Constraint-Induced Movement Therapy (CIMT) and Neural Precursor Cell (NPC) Transplantation Synergistically Promote Anatomical and Functional Recovery in a Hypoxic-Ischemic Mouse Model
Abstract
:1. Introduction
2. Results
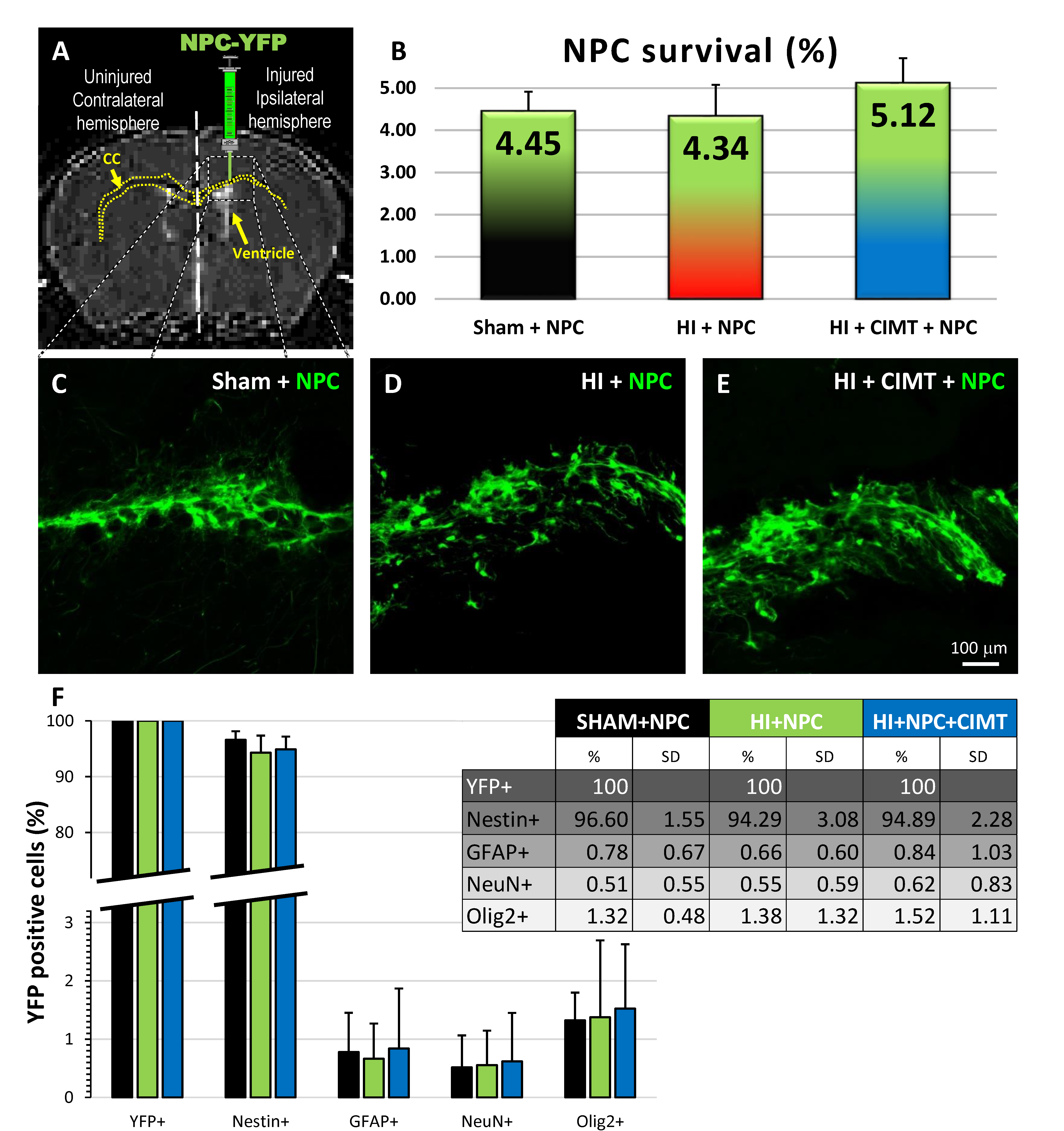
2.1. Transplanted NPCs Survive, Migrate, Differentiate Morphologically, and Integrate in the Corpus Callosum
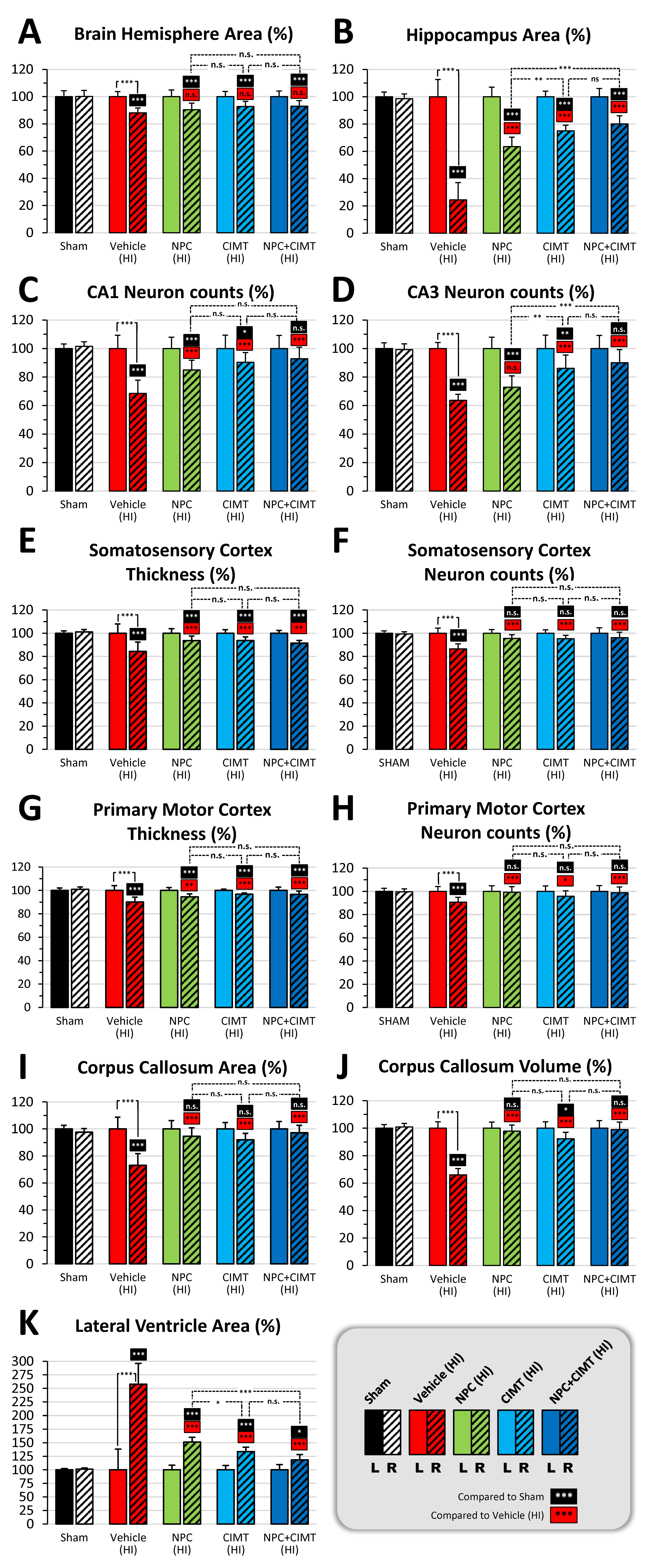
2.2. Transplanted NPCs, CIMT, and NPC + CIMT Lead to Structural Recovery
2.2.1. Neonatal HI Reduces the Overall Brain Size, but No Treatments Restore It
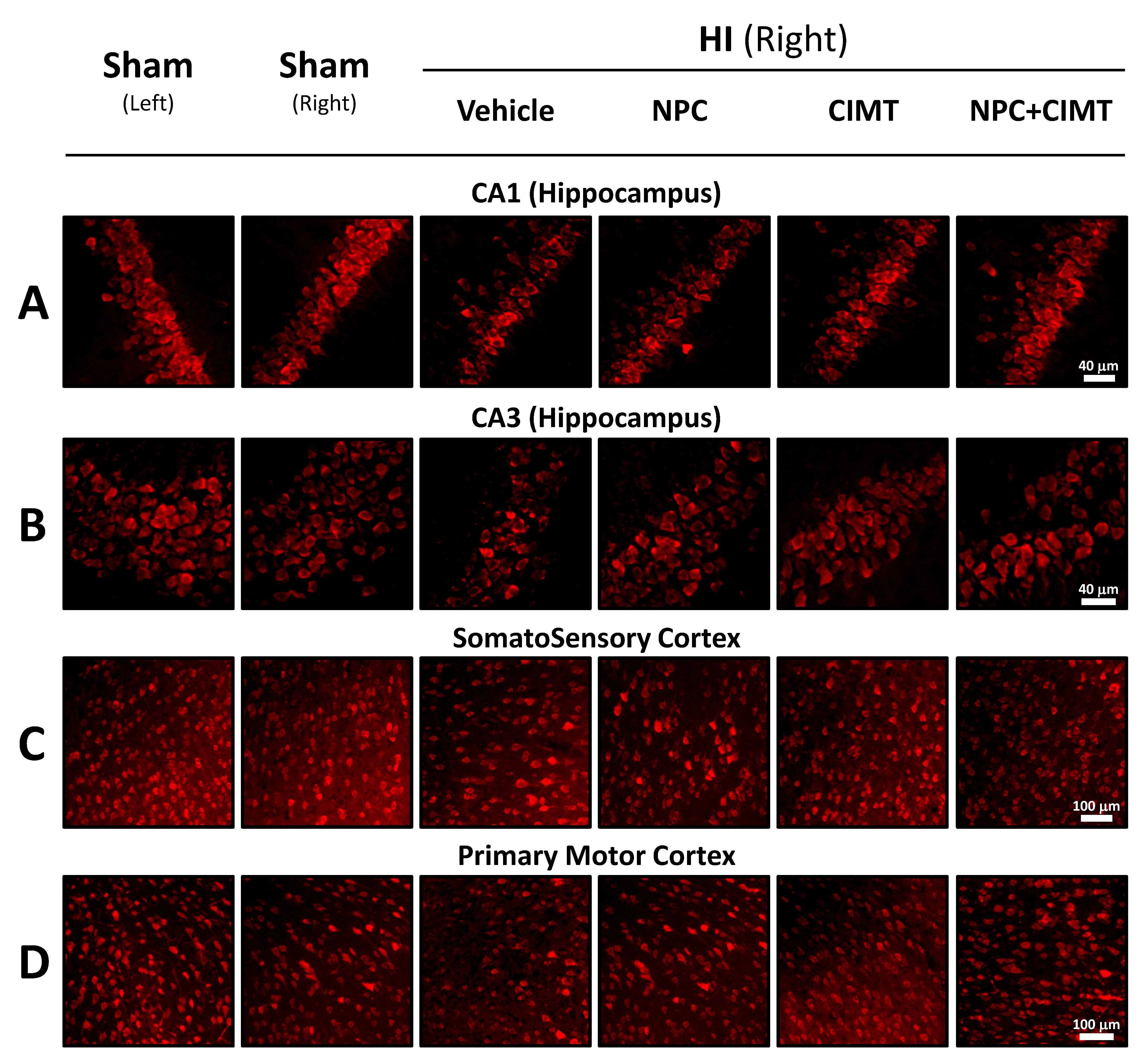
2.2.2. HI Injury Significantly Affects the Hippocampus, and Treatments Mitigate the Damage
2.2.3. HI Injury Affects the Cortex, and Treatment Produces Significant Recovery
2.2.4. HI Injury Significantly Affects the Corpus Callosum, and Treatment with NPCs and CIMT Leads to Recovery
2.2.5. HI Injury Notably Affects the Lateral Ventricle, and Treatment with NPCs and CIMT Leads to Significant Recovery
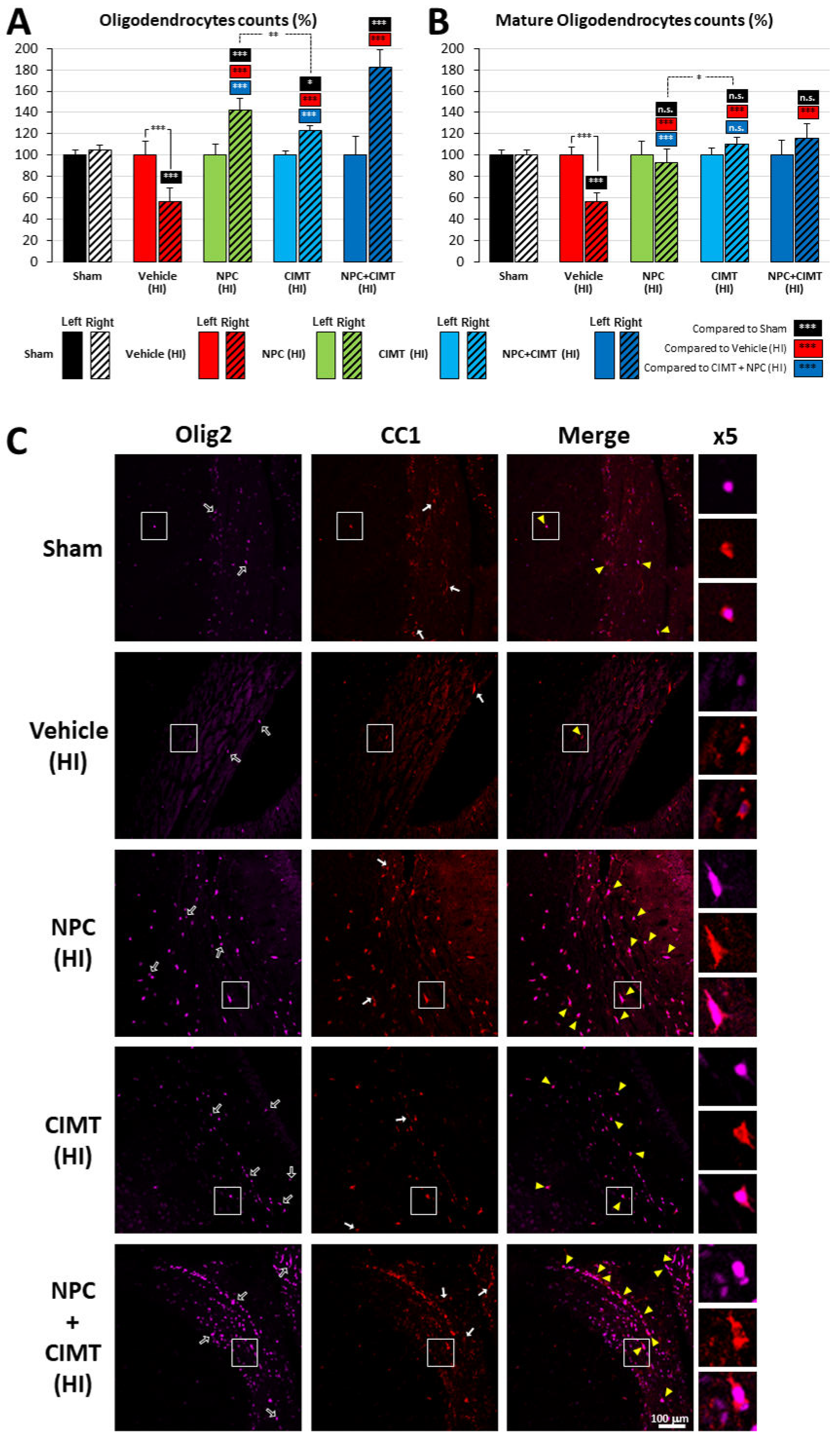
2.3. Transplanted NPCs, CIMT, and the Combination of These Modalities Leads to Myelination
2.3.1. Following HI, Treatment with NPCs and CIMT Restored the Endogenous OL Population above the Normal Level, While the Mature OL Population Was Normalized
2.3.2. HI Injury Significantly Affects Myelination of the CC and Treatment with NPCs and CIMT Leads to Functional Myelination
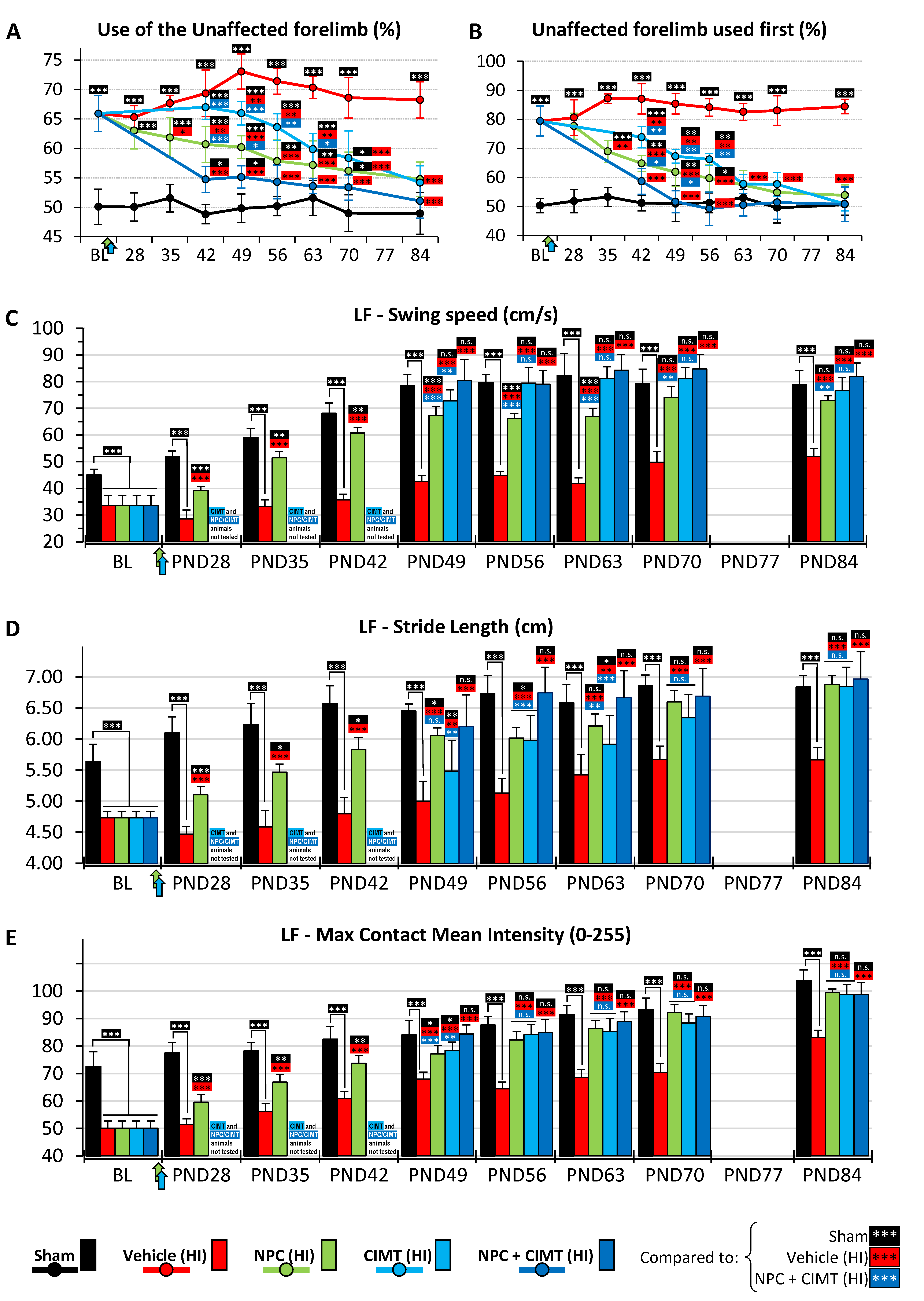
2.4. Transplanted NPC, CIMT, and NPC + CIMT Treatments Led to Functional Recovery
2.4.1. Cylinder Test
2.4.2. CatWalk Gait Assessment
Swing Speed
Stride Length
Paw Intensity
3. Discussion
3.1. Transplanted NPCs Survive, Differentiate Morphologically, and Engraft in Brain Tissue
3.2. NPC Transplantation Leads to Structural and Functional Improvement
3.3. CIMT Also Leads to Structural and Functional Improvement
3.4. NPCs and CIMT in Combination Have a Synergistic Effect on Functional Recovery
4. Materials and Methods
4.1. Animal Use
4.2. Study Design
4.3. Hypoxia-Ischemia Model of Hemiplegic Perinatal Brain Injury
4.4. Adult NPC Transplantation
4.5. Constraint-Induced Movement Therapy (CIMT)
4.6. Structural Assessment
4.6.1. Tissue Preparation
4.6.2. Immunostaining and Histology
4.6.3. Quantification
4.7. Functional Assessment: Electrophysiology
4.8. Functional Assessment: Neurobehavioural Testing
4.8.1. The Cylinder Test
4.8.2. The CatWalk Gait Test
