Google Scholar] [CrossRef]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef]
- Crise, B.; Rose, J. Identification of palmitoylation sites on CD4, the human immunodeficiency virus receptor. J. Biol. Chem. 1992, 267, 13593–13597. [Google Scholar] [CrossRef]
- Turner, J.M.; Brodsky, M.H.; Irving, B.A.; Levin, S.D.; Perlmutter, R.M.; Littman, D.R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell 1990, 60, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Huang, C.-T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in Regulatory T Cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C.; Xiao, W. Expression regulation of co-inhibitory molecules on human natural killer cells in response to cytokine stimulations. Cytokine 2014, 65, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef]
- Maruhashi, T.; Okazaki, I.-M.; Sugiura, D.; Takahashi, S.; Maeda, T.K.; Shimizu, K.; Okazaki, T. LAG-3 inhibits the activation of CD4+ T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat. Immunol. 2018, 19, 1415–1426. [Google Scholar] [CrossRef]
- Okazaki, T.; Okazaki, I.-M.; Wang, J.; Sugiura, D.; Nakaki, F.; Yoshida, T.; Kato, Y.; Fagarasan, S.; Muramatsu, M.; Eto, T.; et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 2011, 208, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Prigent, P.; Tournier, M.; Bruniquel, D.; Triebel, F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995, 25, 2718–2721. [Google Scholar] [CrossRef]
- Workman, C.J.; Rice, D.S.; Dugger, K.J.; Kurschner, C.; Vignali, D.A.A. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur. J. Immunol. 2002, 32, 2255–2263. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e12. [Google Scholar] [CrossRef]
- Maeda, T.K.; Sugiura, D.; Okazaki, I.-M.; Maruhashi, T.; Okazaki, T. Atypical motifs in the cytoplasmic region of the inhibitory immune co-receptor LAG-3 inhibit T cell activation. J. Biol. Chem. 2019, 294, 6017–6026. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, A.; Qiu, C.; Wang, W.; Yang, Y.; Qiu, C.; Liu, A.; Zhu, L.; Yuan, S.; Hu, H.; et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J. Immunol. 2015, 194, 3873–3882. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, X.; Zhang, L.; Zhu, Q.; Chen, C.; Bao, J.; Chen, Y. CD4+ T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunol. 2019, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Bettini, M.; Szymczak-Workman, A.L.; Forbes, K.; Castellaw, A.H.; Selby, M.; Pan, X.; Drake, C.G.; Korman, A.J.; Vignali, D.A.A. Cutting edge: Accelerated autoimmune diabetes in the absence of LAG-3. J. Immunol. 2011, 187, 3493–3498. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Workman, C.J.; McGaha, T.L.; Li, L.; Vas, J.; Vignali, D.A.A.; Monestier, M. Lymphocyte activation gene-3 (LAG-3) negatively regulates environmentally-induced autoimmunity. PLoS ONE 2014, 9, e104484. [Google Scholar] [CrossRef]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting Edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef]
- Boles, K.S.; Vermi, W.; Facchetti, F.; Fuchs, A.; Wilson, T.J.; Diacovo, T.G.; Cella, M.; Colonna, M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur. J. Immunol. 2009, 39, 695–703. [Google Scholar] [CrossRef]
- Levin, S.D.; Taft, D.W.; Brandt, C.S.; Bucher, C.; Howard, E.D.; Chadwick, E.M.; Johnston, J.; Hammond, A.; Bontadelli, K.; Ardourel, D.; et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur. J. Immunol. 2011, 41, 902–915. [Google Scholar] [CrossRef]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg Cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8 + T Cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C.; Grassi, J.; Marcenaro, S.; Reymond, N.; et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 2003, 198, 557–567. [Google Scholar] [CrossRef]
- Chan, C.J.; Martinet, L.; Gilfillan, S.; Souza-Fonseca-Guimaraes, F.; Chow, M.T.; Town, L.; Ritchie, D.S.; Colonna, M.; Andrews, D.M.; Smyth, M.J. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 2014, 15, 431–438. [Google Scholar] [CrossRef]
