Potential of Coffee Cherry Pulp Extract against Polycyclic Aromatic Hydrocarbons in Air Pollution Induced Inflammation and Oxidative Stress for Topical Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Antioxidant Activities
2.2. Lipoxygenase and Protease Inhibitory Effects
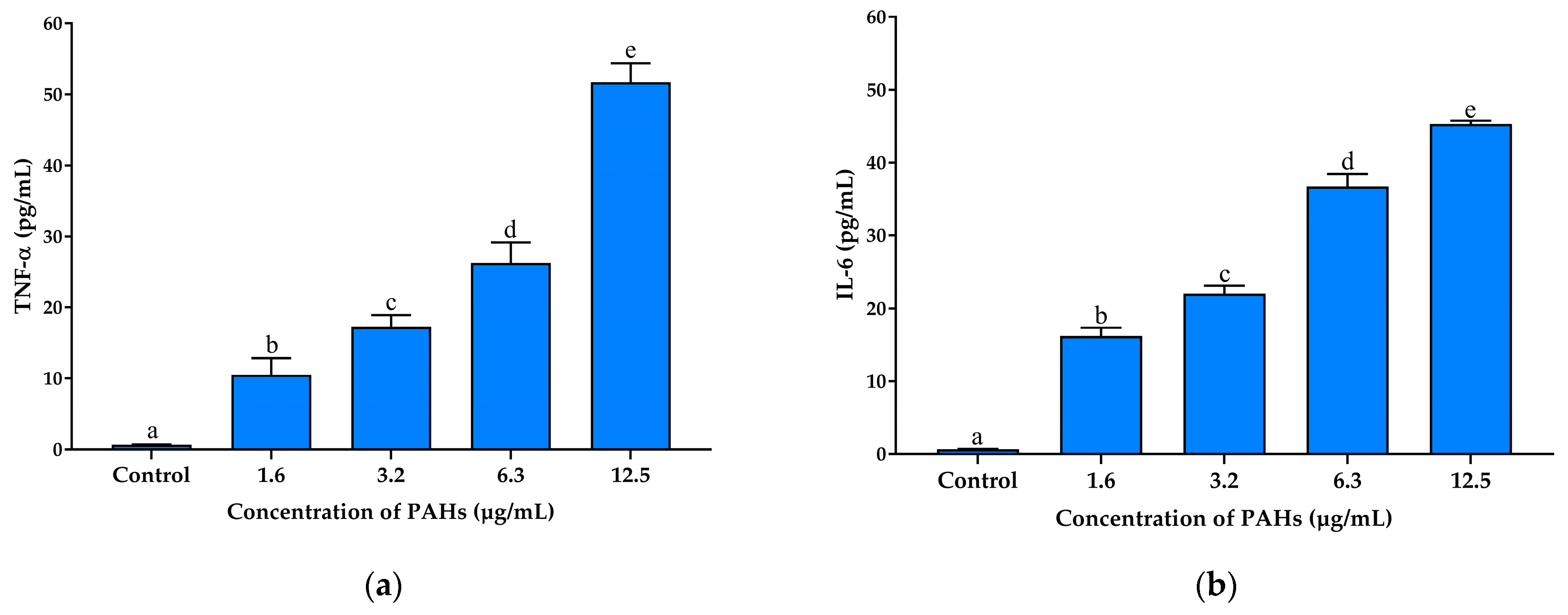
2.3. Anti-Inflammatory Effect of Coffee Cherry Pulp Extract, Chlorogenic Acid, Caffeine, and Theophylline against PAH-Induced Oxidative Stress and Inflammation
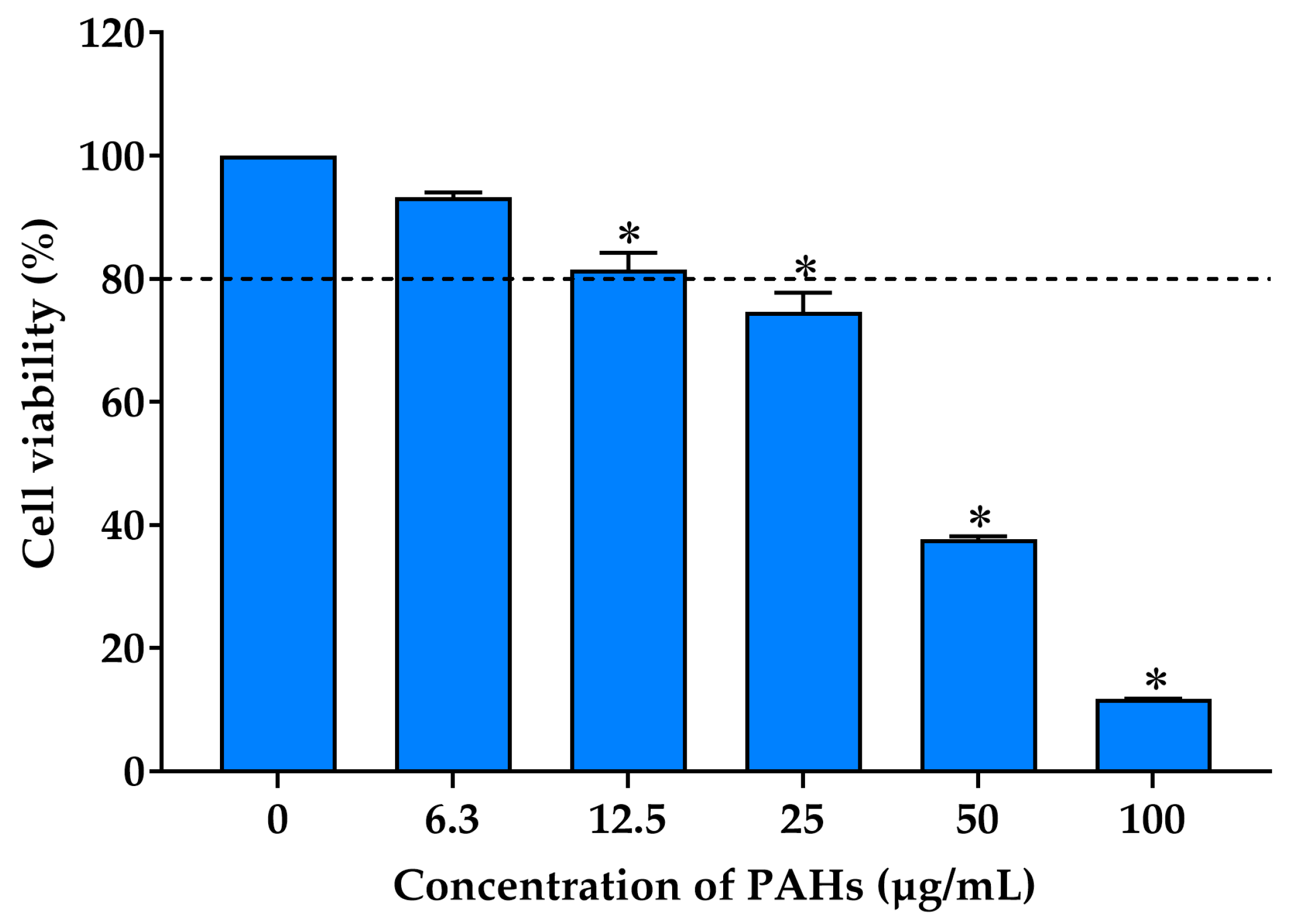
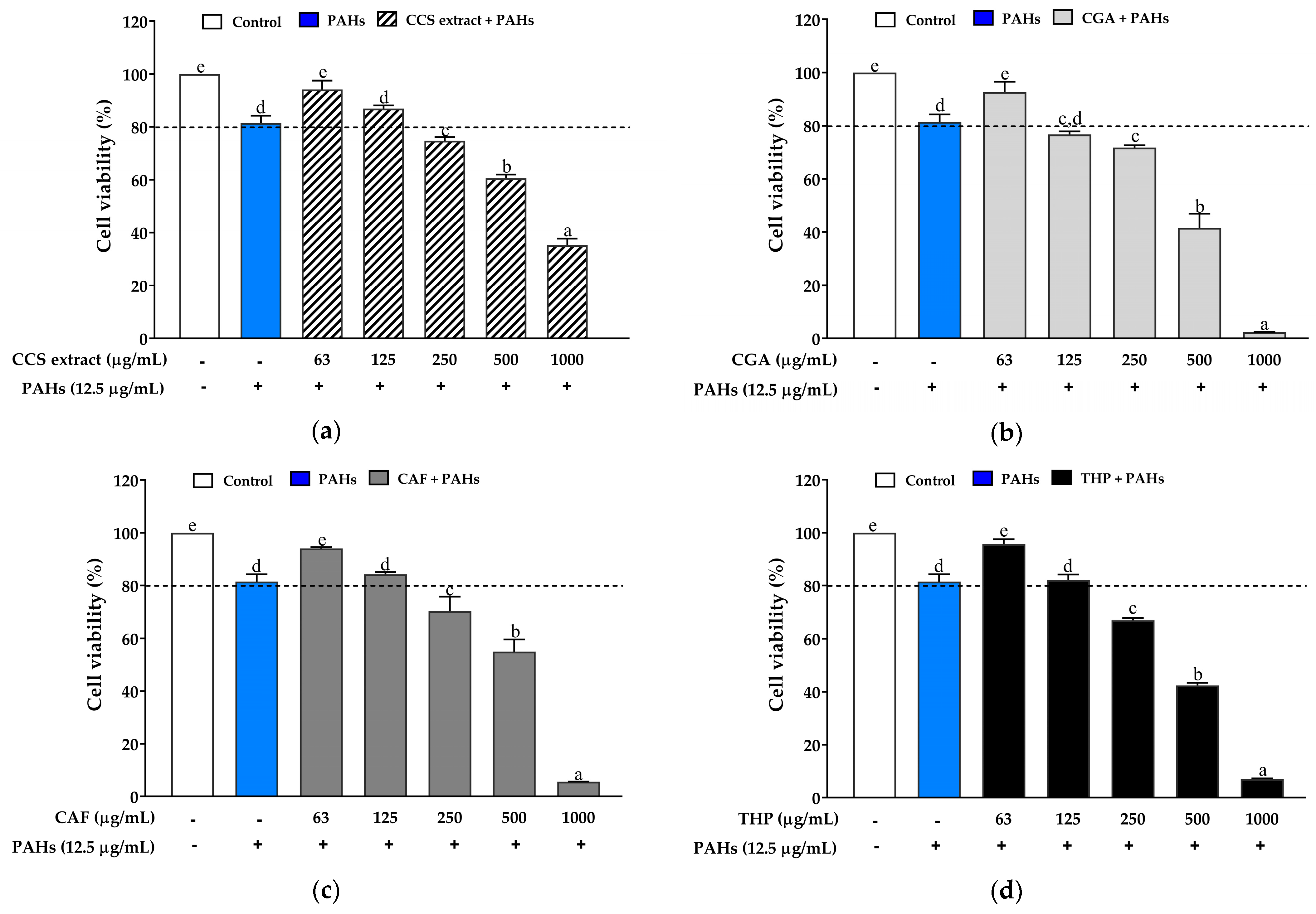
2.3.1. Effect on the Viability of RAW 264.7 Cells Exposed to PAHs
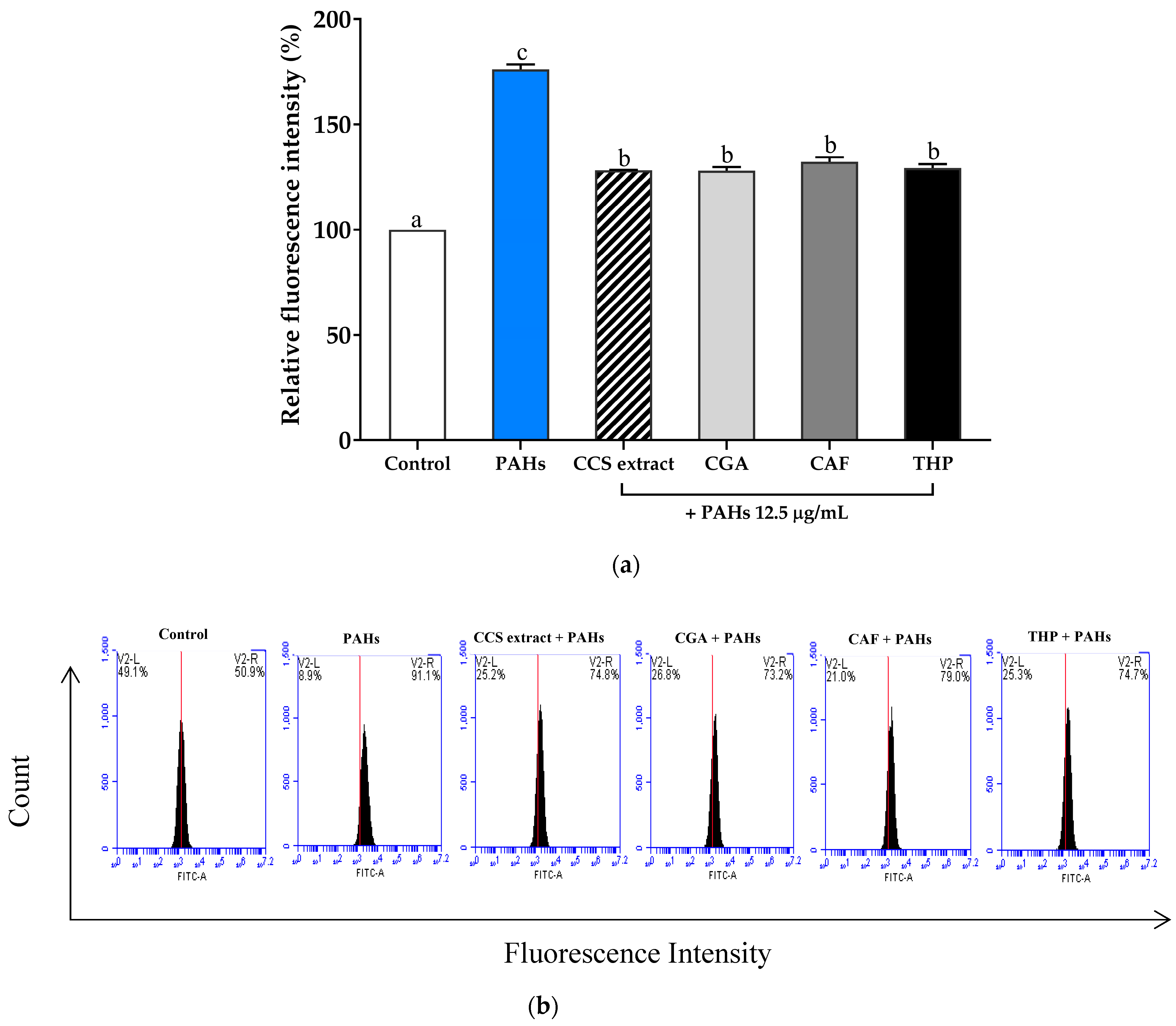
2.3.2. Effect of Inhibiting Intracellular Reactive Oxygen Species Production
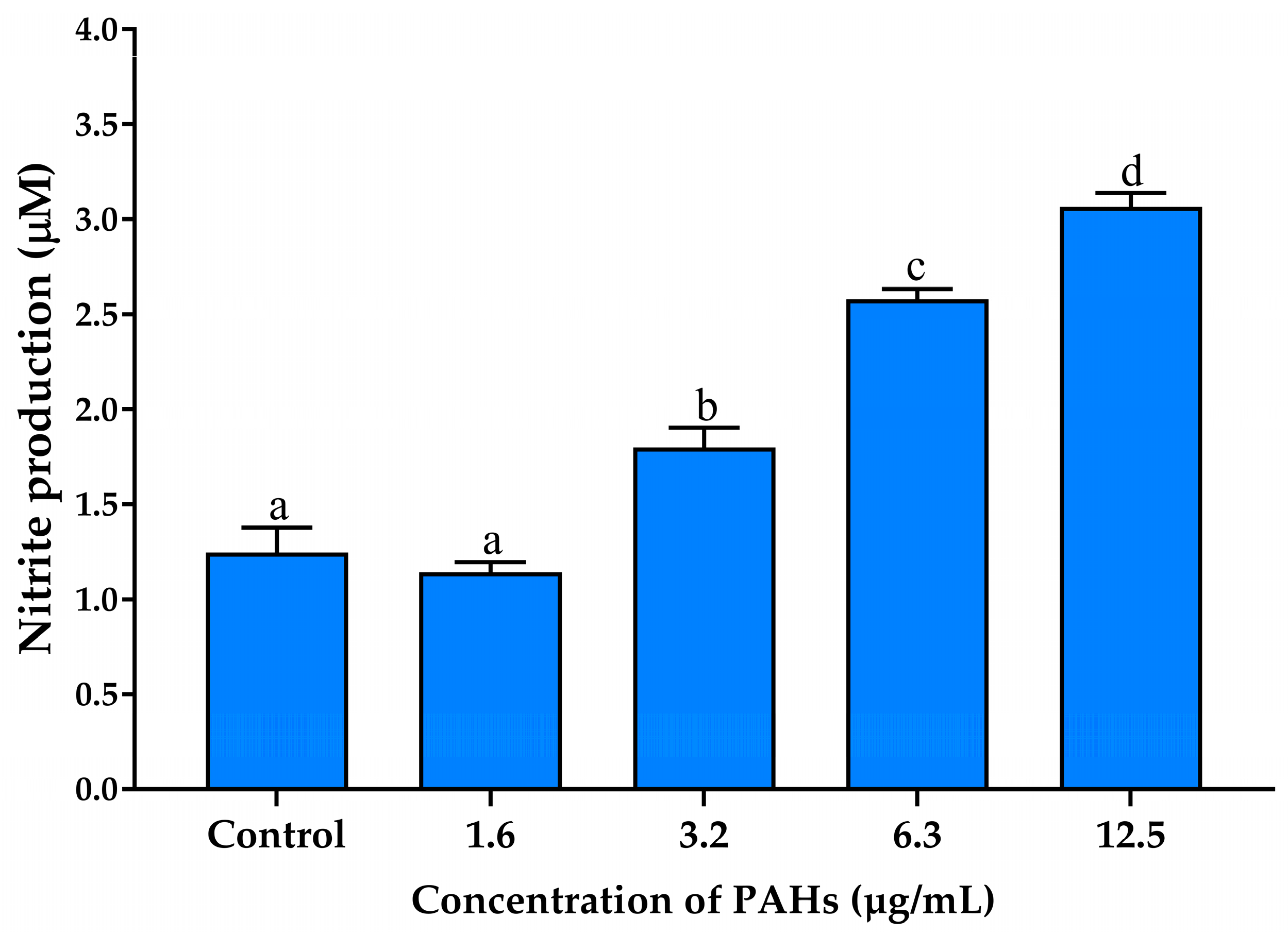
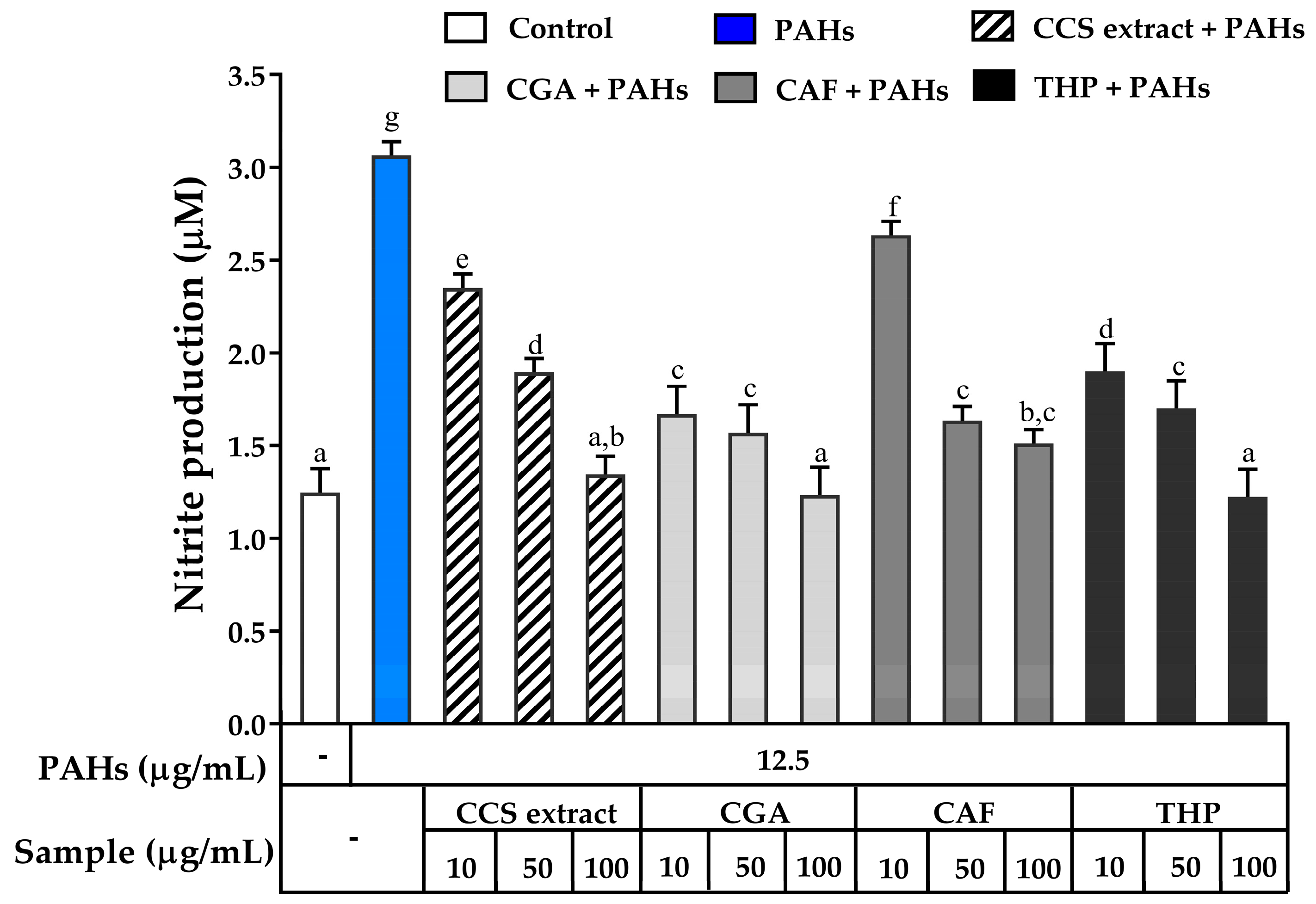
2.3.3. The Effects on Inhibiting Nitric Oxide Production
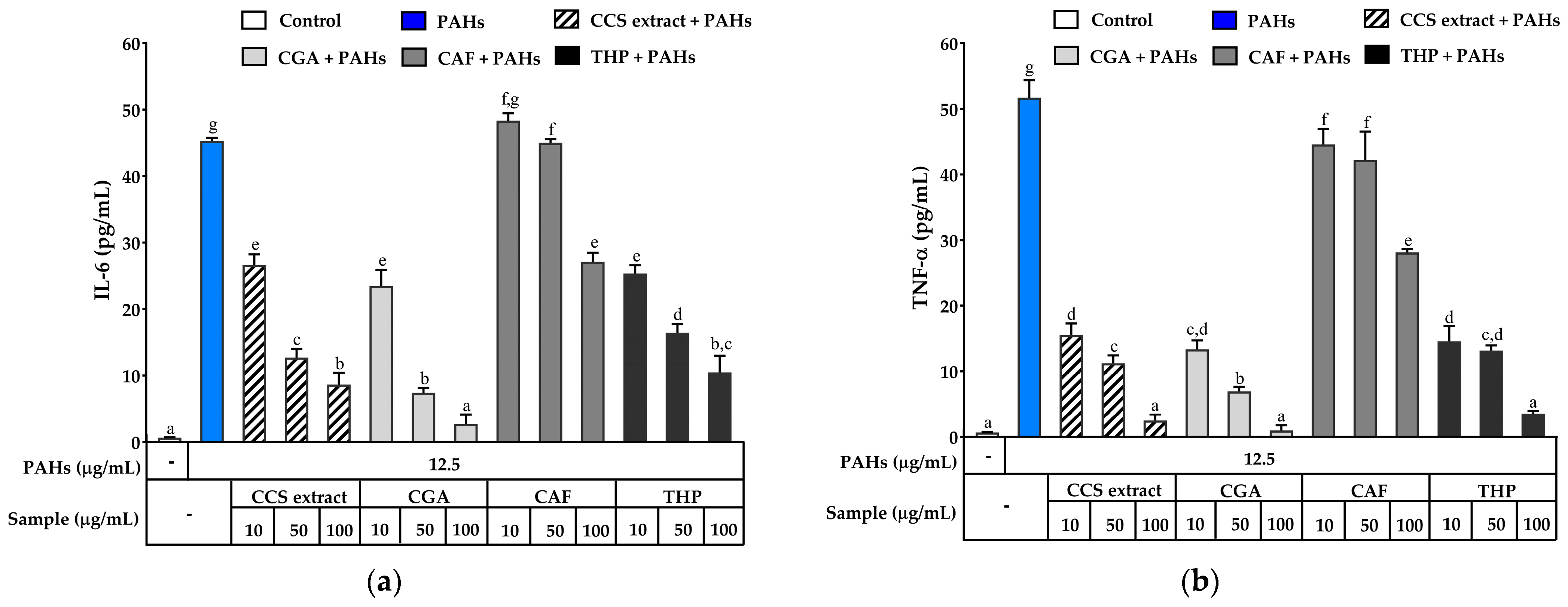
2.3.4. Effect on Inhibiting Secretion and Gene Expression of Pro-Inflammatory Mediators