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 15082. [Google Scholar] [CrossRef]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent Hypoxia-Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol Suppl. 2007, 109, 8–14. [Google Scholar] [CrossRef]
- Thoresen, M. Who should we cool after perinatal asphyxia? Semin. Fetal Neonatal Med. 2015, 20, 66–71. [Google Scholar] [CrossRef]
- Faulkner, S.D.; Ruff, C.A.; Fehlings, M.G. The potential for stem cells in cerebral palsy--piecing together the puzzle. Semin. Pediatr. Neurol. 2013, 20, 146–153. [Google Scholar] [CrossRef]
- Morris, D.M.; Taub, E. Constraint-induced therapy approach to restoring function after neurological injury. Top. Stroke Rehabil. 2001, 8, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Taub, E.; Morris, D.M. Constraint-induced movement therapy to enhance recovery after stroke. Curr. Atheroscler. Rep. 2001, 3, 279–286. [Google Scholar] [CrossRef]
- Charles, J.; Gordon, A.M. A critical review of constraint-induced movement therapy and forced use in children with hemiplegia. Neural Plast. 2005, 12, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Hoare, B.; Imms, C.; Carey, L.; Wasiak, J. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy: A Cochrane systematic review. Clin. Rehabil. 2007, 21, 675–685. [Google Scholar] [CrossRef]
- Brady, K.; Garcia, T. Constraint-induced movement therapy (CIMT): Pediatric applications. Dev. Disabil. Res. Rev. 2009, 15, 102–111. [Google Scholar] [CrossRef]
- Novak, I.; McIntyre, S.; Morgan, C.; Campbell, L.; Dark, L.; Morton, N.; Stumbles, E.; Wilson, S.A.; Goldsmith, S. A systematic review of interventions for children with cerebral palsy: State of the evidence. Dev. Med. Child Neurol. 2013, 55, 885–910. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Kang, L.J.; Chen, C.L.; Lin, K.C.; Chen, F.C.; Wu, K.P. Younger Children with Cerebral Palsy Respond Better Than Older Ones to Therapist-Based Constraint-Induced Therapy at Home on Functional Outcomes and Motor Control. Phys. Occup. Ther. Pediatr. 2016, 36, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Manning, K.Y.; Menon, R.S.; Gorter, J.W.; Mesterman, R.; Campbell, C.; Switzer, L.; Fehlings, D. Neuroplastic Sensorimotor Resting State Network Reorganization in Children With Hemiplegic Cerebral Palsy Treated With Constraint-Induced Movement Therapy. J. Child Neurol. 2016, 31, 220–226. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, S.C.; Trucks, M.R.; Wallace, D.A.; Ramey, S.L. Practice-based evidence from a clinical cohort that received pediatric constraint- induced movement therapy. J. Pediatr. Rehabil. Med. 2017, 10, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hoare, B.; Greaves, S. Unimanual versus bimanual therapy in children with unilateral cerebral palsy: Same, same, but different. J. Pediatr. Rehabil. Med. 2017, 10, 47–59. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Nordstrand, L.; Ek, L.; Lennartsson, F.; Sjostrand, L.; Tedroff, K.; Krumlinde-Sundholm, L. The effectiveness of Baby-CIMT in infants younger than 12 months with clinical signs of unilateral-cerebral palsy; an explorative study with randomized design. Res. Dev. Disabil. 2018, 72, 191–201. [Google Scholar] [CrossRef]
- Jones, T.A.; Adkins, D.L. Motor System Reorganization After Stroke: Stimulating and Training Toward Perfection. Physiology 2015, 30, 358–370. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, M.; Xiao, T.; Jolkkonen, J.; Zhao, C. Constraint-induced movement therapy overcomes the intrinsic axonal growth-inhibitory signals in stroke rats. Stroke 2013, 44, 1698–1705. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, B.; Yu, K.W.; Li, C.; Xie, H.Y.; Bao, W.Q.; Kong, Y.Y.; Jiao, F.Y.; Guan, Y.H.; Bai, Y.L. Effects of constraint-induced movement therapy on brain glucose metabolism in a rat model of cerebral ischemia: A micro PET/CT study. Int. J. Neurosci. 2018, 128, 736–745. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Hua, Y.; Zhang, B.; Gao, B.Y.; Liu, P.L.; Sun, L.M.; Lu, R.R.; Wang, Y.Y.; Bai, Y.L. Constrained-induced movement therapy promotes motor function recovery by enhancing the remodeling of ipsilesional corticospinal tract in rats after stroke. Brain Res. 2019, 1708, 27–35. [Google Scholar] [CrossRef]
- Zhai, Z.Y.; Feng, J. Constraint-induced movement therapy enhances angiogenesis and neurogenesis after cerebral ischemia/reperfusion. Neural Regen. Res. 2019, 14, 1743–1754. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Hua, Y.; Liu, P.; Gao, B.; Wang, Y.; Bai, Y. Constraint-induced movement therapy improves functional recovery after ischemic stroke and its impacts on synaptic plasticity in sensorimotor cortex and hippocampus. Brain Res. Bull. 2020, 160, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, P.L.; Hua, Y.; Gao, B.Y.; Wang, Y.Y.; Bai, Y.L.; Chen, C. Constraint-induced movement therapy enhances AMPA receptor-dependent synaptic plasticity in the ipsilateral hemisphere following ischemic stroke. Neural Regen. Res. 2021, 16, 319–324. [Google Scholar] [CrossRef]
- Ishida, A.; Misumi, S.; Ueda, Y.; Shimizu, Y.; Cha-Gyun, J.; Tamakoshi, K.; Ishida, K.; Hida, H. Early constraint-induced movement therapy promotes functional recovery and neuronal plasticity in a subcortical hemorrhage model rat. Behav. Brain Res. 2015, 284, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Koo, Y.S.; Shin, M.J.; Kim, S.Y.; Shin, Y.B.; Choi, B.T.; Yun, Y.J.; Lee, S.Y.; Shin, H.K. Combination of Constraint-Induced Movement Therapy with Electroacupuncture Improves Functional Recovery following Neonatal Hypoxic-Ischemic Brain Injury in Rats. Biomed. Res. Int. 2018, 2018, 8638294. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.V.; Mahmud, N.; Green-Holland, M.; Vonderwalde, I.; Umebayashi, D.; Sachewsky, N.; Coles, B.L.; van Der Kooy, D.; Morshead, C.M. Constraint-induced movement therapy promotes motor recovery after neonatal stroke in the absence of neural precursor activation. Eur. J. Neurosci. 2021, 53, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.R.; Wang, Y.X.; He, L.; Xu, Y.X.; Huang, J.Y.; Peng, T.T.; Yang, X.B.; Pan, J.; Tang, H.M.; Xu, K.S. Constraint-Induced Movement Therapy Promotes Neural Remodeling and Functional Reorganization by Overcoming Nogo-A/NgR/RhoA/ROCK Signals in Hemiplegic Cerebral Palsy Mice. Neurorehabil. Neural Repair. 2021, 35, 145–157. [Google Scholar] [CrossRef] [PubMed]
- McAdams, R.M.; Juul, S.E. Neonatal Encephalopathy: Update on Therapeutic Hypothermia and Other Novel Therapeutics. Clin. Perinatol. 2016, 43, 485–500. [Google Scholar] [CrossRef]
- Ruff, C.A.; Faulkner, S.D.; Fehlings, M.G. The potential for stem cell therapies to have an impact on cerebral palsy: Opportunities and limitations. Dev. Med. Child Neurol. 2013, 55, 689–697. [Google Scholar] [CrossRef]
- Rumajogee, P.; Altamentova, S.; Li, L.; Li, J.; Wang, J.; Kuurstra, A.; Khazaei, M.; Beldick, S.; Menon, R.S.; van der Kooy, D.; et al. Exogenous Neural Precursor Cell Transplantation Results in Structural and Functional Recovery in a Hypoxic-Ischemic Hemiplegic Mouse Model. eNeuro 2018, 5, ENEURO.0369-18.2018. [Google Scholar] [CrossRef]
- Rice, J.E.; 3rd Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Robert, M.T.; Gutterman, J.; Ferre, C.L.; Chin, K.; Brandao, M.B.; Gordon, A.M.; Friel, K. Corpus Callosum Integrity Relates to Improvement of Upper-Extremity Function Following Intensive Rehabilitation in Children With Unilateral Spastic Cerebral Palsy. Neurorehabil. Neural Repair. 2021, 35, 534–544. [Google Scholar] [CrossRef]
- Clowry, G.J.; Basuodan, R.; Chan, F. What are the Best Animal Models for Testing Early Intervention in Cerebral Palsy? Front. Neurol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell. Mol. Life Sci. CMLS 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [PubMed]
- Nait-Oumesmar, B.; Decker, L.; Lachapelle, F.; Avellana-Adalid, V.; Bachelin, C.; Baron-Van Evercooren, A. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 1999, 11, 4357–4366. [Google Scholar] [CrossRef]
- Carmichael, S.T. Plasticity of cortical projections after stroke. The Neuroscientist: A review journal bringing neurobiology. Neurol. Neurol. Neurol. Psychiatry 2003, 9, 64–75. [Google Scholar] [CrossRef]
- Overman, J.J.; Carmichael, S.T. Plasticity in the injured brain: More than molecules matter. The Neuroscientist: A review journal bringing neurobiology. Neurol. Neurol. Neurol. Psychiatry 2014, 20, 15–28. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tanda, K.; Nishimura, A.; Koshino, S.; Kizaki, Z.; Ohno, K. Diffusion restriction in the corticospinal tract and the corpus callosum of term neonates with hypoxic-ischemic encephalopathy. Pediatr. Radiol. 2022, 52, 1356–1369. [Google Scholar] [CrossRef]
- Hayakawa, K.; Lo, E.H. Brain-peripheral cell crosstalk in white matter damage and repair. Biochim. Biophys. Acta 2016, 1862, 901–908. [Google Scholar] [CrossRef]
- Gensert, J.M.; Goldman, J.E. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 1997, 19, 197–203. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Spano, S.; Chew, D.; Wang, S.; Erwin, M.; Chamankhah, M.; Forgione, N.; Fehlings, M.G. An Examination of the Mechanisms by which Neural Precursors Augment Recovery following Spinal Cord Injury: A Key Role for Remyelination. Cell Transplant. 2014, 23, 365–380. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 2018, 66, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Buono, K.D.; Goodus, M.T.; Guardia Clausi, M.; Jiang, Y.; Loporchio, D.; Levison, S.W. Mechanisms of mouse neural precursor expansion after neonatal hypoxia-ischemia. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 8855–8865. [Google Scholar] [CrossRef]
- Chicha, L.; Smith, T.; Guzman, R. Stem cells for brain repair in neonatal hypoxia-ischemia. Childs Nerv. Syst. 2014, 30, 37–46. [Google Scholar] [CrossRef]
- Yu, J.H.; Seo, J.H.; Lee, J.Y.; Lee, M.Y.; Cho, S.R. Induction of Neurorestoration From Endogenous Stem Cells. Cell Transplant. 2016, 25, 863–882. [Google Scholar] [CrossRef] [PubMed]
