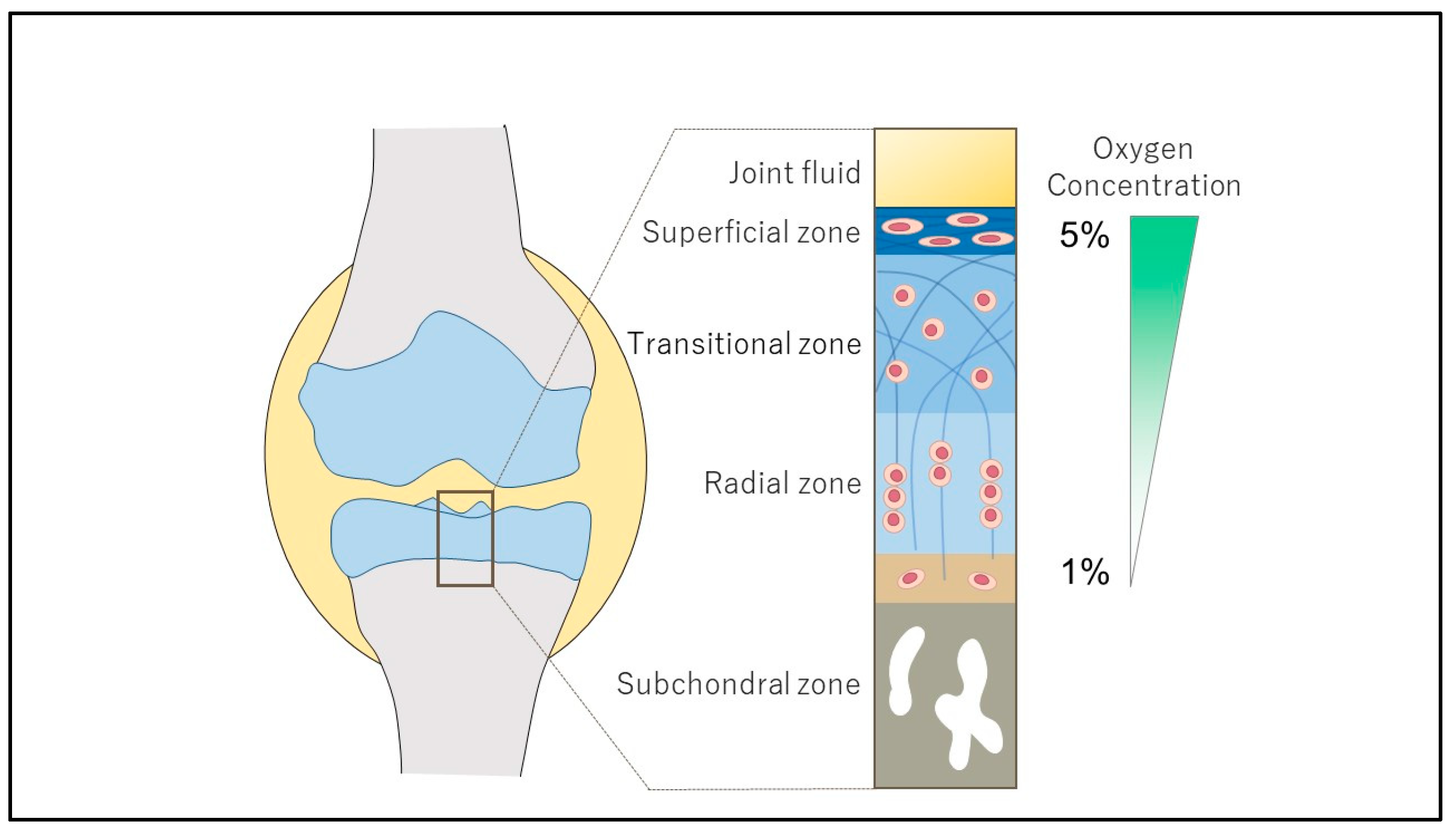
Cartilage Homeostasis under Physioxia
Abstract
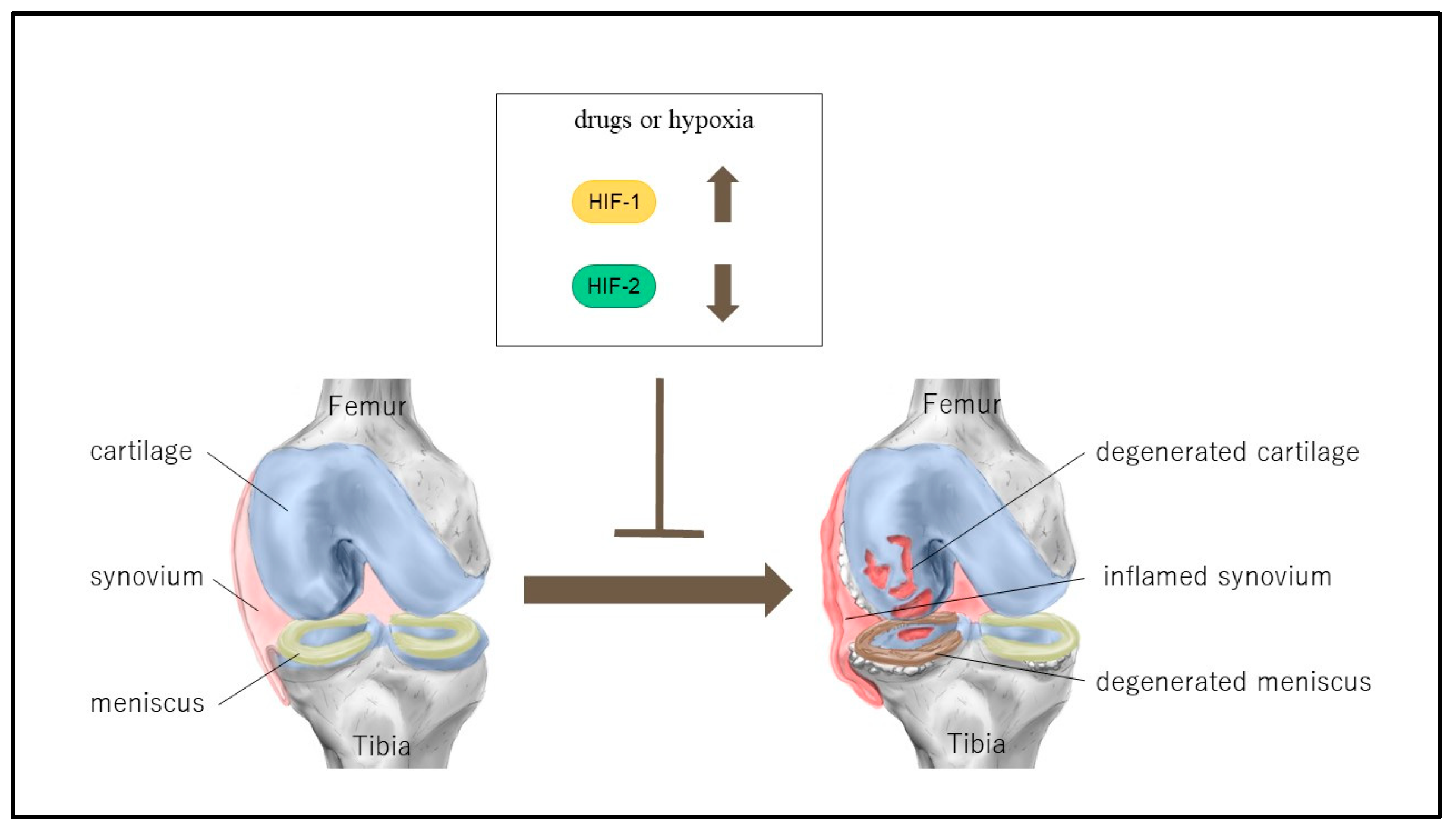
:1. Introduction
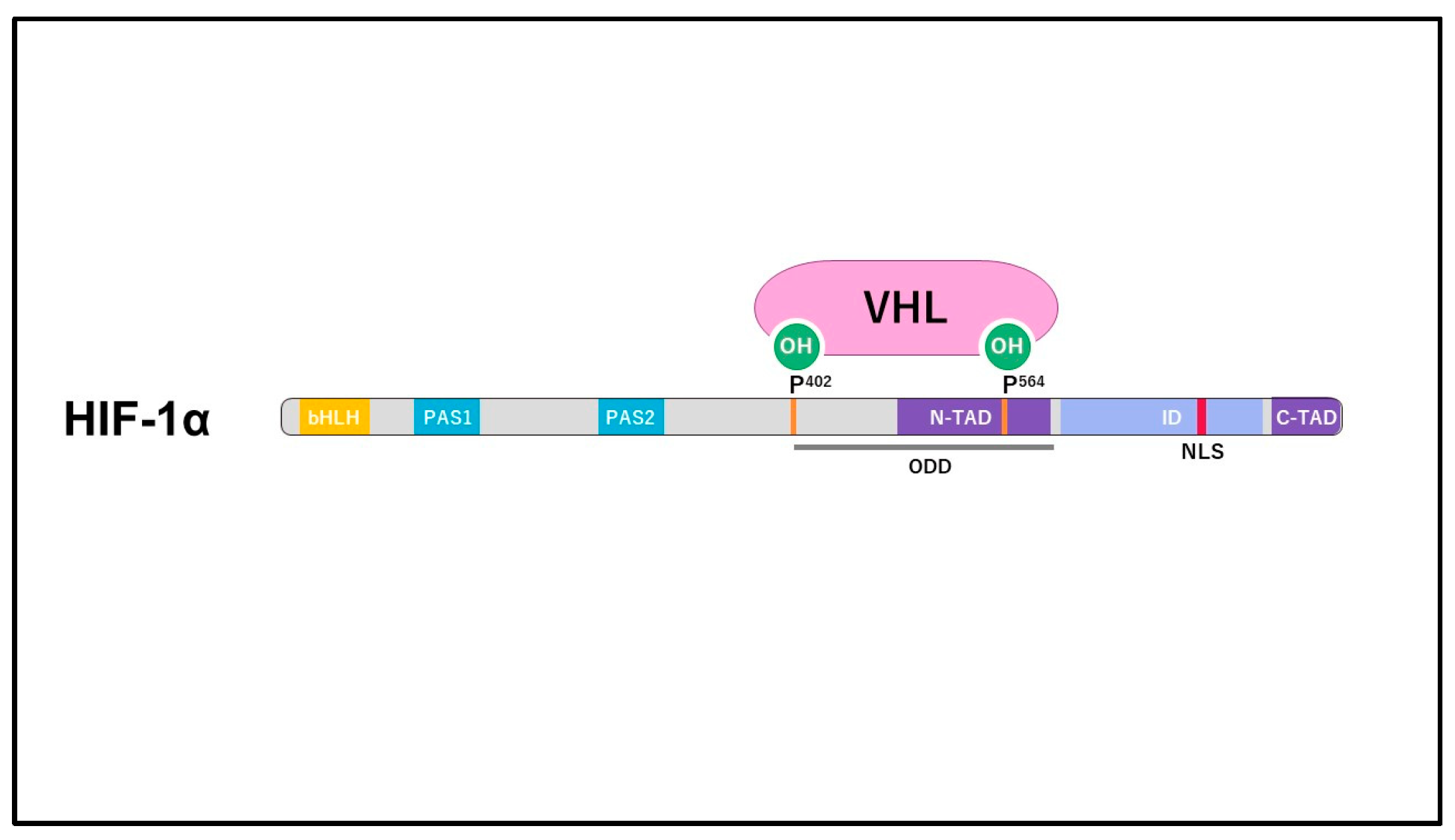
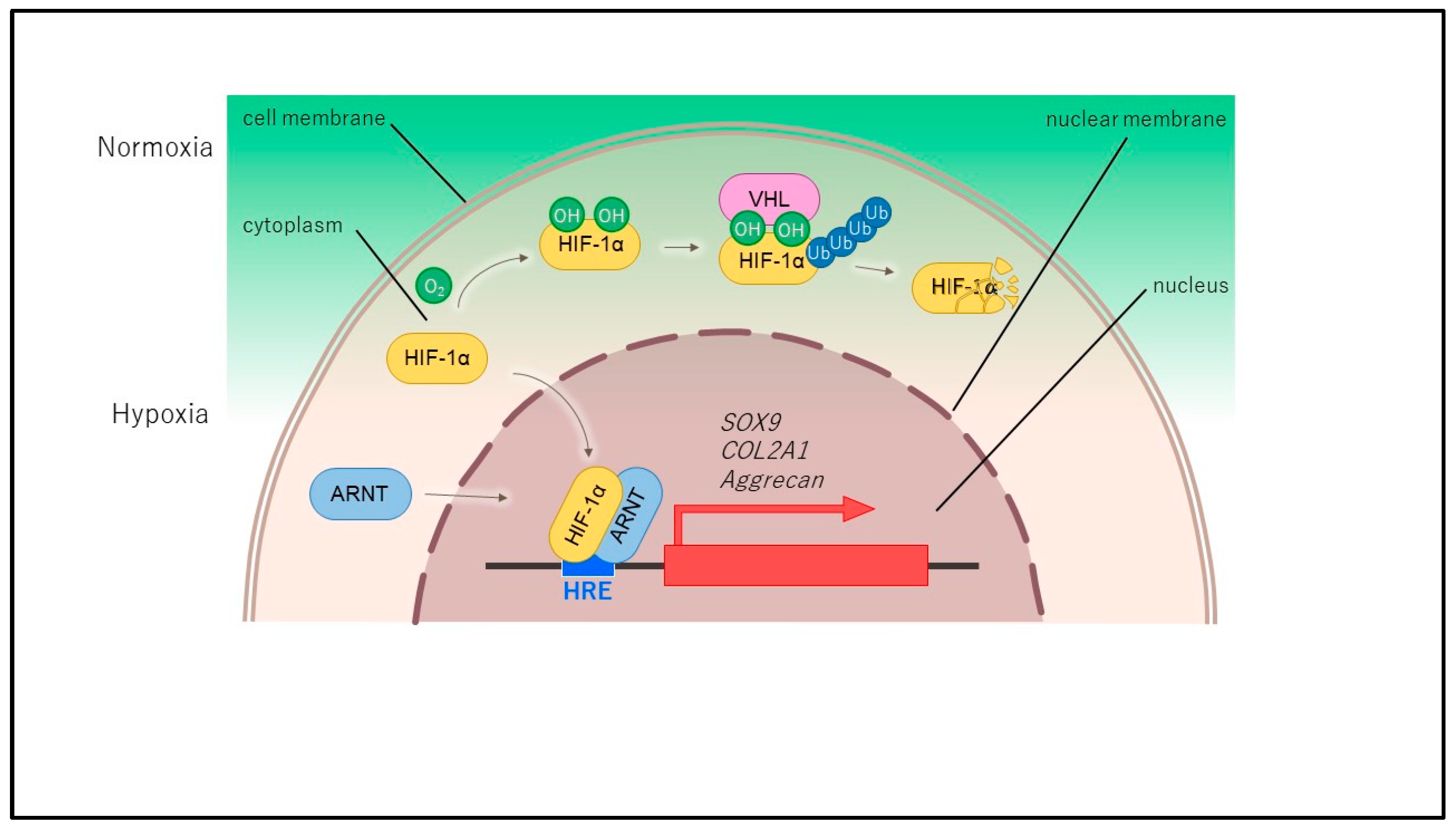
2. Structure and Regulation of HIF
3. HIF-1α in Normal Cartilage and Osteoarthritis
4. The Function of HIF-1α in Chondrocytes
5. The Treatment of Osteoarthritis (OA) with Hypoxia-Inducible Factor 1 Alpha (HIF-1α) Control
6. The Function of HIF-2α in Chondrocytes and the Treatment of OA by HIF-2α Regulation
7. The Function of HIF-3α in Chondrocytes
8. Regulatory Mechanisms of HIF-1α by Duration of Physioxia
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hart, D.A. Osteoarthritis as an umbrella term for different subsets of humans undergoing joint egeneration: The need to address the differences to develop effective conservative treatments and prevention strategies. Int. J. Mol. Sci. 2022, 23, 15365. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37, 3–6. [Google Scholar] [PubMed]
- Guillén, R.; Otero, F.; Mosquera, A.; Vázquez-Mosquera, M.; Rego-Pérez, I.; Blanco, F.J.; Fernández, J.L. Association of accelerated dynamics of telomere sequence loss in peripheral blood leukocytes with incident knee osteoarthritis in Osteoarthritis Initiative cohort. Sci. Rep. 2021, 11, 15914. [Google Scholar] [CrossRef]
- Leifer, V.P.; Katz, J.N.; Losina, E. The burden of OA-health services and economics. Osteoarthr. Cartil. 2022, 30, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Kiaer, T.; Grønlund, J.; Sørensen, K.H. Subchondral pO2, pCO2, pressure, pH, and lactate in human osteoarthritis of the hip. Clin. Orthop. Relat. Res. 1988, 229, 149–155. [Google Scholar] [CrossRef]
- Archer, C.W.; Francis-West, P. The chondrocyte. Int. J. Biochem. Cell Biol. 2003, 35, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.A.; Kong, H. Mechanism of HIFs in osteoarthritis. Front. Immunol. 2023, 14, 1168799. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.Y.; Wang, X.F.; Hua, F.Z. HIF-1alpha in Osteoarthritis: From Pathogenesis to Therapeutic Implications. Front. Pharmacol. 2022, 5, 927126. [Google Scholar]
- Yang, C.; Zhong, Z.F.; Wang, S.P.; Vong, C.T.; Yu, B.; Wang, Y.T. HIF-1: Structure, biology and natural modulators. Chin. J. Nat. Med. 2021, 19, 521–527. [Google Scholar] [CrossRef]
- Yudoh, K.; Nakamura, H.; Masuko-Hongo, K.; Kato, T.; Nishioka, K. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes: Involvement of HIF-1 alpha in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2005, 7, R904–R914. [Google Scholar] [CrossRef]
- Gelse, K.; Pfander, D.; Obier, S.; Knaup, K.X.; Wiesener, M.; Hennig, F.F.; Swoboda, B. Role of hypoxia-inducible factor 1 alpha in the integrity of articular cartilage in murine knee joints. Arthritis Res. Ther. 2008, 10, R111. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, C.; Ni, L.; Huang, C.; Chen, D.; Shi, K.; Jin, H.; Zhang, K.; Li, Y.; Xie, L.; et al. Stabilization of HIF-1a alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020, 11, 481. [Google Scholar] [CrossRef]