- Stengel, K.F.; Harden-Bowles, K.; Yu, X.; Rouge, L.; Yin, J.; Comps-Agrar, L.; Wiesmann, C.; Bazan, J.F.; Eaton, D.L.; Grogan, J.L. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell–cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc. Natl. Acad. Sci. USA 2012, 109, 5399–5404. [Google Scholar] [CrossRef]
- Li, M.; Xia, P.; Du, Y.; Liu, S.; Huang, G.; Chen, J.; Zhang, H.; Hou, N.; Cheng, X.; Zhou, L.; et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-γ production of natural killer cells via β-arrestin 2-mediated negative signaling. J. Biol. Chem. 2014, 289, 17647–17657. [Google Scholar] [CrossRef] [PubMed]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Li, M.; Hu, D.; Li, C.; Ge, B.; Jin, B.; Fan, Z. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013, 20, 456–464. [Google Scholar] [CrossRef]
- Nowak, E.C.; Lines, J.L.; Varn, F.S.; Deng, J.; Sarde, A.; Mabaera, R.; Kuta, A.; Le Mercier, I.; Cheng, C.; Noelle, R.J. Immunoregulatory functions of VISTA. Immunol. Rev. 2017, 276, 66–79. [Google Scholar] [CrossRef]
- Wang, S.-C.; Li, Y.-H.; Piao, H.-L.; Hong, X.-W.; Zhang, D.; Xu, Y.-Y.; Tao, Y.; Wang, Y.; Yuan, M.-M.; Li, D.-J.; et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. 2015, 6, e1738. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Yu, T.; Hu, M.; Xing, J.; Bai, S.; Qu, W.; Tong, X. Dynamics of TIGIT and PD-1 expression on NK cells during the course of normal pregnancy. Immunol. Lett. 2021, 230, 42–48. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Harrington, S.M.; Creedon, D.J.; Ruano, R.; Markovic, S.N.; Dong, H.; Dronca, R.S. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am. J. Reprod. Immunol. 2017, 79, e12795. [Google Scholar] [CrossRef]
- Sun, J.; Yang, M.; Ban, Y.; Gao, W.; Song, B.; Wang, Y.; Zhang, Y.; Shao, Q.; Kong, B.; Qu, X. Tim-3 Is Upregulated in NK Cells during Early Pregnancy and Inhibits NK Cytotoxicity toward Trophoblast in Galectin-9 Dependent Pathway. PLoS ONE 2016, 11, e0147186. [Google Scholar] [CrossRef] [PubMed]
- Veras, E.M.; Kurman, R.J.; Wang, T.-L.; Shih, I.-M.M. PD-L1 Expression in Human Placentas and Gestational Trophoblastic Diseases. Int. J. Gynecol. Pathol. 2017, 36, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.K.; Lenvik, T.R.; McCullar, V.; Felices, M.; O’Brien, M.S.; Cooley, S.A.; Verneris, M.R.; Cichocki, F.; Holman, C.J.; Panoskaltsis-Mortari, A.; et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012, 119, 3064–3072. [Google Scholar] [CrossRef]
- Ndhlovu, L.C.; Lopez-Vergès, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef] [PubMed]
- Meggyes, M.; Miko, E.; Polgar, B.; Bogar, B.; Farkas, B.; Illes, Z.; Szereday, L. Peripheral blood TIM-3 positive NK and CD8+ T cells throughout pregnancy: TIM-3/galectin-9 interaction and its possible role during pregnancy. PLoS ONE 2014, 9, e92371. [Google Scholar] [CrossRef]
- Miko, E.; Meggyes, M.; Bogar, B.; Schmitz, N.; Barakonyi, A.; Varnagy, A.; Farkas, B.; Tamas, P.; Bodis, J.; Szekeres-Bartho, J.; et al. Involvement of Galectin-9/TIM-3 Pathway in the Systemic Inflammatory Response in Early-Onset Preeclampsia. PLoS ONE 2013, 8, e71811. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhou, W.H.; Tao, Y.; Wang, S.-C.; Jiang, Y.-L.; Zhang, D.; Piao, H.-L.; Fu, Q.; Li, D.-J.; Du, M.-R. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy. Cell. Mol. Immunol. 2016, 13, 73–81. [Google Scholar] [CrossRef]
