Viral Factors in Modulation of Host Immune Response: A Route to Novel Antiviral Agents and New Therapeutic Approaches
Abstract
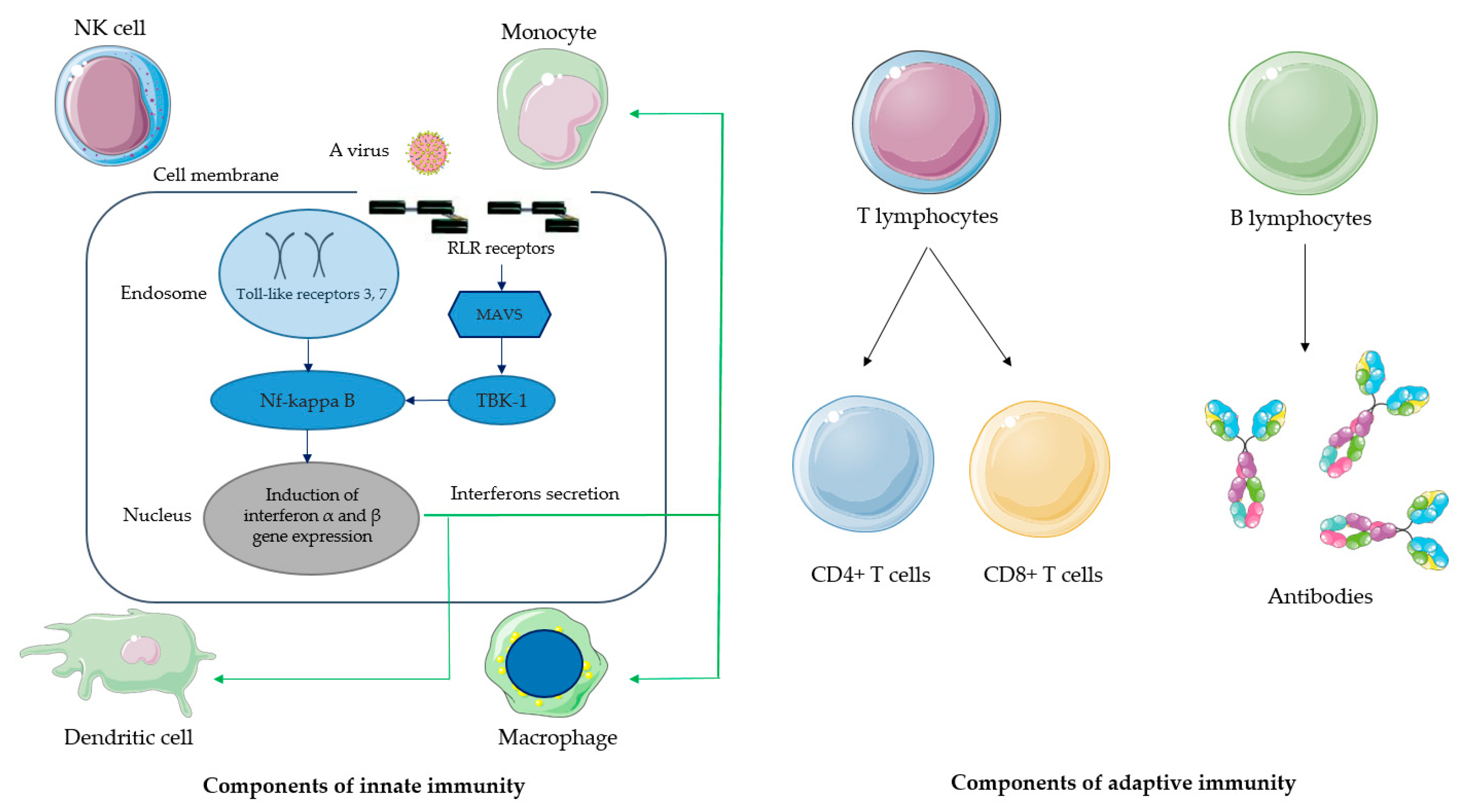
:1. Introduction
2. Molecular Mechanisms of Immune Response to Viral Infection
| Virus | Key Viral Enzyme | Molecular Mechanism | Systemic Effect | Reference |
|---|---|---|---|---|
| SARS-CoV-2 | Papain-like protease (PLpro) | PLpro cleaves ubiquitin-like interferon-stimulated gene 15 protein (ISG15) | Deregulation of host interferon response | [1,21] |
| NSP1, NSP6 | Inhibition of STAT1 phosphorylation antagonizes the IFN signaling | Inhibition of interferon response | [22] | |
| NSP8 | Interaction with MDA5 leads to the inhibition of IFN3 phosphorylation | Inhibition of interferon response | [22] | |
| Hepatitis B | HBc, HBs, HBe antigens | Exhaustion of HBV specific T and B cells. Hepatotropism | Immune tolerance occurring due to HBc, HBs and HBe antigens | [1] |
| IP0, ICP4, US3 | ICP0-mediated translocation of USP7 (Ubiquitin-specific-processing protease7) from the nucleus to cytoplasm | Inhibition of TLR-mediated immune response | [23,24] | |
| Herpes simplex virus type 1 | US11 | US11 interacts with RIG-1 and MDA5 and leads to a blockade of signal transduction | Negative downstream regulation of interferon type 1 transcription | [24] |
| RNA intermediates, host derived RNAs | RNA intermediates interact with RLRs, leading to inhibition of signal transduction | Negative downstream regulation of interferon type 1 transcription | [24] | |
| ICP0 | ICP0 mediates modulation of interferon type 3 response | Inhibition of interferon response | [24] | |
| HSV gE and gC | HSV gE and gC inhibits specific antibody response | Inhibition of antibody response | [25,26] | |
| Kaposi’s Sarcoma-Associated Herpes virus | ORF64 | ORF64 interferes with RIG-I ubiquitination | Inhibition of interferon response | [27] |
| Epstein–Barr virus | BPLF1 | BPLF1 interferes with RIG-I ubiquitination and downregulates interferon response; BPLF1 interacts with and deubiquitinates TRAF6 | Inhibition of interferon response | [28,29,30] |
| Influenza A virus | NS1, NS2 | NS1 inhibits RIG-1 activation; NS2 interacts with IRF7 | Inhibition of interferon production | [31] |
| PB1 | Interaction with MAVS | Inhibition of interferon production | [31] | |
| PB2, HA | Inhibition of JAK1/STAT pathway | Inhibition of interferon signaling | [31] | |
| Human immuno-deficiency virus | Nef | HIV Nef protein interacts with naïve B cells and macrophages and inhibits interferon response | Inhibition of interferon response | [32] |
| Vif | HIV Vif inhibits ubiquitination of STING influencing on STING-TBK1-IRF3 pathway | Inhibition of the production of type I interferon | [33] |
3. Development of Therapeutic Strategies Based on the Current Knowledge on Virus–Host Interactions and Modulation of Immune Response to Viral Infections
4. New Approaches to HIV Treatment and Cure Based on Fighting Latency and Targeting CD4+ T Lymphocyte Proliferation
5. Recent Efforts in the Development of Monoclonal Antibodies in Treatment of Viral Infections
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kuipery, A.; Gehring, A.J.; Isogawa, M. Mechanisms of HBV Immune Evasion. Antivir. Res. 2020, 179, 104816. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.G.; Wang, N.; Chen, Z.; Chen, Z.; Tseng, M.; Barretto, N.; Lin, R.; Peters, C.J.; Tseng, C.-T.K.; Baker, S.C.; et al. Regulation of IRF-3-Dependent Innate Immunity by the Papain-like Protease Domain of the Severe Acute Respiratory Syndrome Coronavirus. J. Biol. Chem. 2007, 282, 32208–32221. [Google Scholar] [CrossRef]
- Frieman, M.; Ratia, K.; Johnston, R.E.; Mesecar, A.D.; Baric, R.S. Severe Acute Respiratory Syndrome Coronavirus Papain-like Protease Ubiquitin-like Domain and Catalytic Domain Regulate Antagonism of IRF3 and NF-KappaB Signaling. J. Virol. 2009, 83, 6689–6705. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like Protease Regulates SARS-CoV-2 Viral Spread and Innate Immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef]
- Locci, M.; Havenar-Daughton, C.; Landais, E.; Wu, J.; Kroenke, M.A.; Arlehamn, C.L.; Su, L.F.; Cubas, R.; Davis, M.M.; Sette, A.; et al. Human Circulating PD-1+CXCR3-CXCR5+ Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity 2013, 39, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Ohlson, M.B.; Eitson, J.L.; Wells, A.I.; Kumar, A.; Jang, S.; Ni, C.; Xing, C.; Buszczak, M.; Schoggins, J.W. Genome-Scale CRISPR Screening Reveals Host Factors Required for Ribosome Formation and Viral Replication. mBio 2023, 14, e0012723. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Yang, S.; Yang, X.; Lian, X.; Huang, Y.; Dong, X.; Zhang, Z. Human Gene Functional Network-Informed Prediction of HIV-1 Host Dependency Factors. mSystems 2020, 5, e00960-20. [Google Scholar] [CrossRef]
- Ivanov, S.; Lagunin, A.; Filimonov, D.; Tarasova, O. Network-Based Analysis of OMICs Data to Understand the HIV-Host Interaction. Front. Microbiol. 2020, 11, 1314. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-Adaptive Immunity Interplay and Redox Regulation in Immune Response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Loo, Y.-M.; Gale, M. Immune Signaling by RIG-I-like Receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, W. TANK-Binding Kinase 1 as a Novel Therapeutic Target for Viral Diseases. Expert. Opin. Ther. Targets 2019, 23, 437–446. [Google Scholar] [CrossRef]
- Soto, J.A.; Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Bohmwald, K.; Berrios, R.V.; Bueno, S.M.; Kalergis, A.M. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front. Immunol. 2020, 11, 1513. [Google Scholar] [CrossRef]
- Gelmez, M.Y.; Oktelik, F.B.; Tahrali, I.; Yilmaz, V.; Kucuksezer, U.C.; Akdeniz, N.; Cetin, E.A.; Kose, M.; Cinar, C.; Oguz, F.S.; et al. Immune Modulation as a Consequence of SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 954391. [Google Scholar] [CrossRef]
- Gadotti, A.C.; de Castro Deus, M.; Telles, J.P.; Wind, R.; Goes, M.; Garcia Charello Ossoski, R.; de Padua, A.M.; de Noronha, L.; Moreno-Amaral, A.; Baena, C.P.; et al. IFN-γ Is an Independent Risk Factor Associated with Mortality in Patients with Moderate and Severe COVID-19 Infection. Virus Res. 2020, 289, 198171. [Google Scholar] [CrossRef] [PubMed]
- Trevejo, J.M.; Marino, M.W.; Philpott, N.; Josien, R.; Richards, E.C.; Elkon, K.B.; Falck-Pedersen, E. TNF-α-Dependent Maturation of Local Dendritic Cells Is Critical for Activating the Adaptive Immune Response to Virus Infection. Proc. Natl. Acad. Sci. USA 2001, 98, 12162–12167. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Coquard, L.; Herbein, G. Targeting TNF-Alpha in HIV-1 Infection. Curr. Drug Targets 2016, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.D.; Salinas, E.; Grakoui, A. Immune System Control of Hepatitis C Virus Infection. Curr. Opin. Virol. 2021, 46, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of Adaptive Immunity by the Innate Immune System. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Koh, C.-H.; Lee, S.; Kwak, M.; Kim, B.-S.; Chung, Y. CD8 T-Cell Subsets: Heterogeneity, Functions, and Therapeutic Potential. Exp. Mol. Med. 2023, 55, 2287–2299. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Yan, H.; Huang, J.; Wang, F.; Liu, T.; Zeng, L.; Zhou, F. ISGylation in Innate Antiviral Immunity and Pathogen Defense Responses: A Review. Front. Cell Dev. Biol. 2021, 9, 788410. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Xie, Z.; Suleman, M.; Shah, A.; Khan, S.; Luo, S. Roles and Functions of SARS-CoV-2 Proteins in Host Immune Evasion. Front. Immunol. 2022, 13, 940756. [Google Scholar] [CrossRef] [PubMed]
- Daubeuf, S.; Singh, D.; Tan, Y.; Liu, H.; Federoff, H.J.; Bowers, W.J.; Tolba, K. HSV ICP0 Recruits USP7 to Modulate TLR-Mediated Innate Response. Blood 2009, 113, 3264–3275. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, C. The Race between Host Antiviral Innate Immunity and the Immune Evasion Strategies of Herpes Simplex Virus 1. Microbiol. Mol. Biol. Rev. 2020, 84, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, J.M.; Jiang, M.; Hook, L.; Chang, Y.; Sarver, C.; Mastellos, D.; Lambris, J.D.; Cohen, G.H.; Eisenberg, R.J.; Friedman, H.M. Herpes Simplex Virus Type 1 Evades the Effects of Antibody and Complement in Vivo. J. Virol. 2002, 76, 9232–9241. [Google Scholar] [CrossRef]
- Collins, W.J.; Johnson, D.C. Herpes Simplex Virus GE/GI Expressed in Epithelial Cells Interferes with Cell-to-Cell Spread. J. Virol. 2003, 77, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Inn, K.-S.; Lee, S.-H.; Rathbun, J.Y.; Wong, L.-Y.; Toth, Z.; Machida, K.; Ou, J.-H.J.; Jung, J.U. Inhibition of RIG-I-Mediated Signaling by Kaposi’s Sarcoma-Associated Herpesvirus-Encoded Deubiquitinase ORF64. J. Virol. 2011, 85, 10899–10904. [Google Scholar] [CrossRef]
- Atkins, S.L.; Motaib, S.; Wiser, L.C.; Hopcraft, S.E.; Hardy, P.B.; Shackelford, J.; Foote, P.; Wade, A.H.; Damania, B.; Pagano, J.S.; et al. Small Molecule Screening Identifies Inhibitors of the Epstein-Barr Virus Deubiquitinating Enzyme, BPLF1. Antivir. Res. 2020, 173, 104649. [Google Scholar] [CrossRef]
- Gupta, S.; Ylä-Anttila, P.; Callegari, S.; Tsai, M.-H.; Delecluse, H.-J.; Masucci, M.G. Herpesvirus Deconjugases Inhibit the IFN Response by Promoting TRIM25 Autoubiquitination and Functional Inactivation of the RIG-I Signalosome. PLoS Pathog. 2018, 14, e1006852. [Google Scholar] [CrossRef]
- Lui, W.-Y.; Bharti, A.; Wong, N.-H.M.; Jangra, S.; Botelho, M.G.; Yuen, K.-S.; Jin, D.-Y. Suppression of cGAS- and RIG-I-Mediated Innate Immune Signaling by Epstein-Barr Virus Deubiquitinase BPLF1. PLoS Pathog. 2023, 19, e1011186. [Google Scholar] [CrossRef]
- Rashid, F.; Xie, Z.; Li, M.; Xie, Z.; Luo, S.; Xie, L. Roles and Functions of IAV Proteins in Host Immune Evasion. Front. Immunol. 2023, 14, 1323560. [Google Scholar] [CrossRef]