- Fernández-Torres, J.; Hernández-Díaz, C.; Espinosa-Morales, R.; Camacho-Galindo, J.; Galindo-Sevilla, N.D.C.; López-Macay, Á.; Zamudio-Cuevas, Y.; Martínez-Flores, K.; Santamaría-Olmedo, M.G.; Pineda, C.; et al. Polymorphic variation of hypoxia inducible factor-1 A (HIF1A) gene might contribute to the development of knee osteoarthritis: A pilot study. BMC Musculoskelet Disord. 2015, 16, 218. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Huang, Z.; Xing, R.; Li, X.; Yin, S.; Mao, J.; Zhang, N.; Mei, W.; Ding, L.; et al. Increased HIF-1alpha in knee osteoarthritis aggravate synovial fibrosis via fibroblastlike synoviocyte pyroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 6326517. [Google Scholar]
- Zhang, H.; Wang, L.; Cui, J.; Wang, S.; Han, Y.; Shao, H.; Wang, C.; Hu, Y.; Li, X.; Zhou, Q.; et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci. Adv. 2023, 9, eabo7868. [Google Scholar] [CrossRef]
- Li, F.; Tan, Q.; Li, F.; Zhang, K.; Liu, Z.; Tian, Y.; Zhu, T. Hypoxia-induced Wnt/beta-catenin signaling activation in subchondral bone osteoblasts leads to an osteoarthritis-like phenotype of chondrocytes in articular cartilage. Front. Mol. Biosci. 2023, 21, 1057154. [Google Scholar]
- Zhang, F.J.; Luo, W.; Lei, G.H. Role of HIF-1alpha and HIF-2alpha in osteoarthritis. Jt. Bone Spine 2015, 82, 144–147. [Google Scholar] [CrossRef]
- Fernández-Torres, J.; Zamudio-Cuevas, Y.; Martínez-Nava, G.A.; López-Reyes, A.G. Hypoxia-Inducible Factors (HIFs) in the articular cartilage: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2800–2810. [Google Scholar]
- Kong, P.; Chen, R.; Zou, F.Q.; Wang, Y.; Liu, M.C.; Wang, W.G. HIF-1alpha repairs degenerative chondrocyte glycolytic metabolism by the transcriptional regulation of Runx2. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1206–1214. [Google Scholar]
- Myllyharju, J.; Schipani, E. Extracellular matrix genes as hypoxia-inducible targets. Cell Tissue Res. 2010, 339, 19–29. [Google Scholar] [CrossRef]
- Taheem, D.K.; Jell, G.; Gentleman, E. Hypoxia inducible factor-1alpha in osteochondral tissue engineering. Tissue Eng. Part B Rev. 2020, 26, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Cho, H.; Johnstone, B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis Res. Ther. 2013, 15, R92. [Google Scholar] [CrossRef]
- Yang, X.; Chen, W.; Zhao, X.; Chen, L.; Li, W.; Ran, J.; Wu, L. Pyruvate kinase M2 modulates the glycolysis of chondrocyte and extracellular matrix in osteoarthritis. DNA Cell Biol. 2018, 37, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, W.; Sigaux, J.; Modrowski, D.; Devignes, C.S.; Funck-Brentano, T.; Richette, P.; Ea, H.K.; Provot, S.; Cohen-Solal, M.; Haÿ, E. Interaction of HIF1α and β-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5453–5458. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Mori, D.; Makii, Y.; Nakamoto, H.; Murahashi, Y.; Yano, F.; Chang, S.H.; Taniguchi, Y.; Kobayashi, H.; Semba, H.; et al. Hypoxia-inducible factor-1 alpha maintains mouse articular cartilage through suppression of NF-kappaB signaling. Sci. Rep. 2020, 10, 5425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Chang, B.Y.; Wang, X.F.; Zang, Y.F.; Zheng, Z.X.; Zhao, H.J.; Cui, Q.D. FBW7 regulates HIF-1alpha/VEGF pathway in the IL-1beta induced chondrocytes degeneration. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5914–5924. [Google Scholar]
- Li, Z.; Xie, L.; Zou, L.; Xiao, S.; Tao, J. Overexpression of RAD54L attenuates osteoarthritis by suppressing the HIF-1alpha/VEGF signaling pathway: Bioinformatics analysis and experimental validation. PLoS ONE 2024, 19, e0298575. [Google Scholar]
- Zhang, F.; Wang, J.; Chu, J.; Yang, C.; Xiao, H.; Zhao, C.; Sun, Z.; Gao, X.; Chen, G.; Han, Z.; et al. MicroRNA-146a induced by hypoxia promotes chondrocyte autophagy through bcl-2. Cell. Physiol. Biochem. 2015, 37, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gao, X.; Wang, J.; Yang, C.; Wang, Y.; Liu, Y.; Zou, W.; Liu, T. Hypoxia-induced microRNA-146a represses bcl-2 through Traf6/IRAK1 but not Smad4 to promote chondrocyte autophagy. Biol. Chem. 2017, 398, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, R.; Ma, H.; Zhao, X.; Wang, G. miRNA-411 regulates chondrocyte autophagy in osteoarthritis by targeting hypoxia-inducible factor 1 alpha (HIF-1alpha). Med. Sci. Monit. 2020, 19, e921155. [Google Scholar]
- Tsuchida, S.; Arai, Y.; Takahashi, K.A.; Kishida, T.; Terauchi, R.; Honjo, K.; Nakagawa, S.; Inoue, H.; Ikoma, K.; Ueshima, K.; et al. HIF-1α-induced HSP70 regulates anabolic responses in articular chondrocytes under hypoxic conditions. J. Orthop. Res. 2014, 32, 975–980. [Google Scholar] [CrossRef]
- Velard, F.; Chatron-Colliet, A.; Côme, D.; Ah-Kioon, M.D.; Lin, H.; Hafsia, N.; Cohen-Solal, M.; Ea, H.K.; Lioté, F. Adrenomedullin and truncated peptide adrenomedullin (22-52) affect chondrocyte response to apoptotis in vitro: Downregulation of FAS protects chondrocyte from cell death. Sci. Rep. 2020, 10, 16740. [Google Scholar] [CrossRef]
- Lu, J.; Peng, Y.; Zou, J.; Wang, J.; Lu, S.; Fu, T.; Jiang, L.; Zhang, C.; Zhang, J. Hypoxia inducible factor-1a is a regulator of autophagy in osteoarthritic chondrocytes. Cartilage 2021, 13, 1030S–1040S. [Google Scholar] [CrossRef]
- Juhász, K.Z.; Hajdú, T.; Kovács, P.; Vágó, J.; Matta, C.; Takács, R. Hypoxic conditions modulate chondrogenesis through the circadian clock: The role of hypoxia-inducible factor-1alpha. Cells 2024, 13, 512. [Google Scholar] [CrossRef]
- Ma, Z.; Jin, X.; Qian, Z.; Li, F.; Xu, M.; Zhang, Y.; Kang, X.; Li, H.; Gao, X.; Zhao, L.; et al. Deletion of clock gene Bmal1 impaired the chondrocyte function due to disruption of the HIF1a-VEGF signaling pathway. Cell Cycle 2019, 18, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, X.; Su, L.; Cao, S. Long non-coding RNA LncHIFCAR promotes osteoarthritis development via positively regulating HIF-1a and activating the PI3K/AKT/mTOR pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 3000–3009. [Google Scholar] [PubMed]
- Ni, W.; Zhang, H.; Mei, Z.; Hongyi, Z.; Wu, Y.; Xu, W.; Ma, Y.; Yang, W.; Liang, Y.; Gu, T.; et al. An inducible long noncoding RNA, LncZFHX2, facilitates DNA repair to mediate osteoarthritis pathology. Redox Biol. 2023, 66, 102858. [Google Scholar] [CrossRef]
- Chen, G.; Liu, T.; Yu, B.; Wang, B.; Peng, Q. CircRNA-UBE2G1 regulates LPS induced osteoarthritis through miR-373/HIF-1a axis. Cell Cycle 2020, 19, 1696–1705. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, F.; Cornelia, R.; Tang, W.; Swisher, S.; Kim, H. Hypoxia-inducible factor-1 is a positive regulator of Sox9 activity in femoral head osteonecrosis. Bone 2011, 48, 507–513. [Google Scholar] [CrossRef]
- Robins, J.C.; Akeno, N.; Mukherjee, A.; Dalal, R.R.; Aronow, B.J.; Koopman, P.; Clemens, T.L. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 2005, 37, 313–322. [Google Scholar] [CrossRef]
- Henrionnet, C.; Liang, G.; Roeder, E.; Dossot, M.; Wang, H.; Magdalou, J.; Gillet, P.; Pinzano, A. Hypoxia for Mesenchymal Stem Cell Expansion and Differentiation: The Best Way for Enhancing TGFβ-Induced Chondrogenesis and Preventing Calcifications in Alginate Beads. Tissue Eng. Part A 2017, 23, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, K.; Aggelidou, E.; Manthou, M.E.; Kritis, A. Hypoxia Promotes Cartilage Regeneration in Cell-Seeded 3D-Printed Bioscaffolds Cultured with a Bespoke 3D Culture Device. Int. J. Mol. Sci. 2023, 24, 6040. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; Ma, Z.; Szojka, A.R.; Lan, X.; Kunze, M.; Mulet-Sierra, A.; Westover, L.; Adesida, A.B. Non-hypertrophic chondrogenesis of mesenchymal stem cells through mechano-hypoxia programing. J. Tissue Eng. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Feng, K.; Yu, Y.; Chen, Z.; Wang, F.; Zhang, K.; Chen, H.; Xu, J.; Kang, Q. Injectable hypoxia-preconditioned cartilage progenitor cells-laden GelMA microspheres system for enhanced osteoarthritis treatment. Mater. Today Bio 2023, 20, 100637. [Google Scholar] [CrossRef]
- Shimomura, S.; Inoue, H.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Kishida, T.; Shin-Ya, M.; Ichimaru, S.; Tsuchida, S.; Mazda, O.; et al. Hypoxia promotes differentiation of pure cartilage from human induced pluripotent stem cells. Mol. Med. Rep. 2022, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Wei, L.; Li, W.; Yang, W.; Cai, L.; Qian, Z.; Wu, S. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef]
- Guan, Z.; Jin, X.; Guan, Z.; Liu, S.; Tao, K.; Luo, L. The gut microbiota metabolite capsiate regulate SLC2A1 expression by targeting HIF-1alpha to inhibit knee osteoarthritis-induced ferroptosis. Aging Cell 2023, 22, e13807. [Google Scholar] [CrossRef]
- Ichimaru, S.; Nakagawa, S.; Arai, Y.; Kishida, T.; Shin-Ya, M.; Honjo, K.; Tsuchida, S.; Inoue, H.; Fujiwara, H.; Shimomura, S.; et al. Hypoxia Potentiates Anabolic Effects of Exogenous Hyaluronic Acid in Rat Articular Cartilage. Int. J. Mol. Sci. 2016, 17, 1013. [Google Scholar] [CrossRef] [PubMed]
- Oeding, J.F.; Varady, N.H.; Fearington, F.W.; Pareek, A.; Strickland, S.M.; Nwachukwu, B.U.; Camp, C.L.; Krych, A.J. Platelet-rich plasma versus alternative injections for osteoarthritis of the knee: A systematic review and statistical fragility index-based meta-analysis of randomized controlled trials. Am. J. Sports Med. 2024, 29, 03635465231224463. [Google Scholar] [CrossRef]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, antiinflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef]
- Takahashi, K.; Kubo, T.; Kobayashi, K.; Imanishi, J.; Takigawa, M.; Arai, Y.; Hirasawa, Y. Hydrostatic pressure influences mRNA expression of transforming growth factor-beta 1 and heat shock protein 70 in chondrocyte-like cell line. J. Orthop. Res. 1997, 15, 150–158. [Google Scholar] [CrossRef]
- Nakamura, S.; Arai, Y.; Takahashi, K.A.; Terauchi, R.; Ohashi, S.; Mazda, O.; Imanishi, J.; Inoue, A.; Tonomura, H.; Kubo, T. Hydrostatic pressure induces apoptosis of chondrocytes cultured in alginate beads. J. Orthop. Res. 2006, 24, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, S.; Inoue, H.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Kishida, T.; Shin-Ya, M.; Ichimaru, S.; Tsuchida, S.; Mazda, O.; et al. Mechanical stimulation of chondrocytes regulates HIF-1α under hypoxic conditions. Tissue Cell 2021, 71, 101574. [Google Scholar] [CrossRef]
- Coimbra, I.B.; Jimenez, S.A.; Hawkins, D.F.; Piera-Velazquez, S.; Stokes, D.G. Hypoxia inducible factor-1 alpha expression in human normal and osteoarthritic chondrocytes. Osteoarthr. Cartil. 2004, 12, 336–345. [Google Scholar] [CrossRef]