- Zhuang, X.; Xia, X.; Liu, L.; Zhang, Y.; Zhang, X.; Wang, C. Expression of Tim-3 in peripheral blood mononuclear cells and placental tissue in unexplained recurrent spontaneous abortion. Medicine 2018, 97, e12099. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, X.; Xu, Y.; Zhang, D.; Li, Y.; Tao, Y.; Piao, H.; Li, D.; Du, M. Programmed cell death-1 (PD-1) and T-cell immunoglobulin mucin-3 (Tim-3) regulate CD4+T cells to induce Type 2 helper T cell (Th2) bias at the maternal–fetal interface. Hum. Reprod. 2016, 31, 700–711. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Wang, S.-C.; Lin, Y.-K.; Li, D.-J.; DU, M.-R. Tim-3 and PD-1 regulate CD8+ T cell function to maintain early pregnancy in mice. J. Reprod. Dev. 2017, 63, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dang, J.; Jiang, M.; He, M.; Yang, M.; Li, J.; Hao, H.; Zhou, Y.; Zuo, W.; Xie, Y.; et al. Upregulation of Tim-3 expression at feto-maternal interface may explain embryo survival in the CBAxDBA/2 model of abortion. Am. J. Reprod. Immunol. 2018, 79, e12775. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Z.; Liu, Y.; Li, B.; Zhang, L.; Fang, H.; Song, C.; Wang, X.; Zhang, G.-M.; Feng, Z.-H.; et al. Human pregnancy up-regulates Tim-3 in innate immune cells for systemic immunity. J. Immunol. 2009, 182, 6618–6624. [Google Scholar] [CrossRef]
- Khalaf, W.S.; Mahmoud, M.R.A.; Elkhatib, W.F.; Hashem, H.R.; Soliman, W.E. Phenotypic characterization of NKT-like cells and evaluation of specifically related cytokines for the prediction of unexplained recurrent miscarriage. Heliyon 2021, 7, e08409. [Google Scholar] [CrossRef]
- Liang, Q.; Tong, L.; Xiang, L.; Shen, S.; Pan, C.; Liu, C.; Zhang, H. Distinct changes of in BTLA, ICOS, PD-1, and TIGIT expression on peripheral blood and decidual CD8+ T cells in women with unexplained recurrent spontaneous abortion. Biol. Reprod. 2020, 103, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Granne, I.; Shen, M.; Rodriguez-Caro, H.; Chadha, G.; O’Donnell, E.; Brosens, J.J.; Quenby, S.; Child, T.; Southcombe, J.H. Characterisation of peri-implantation endometrial Treg and identification of an altered phenotype in recurrent pregnancy loss. Mucosal Immunol. 2022, 15, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, X.; Li, E.; Zhang, G.; Wang, X.; Tang, T.; Bai, X.; Liang, T. VISTA: An immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef]
- Lines, J.L.; Sempere, L.F.; Broughton, T.; Wang, L.; Noelle, R. VISTA Is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol. Res. 2014, 2, 510–517. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G.; Manick, B.; Hernandez, V.; Renelt, M.; Erickson, C.; Guan, J.; Singh, R.; Rollins, S.; Solorz, A.; et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 2019, 156, 74–85. [Google Scholar] [CrossRef]
- Ohno, T.; Zhang, C.; Kondo, Y.; Kang, S.; Furusawa, E.; Tsuchiya, K.; Miyazaki, Y.; Azuma, M. The immune checkpoint molecule VISTA regulates allergen-specific Th2-mediated immune responses. Int. Immunol. 2018, 30, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal–Fetal Immunity. Front. Immunol. 2020, 11, 458. [Google Scholar] [CrossRef]
- Flies, D.B.; Wang, S.; Xu, H.; Chen, L. Cutting edge: A monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J. Immunol. 2011, 187, 1537–1541. [Google Scholar] [CrossRef]
- ElTanbouly, M.A.; Schaafsma, E.; Noelle, R.J.; Lines, J.L. VISTA: Coming of age as a multi-lineage immune checkpoint. Clin. Exp. Immunol. 2020, 200, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Flies, D.B.; Han, X.; Higuchi, T.; Zheng, L.; Sun, J.; Ye, J.J.; Chen, L. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell–mediated immunity. J. Clin. Investig. 2014, 124, 1966–1975. [Google Scholar] [CrossRef]
- Cadena, R.H.; Reitsema, R.D.; Huitema, M.G.; van Sleen, Y.; van der Geest, K.S.M.; Heeringa, P.; Boots, A.M.H.; Abdulahad, W.H.; Brouwer, E. Decreased Expression of Negative Immune Checkpoint VISTA by CD4+ T Cells Facilitates T Helper 1, T Helper 17, and T Follicular Helper Lineage Differentiation in GCA. Front. Immunol. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H. Immune checkpoint molecules in pregnancy: Focus on regulatory T cells. Eur. J. Immunol. 2020, 50, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Dimova, T.; Nagaeva, O.; Stenqvist, A.-C.; Hedlund, M.; Kjellberg, L.; Strand, M.; Dehlin, E.; Mincheva-Nilsson, L. Maternal Foxp3 Expressing CD4+CD25+ and CD4-CD25− Regulatory T-Cell Populations are Enriched in Human Early Normal Pregnancy Decidua: A Phenotypic Study of Paired Decidual and Peripheral Blood Samples. Am. J. Reprod. Immunol. 2011, 66, 44–56. [Google Scholar] [CrossRef]