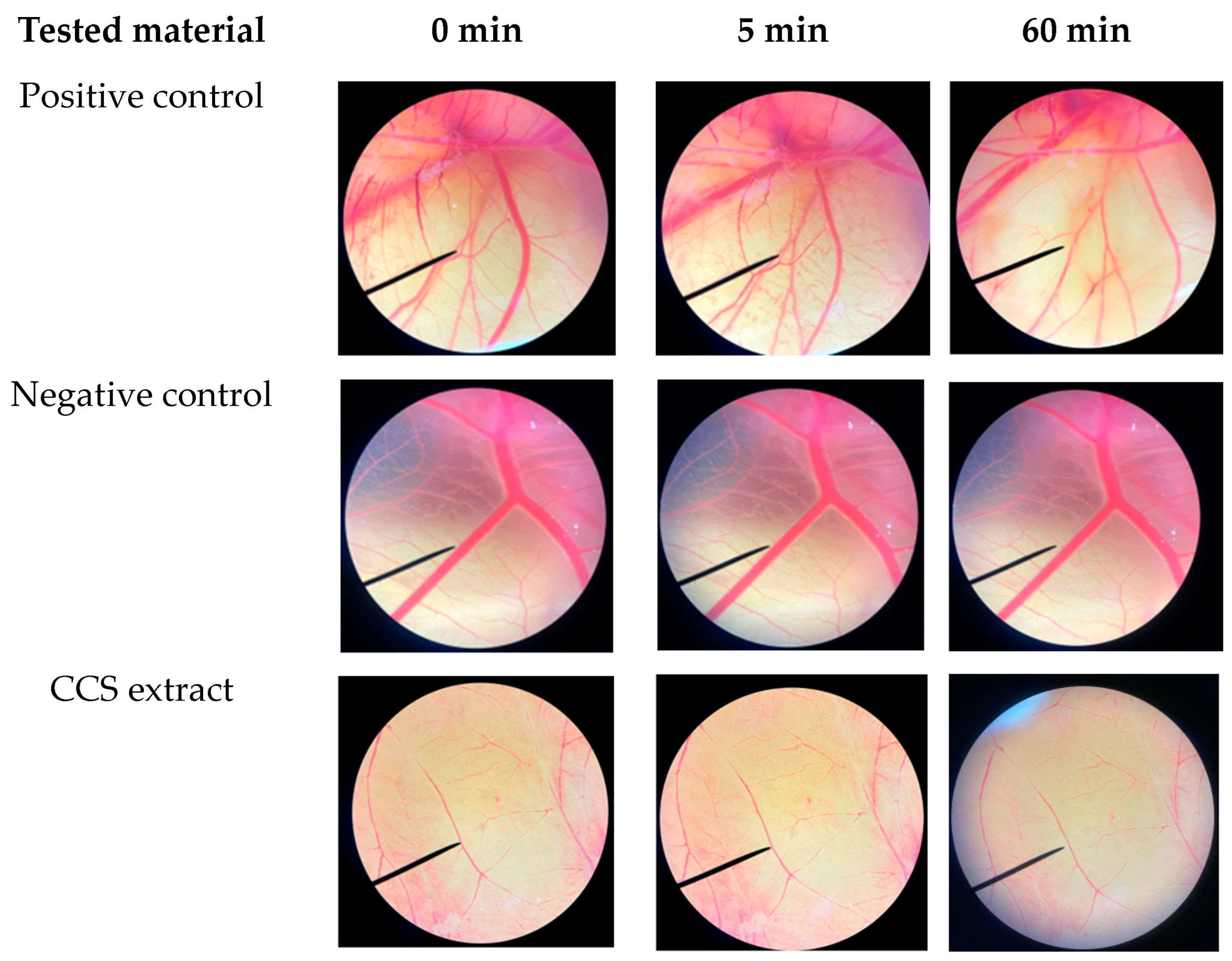
2.4. Irritation Assessment of Coffee Cherry Pulp Extract Using Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM) Assay
3. Materials and Methods
3.1. Materials
3.2. Preparation of Coffee Cherry Pulp Extract
3.3. Determination of Antioxidant Activities
3.3.1. ABTS Free Radical Scavenging Assay
3.3.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.4. Determination of Anti-Inflammatory Activities
3.4.1. Protease Inhibition Assay
3.4.2. Lipoxygenase (LOX) Inhibition Assay
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. Reactive Oxygen Species Production Inhibition (H2DCFDA Assay)
3.8. Intracellular Nitric Oxide Production
3.9. Enzyme-Linked Immunoassay (ELISA) Analysis
3.10. Semi-Quantitative Reverse Transcription and Polymerase Chain Reaction Analysis (RT-PCR)
3.11. Irritation Property by Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM) Assay
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menichini, E. Urban air pollution by polycyclic aromatic hydrocarbons: Levels and sources of variability. Sci. Total Environ. 1992, 116, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, X.; Li, W.; Zu, Y.; Zhou, F.; Shou, Q.; Ding, Z. PM2.5 Exposure Induces Inflammatory Response in Macrophages via the TLR4/COX-2/NF-κB Pathway. Inflammation 2020, 43, 1948–1958. [Google Scholar] [CrossRef]
- Tay, S.S.; Roediger, B.; Tong, P.L.; Tikoo, S.; Weninger, W. The Skin-Resident Immune Network. Curr. Dermatol. Rep. 2014, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, K.; van Eeden, S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (-)-epigallocatechin-3-gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Skin. Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Bahri, R.; Saidane-Mosbahi, D.; Rouabhia, M. Cytokine release and cytotoxicity in human keratinocytes induced by polycyclic aromatic hydrocarbons (1-methylpyrene and perylene). J. Toxicol. Environ. Health A 2010, 73, 552–564. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-κB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Magnani, N.D.; Muresan, X.M.; Belmonte, G.; Cervellati, F.; Sticozzi, C.; Pecorelli, A.; Miracco, C.; Marchini, T.; Evelson, P.; Valacchi, G. Skin Damage Mechanisms Related to Airborne Particulate Matter Exposure. Toxicol. Sci. 2016, 149, 227–236. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Wang, L.; Kim, W.-S.; Lee, T.-K.; Kim, Y.-T.; Jeon, Y.-J. Alginic Acid from Padina boryana Abate Particulate Matter-Induced Inflammatory Responses in Keratinocytes and Dermal Fibroblasts. Molecules 2020, 25, 5746. [Google Scholar] [CrossRef]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Fernando, P.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol Inhibits Particulate Matter 2.5-Induced Skin Keratinocyte Damage via MAPK Signaling Pathway. Mar. Drugs 2019, 17, 444. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Lee, H.G.; Nagahawatta, D.P.; Yang, H.W.; Kang, M.C.; Jeon, Y.J. Particulate Matter-Induced