- Saito, T.; Fukai, A.; Mabuchi, A.; Ikeda, T.; Yano, F.; Ohba, S.; Nishida, N.; Akune, T.; Yoshimura, N.; Nakagawa, T.; et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 2010, 16, 678–686. [Google Scholar] [CrossRef]
- Yang, S.; Kim, J.; Ryu, J.H.; Oh, H.; Chun, C.H.; Kim, B.J.; Min, B.H.; Chun, J.S. Hypoxia-inducible factor-2 alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef]
- Saito, T.; Kawaguchi, H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthr. Cartil. 2010, 18, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Bohensky, J.; Terkhorn, S.P.; Freeman, T.A.; Adams, C.S.; Garcia, J.A.; Shapiro, I.M.; Srinivas, V. Regulation of autophagy in human and murine cartilage: Hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009, 60, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Shin, Y.; Huh, Y.H.; Yang, S.; Chun, C.H.; Chun, J.S. Hypoxia-inducible factor2a regulates fas-mediated chondrocyte apoptosis during osteoarthritic cartilage destruction. Cell Death Differ. 2012, 19, 440–450. [Google Scholar] [CrossRef]
- Oh, H.; Kwak, J.S.; Yang, S.; Gong, M.K.; Kim, J.H.; Rhee, J.; Kim, S.K.; Kim, H.E.; Ryu, J.H.; Chun, J.S. Reciprocal regulation by hypoxia-inducible factor-2alpha and the NAMPT-NAD (+)-SIRT axis in articular chondrocytes is involved in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ryu, J.H.; Oh, H.; Jeon, J.; Kwak, J.S.; Kim, J.H.; Kim, H.A.; Chun, C.H.; Chun, J.S. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2alpha, is an essential catabolic regulator of osteoarthritis. Ann. Rheum. Dis. 2015, 74, 595–602. [Google Scholar] [CrossRef]
- Lee, M.; Won, Y.; Shin, Y.; Kim, J.H.; Chun, J.S. Reciprocal activation of hypoxia-inducible factor (HIF)-2alpha and the zinc-ZIP8-MTF1 axis amplifies catabolic signaling in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 134–145. [Google Scholar] [CrossRef]
- Yang, J.; Shin, Y.; Kim, H.J.; Kim, H.E.; Chun, J.S. Prokineticin 2 is a catabolic regulator of osteoarthritic cartilage destruction in mouse. Arthritis Res. Ther. 2023, 25, 236. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Cai, P.; Fu, W.; Wang, J.; Wei, Q.; Li, X. Up-regulated HIF-2alpha contributes to the Osteoarthritis development through mediating the primary cilia loss. Int. Immunopharmacol. 2019, 75, 105762. [Google Scholar] [CrossRef]
- Inoue, H.; Arai, Y.; Kishida, T.; Terauchi, R.; Honjo, K.; Nakagawa, S.; Tsuchida, S.; Matsuki, T.; Ueshima, K.; Fujiwara, H.; et al. Hydrostatic pressure influences HIF-2 alpha expression in chondrocytes. Int. J. Mol. Sci. 2015, 16, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.; Liu, Q.; Su, J.; Wang, T.; Li, T. Effect of whole body vibration on HIF-2alpha expression in SD rats with early knee osteoarthritis. J. Bone Miner. Metab. 2020, 38, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Zhang, X.; Shao, Z.; Zhao, F.; Hu, X.; Ao, Y. Intra-articular delivery of anti-Hif-2alpha siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 2015, 22, 439–448. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Hou, Y.K.; Chen, M.W.; Yu, X.Z.; Chen, S.Y.; Yue, Y.R.; Guo, X.T.; Chen, J.X.; Zhou, Q. A pH-responsive metal-organic framework for the co-delivery of HIF-2alpha siRNA and curcumin for enhanced therapy of osteoarthritis. J. Nanobiotechnology 2023, 21, 18. [Google Scholar]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2alpha. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, F.J.; Tian, J.; Tu, M.; Xiong, Y.L.; Luo, W.; Li, Y.S.; Song, B.B.; Gao, S.G.; Lei, G.H. Osteopontin inhibits HIF-2alpha mRNA expression in osteoarthritic chondrocytes. Exp. Ther. Med. 2015, 9, 2415–2419. [Google Scholar] [CrossRef]
- Zhang, X.; Prasadam, I.; Fang, W.; Crawford, R.; Xiao, Y. Chondromodulin-1 ameliorates osteoarthritis progression by inhibiting HIF-2alpha activity. Osteoarthr. Cartil. 2016, 24, 1970–1980. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Y.; Sun, W.; Zhang, Z.; Liu, J.; Yang, W.; Yuan, W.; Yi, Y.; Wang, J.; Liu, J. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2alpha-dependent manner. Cell Prolif. 2021, 54, e13134. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.S.; Lee, Y.; Yang, J.; Kim, S.K.; Shin, Y.; Kim, H.J.; Choi, J.H.; Im, Y.J.; Kim, M.J.; Yu, K.L.; et al. Characterization of rhodanine derivatives as potential disease-modifying drugs for experimental mouse osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1210–1221. [Google Scholar] [CrossRef]
- Yang, J.; Guo, A.; Li, Q.; Wu, J. Platelet-rich plasma attenuates interleukin-1beta-induced apoptosis and inflammation in chondrocytes through targeting hypoxia-inducible factor-2alpha. Tissue Cell 2021, 73, 101646. [Google Scholar] [CrossRef]
- Hwang, H.S.; Park, S.J.; Lee, M.H.; Kim, H.A. MicroRNA-365 regulates IL-1b-induced catabolic factor expression by targeting HIF-2a in primary chondrocytes. Sci. Rep. 2017, 7, 17889. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Matsuzaki, T.; Ayabe, F.; Mokuda, S.; Kurimoto, R.; Matsushima, T.; Tabata, Y.; Inotsume, M.; Tsutsumi, H.; Liu, L.; et al. Both microRNA-455-5p and -3p repress hypoxia-inducible factor-2a expression and coordinately regulate cartilage homeostasis. Nat. Commun. 2021, 12, 4148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; He, S.; Yu, H.; Pei, F.; Zhou, Z. Inhibition of syndecan-4 reduces cartilage degradation in murine models of osteoarthritis through the downregulation of HIF-2alpha by miR-96-5p. Lab. Investig. 2021, 101, 1060–1070. [Google Scholar] [CrossRef]
- Schrobback, K.; Klein, T.J.; Crawford, R.; Upton, Z.; Malda, J.; Leavesley, D.I. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2012, 347, 649–663. [Google Scholar] [CrossRef]
- Maynard, M.A.; Evans, A.J.; Hosomi, T.; Hara, S.; Jewett, M.A.; Ohh, M. Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma. FASEB J. 2005, 19, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Cho, H.; Zilberman-Rudenko, J.; Holden, P.; McAlinden, A.; Johnstone, B. Hypoxia-inducible factor 3-alpha expression is associated with the stable chondrocyte phenotype. J. Orthop. Res. 2015, 33, 1561–1570. [Google Scholar] [CrossRef]
- Li, Z.; Meng, D.; Li, G.; Xu, J.; Tian, K.; Li, Y. Overexpression of microRNA-210 promotes chondrocyte proliferation and extracellular matrix deposition by targeting HIF-3alpha in osteoarthritis. Mol. Med. Rep. 2016, 13, 2769–2776. [Google Scholar] [CrossRef]
- Bader, S.B.; Dewhirst, M.W.; Hammond, E.M. Cyclic hypoxia: An update on its characteristics, methods to measure it and biological implications in cancer. Cancers 2020, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Kaihara, K.; Nakagawa, S.; Arai, Y.; Inoue, H.; Tsuchida, S.; Fujii, Y.; Kamada, Y.; Kishida, T.; Mazda, O.; Takahashi, K. Sustained hypoxia suppresses joint destruction in a rat model of rheumatoid arthritis via negative feedback of hypoxia inducible factor-1α. Int. J. Mol. Sci. 2021, 22, 3898. [Google Scholar] [CrossRef]
- Kamada, Y.; Arai, Y.; Toyama, S.; Inoue, A.; Nakagawa, S.; Fujii, Y.; Kaihara, K.; Cha, R.; Mazda, O.; Takahashi, K. Hypoxia with or without treadmill exercises affects slow-twitch muscle atrophy and joint destruction in a rat model of rheumatoid arthritis. Int. J. Mol. Sci. 2023, 24, 9761. [Google Scholar] [CrossRef]
- Uchida, T.; Rossignol, F.; Matthay, M.A.; Mounier, R.; Couette, S.; Clottes, E.; Clerici, C. Prolonged Hypoxia Differentially Regulates Hypoxia-inducible Factor (HIF) -1alpha and HIF-2alpha Expression in Lung Epithelial Cells: Implications of natural antisense HIF-1alpha. J. Biol. Chem. 2004, 279, 14871–14878. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, Z.; Zhu, M.; Zhang, W.; Chen, L.; Chen, H.; Kang, P. Hypobaric hypoxia aggravates osteoarthritis via the alteration of the oxygen environment and bone remodeling in the subchondral zone. FASEB J. 2024, 38, e23594. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Sceneay, J.; Gödde, N.; Kinwel, T.; Ham, S.; Thompson, E.W.; Humbert, P.O.; Möller, A. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene 2018, 37, 4214–4225. [Google Scholar] [CrossRef] [PubMed]
- Minoves, M.; Hazane-Puch, F.; Moriondo, G.; Boutin-Paradis, A.; Lemarié, E.; Pépin, J.L.; Godin-Ribuot, D.; Briançon-Marjollet, A. Differential impact of intermittent vs. sustained hypoxia on HIF-1, VEGF and proliferation of HepG2 cells. Int. J. Mol. Sci. 2023, 24, 6875. [Google Scholar] [CrossRef]
- Toffoli, S.; Roegiers, A.; Feron, O.; Van Steenbrugge, M.; Ninane, N.; Raes, M.; Michiels, C. Intermittent hypoxia is an angiogenic inducer for endothelial cells: Role of HIF-1. Angiogenesis 2009, 12, 47–67. [Google Scholar] [CrossRef]
- Martinive, P.; Defresne, F.; Quaghebeur, E.; Daneau, G.; Crokart, N.; Grégoire, V.; Gallez, B.; Dessy, C.; Feron, O. Impact of cyclic hypoxia on HIF-1alpha regulation in endothelial cells--new insights for anti-tumor treatments. FEBS J. 2009, 276, 509–518. [Google Scholar] [CrossRef]
- Toffoli, S.; Feron, O.; Raes, M.; Michiels, C. Intermittent hypoxia changes HIF-1alpha phosphorylation pattern in endothelial cells: Unravelling of a new PKA-dependent regulation of HIF-1alpha. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1558–1571. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Malec, V.; Gottschald, O.R.; Li, S.; Rose, F.; Seeger, W.; Hänze, J. HIF-1 alpha signaling is augmented during intermittent hypoxia by induction of the Nrf2 pathway in NOX1-expressing adenocarcinoma A549 cells. Free Radic. Biol. Med. 2010, 48, 1626–1635. [Google Scholar] [CrossRef]
- Mazza, F.; Cicciarelli, A.; Rubino, F.; Apponi, F.; Cioffi, E.A.; Simonelli, R. HIF-α: New target for treatment of renal anemia. Molecular aspects and activation of pathway HREs. G. Ital. Nefrol. Organo Uff. Della Soc. Ital. Nefrol. 2024, 3, 1–13. [Google Scholar]
- Jonasch, E.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; Dror Michaelson, M.; Appleman, L.J.; Roy, A.; Perini, R.F.; et al. Phase I LITESPARK-001 study of belzutifan for advanced solid tumors: Extended 41-month follow-up in the clear cell renal cell carcinoma cohort. Eur. J. Cancer. 2024, 196, 113434. [Google Scholar] [CrossRef] [PubMed]
| Regulators | HIFs | Tissue (Cell) | Mechanism | References |
|---|---|---|---|---|
| DMOG | HIF-1α | mouse chondrocytes, mouse OA model | activation of mitophagy | Hu et al. (2020) [18] |
| FBW7 | HIF-1α | human chondrocytes | maintenance of chondrogenic phenotype | Zhu et al. (2020) [32] |
| RAD54L | HIF-1α | human chondrocytes, rat OA model | anti-inflammation | Li et al. (2024) [33] |
| miR-146a | HIF-1α | mouse chondrocytes | activation of autophagy | Zhang et al. (2015), Chen et al. (2017) [34,35] |
| miR-411 | HIF-1α | human chondrocytes | activation of autophagy | Yang et al. (2020) [36] |
| HSP70 | HIF-1α | rabbit chondrocytes | inhibition of apoptosis | Tsuchida et al. (2014) [37] |
| AM | HIF-1α | bovine chondrocytes | inhibition of apoptosis | Velard et al. (2010) [38] |
| Bmal1 | HIF-1α | mouse chondrocytes | inhibition of apoptosis | Ma et al. (2019) [41] |
| resveratrol | HIF-1α,2α | mouse OA model | activation of autophagy | Qin et al. (2017) [52] |
| capsiate | HIF-1α | mouse OA model | inhibition of ferroptosis | Guan et al. (2023) [53] |
| hyaluronic acid | HIF-1α | rat cartilage tissue | cartilage metabolism | Ichimaru et al. (2016) [54] |
| PRP | HIF-1α | human chondrocytes | activation of autophagy | Moussa et al. (2017) [56] |
| mechanical stimulation | HIF-1α | rat chondrocytes | cartilage homeostasis | Shimomura et al. (2021) [59] |
| WBV | HIF-2α | rat OA model | anti-inflammation, cartilage hometostasis | Wang et al. (2020) [72] |
| HIF-2α siRNA | HIF-2α | mouse OA model | anti-inflammation, cartilage regeneration | Pi et al. (2015), Zhang et al. (2023) [73,74] |
| resveratrol | HIF-2α | mouse OA model | inhibition of cartilage degradation | Li et al. (2015) [75] |
| Osteopontin | HIF-2α | human chondrocytes | CD44 interaction | Cheng et al. (2015) [76] |
| Chondromodulin-1 | HIF-2α | rat OA model | cartilage homeostasis | Zhang et al. (2016) [77] |
| D-mannose | HIF-2α | mouse OA model | inhibition of ferroptosis | Zhou et al. (2021) [78] |
| rhodanine | HIF-2α | mouse OA model | inhibition of cartilage degradation | Kwak et al. (2022) [79] |
| PRP | HIF-2α | rat chondrocytes | inhibition of apoptosis, anti-inflammation | Yang et al. (2021) [80] |
| miR-365 | HIF-2α | human chondrocytes | inhibition of catabolic factor | Hwang et al. (2017) [81] |
| miR-96-5p and -3P | HIF-2α | mouse OA model | cartilage homeostasis | Ito et al. (2021) [82] |
| miR-96-5p | HIF-2α | mouse OA model | cartilage homeostasis | Zhou et al. (2021) [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, Y.; Cha, R.; Nakagawa, S.; Inoue, A.; Nakamura, K.; Takahashi, K. Cartilage Homeostasis under Physioxia. Int. J. Mol. Sci. 2024, 25, 9398. https://doi.org/10.3390/ijms25179398
Arai Y, Cha R, Nakagawa S, Inoue A, Nakamura K, Takahashi K. Cartilage Homeostasis under Physioxia. International Journal of Molecular Sciences. 2024; 25(17):9398. https://doi.org/10.3390/ijms25179398
Chicago/Turabian StyleArai, Yuji, Ryota Cha, Shuji Nakagawa, Atsuo Inoue, Kei Nakamura, and Kenji Takahashi. 2024. "Cartilage Homeostasis under Physioxia" International Journal of Molecular Sciences 25, no. 17: 9398. https://doi.org/10.3390/ijms25179398
APA StyleArai, Y., Cha, R., Nakagawa, S., Inoue, A., Nakamura, K., & Takahashi, K. (2024). Cartilage Homeostasis under Physioxia. International Journal of Molecular Sciences, 25(17), 9398. https://doi.org/10.3390/ijms25179398