- Madadi, S.; Mohammadinejad, S.; Alizadegan, A.; Hojjat-Farsangi, M.; Dolati, S.; Kafil, H.S.; Jadidi-Niaragh, F.; Soltani-Zangbar, M.S.; Motavalli, R.; Etemadi, J.; et al. Expression level of immune checkpoint inhibitory factors in preeclampsia. Hum. Immunol. 2022, 83, 628–636. [Google Scholar] [CrossRef]


| Median and Q1–Q4 Quartile | Non-Pregnant Multiparous Women | Pregnant Women | RSA Women | p-Value 1 RSA vs. Pregnant 2 RSA vs. Multiparous 3 Multiparous vs. Pregnant | |
|---|---|---|---|---|---|
| Age | Median | 33.5 | 30 | 34 | 1 p = 0.26 |
| Q1 | 26 | 25 | 22 | 2 p = 0.26 | |
| Q4 | 40 | 39 | 40 | 3 p = 0.4 | |
| BMI (body mass index) | Median | 25 | 21.6 | 21.8 | 1 p = 0.5 |
| Q1 | 18.6 | 16.7 | 17.9 | 2 p = 0.48 | |
| Q4 | 36.2 | 31.4 | 37.2 | 3 p = 0.28 | |
| Number of full-term pregnancies | Median | 2 | 1 | 0 | 1 p = 0.027 * |
| Q1 | 1 | 1 | 0 | 2 p = 0.00001 ** | |
| Q4 | 3 | 3 | 0 | 3 p = 0.007 * | |
| Number of miscarriages | Median | 0 | 0 | 3 | 1 p = 0.0001 ** |
| Q1 | 0 | 0 | 2 | 2 p = 0.0001 ** | |
| Q4 | 0 | 0 | 5 | 3 p = 0.5 | |
| Pregnancy duration (weeks) | Median | N/A | 12.8 | 8.8 | 1 p = 0.1 |
| Q1 | N/A | 11.7 | 4.0 | 2 N/A | |
| Q4 | N/A | 14.6 | 13.6 | 3 N/A | |
| The occurrence of chronic diseases | |||||
| Non-Pregnant multiparous women | Pregnant women | RSA women | |||
| Diabetes | 0% | 10% | 5% | ||
| Endometriosis | 0% | 0% | 0% | ||
| Insulin resistance | 1% | 5% | 5% | ||
| Hashimoto disease | 0% | 0% | 0% | ||
| Polycystic ovary syndrome | 0% | 10% | 0% | ||
| Diet supplements and folic acid administration before pregnancy and during pregnancy | |||||
| Folic acid administration | 60% | 65% | 80% | ||
| Medicine and dietary supplement administration before pregnancy | 30% | 60% | 75% | ||
| Salicylic acid and dietary supplement administration during pregnancy | 40% | 90% | 75% | ||
| No medicine and dietary supplement administration before pregnancy | 70% | 40% | 25% | ||
| No medicine and dietary supplement administration during pregnancy | 60% | 10% | 25% | ||
| Antibody | Fluorochrome | Clone | Volume after Titration | Manufacturer |
|---|---|---|---|---|
| Anti-CD3 | PreCP | SK7 | 2 µL | Becton Dickinson |
| Anti-CD4 | APC-Cy7 | RPA-T4 | 0.5 µL | Becton Dickinson |
| Anti-CD8 | APC | SK1 | 0.5 µL | Becton Dickinson |
| Anti-CD25 | FITC | CD25-4E3 | 1 µL | Becton Dickinson |
| Anti-CD127 | BV450 | HIL-7R-M21 | 0.5 µL | Becton Dickinson |
| Anti-CD56 | PE-Cy7 | B159 | 1 µL | Becton Dickinson |
| Experiment | Antibody | Fluorochrome | Clone | Volume after Titration | Manufacturer |
|---|---|---|---|---|---|
| 1st tube | - | - | - | - | - |
| 2nd tube | Anti-PD1 | BV480 | EH12.1 | 1 µL | Becton Dickinson |
| Anti-Tim3 | PE | 7D3 | 0.5 µL | Becton Dickinson | |
| 3rd tube | Anti-Lag3 | BV480 | T47-530 | 1 µL | Becton Dickinson |
| 4th tube | Anti -TIGIT | PE | 741182 | 1 µL | Becton Dickinson |
| Anti-VISTA | BV480 | 1 µL | Becton Dickinson |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, M.; Kniotek, M.; Roszczyk, A.; Dąbrowski, F.; Jędra, R.; Zagożdżon, R. Surface Immune Checkpoints as Potential Biomarkers in Physiological Pregnancy and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 9378. https://doi.org/10.3390/ijms25179378
Zych M, Kniotek M, Roszczyk A, Dąbrowski F, Jędra R, Zagożdżon R. Surface Immune Checkpoints as Potential Biomarkers in Physiological Pregnancy and Recurrent Pregnancy Loss. International Journal of Molecular Sciences. 2024; 25(17):9378. https://doi.org/10.3390/ijms25179378
Chicago/Turabian StyleZych, Michał, Monika Kniotek, Aleksander Roszczyk, Filip Dąbrowski, Robert Jędra, and Radosław Zagożdżon. 2024. "Surface Immune Checkpoints as Potential Biomarkers in Physiological Pregnancy and Recurrent Pregnancy Loss" International Journal of Molecular Sciences 25, no. 17: 9378. https://doi.org/10.3390/ijms25179378