Inflammation/Oxidative Stress in Macrophages: Fucosterol from Padina boryana as a Potent Protector, Activated via NF-κB/MAPK Pathways and Nrf2/HO-1 Involvement. Mar. Drugs 2020, 18, 628. [Google Scholar] [CrossRef] [PubMed]
- Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Potprommanee, S.; Kiattisin, K. Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts. Foods 2023, 12, 4292. [Google Scholar] [CrossRef]
- Hu, S.; Gil-Ramírez, A.; Martín-Trueba, M.; Benítez, V.; Aguilera, Y.; Martín-Cabrejas, M.A. Valorization of coffee pulp as bioactive food ingredient by sustainable extraction methodologies. Curr. Res. Food Sci. 2023, 6, 100475. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. Chlorogenic acid and its role in biological functions: An up to date. EXCLI J. 2019, 18, 310–316. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Reddy, V.S.; Shiva, S.; Manikantan, S.; Ramakrishna, S. Pharmacology of caffeine and its effects on the human body. Eur. J. Med. Chem. Rep. 2024, 10, 100138. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Jacinto, T.A.; Gonçalves, A.C.; Gaspar, D.; Silva, L.R. Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies. Pharmaceuticals 2023, 16, 1067. [Google Scholar] [CrossRef]
- Gulati, K.; Ray, A.; Vijayan, V.K. Free radicals and theophylline neurotoxicity: An experimental study. Cell. Mol. Biol. 2007, 53, 42–52. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Mao, S.; Wang, K.; Lei, Y.; Yao, S.; Lu, B.; Huang, W. Antioxidant synergistic effects of Osmanthus fragrans flowers with green tea and their major contributed antioxidant compounds. Sci. Rep. 2017, 7, 46501. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.; Porto, G.; Cabrita, E.J.; Marques, M.M.; Fernandes, E. Inhibition of LOX by flavonoids: A structure-activity relationship study. Eur. J. Med. Chem. 2014, 72, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Procopio, A. New aspects on the role of lipoxygenases in cancer progression. Histol. Histopathol. 