- Moir, S.; Fauci, A.S. B-Cell Responses to HIV Infection. Immunol. Rev. 2017, 275, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, G.; Zhu, L.; Zhao, Z.; Liu, Y.; Han, W.; Zhang, X.; Zhang, Y.; Xiong, T.; Zeng, H.; et al. HIV-1 Vif Suppresses Antiviral Immunity by Targeting STING. Cell Mol. Immunol. 2022, 19, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Majerová, T.; Konvalinka, J. Viral Proteases as Therapeutic Targets. Mol. Aspects Med. 2022, 88, 101159. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chan, J.F.-W.; Wang, Y.; Yuen, T.T.-T.; Chai, Y.; Hou, Y.; Shuai, H.; Yang, D.; Hu, B.; Huang, X.; et al. Comparative Replication and Immune Activation Profiles of SARS-CoV-2 and SARS-CoV in Human Lungs: An Ex Vivo Study With Implications for the Pathogenesis of COVID-19. Clin. Infect. Dis. 2020, 71, 1400–1409. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Ng, T.I.; Correia, I.; Seagal, J.; DeGoey, D.A.; Schrimpf, M.R.; Hardee, D.J.; Noey, E.L.; Kati, W.M. Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses 2022, 14, 961. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Sloan, D.D.; Han, J.-Y.; Sandifer, T.K.; Stewart, M.; Hinz, A.J.; Yoon, M.; Johnson, D.C.; Spear, P.G.; Jerome, K.R. Inhibition of TCR Signaling by Herpes Simplex Virus. J. Immunol. 2006, 176, 1825–1833. [Google Scholar] [CrossRef]
- Tian, Y.; Kuo, C.; Akbari, O.; Ou, J.J. Maternal-Derived Hepatitis B Virus e Antigen Alters Macrophage Function in Offspring to Drive Viral Persistence after Vertical Transmission. Immunity 2016, 44, 1204–1214. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Virus Entry: Molecular Mechanisms and Biomedical Applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef]
- Wei, X.; Decker, J.M.; Wang, S.; Hui, H.; Kappes, J.C.; Wu, X.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Kilby, J.M.; Saag, M.S.; et al. Antibody Neutralization and Escape by HIV-1. Nature 2003, 422, 307–312. [Google Scholar] [CrossRef]
- Kwong, P.D.; Doyle, M.L.; Casper, D.J.; Cicala, C.; Leavitt, S.A.; Majeed, S.; Steenbeke, T.D.; Venturi, M.; Chaiken, I.; Fung, M.; et al. HIV-1 Evades Antibody-Mediated Neutralization through Conformational Masking of Receptor-Binding Sites. Nature 2002, 420, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, S.; Xu, X.; Ma, C.; Zhang, P.; Ji, W.; Liu, X. HIV-1 P17 Matrix Protein Enhances Type I Interferon Responses through the P17-OLA1-STING Axis. J. Cell Sci. 2024, 137, jcs261500. [Google Scholar] [CrossRef]
- Moir, S.; Ho, J.; Malaspina, A.; Wang, W.; DiPoto, A.C.; O’Shea, M.A.; Roby, G.; Kottilil, S.; Arthos, J.; Proschan, M.A.; et al. Evidence for HIV-Associated B Cell Exhaustion in a Dysfunctional Memory B Cell Compartment in HIV-Infected Viremic Individuals. J. Exp. Med. 2008, 205, 1797–1805. [Google Scholar] [CrossRef]
- Levesque, M.C.; Moody, M.A.; Hwang, K.-K.; Marshall, D.J.; Whitesides, J.F.; Amos, J.D.; Gurley, T.C.; Allgood, S.; Haynes, B.B.; Vandergrift, N.A.; et al. Polyclonal B Cell Differentiation and Loss of Gastrointestinal Tract Germinal Centers in the Earliest Stages of HIV-1 Infection. PLoS Med. 2009, 6, e1000107. [Google Scholar] [CrossRef] [PubMed]
- Speck, S.H.; Ganem, D. Viral Latency and Its Regulation: Lessons from the Gamma-Herpesviruses. Cell Host Microbe 2010, 8, 100–115. [Google Scholar] [CrossRef]
- Murata, T.; Sugimoto, A.; Inagaki, T.; Yanagi, Y.; Watanabe, T.; Sato, Y.; Kimura, H. Molecular Basis of Epstein-Barr Virus Latency Establishment and Lytic Reactivation. Viruses 2021, 13, 2344. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; Mogensen, T.H.; Cohrs, R.J. Recent Issues in Varicella-Zoster Virus Latency. Viruses 2021, 13, 2018. [Google Scholar] [CrossRef] [PubMed]
- Dahabieh, M.S.; Battivelli, E.; Verdin, E. Understanding HIV Latency: The Road to an HIV Cure. Annu. Rev. Med. 2015, 66, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Ameya, G.; Birri, D.J. The Molecular Mechanisms of Virus-Induced Human Cancers. Microb. Pathog. 2023, 183, 106292. [Google Scholar] [CrossRef] [PubMed]
- Clé, M.; Eldin, P.; Briant, L.; Lannuzel, A.; Simonin, Y.; Van de Perre, P.; Cabié, A.; Salinas, S. Neurocognitive Impacts of Arbovirus Infections. J. Neuroinflamm. 2020, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Greene, C.; Dayaramani, C.; Rauchman, S.H.; Stecker, M.M.; De Leon, J.; Pinkhasov, A. Long COVID, the Brain, Nerves, and Cognitive Function. Neurol. Int. 2023, 15, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Aguocha, C.M.; Okeke, C.V.; Okoro, C.B.; Peterson, J.C. HIV-Related Neurocognitive Disorders: Diagnosis, Treatment, and Mental Health Implications: A Review. Medicine 2023, 102, e35652. [Google Scholar] [CrossRef]
- Wang, X.; Zou, P.; Wu, F.; Lu, L.; Jiang, S. Development of Small-Molecule Viral Inhibitors Targeting Various Stages of the Life Cycle of Emerging and Re-Emerging Viruses. Front. Med. 2017, 11, 449–461. [Google Scholar] [CrossRef]
- Liang, R.; Wang, L.; Zhang, N.; Deng, X.; Su, M.; Su, Y.; Hu, L.; He, C.; Ying, T.; Jiang, S.; et al. Development of Small-Molecule MERS-CoV Inhibitors. Viruses 2018, 10, 721. [Google Scholar] [CrossRef]
- Picazo, E.; Giordanetto, F. Small Molecule Inhibitors of Ebola Virus Infection. Drug Discov. Today 2015, 20, 277–286. [Google Scholar] [CrossRef]
- Haese, N.; Powers, J.; Streblow, D.N. Small Molecule Inhibitors Targeting Chikungunya Virus. In Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Ivanenkov, Y.A.; Aladinskiy, V.A.; Bushkov, N.A.; Ayginin, A.A.; Majouga, A.G.; Ivachtchenko, A.V. Small-Molecule Inhibitors of Hepatitis C Virus (HCV) Non-Structural Protein 5A (NS5A): A Patent Review (2010–2015). Expert. Opin. Ther. Pat. 2017, 27, 401–414. [Google Scholar] [CrossRef]
- Lu, L.; Yu, F.; Cai, L.; Debnath, A.K.; Jiang, S. Development of Small-Molecule HIV Entry Inhibitors Specifically Targeting Gp120 or Gp41. Curr. Top. Med. Chem. 2016, 16, 1074–1090. [Google Scholar] [CrossRef]
- Belda, O.; Targett-Adams, P. Small Molecule Inhibitors of the Hepatitis C Virus-Encoded NS5A Protein. Virus Res. 2012, 170, 1–14. [Google Scholar] [CrossRef]
- Elseginy, S.A.; Massarotti, A.; Nawwar, G.A.; Amin, K.M.; Brancale, A. Small Molecule Inhibitors of West Nile Virus. Antivir. Chem. Chemother. 2014, 23, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Pitts, J.; Hsia, C.-Y.; Lian, W.; Wang, J.; Pfeil, M.-P.; Kwiatkowski, N.; Li, Z.; Jang, J.; Gray, N.S.; Yang, P.L. Identification of Small Molecule Inhibitors Targeting the Zika Virus Envelope Protein. Antivir. Res. 2019, 164, 147–153. [Google Scholar] [CrossRef]
- Yuan, Y.; Miao, Y.; Zeng, C.; Liu, J.; Chen, X.; Qian, L.; Wang, X.; Qian, F.; Yu, Z.; Wang, J.; et al. Small-Molecule Inhibitors of Ubiquitin-Specific Protease 7 Enhance Type-I Interferon Antiviral Efficacy by Destabilizing SOCS1. Immunology 2020, 159, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Ibba, R.; Corona, P.; Nonne, F.; Caria, P.; Serreli, G.; Palmas, V.; Riu, F.; Sestito, S.; Nieddu, M.; Loddo, R.; et al. Design, Synthesis, and Antiviral Activities of New Benzotriazole-Based Derivatives. Pharmaceuticals 2023, 16, 429. [Google Scholar] [CrossRef]
- Bugatti, K.; Sartori, A.; Battistini, L.; Coppa, C.; Vanhulle, E.; Noppen, S.; Provinciael, B.; Naesens, L.; Stevaert, A.; Contini, A.; et al. Novel Polymyxin-Inspired Peptidomimetics Targeting the SARS-CoV-2 Spike:hACE2 Interface. Int. J. Mol. Sci. 2023, 24, 8765. [Google Scholar] [CrossRef]
- Haas, K.M.; McGregor, M.J.; Bouhaddou, M.; Polacco, B.J.; Kim, E.-Y.; Nguyen, T.T.; Newton, B.W.; Urbanowski, M.; Kim, H.; Williams, M.A.P.; et al. Proteomic and Genetic Analyses of Influenza A Viruses Identify Pan-Viral Host Targets. Nat. Commun. 2023, 14, 6030. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, X.; Wang, X.; Liu, T.; Zhao, Y.; Chen, L.; Luo, Y.; Du, H.; Li, Y.; Liu, T.; et al. TBK1-METTL3 Axis Facilitates Antiviral Immunity. Cell Rep. 2022, 38, 110373. [Google Scholar] [CrossRef]
- Dang, W.; Xie, Y.; Cao, P.; Xin, S.; Wang, J.; Li, S.; Li, Y.; Lu, J. N6-Methyladenosine and Viral Infection. Front. Microbiol. 2019, 10, 417. [Google Scholar] [CrossRef]
- Du, Y.; Yuan, Y.; Xu, L.; Zhao, F.; Wang, W.; Xu, Y.; Tian, X. Discovery of METTL3 Small Molecule Inhibitors by Virtual Screening of Natural Products. Front. Pharmacol. 2022, 13, 878135. [Google Scholar] [CrossRef]
- Burgess, H.M.; Depledge, D.P.; Thompson, L.; Srinivas, K.P.; Grande, R.C.; Vink, E.I.; Abebe, J.S.; Blackaby, W.P.; Hendrick, A.; Albertella, M.R.; et al. Targeting the m6A RNA Modification Pathway Blocks SARS-CoV-2 and HCoV-OC43 Replication. Genes Dev. 2021, 35, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Kostyusheva, A.; Brezgin, S.; Glebe, D.; Kostyushev, D.; Chulanov, V. Host-Cell Interactions in HBV Infection and Pathogenesis: The Emerging Role of m6A Modification. Emerg. Microbes Infect. 2021, 10, 2264–2275. [Google Scholar] [CrossRef]
- Winkler, R.; Gillis, E.; Lasman, L.; Safra, M.; Geula, S.; Soyris, C.; Nachshon, A.; Tai-Schmiedel, J.; Friedman, N.; Le-Trilling, V.T.K.; et al. m6A Modification Controls the Innate Immune Response to Infection by Targeting Type I Interferons. Nat. Immunol. 2019, 20, 173–182. [Google Scholar] [CrossRef]
- Zagaliotis, P.; Petrou, A.; Mystridis, G.; Geronikaki, A.; Vizirianakis, I.; Walsh, T. Developing New Treatments for COVID-19 through Dual-Action Antiviral/Anti-Inflammatory Small Molecules and Physiologically Based Pharmacokinetic Modeling. Int. J. Mol. Sci. 2022, 23, 8006. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Sugi, T.; Iida, S.; Hirata, Y.; Kusakabe, S.; Konishi, K.; Itakura, Y.; Tabata, K.; Kishimoto, M.; Kobayashi, H.; et al. Combination Therapy with Oral Antiviral and Anti-Inflammatory Drugs Improves the Efficacy of Delayed Treatment in a COVID-19 Hamster Model. eBioMedicine 2024, 99, 104950. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Shitu, Z.; Mostafa, A. Drug Repurposing of Nitazoxanide: Can It Be an Effective Therapy for COVID-19? J. Genet. Eng. Biotechnol. 2020, 18, 35. [Google Scholar] [CrossRef]
- Bharti, C.; Sharma, S.; Goswami, N.; Sharma, H.; Rabbani, S.A.; Kumar, S. Role of Nitazoxanide as a Repurposed Drug in the Treatment and Management of Various Diseases. Drugs Today 2021, 57, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Nair, M.S.; Masuda, K.; Castagna, C.; Chong, Z.; Darling, T.L.; Seehra, K.; Hwang, Y.; Ribeiro, Á.L.; Ferreira, G.M.; et al. An Immunostimulatory Glycolipid That Blocks SARS-CoV-2, RSV, and Influenza Infections in Vivo. Nat. Commun. 2023, 14, 3959. [Google Scholar] [CrossRef]
- Salerno, M.; Varricchio, C.; Bevilacqua, F.; Jochmans, D.; Neyts, J.; Brancale, A.; Ferla, S.; Bassetto, M. Rational Design of Novel Nucleoside Analogues Reveals Potent Antiviral Agents for EV71. Eur. J. Med. Chem. 2023, 246, 114942. [Google Scholar] [CrossRef]
- Phillips, S.; Jagatia, R.; Chokshi, S. Novel Therapeutic Strategies for Chronic Hepatitis B. Virulence 2022, 13, 1111–1132. [Google Scholar] [CrossRef]
- Dawood, A.; Abdul Basit, S.; Jayaraj, M.; Gish, R.G. Drugs in Development for Hepatitis B. Drugs 2017, 77, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Thapa, R.J.; Guo, H.; Block, T.M. Host Functions Used by Hepatitis B Virus to Complete Its Life Cycle: Implications for Developing Host-Targeting Agents to Treat Chronic Hepatitis B. Antivir. Res. 2018, 158, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.S.; Jones, C. Wnt Antagonists Suppress Herpes Simplex Virus Type 1 Productive Infection. Antivir. Res. 2021, 191, 105082. [Google Scholar] [CrossRef] [PubMed]
- Koujah, L.; Madavaraju, K.; Agelidis, A.M.; Patil, C.D.; Shukla, D. Heparanase-Induced Activation of AKT Stabilizes β-Catenin and Modulates Wnt/β-Catenin Signaling during Herpes Simplex Virus 1 Infection. mBio 2021, 12, e0279221. [Google Scholar] [CrossRef]
- Zhu, L.; Jones, C. The Canonical Wnt/β-Catenin Signaling Pathway Stimulates Herpes Simplex Virus 1 Productive Infection. Virus Res. 2018, 256, 29–37. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, R.; Cao, Q.; Yang, X.; Huang, Z.; An, J. Discoveries and Developments of CXCR4-Targeted HIV-1 Entry Inhibitors. Exp. Biol. Med. 2020, 245, 477–485. [Google Scholar] [CrossRef]
- Armani-Tourret, M.; Zhou, Z.; Gasser, R.; Staropoli, I.; Cantaloube-Ferrieu, V.; Benureau, Y.; Garcia-Perez, J.; Pérez-Olmeda, M.; Lorin, V.; Puissant-Lubrano, B.; et al. Mechanisms of HIV-1 Evasion to the Antiviral Activity of Chemokine CXCL12 Indicate Potential Links with Pathogenesis. PLoS Pathog. 2021, 17, e1009526. [Google Scholar] [CrossRef]
- Mohamed, H.; Gurrola, T.; Berman, R.; Collins, M.; Sariyer, I.K.; Nonnemacher, M.R.; Wigdahl, B. Targeting CCR5 as a Component of an HIV-1 Therapeutic Strategy. Front. Immunol. 2021, 12, 816515. [Google Scholar] [CrossRef]
- Weichseldorfer, M.; Affram, Y.; Heredia, A.; Tagaya, Y.; Benedetti, F.; Zella, D.; Reitz, M.; Romerio, F.; Latinovic, O.S. Anti-HIV Activity of Standard Combined Antiretroviral Therapy in Primary Cells Is Intensified by CCR5-Targeting Drugs. AIDS Res. Hum. Retroviruses 2020, 36, 835–841. [Google Scholar] [CrossRef]
- Rossi, R.; Lichtner, M.; De Rosa, A.; Sauzullo, I.; Mengoni, F.; Massetti, A.P.; Mastroianni, C.M.; Vullo, V. In Vitro Effect of Anti-Human Immunodeficiency Virus CCR5 Antagonist Maraviroc on Chemotactic Activity of Monocytes, Macrophages and Dendritic Cells. Clin. Exp. Immunol. 2011, 166, 184–190. [Google Scholar] [CrossRef]
- Haneklaus, M.; Gerlic, M.; O’Neill, L.A.J.; Masters, S.L. MiR-223: Infection, Inflammation and Cancer. J. Intern. Med. 2013, 274, 215–226. [Google Scholar] [CrossRef]
- Duan, S.; Wang, S.; Song, Y.; Gao, N.; Meng, L.; Gai, Y.; Zhang, Y.; Wang, S.; Wang, C.; Yu, B.; et al. A Novel HIV-1 Inhibitor That Blocks Viral Replication and Rescues APOBEC3s by Interrupting Vif/CBFβ Interaction. J. Biol. Chem. 2020, 295, 14592–14605. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.A.; Tolstrup, M.; Brinkmann, C.R.; Olesen, R.; Erikstrup, C.; Solomon, A.; Winckelmann, A.; Palmer, S.; Dinarello, C.; Buzon, M.; et al. Panobinostat, a Histone Deacetylase Inhibitor, for Latent-Virus Reactivation in HIV-Infected Patients on Suppressive Antiretroviral Therapy: A Phase 1/2, Single Group, Clinical Trial. Lancet HIV 2014, 1, e13–e21. [Google Scholar] [CrossRef]
- Castelli, V.; Lombardi, A.; Palomba, E.; Bozzi, G.; Ungaro, R.; Alagna, L.; Mangioni, D.; Muscatello, A.; Bandera, A.; Gori, A. Immune Checkpoint Inhibitors in People Living with HIV/AIDS: Facts and Controversies. Cells 2021, 10, 2227. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.-R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 Expression on HIV-Specific CD8+ T Cells Leads to Reversible Immune Dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Abbar, B.; Baron, M.; Katlama, C.; Marcelin, A.-G.; Veyri, M.; Autran, B.; Guihot, A.; Spano, J.-P. Immune Checkpoint Inhibitors in People Living with HIV: What about Anti-HIV Effects? Aids 2020, 34, 167–175. [Google Scholar] [CrossRef]
- Archin, N.M.; Liberty, A.L.; Kashuba, A.D.; Choudhary, S.K.; Kuruc, J.D.; Crooks, A.M.; Parker, D.C.; Anderson, E.M.; Kearney, M.F.; Strain, M.C.; et al. Administration of Vorinostat Disrupts HIV-1 Latency in Patients on Antiretroviral Therapy. Nature 2012, 487, 482–485. [Google Scholar] [CrossRef]
- Elliott, J.H.; McMahon, J.H.; Chang, C.C.; Lee, S.A.; Hartogensis, W.; Bumpus, N.; Savic, R.; Roney, J.; Hoh, R.; Solomon, A.; et al. Short-Term Administration of Disulfiram for Reversal of Latent HIV Infection: A Phase 2 Dose-Escalation Study. Lancet HIV 2015, 2, e520–e529. [Google Scholar] [CrossRef]
- Elliott, J.H.; Wightman, F.; Solomon, A.; Ghneim, K.; Ahlers, J.; Cameron, M.J.; Smith, M.Z.; Spelman, T.; McMahon, J.; Velayudham, P.; et al. Activation of HIV Transcription with Short-Course Vorinostat in HIV-Infected Patients on Suppressive Antiretroviral Therapy. PLoS Pathog. 2014, 10, e1004473. [Google Scholar] [CrossRef]
- Rasmussen, T.A.; Tolstrup, M.; Søgaard, O.S. Reversal of Latency as Part of a Cure for HIV-1. Trends Microbiol. 2016, 24, 90–97. [Google Scholar] [CrossRef]
- Moranguinho, I.; Valente, S.T. Block-And-Lock: New Horizons for a Cure for HIV-1. Viruses 2020, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Ahlenstiel, C.L.; Symonds, G.; Kent, S.J.; Kelleher, A.D. Block and Lock HIV Cure Strategies to Control the Latent Reservoir. Front. Cell. Infect. Microbiol. 2020, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.A. Mitochondrial Exhaustion of Memory CD4 T-Cells in Treated HIV-1 Infection. Immunometabolism 2022, 4, e220013. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.R.; Chen, B.; Mudd, J.C.; Panigrahi, S.; Shive, C.L.; Sieg, S.F.; Cameron, C.M.; Zidar, D.A.; Funderburg, N.T.; Younes, S.-A.; et al. Inflammescent CX3CR1+CD57+CD8+ T Cells Are Generated and Expanded by IL-15. JCI Insight 2020, 5, e132963. [Google Scholar] [CrossRef]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. MTOR Controls Mitochondrial Oxidative Function through a YY1-PGC-1alpha Transcriptional Complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef]
- Gardiner, D.; Lalezari, J.; Lawitz, E.; DiMicco, M.; Ghalib, R.; Reddy, K.R.; Chang, K.-M.; Sulkowski, M.; Marro, S.O.; Anderson, J.; et al. A Randomized, Double-Blind, Placebo-Controlled Assessment of BMS-936558, a Fully Human Monoclonal Antibody to Programmed Death-1 (PD-1), in Patients with Chronic Hepatitis C Virus Infection. PLoS ONE 2013, 8, e63818. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-Directed Antiviral Therapy. Clin. Microbiol. Rev. 2020, 33, e00168-19. [Google Scholar] [CrossRef]
- Funderburg, N.; Kalinowska, M.; Eason, J.; Goodrich, J.; Heera, J.; Mayer, H.; Rajicic, N.; Valdez, H.; Lederman, M.M. Effects of Maraviroc and Efavirenz on Markers of Immune Activation and Inflammation and Associations with CD4+ Cell Rises in HIV-Infected Patients. PLoS ONE 2010, 5, e13188. [Google Scholar] [CrossRef]
- Iacob, S.A.; Iacob, D.G. Ibalizumab Targeting CD4 Receptors, An Emerging Molecule in HIV Therapy. Front. Microbiol. 2017, 8, 2323. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Jiang, S. Peptide Fusion Inhibitors Targeting the HIV-1 Gp41: A Patent Review (2009–2014). Expert. Opin. Ther. Pat. 2015, 25, 159–173. [Google Scholar] [CrossRef]
- Cobos Jiménez, V.; Booiman, T.; de Taeye, S.W.; van Dort, K.A.; Rits, M.A.N.; Hamann, J.; Kootstra, N.A. Differential Expression of HIV-1 Interfering Factors in Monocyte-Derived Macrophages Stimulated with Polarizing Cytokines or Interferons. Sci. Rep. 2012, 2, 763. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The MTOR Kinase Differentially Regulates Effector and Regulatory T Cell Lineage Commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, Y.; Nagai, S.; Ikejiri, A.; Ohtani, M.; Ichiyama, K.; Baba, Y.; Yamada, T.; Egami, S.; Hoshii, T.; Hirao, A.; et al. PI3K-Akt-MTORC1-S6K1/2 Axis Controls Th17 Differentiation by Regulating Gfi1 Expression and Nuclear Translocation of RORγ. Cell Rep. 2012, 1, 360–373. [Google Scholar] [CrossRef]
- Yang, K.; Shrestha, S.; Zeng, H.; Karmaus, P.W.F.; Neale, G.; Vogel, P.; Guertin, D.A.; Lamb, R.F.; Chi, H. T Cell Exit from Quiescence and Differentiation into Th2 Cells Depend on Raptor-MTORC1-Mediated Metabolic Reprogramming. Immunity 2013, 39, 1043–1056. [Google Scholar] [CrossRef]
- Park, Y.; Jin, H.-S.; Lopez, J.; Elly, C.; Kim, G.; Murai, M.; Kronenberg, M.; Liu, Y.-C. TSC1 Regulates the Balance between Effector and Regulatory T Cells. J. Clin. Investig. 2013, 123, 5165–5178. [Google Scholar] [CrossRef]
- Nakaya, M.; Xiao, Y.; Zhou, X.; Chang, J.-H.; Chang, M.; Cheng, X.; Blonska, M.; Lin, X.; Sun, S.-C. Inflammatory T Cell Responses Rely on Amino Acid Transporter ASCT2 Facilitation of Glutamine Uptake and MTORC1 Kinase Activation. Immunity 2014, 40, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Turner, A.P.; Shaffer, V.O.; Gangappa, S.; Keller, S.A.; Bachmann, M.F.; Larsen, C.P.; Ahmed, R. MTOR Regulates Memory CD8 T-Cell Differentiation. Nature 2009, 460, 108–112. [Google Scholar] [CrossRef]
- Pearce, E.L.; Walsh, M.C.; Cejas, P.J.; Harms, G.M.; Shen, H.; Wang, L.-S.; Jones, R.G.; Choi, Y. Enhancing CD8 T-Cell Memory by Modulating Fatty Acid Metabolism. Nature 2009, 460, 103–107. [Google Scholar] [CrossRef]
- Rao, R.R.; Li, Q.; Odunsi, K.; Shrikant, P.A. The MTOR Kinase Determines Effector versus Memory CD8+ T Cell Fate by Regulating the Expression of Transcription Factors T-Bet and Eomesodermin. Immunity 2010, 32, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Rasmussen, T.A.; Lewin, S.R. Shocking HIV out of Hiding: Where Are We with Clinical Trials of Latency Reversing Agents? Curr. Opin. HIV AIDS 2016, 11, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Paik, J. Lenacapavir: First Approval. Drugs 2022, 82, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Dvory-Sobol, H.; Shaik, N.; Callebaut, C.; Rhee, M.S. Lenacapavir: A First-in-Class HIV-1 Capsid Inhibitor. Curr. Opin. HIV AIDS 2022, 17, 15–21. [Google Scholar] [CrossRef]
- Beninger, P. Lenacapavir. Clin. Ther. 2024, 46, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Tuan, J.; Ogbuagu, O. Lenacapavir: A Twice-Yearly Treatment for Adults with Multidrug-Resistant HIV Infection and Limited Treatment Options. Expert. Rev. Anti-Infect. Ther. 2023, 21, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Ogbuagu, O.; Segal-Maurer, S.; Ratanasuwan, W.; Avihingsanon, A.; Brinson, C.; Workowski, K.; Antinori, A.; Yazdanpanah, Y.; Trottier, B.; Wang, H.; et al. Efficacy and Safety of the Novel Capsid Inhibitor Lenacapavir to Treat Multidrug-Resistant HIV: Week 52 Results of a Phase 2/3 Trial. Lancet HIV 2023, 10, e497–e505. [Google Scholar] [CrossRef]
- Tailor, M.W.; Chahine, E.B.; Koren, D.; Sherman, E.M. Lenacapavir: A Novel Long-Acting Capsid Inhibitor for HIV. Ann. Pharmacother. 2024, 58, 185–195. [Google Scholar] [CrossRef]
- Segal-Maurer, S.; DeJesus, E.; Stellbrink, H.-J.; Castagna, A.; Richmond, G.J.; Sinclair, G.I.; Siripassorn, K.; Ruane, P.J.; Berhe, M.; Wang, H.; et al. Capsid Inhibition with Lenacapavir in Multidrug-Resistant HIV-1 Infection. N. Engl. J. Med. 2022, 386, 1793–1803. [Google Scholar] [CrossRef]
- Di Perri, G. Pharmacological Outlook of Lenacapavir: A Novel First-in-Class Long-Acting HIV-1 Capsid Inhibitor. Infez. Med. 2023, 31, 495–499. [Google Scholar] [CrossRef]
- Hsu, D.C.; O’Connell, R.J. Progress in HIV Vaccine Development. Hum. Vaccin. Immunother. 2017, 13, 1018–1030. [Google Scholar] [CrossRef]
- Sok, D.; Burton, D.R. Recent Progress in Broadly Neutralizing Antibodies to HIV. Nat. Immunol. 2018, 19, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Brett-Major, D.M.; Crowell, T.A.; Michael, N.L. Prospecting for an HIV Vaccine. Trop. Dis. Travel. Med. Vaccines 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Advancing an HIV Vaccine; Advancing Vaccinology. Nat. Rev. Immunol. 2019, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Hokello, J.; Sharma, A.L.; Tyagi, M. An Update on the HIV DNA Vaccine Strategy. Vaccines 2021, 9, 605. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to Combat Viral Infections: Development Strategies and Progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef]
- Mitra, S.; Tomar, P.C. Hybridoma Technology; Advancements, Clinical Significance, and Future Aspects. J. Genet. Eng. Biotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Ryu, D.-K.; Song, R.; Kim, M.; Kim, Y.-I.; Kim, C.; Kim, J.-I.; Kwon, K.-S.; Tijsma, A.S.; Nuijten, P.M.; van Baalen, C.A.; et al. Therapeutic Effect of CT-P59 against SARS-CoV-2 South African Variant. Biochem. Biophys. Res. Commun. 2021, 566, 135–140. [Google Scholar] [CrossRef]
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (Bebtelovimab) Potently Neutralizes SARS-CoV-2 Variants. Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef]
- Focosi, D.; McConnell, S.; Casadevall, A.; Cappello, E.; Valdiserra, G.; Tuccori, M. Monoclonal Antibody Therapies against SARS-CoV-2. Lancet Infect. Dis. 2022, 22, e311–e326. [Google Scholar] [CrossRef]
- Ordaya, E.E.; Razonable, R.R. Emerging Anti-Spike Monoclonal Antibodies against SARS-CoV-2. Expert. Opin. Biol. Ther. 2024, 24, 191–201. [Google Scholar] [CrossRef]
- ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Efficacy and Safety of Two Neutralising Monoclonal Antibody Therapies, Sotrovimab and BRII-196 plus BRII-198, for Adults Hospitalised with COVID-19 (TICO): A Randomised Controlled Trial. Lancet Infect. Dis. 2022, 22, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Sakharkar, M.; Rappazzo, C.G.; Wieland-Alter, W.F.; Hsieh, C.-L.; Wrapp, D.; Esterman, E.S.; Kaku, C.I.; Wec, A.Z.; Geoghegan, J.C.; McLellan, J.S.; et al. Prolonged Evolution of the Human B Cell Response to SARS-CoV-2 Infection. Sci. Immunol. 2021, 6, eabg6916. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J.; et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of COVID-19. N. Engl. J. Med. 2022, 386, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- Both, L.; Banyard, A.C.; van Dolleweerd, C.; Wright, E.; Ma, J.K.-C.; Fooks, A.R. Monoclonal Antibodies for Prophylactic and Therapeutic Use against Viral Infections. Vaccine 2013, 31, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; McMahon, M.; Turner, H.L.; Zhu, X.; Turner, J.S.; Ozorowski, G.; Stadlbauer, D.; Vahokoski, J.; Schmitz, A.J.; Rizk, A.A.; et al. Human Anti-N1 Monoclonal Antibodies Elicited by Pandemic H1N1 Virus Infection Broadly Inhibit HxN1 Viruses in Vitro and in Vivo. Immunity 2023, 56, 1927–1938.e8. [Google Scholar] [CrossRef]
- Esposito, S.; Abu Raya, B.; Baraldi, E.; Flanagan, K.; Martinon Torres, F.; Tsolia, M.; Zielen, S. RSV Prevention in All Infants: Which Is the Most Preferable Strategy? Front. Immunol. 2022, 13, 880368. [Google Scholar] [CrossRef]
- Langedijk, A.C.; Harding, E.R.; Konya, B.; Vrancken, B.; Lebbink, R.J.; Evers, A.; Willemsen, J.; Lemey, P.; Bont, L.J. A Systematic Review on Global RSV Genetic Data: Identification of Knowledge Gaps. Rev. Med. Virol. 2022, 32, e2284. [Google Scholar] [CrossRef]
- Liu, G.; He, S.; Chan, M.; Zhang, Z.; Schulz, H.; Cao, W.; Rahim, M.N.; Audet, J.; Garnett, L.; Wec, A.; et al. A Pan-Ebolavirus Monoclonal Antibody Cocktail Provides Protection Against Ebola and Sudan Viruses. J. Infect. Dis. 2023, 228, S691–S700. [Google Scholar] [CrossRef]
- Cabán, M.; Rodarte, J.V.; Bibby, M.; Gray, M.D.; Taylor, J.J.; Pancera, M.; Boonyaratanakornkit, J. Cross-Protective Antibodies against Common Endemic Respiratory Viruses. Nat. Commun. 2023, 14, 798. [Google Scholar] [CrossRef]
- Waldmann, H. Human Monoclonal Antibodies: The Benefits of Humanization. Methods Mol. Biol. 2019, 1904, 1–10. [Google Scholar] [CrossRef]
| Virus | Key Host Target | Drug | Effect | Reference |
|---|---|---|---|---|
| SARS-CoV-2 | TBK1, METTL3 | quercetin, nucleoside analogues | Stabilization of interferon 3 (IRF3) mRNA that influence interferon production | [1,69,70,71,72,73,74] |
| Cytokines: TNF-α, IL-3, IL-6. | sabizabulin, opaganib, selinexor, atazanavir, nitazoxanide | Stabilizing of inflammatory response/antiviral effect | [22,75,76,77,78] | |
| CD1d | 7DW8-5 | Stimulation of NKT cells to release the cascade of cytokines and chemokines/inhibition of virus replication | [79] | |
| Enterovirus-71 | METTL3 | methylthioadenosine & S-tubercidinyl-l-homocysteine analogs | Inhibition of METTL3 | [80] |
| Hepatitis B | TLRs 3,7,8,9 | GS-9620 | Induction of peripheral IFN-stimulated gene 15 (ISG15) expression | [81,82] |
| NTCP | myrcludex | binding to NTCP and blockade of de novo HBV infection | [83] | |
| Herpes simplex virus type 1 | Wnt/β-catenin | iCRT14 KYA1797K | Inhibition of β-catenin-dependent transcription/enhancement of the formation of the β-catenin destruction complex. Inhibition of virus penetration and virus replication | [84,85,86] |
| Kaposi’s Sarcoma-Associated Herpes virus | NF-κB signaling | suramin | Inhibitors of ORF64 prevent inhibition of TLR-mediated activation of NF-κB signaling, preventing inhibition of interferon response | [28] |
| Epstein–Barr virus | USP7/RIG family, NF-κB signaling, TRAF6 | suramin | Inhibitors of BPLF1 prevent inhibition of TLR-mediated activation of NF-κB signaling, preventing inhibition of interferon response | [28] |
| Influenza A | MAP2K3, MAP2K6, CDK2, ILK, PRKDC | daunorubicin, verdinexor, selinexor, bafilomycin A1, alexidine, lestaurtinib, MRT68921 | RNA splicing and processing, cellular and nuclear membranes, regulation of gene silencing, and innate immune response | [68] |
| Human immuno-deficiency virus | CCR5 | maraviroc | Antagonists of CCR5 prevent entry of HIV into the host cell | [87,88,89,90,91] |
| Granzyme B, IKKα, Roquin and STAT3 | miR-223 | Prevention of chronic inflammation | [92] | |
| CBFβ/Vif-3 complex | CV-3 | inhibition of HIV-1 replication | [93] | |
| Human immunodeficiency virus | Histone deacetylase/Histone methyltransferase, DNA methyltransferase, bromodomain inhibitors; protein kinase C (PKC) agonists | vorinostat, panobinostat, romidepsin, disulfiram, | “shock and kill” Activation of lymphocytes infected with HIV in the latent stage for further application of antiretroviral therapy | [94,95,96,97,98,99,100,101] |
| LEDGF/p75 FACT HSP90 Jak-STAT BRD4 mTOR | didehydro-cortistatin A (dCA), LEDGINs curaxin CBL0100, AUY922, 17-AAG ruxolitinib, tofacitinib, ZL0580 PF-3758309, danusertib, AZ628, P276-00 | “block and lock”, prevention of HIV transcription and reactivation in latently infected cells | [102,103] | |
| PPARα and PPARγ agonists | bezafibrate, GW7647, pioglitazone | Activation of CD4+ T lymphocytes proliferation | [104,105,106] |
| Virus | Drug | Molecular Target | Reference |
|---|---|---|---|
| SARS-CoV-2 | tixagevimab, cilgavimab | SARS-CoV-2 spike protein | [138,139,140,141,142,143,144] |
| Respiratory syncytial virus (RSV) | palivisumab, nirsevimab, MK-1654 | An epitope of the F protein of RSV | [147,148] |
| Ebola virus, Sudan virus | 4F9, 6H8 | Ebola virus glycoprotein Sudan virus glycoprotein | [149] |
| Human parainfluenza virus | 3 × 1 | F protein of HPIV3 | [150] |
| Respiratory syncytial virus Human pneumovirus | M×R | F protein of RSV | [150] |
| Hepatitis B infection (Immune checkpoint inhibitor) | nivolumab | Programmed cell death (PD-1) inhibitor | [107] |
| Human Immunodeficiency virus 1 (Immune checkpoint inhibitor) | nivolumab, ipilimumab | Cytotoxic T lymphocyte associated protein 4 (CTLA-4) | [95,96,97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, O.; Petrou, A.; Ivanov, S.M.; Geronikaki, A.; Poroikov, V. Viral Factors in Modulation of Host Immune Response: A Route to Novel Antiviral Agents and New Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 9408. https://doi.org/10.3390/ijms25179408
Tarasova O, Petrou A, Ivanov SM, Geronikaki A, Poroikov V. Viral Factors in Modulation of Host Immune Response: A Route to Novel Antiviral Agents and New Therapeutic Approaches. International Journal of Molecular Sciences. 2024; 25(17):9408. https://doi.org/10.3390/ijms25179408
Chicago/Turabian StyleTarasova, Olga, Anthi Petrou, Sergey M. Ivanov, Athina Geronikaki, and Vladimir Poroikov. 2024. "Viral Factors in Modulation of Host Immune Response: A Route to Novel Antiviral Agents and New Therapeutic Approaches" International Journal of Molecular Sciences 25, no. 17: 9408. https://doi.org/10.3390/ijms25179408