- Kokaia, Z.; Martino, G.; Schwartz, M.; Lindvall, O. Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat. Neurosci. 2012, 15, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Braccioli, L.; Heijnen, C.J.; Coffer, P.J.; Nijboer, C.H. Delayed administration of neural stem cells after hypoxia-ischemia reduces sensorimotor deficits, cerebral lesion size, and neuroinflammation in neonatal mice. Pediatr. Res. 2017, 81, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Altamentova, S.; Rumajogee, P.; Hong, J.; Beldick, S.R.; Park, S.J.; Yee, A.; Fehlings, M.G. Methylprednisolone Reduces Persistent Post-ischemic Inflammation in a Rat Hypoxia-Ischemia Model of Perinatal Stroke. Transl. Stroke Res. 2020, 11, 1117–1136. [Google Scholar] [CrossRef]
- Ruddy, R.M.; Morshead, C.M. Home sweet home: The neural stem cell niche throughout development and after injury. Cell Tissue Res. 2018, 371, 125–141. [Google Scholar] [CrossRef]
- Dadwal, P.; Mahmud, N.; Sinai, L.; Azimi, A.; Fatt, M.; Wondisford, F.E.; Miller, F.D.; Morshead, C.M. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem Cell Rep. 2015, 5, 166–173. [Google Scholar] [CrossRef]
- Liska, M.G.; Crowley, M.G.; Nguyen, H.; Borlongan, C.V. Biobridge concept in stem cell therapy for ischemic stroke. J. Neurosurg. Sci. 2017, 61, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Xu, K.; Nguyen, H.; Guedes, V.A.; Borlongan, C.V.; Acosta, S.A. Stem Cell-Induced Biobridges as Possible Tools to Aid Neuroreconstruction after CNS Injury. Front. Cell Dev. Biol. 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.; Gonzales-Portillo, G.S.; Acosta, S.A.; Kaneko, Y.; Borlongan, C.V.; Tajiri, N. Stem cell-paved biobridges facilitate stem transplant and host brain cell interactions for stroke therapy. Brain Res. 2015, 1623, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Xu, Y.; Liu, L.; Tang, H.; He, L.; Zhang, J.; Zhou, H.; Xu, Y.; Zhao, J.; et al. Proteomic changes of the bilateral M1 and spinal cord in hemiplegic cerebral palsy mouse: Effects of constraint-induced movement therapy. Behav. Brain Res. 2023, 452, 114583. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Pan, J.; Xu, Y.; Liu, L.; Yang, X.; Huang, S.; Peng, T.; Huang, Y.; Zhao, Y.; Fu, C.; et al. Constraint therapy promotes motor cortex remodeling and functional improvement by regulating c-Jun/miR-182-5p/Nogo—A signals in hemiplegic cerebral palsy mice. Ann. Anat. 2023, 250, 152136. [Google Scholar] [CrossRef]
- Fu, C.; Tang, H.; Liu, L.; Huang, Y.; Zhou, H.; Huang, S.; Peng, T.; Zeng, P.; Yang, X.; He, L.; et al. Constraint-Induced Movement Therapy Promotes Myelin Remodeling and Motor Function by Mediating Sox2/Fyn Signals in Rats with Hemiplegic Cerebral Palsy. Phys. Ther. 2024, 104, pzae011. [Google Scholar] [CrossRef]
- Pusic, K.M.; Pusic, A.D.; Kraig, R.P. Environmental Enrichment Stimulates Immune Cell Secretion of Exosomes that Promote CNS Myelination and May Regulate Inflammation. Cell Mol. Neurobiol. 2016, 36, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Schuch, C.P.; Diaz, R.; Deckmann, I.; Rojas, J.J.; Deniz, B.F.; Pereira, L.O. Early environmental enrichment affects neurobehavioral development and prevents brain damage in rats submitted to neonatal hypoxia-ischemia. Neurosci. Lett. 2016, 617, 101–107. [Google Scholar] [CrossRef]
- Rha, D.W.; Kang, S.W.; Park, Y.G.; Cho, S.R.; Lee, W.T.; Lee, J.E.; Nam, C.M.; Han, K.H.; Park, E.S. Effects of constraint-induced movement therapy on neurogenesis and functional recovery after early hypoxic-ischemic injury in mice. Dev. Med. Child Neurol. 2011, 53, 327–333. [Google Scholar] [CrossRef]
- Palmer, C.; Vannucci, R.C.; Towfighi, J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr. Res. 1990, 27, 332–336. [Google Scholar] [CrossRef]
- Okusa, C.; Oeschger, F.; Ginet, V.; Wang, W.Z.; Hoerder-Suabedissen, A.; Matsuyama, T.; Truttmann, A.C.; Molnár, Z. Subplate in a rat model of preterm hypoxia-ischemia. Ann. Clin. Transl. Neurol. 2014, 1, 679–691. [Google Scholar] [CrossRef] [PubMed]
- McQuillen, P.S.; Sheldon, R.A.; Shatz, C.J.; Ferriero, D.M. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 3308–3315. [Google Scholar] [CrossRef]
- Balduini, W.; De Angelis, V.; Mazzoni, E.; Cimino, M. Long-lasting behavioral alterations following a hypoxic/ischemic brain injury in neonatal rats. Brain Res. 2000, 859, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Coq, J.O.; Delcour, M.; Massicotte, V.S.; Baud, O.; Barbe, M.F. Prenatal ischemia deteriorates white matter, brain organization, and function: Implications for prematurity and cerebral palsy. Dev. Med. Child Neurol. 2016, 58 (Suppl. S4), 7–11. [Google Scholar] [CrossRef]
- Johnston, M.V.; Ferriero, D.M.; Vannucci, S.J.; Hagberg, H. Models of cerebral palsy: Which ones are best? J. Child Neurol. 2005, 20, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Tropepe, V.; Sibilia, M.; Ciruna, B.G.; Rossant, J.; Wagner, E.F.; van der Kooy, D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev. Biol. 1999, 208, 166–188. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.A.; Ye, H.; Legasto, J.M.; Stribbell, N.A.; Wang, J.; Zhang, L.; Fehlings, M.G. Effects of adult neural precursor-derived myelination on axonal function in the perinatal congenitally dysmyelinated brain: Optimizing time of intervention, developing accurate prediction models, and enhancing performance. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 11899–11915. [Google Scholar] [CrossRef]
- Goto, N. Discriminative staining methods for the nervous system: Luxol fast blue—periodic acid-Schiff–hematoxylin triple stain and subsidiary staining methods. Stain. Technol. 1987, 62, 305–315. [Google Scholar] [CrossRef]
- Li, L.; Velumian, A.A.; Samoilova, M.; Fehlings, M.G. A Novel Approach for Studying the Physiology and Pathophysiology of Myelinated and Non-Myelinated Axons in the CNS White Matter. PLoS ONE 2016, 11, e0165637. [Google Scholar] [CrossRef]
- Chen, H.; Du, J.; Zhang, Y.; Barnes, K.; Jia, X. Establishing a Reliable Gait Evaluation Method for Rodent Studies. J. Neurosci. Methods 2017, 283, 92–100. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumajogee, P.; Altamentova, S.; Li, J.; Puvanenthirarajah, N.; Wang, J.; Asgarihafshejani, A.; Van Der Kooy, D.; Fehlings, M.G. Constraint-Induced Movement Therapy (CIMT) and Neural Precursor Cell (NPC) Transplantation Synergistically Promote Anatomical and Functional Recovery in a Hypoxic-Ischemic Mouse Model. Int. J. Mol. Sci. 2024, 25, 9403. https://doi.org/10.3390/ijms25179403
Rumajogee P, Altamentova S, Li J, Puvanenthirarajah N, Wang J, Asgarihafshejani A, Van Der Kooy D, Fehlings MG. Constraint-Induced Movement Therapy (CIMT) and Neural Precursor Cell (NPC) Transplantation Synergistically Promote Anatomical and Functional Recovery in a Hypoxic-Ischemic Mouse Model. International Journal of Molecular Sciences. 2024; 25(17):9403. https://doi.org/10.3390/ijms25179403
Chicago/Turabian StyleRumajogee, Prakasham, Svetlana Altamentova, Junyi Li, Nirushan Puvanenthirarajah, Jian Wang, Azam Asgarihafshejani, Derek Van Der Kooy, and Michael G. Fehlings. 2024. "Constraint-Induced Movement Therapy (CIMT) and Neural Precursor Cell (NPC) Transplantation Synergistically Promote Anatomical and Functional Recovery in a Hypoxic-Ischemic Mouse Model" International Journal of Molecular Sciences 25, no. 17: 9403. https://doi.org/10.3390/ijms25179403
APA StyleRumajogee, P., Altamentova, S., Li, J., Puvanenthirarajah, N., Wang, J., Asgarihafshejani, A., Van Der Kooy, D., & Fehlings, M. G. (2024). Constraint-Induced Movement Therapy (CIMT) and Neural Precursor Cell (NPC) Transplantation Synergistically Promote Anatomical and Functional Recovery in a Hypoxic-Ischemic Mouse Model. International Journal of Molecular Sciences, 25(17), 9403. https://doi.org/10.3390/ijms25179403