2005, 20, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Aiyelabegan, H.T.; Negahdari, B.; Mazlomi, M.A.; Daraee, H.; Daraee, N.; Eatemadi, R.; Sadroddiny, E. Role of protease and protease inhibitors in cancer pathogenesis and treatment. Biomed. Pharmacother. 2017, 86, 221–231. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Thakur, K.; Wei, C.K.; Wang, H.; Zhang, J.G.; Wei, Z.J. Evaluation of inhibitory activity of natural plant polyphenols on Soybean lipoxygenase by UFLC-mass spectrometry. South. Afr. J. Bot. 2019, 120, 179–185. [Google Scholar] [CrossRef]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Huang, Y.; Zhu, Q.-F.; Song, M.; Xiong, S.; Manyande, A.; Du, H. The mechanism of chlorogenic acid inhibits lipid oxidation: An investigation using multi-spectroscopic methods and molecular docking. Food Chem. 2020, 333, 127528. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Din, K.M.; Rasheed, R.; Sadiq, A.; Jan, M.S.; Minhas, A.M.; Khan, A. Phytochemical investigation, anti-inflammatory, antipyretic and antinociceptive activities of Zanthoxylum armatum DC extracts-in vivo and in vitro experiments. Heliyon 2020, 6, e05571. [Google Scholar] [CrossRef]
- Jin, U.H.; Lee, J.Y.; Kang, S.K.; Kim, J.K.; Park, W.H.; Kim, J.G.; Moon, S.K.; Kim, C.H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloProtease-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005, 77, 2760–2769. [Google Scholar] [CrossRef]
- Jeffy, B.D.; Chen, E.J.; Gudas, J.M.; Romagnolo, D.F. Disruption of cell cycle kinetics by benzo[a]pyrene: Inverse expression patterns of BRCA-1 and p53 in MCF-7 cells arrested in S and G2. Neoplasia 2000, 2, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.L.; Wang, P.W.; Aljuffali, I.A.; Huang, C.T.; Lee, C.W.; Fang, J.Y. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015, 78, 51–60. [Google Scholar] [CrossRef]
- Smith, J.; Neupane, R.; McAmis, W.; Singh, U.; Chatterjee, S.; Raychoudhury, S. Toxicity of polycyclic aromatic hydrocarbons involves NOX2 activation. Toxicol. