The Role of Serum Albumin and Secretory Phospholipase A2 in Sepsis
Abstract
1. Introduction
2. Results
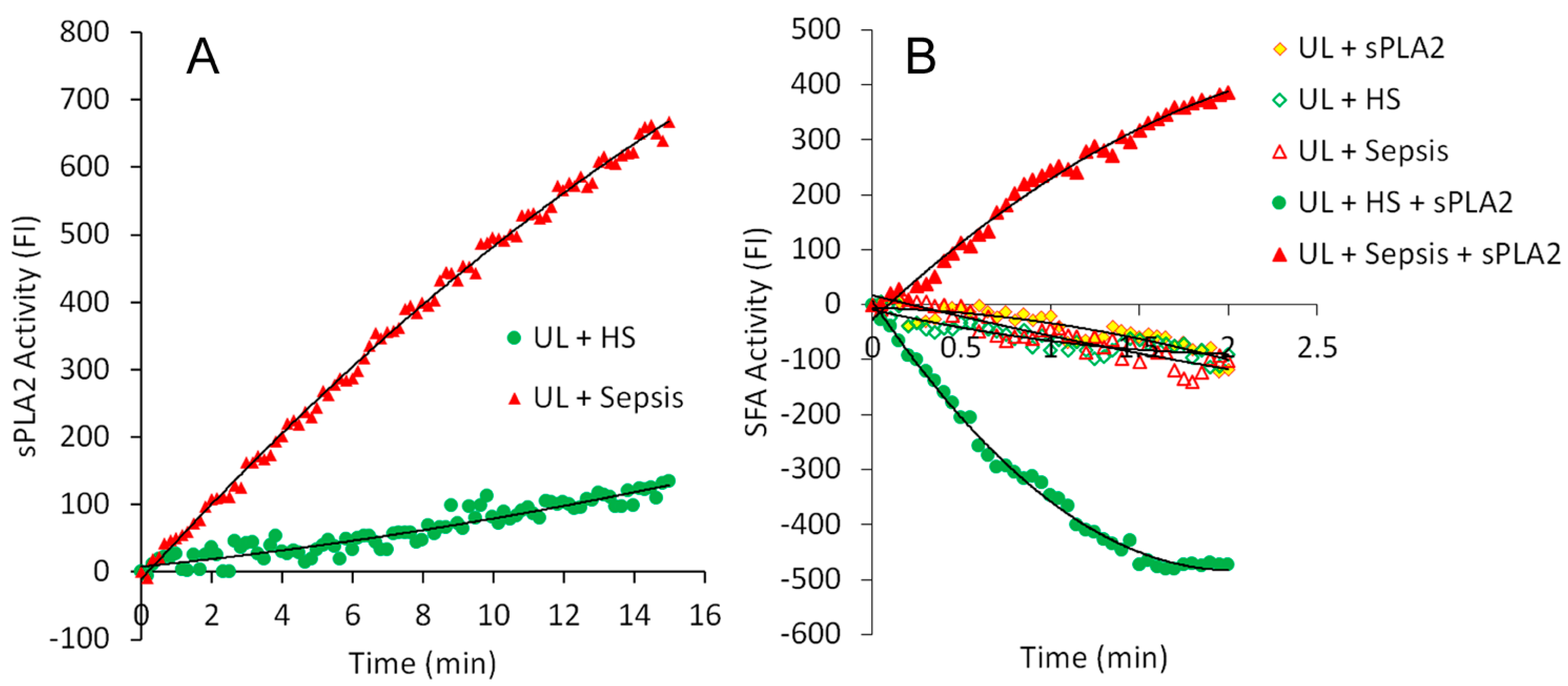
2.1. Real-Time Determination of the Activities of sPLA2 and SFA in the Sera of Healthy Subjects and Patients with Sepsis
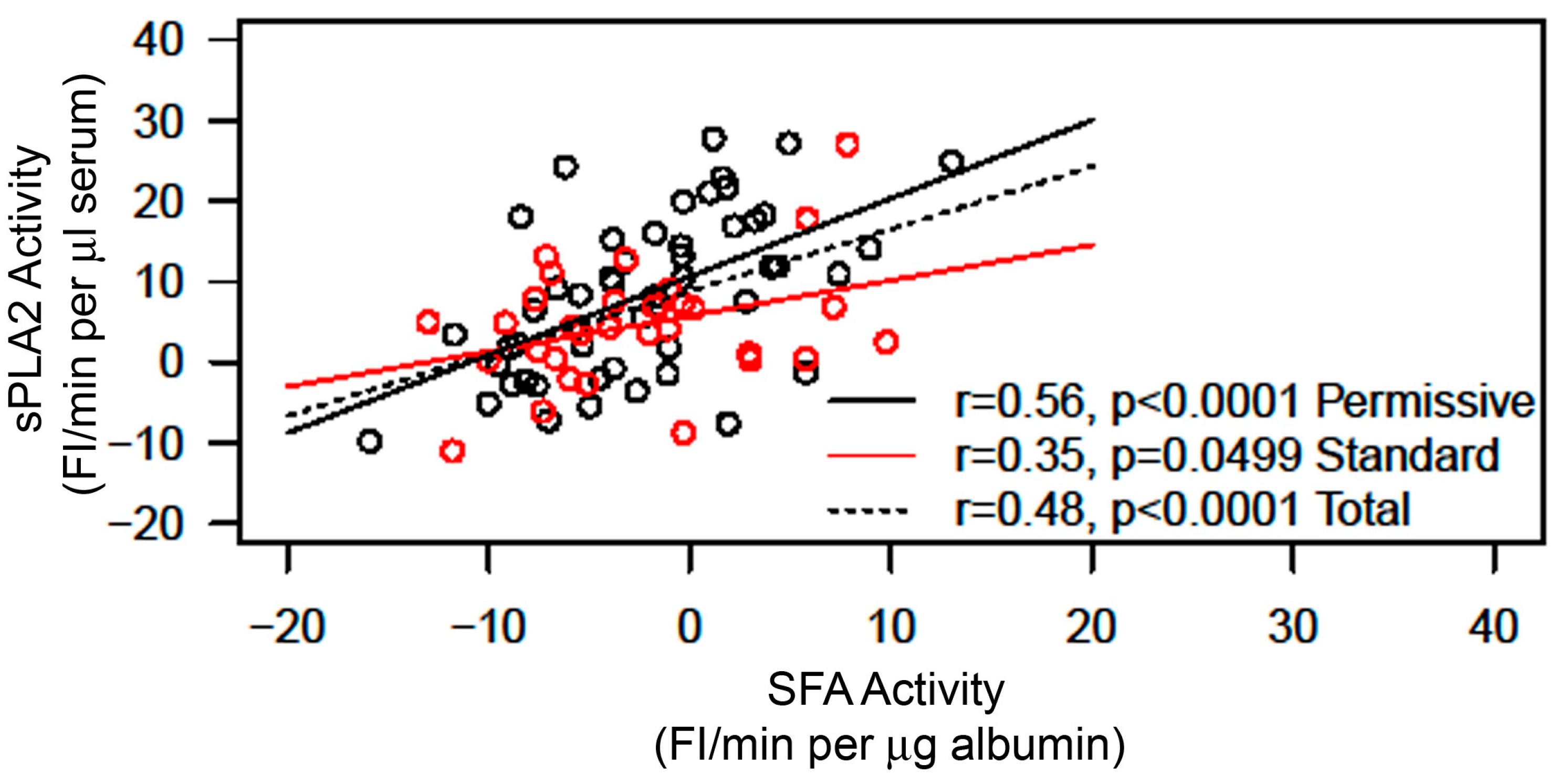
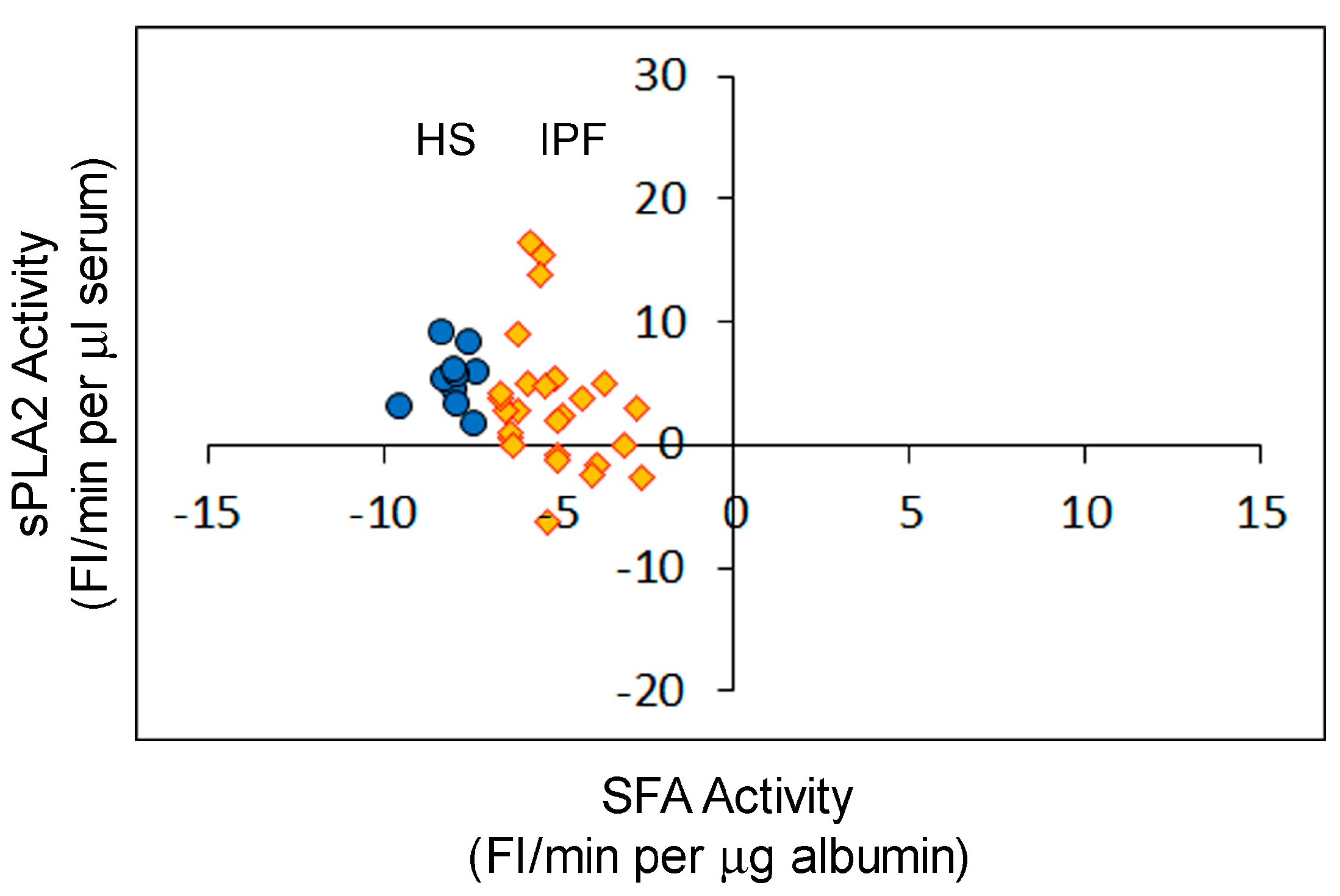
2.2. Correlation between sPLA2 Activity and SFA Activity in the Sera of Patients with Sepsis
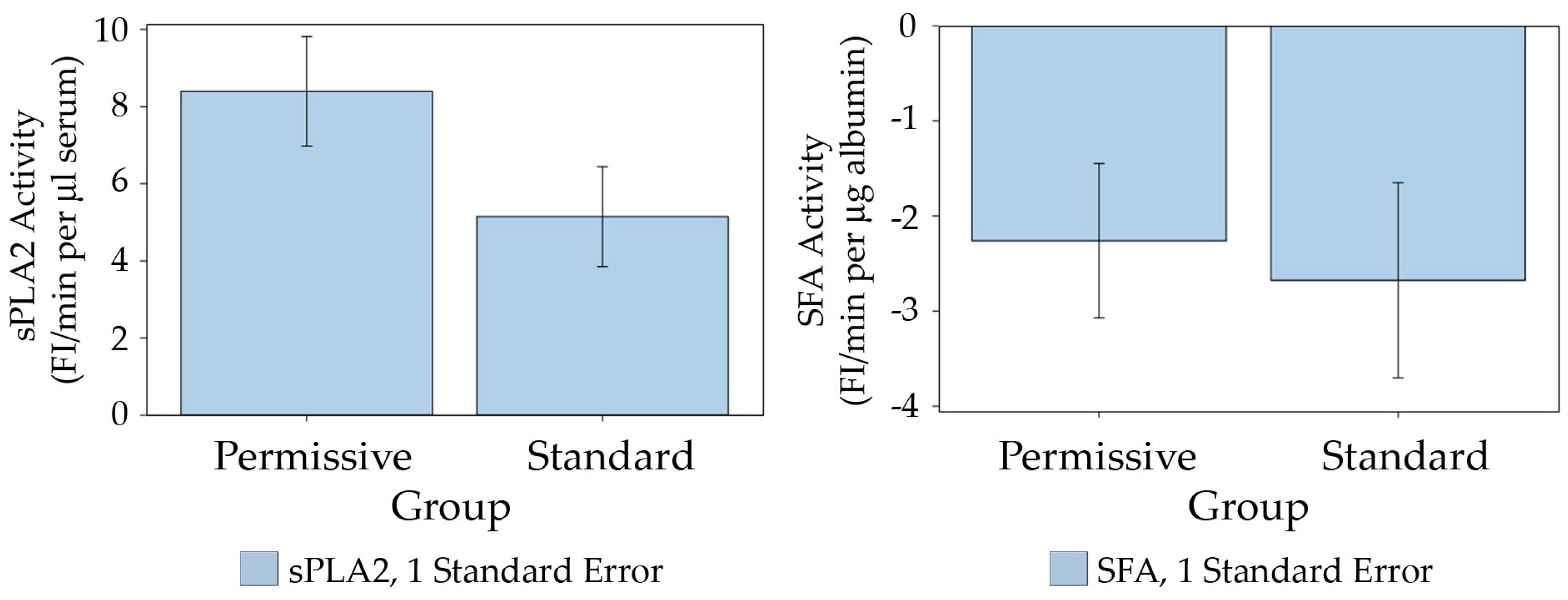
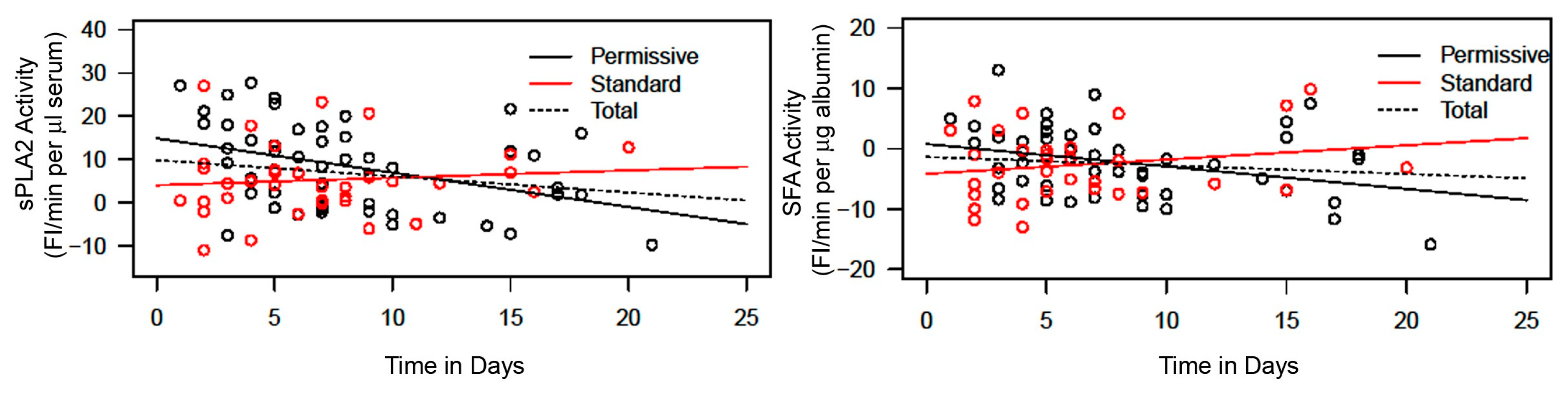
2.3. Comparison of sPLA2 Activity and SFA Activity Returning to Normal Values between Permissive Feeding and Standard Feeding
2.4. Determination of sPLA2 Activity and SFA Activity in the Sera of Healthy Subjects and Non-Infectious, Non-Septic Patients
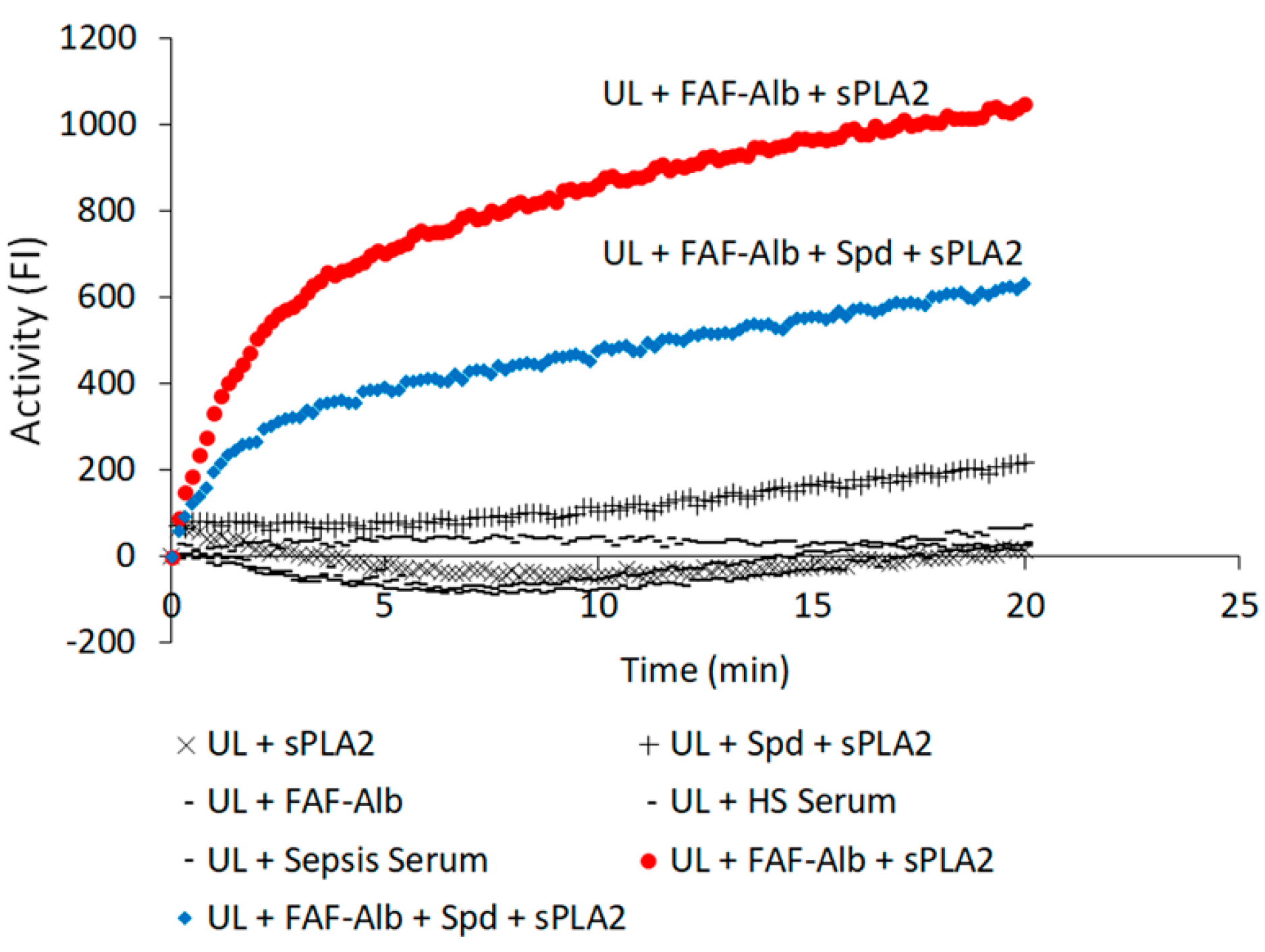
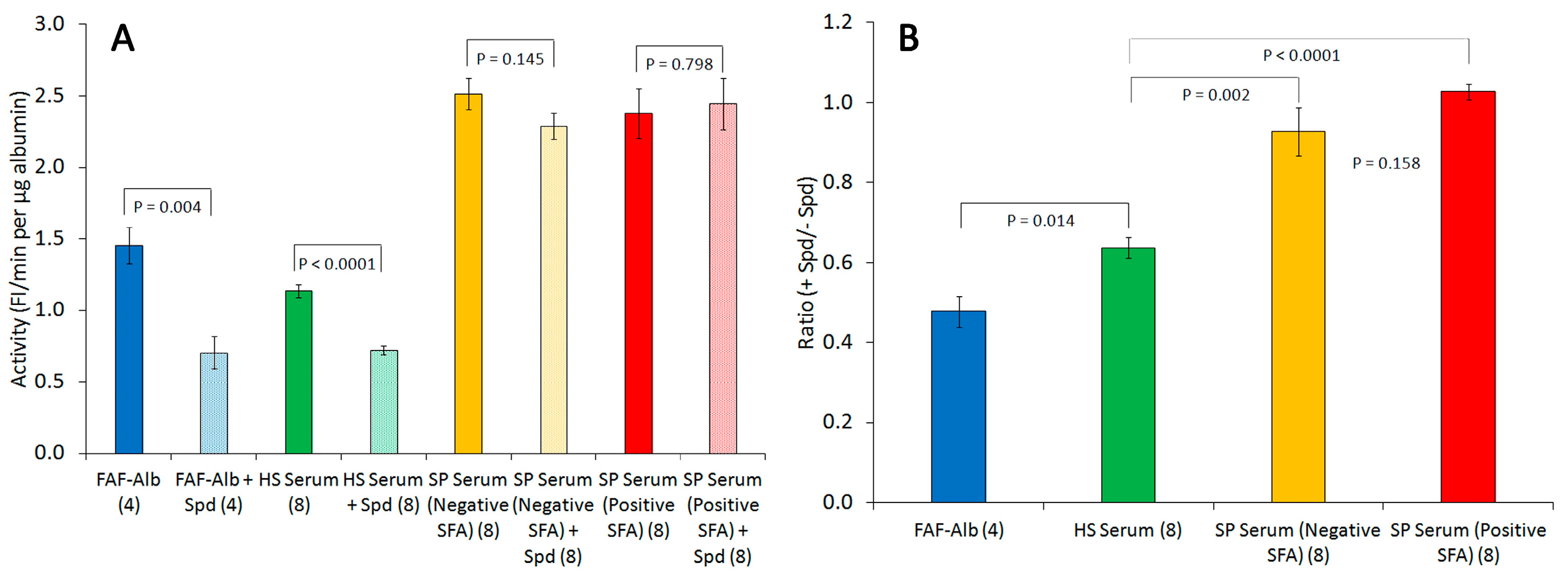
2.5. Effects of Spermidine on Albumin–FA Binding Activity (Alb-FA-BA)
- Hypothesis:
- Hypothesis 1.An increase in serum sPLA2 activity is associated with a decrease in serum albumin–membrane binding (SFA) activity in sepsis, which may serve as a useful biomarker for assessing patient prognosis under different feeding strategies, such as permissive underfeeding versus standard feeding.Hypothesis 2.The deficiency in albumin–membrane binding capacity in the serum of patients with sepsis is caused by the binding sites on albumin being occupied by prebound fatty acids.
- Methods:
- Hypothesis 1 was evaluated by employing fluorescent sPLA2 and SFA assays to examine 88 sequential serum samples collected on various days from 20 patients with sepsis. Each sample was identified by a number only, and the lab personnel conducting the assays were unaware of any information regarding the sample identities.Hypothesis 2 was tested by incorporating spermidine into the sPLA2-albumin assay mixtures to detect the presence of prebound fatty acids in the albumin–FA binding sites.
- Results:
- Hypothesis 1—The analysis of sPLA2 activity and albumin–membrane binding (SFA) activity in 88 serum samples revealed an inverse relationship between the activities of these two proteins. Elevated sPLA2 activity was associated with a reduction in SFA activity, resulting in a change in SFA activity from negative to positive (Figure 1, Figure 2 and Figure 3). The correlation between these protein activities and patient outcomes under permissive versus standard feeding suggests that they could serve as potential markers for evaluating the presence and severity of sepsis in patients (Figure 3 and Figure 4).
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Serum Samples
4.3. Preparation of DOPC-PG Fluorescent Liposome Substrate
4.4. Serum Endogenous sPLA2 Activity Assay
4.5. Serum Specific Fraction of Albumin (SFA) Assay
4.5.1. Preparation of sPLA2 Working Solution for the SFA Assay
4.5.2. SFA Assay
4.6. Determination of Spermidine Effect on Albumin–FA Binding Activity (Alb-FA-BA)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in Sepsis: Potent Immunoregulators and Potential Therapeutic Targets-An Updated View. Mediators Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Bantel, H.; Schulze-Osthoff, K. Cell death in sepsis: A matter of how, when, and where. Crit. Care 2009, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Namendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.N.; Vincent, J.L. Sepsis in intensive care unit patients: Worldwide data from the intensive care over nations audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Genga, K.R.; Russell, J.A. Update of sepsis in the intensive care unit. J. Innate. Immunol. 2017, 9, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Dolin, H.H.; Papadimos, T.J.; Chen, X.H.; Pan, Z.K. Characterization of Pathogenic Sepsis Etiologies and Patient Profiles: A Novel Approach to Triage and Treatment. Microbiol. Insights 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Randolph, A.G.; McCulloh, R.J. Important considerations for diagnosing and managing severe infections in infants, children, and adolescents. Virulence 2014, 5, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Langley, R.J.; Wong, H.R. Early Diagnosis of Sepsis: Is an Integrated Omics Approach the Way Forward? Mol Diagn. Ther. 2017, 21, 525–537. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, S.H. Sepsis: Early Recognition and Optimized Treatment. Tuberc. Respir. Dis. 2019, 82, 6–14. [Google Scholar] [CrossRef]
- Dolin, H.H.; Papadimos, T.J.; Stepkowski, S.; Chen, X.H.; Pan, Z.K. A novel combination of biomarkers to herald the onset of sepsis prior to the manifestation of symptoms. Shock 2018, 49, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Haddad, H.E.; Chaftari, A.M.; Hachem, R.; Chaftari, P.; Raad, I.I. Biomarkers of Sepsis and Bloodstream Infections: The role of procalcitonin and proadrenomedullin with emphasis in patients with cancer. Clin. Infect. Dis. 2018, 67, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Kumar, A.; Gangadharan, S. Biomarkers and newer laboratory investigations in the diagnosis of sepsis. J. R. Coll. Physicians Edinb. 2019, 49, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Parrillo, J.E.; Seymour, C.W.; Angus, D.C.; Bicking, K.; Esguerra, V.G.; Peck-Palmer, O.M.; Magari, R.T.; Julian, M.W.; Kleven, J.M.; et al. Monocyte Distribution Width: A Novel Indicator of Sepsis-2 and Sepsis-3 in High-Risk Emergency Department Patients. Crit. Care Med. 2019, 47, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Polilli, E.; Sozio, F.; Frattari, A.; Persichitti, L.; Sensi, M.; Posata, R.; Di Gregorio, M.; Sciacca, A.; Flacco, M.E.; Manzoli, L.; et al. Comparison of Monocyte Distribution Width (MDW) and Procalcitonin for early recognition of sepsis. PLoS ONE 2020, 15, e0227300. [Google Scholar] [CrossRef]
- Pinheiro da Silva, F.; Nizet, V. Cell death during sepsis: Integration of disintegration in the inflammatory response to overwhelming infection. Apoptosis 2009, 14, 509–521. [Google Scholar] [CrossRef]
- Yoon, K.W. Dead cell phagocytosis and innate immune checkpoint. BMB Rep. 2017, 50, 496–503. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chen, X.; Gueydan, C.; Han, J.H. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Westman, J.; Grinstein, S.; Elias Marques, P. Phagocytosis of necrotic debris at sites of injury and inflammation. Front Immunol. 2020, 10, 3030. [Google Scholar] [CrossRef]
- Madiraju, C.; Mallarpu, C.S.; Prasad, S.; Singarapu, M.; Kim, J.H.; Haririparsa, N.; Bratic, N.; Brar, H.; Cashman, J.R.; Chelluri, L.K. Cell death markers in sepsis. J. Immunol. 2018, 200 (Suppl. S1), 166.33. [Google Scholar] [CrossRef]
- Bailey, R.W.; Olson, E.D.; Vu, M.P.; Brueseke, T.J.; Robertson, L.; Christensen, R.E.; Parker, K.H.; Judd, A.M.; Bell, J.D. Relationship between Membrane Physical Properties and Secretory Phospholipase A2 Hydrolysis Kinetics in S49 Cells during Ionophore-Induced Apoptosis. Biophys. J. 2007, 93, 2350–2362. [Google Scholar] [CrossRef] [PubMed]
- Nielson, K.H.; Olsen, C.A.; Allred, D.V.; O’Neill, K.L.; Burton, G.F.; Bell, J.D. Susceptibility of S49 lymphoma cell membranes to hydrolysis by secretory phospholipase A2 during early phase of apoptosis. Biochim. Biophys. Acta 2000, 1484, 163–174. [Google Scholar] [CrossRef]
- Olson, E.D.; Nelson, J.; Griffith, K.; Nguyen, T.; Streeter, M.; Wilson-Ashworth, H.A.; Gelb, M.H.; Judd, A.M.; Bell, J.D. Kinetic Evaluation of Cell Membrane Hydrolysis during Apoptosis by Human Isoforms of Secretory Phospholipase A2. J. Biol. Chem. 2010, 285, 10993–11002. [Google Scholar] [CrossRef]
- Hamilton, B.; Ware, L.B.; Matthay, M.A. Lipid mediators in the pathogenesis and resolution of sepsis and ARDS. In Annual Update in Intensive Care and Emergency Medicine; Vincent, J.L., Ed.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 3–11. [Google Scholar]
- Padovan, M.G.; Norling, L.V. Pro-resolving lipid mediators in sepsis and critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 76–81. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Miki, Y.; Yamamoto, K.; Taketomi, Y. A new era of secreted phospholipase A 2. J. Lipid Res. 2015, 56, 1248–1261. [Google Scholar] [CrossRef]
- Menschikowski, M.; Hagelgans, A.; Siegert, G. Secretory phospholipase A2 of group IIA: Is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostaglandins Other Lipid Mediat. 2006, 79, 1–33. [Google Scholar] [CrossRef]
- Tsao, F.H.C.; Shanmuganayagam, D.; Zachman, D.K.; Khosravi, M.; Folts, J.D.; Meyer, K.C. A continuous fluorescence assay for the determination of calcium-dependent secretory phospholipase A2 activity in serum. Clin. Chim. Acta 2007, 379, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Tsao, F.H.C.; Culver, B.J.; Pierre, J.F.; Shanmuganayagam, D.; Patten Jr, C.C.; Meyer, K.C. Effect of prophylactic supplementation with grape polyphenolics on endotoxin-induced serum secretory phospholipase A2 activity in rats. Comp. Med. 2012, 62, 271–278. [Google Scholar] [PubMed]
- Grönroos, J.O.; Salonen, J.H.; Viander, M.; Nevalainen, T.J.; Laine, V.J. Roles of group IIA phospholipase A2 and complement in killing of bacteria by acute phase serum. Scand. J. Immunol. 2005, 62, 413–419. [Google Scholar] [CrossRef]
- Ogawa, M.; Arakawa, H.; Yamashita, S.; Sakamoto, K.; Ikei, S. Postoperative elevations of serum interleukin 6 and group II phospholipase A2: Group II phospholipase A2 in serum is an acute phase reactant. Res. Commun. Chem. Pathol. Pharmacol. 1992, 75, 109–115. [Google Scholar] [PubMed]
- Tsao, F.H.C.; Xiang, Z.; Meyer, K.C. Fluorescent determination of secretory phospholipase A2 (sPLA2)-mediated human serum albumin binding activity with membrane phospholipids and fatty acids. Transl. Med. 2015, 5, 2161-1025. [Google Scholar]
- Tan, T.L.; Goh, Y.Y. The role of group IIA secretory phospholipase A2 (sPLA2-IIA) as a biomarker for the diagnosis of sepsis and bacterial infection in adults—A systematic review. PLoS ONE 2017, 12, e0180554. [Google Scholar] [CrossRef]
- Nandi, U.; Jones, A.E.; Puskarich, M.A. Group IIA secretory phospholipase 2 independently predicts mortality and positive blood culture in emergency department sepsis patients. JACEP Open 2021, 2, e12460. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Aldawood, A.S.; Haddad, S.H.; Al-Dorzi, H.M.; Tamim, H.M.; Jones, G.; Mehta, S.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; et al. Permissive underfeeding or standard enteral Feeding in critically ill adults. N. Engl. J. Med. 2015, 372, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Al-Dorzi, H.M.; Albarrak, A.; Ferwana, M.; Murad, M.H.; Arabi, Y.M. Lower versus higher dose of enteral caloric intake in adult critically ill patients: A systematic review and meta-analysis. Crit. Care 2016, 20, 358. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Aldawood, A.S.; Al-Dorzi, H.M.; Tamim, H.M.; Haddad, S.H.; Jones, G.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; Sadat, M.; et al. Permissive underfeeding or standard enteral feeding in High- and low-nutritional-risk critically ill adults. Post hoc analysis of the PermiT Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 652–662. [Google Scholar] [CrossRef]
- Wu, C.D.; Xie, J.F.; Zhang, X.W.; Yang, C.S.; Huang, Y.Z.; Liu, S.Q.; Qiu, H.B.; Yang, Y. Compare permissive underfeeding enteral nutrition with stand feeding under enough protein intakes on mortality in critically ill patients: A systematic review and meta-analysis planning councils in the United States. J. Clin. Respir. Med. 2017, 1, 3–11. [Google Scholar] [CrossRef]
- Tsao, F.H.C.; Barnes, J.N.; Amessoudji, A.; Li, Z.H.; Meyer, K.C. Aging-related and gender specific albumin misfolding in Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2020, 4, 67–77. [Google Scholar] [CrossRef]
- Tsao, F.H.C.; Meyer, K.C. Human serum albumin misfolding in aging and disease. Int. J. Mol. Sci. 2022, 23, 11675. [Google Scholar] [CrossRef]
- Dalli, J.; Colas, R.A.; Quintana, C.; Barragan-Bradford, D.; Hurwitz, S.; Levy, B.D.; Choi, A.M.; Serhan, C.N.; Baron, R.M. Human sepsis eicosanoid and pro-resolving lipid mediator temporal profiles: Correlations with survival and clinical outcomes. Crit. Care Med. 2017, 45, 58–68. [Google Scholar] [CrossRef]
- Amunugama, K.; Pike, D.P.; Ford, D.A. The lipid biology of sepsis. J. Lipid Res. 2021, 62, 100090. [Google Scholar] [CrossRef]
- Hurley, B.P.; McCormick, B.A. Multiple Roles of Phospholipase A2 during Lung Infection and Inflammation. Infect. Immun. 2008, 76, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, S.; Spadaro, F.; Gessani, S.; Podo, F.; Fantuzzi, L. Phospholipases: At the crossroads of the immune system and the pathogenesis of HIV-1 infection. J. Leukoc. Biol. 2017, 101, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Arbibe, L.; Vial, D.; Rosinski-Chupin, I.; Havet, N.; Huerre, M.; Vargaftig, B.B.; Touqui, L. Endotoxin induces expression of type II phospholipase A2 in macrophages during acute lung injury in guinea pigs: Involvement of TNF-alpha in lipopolysaccharide-induced type II phospholipase A2 synthesis. J. Immunol. 1997, 159, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.F.; Heneghan, A.F.; Tsao, F.H.C.; Sano, Y.; Jonker, M.A.; Omata, J.; Lan, J.G.; Kudsk, K.A. Route and Type of Nutrition and Surgical Stress Influence Secretory Phospholipase A2 Secretion of the Murine Small Intestine. J. Parenter. Enteral. Nutr. 2011, 35, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Omata, J.; Pierre, J.F.; Heneghan, A.F.; Tsao, F.H.C.; Sano, Y.; Jonker, M.A.; Kudsk, K.A. Parenteral Nutrition Suppresses the Bactericidal Response of the Small Intestine. Surgery 2013, 153, 17–24. [Google Scholar] [CrossRef]
- Vadas, P.; Pruzanski, W.; Stefanski, E.; Ellies, L.G.; Aubin, J.E.; Sos, A.; Melcher, A. Extracellular phospholipase A2 secretion is a common effector pathway of interleukin 1 and tumour necrosis factor action. Immunol. Lett. 1991, 28, 187–193. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Mühl, H.; Pignat, W.; Märki, F.; van den Bosch, H. Cytokine regulation of group II phospholipase A2 expression in glomerular mesangial cells. Eur. J. Clin. Pharmacol. 1993, 44 (Suppl. S1), S7–S9. [Google Scholar] [CrossRef]
- Pfeilschifter, J.M.; Schalkwijk, C.; Briner, V.A.; van den Bosch, H. Cytokine-stimulated Secretion of Group II Phospholipase A2 by Rat Mesangial Cells Its Contribution to Arachidonic Acid Release and Prostaglandin Synthesis by Cultured Rat Glomerular Cells. J. Clin. Investig. 1993, 92, 2516–2523. [Google Scholar] [CrossRef]
- Cummings, B.S.; Mchowat, J.; Schnellmann, R.G. Phospholipase A2s in Cell Injury and Death. J. Pharmacol. Exp. Ther. 2000, 294, 793–799. [Google Scholar] [PubMed]
- Birge, R.B.; Boeltz, S.; Kumar, S.; Carlson, J.; Wanderley, J.; Calianese, D.; Barcinski, M.; Brekken, R.A.; Huang, X.; Hutchins, J.T.; et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016, 23, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Shlomovitz, I.; Speir, M.; Gerlic, M. Flipping the dogma—Phosphatidylserine in non-apoptotic cell death. Cell Commun. Signal 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.M.; You, J.K.; Wang, X.; Snider, A.J.; Hallmark, B.; Zec, M.M.; Seeds, M.C.; Sergeant, S.; Johnstone, L.; Wang, Q.M.; et al. Group IIA secreted phospholipase A2 is associated with the pathobiology leading to COVID-19 mortality. J. Clin. Investig. 2021, 131, e149236. [Google Scholar] [CrossRef]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, F.H.C.; Li, Z.; Amessoudji, A.W.; Jawdat, D.; Sadat, M.; Arabi, Y.; Meyer, K.C. The Role of Serum Albumin and Secretory Phospholipase A2 in Sepsis. Int. J. Mol. Sci. 2024, 25, 9413. https://doi.org/10.3390/ijms25179413
Tsao FHC, Li Z, Amessoudji AW, Jawdat D, Sadat M, Arabi Y, Meyer KC. The Role of Serum Albumin and Secretory Phospholipase A2 in Sepsis. International Journal of Molecular Sciences. 2024; 25(17):9413. https://doi.org/10.3390/ijms25179413
Chicago/Turabian StyleTsao, Francis H. C., Zhanhai Li, Amy W. Amessoudji, Dunia Jawdat, Musharaf Sadat, Yaseen Arabi, and Keith C. Meyer. 2024. "The Role of Serum Albumin and Secretory Phospholipase A2 in Sepsis" International Journal of Molecular Sciences 25, no. 17: 9413. https://doi.org/10.3390/ijms25179413
APA StyleTsao, F. H. C., Li, Z., Amessoudji, A. W., Jawdat, D., Sadat, M., Arabi, Y., & Meyer, K. C. (2024). The Role of Serum Albumin and Secretory Phospholipase A2 in Sepsis. International Journal of Molecular Sciences, 25(17), 9413. https://doi.org/10.3390/ijms25179413





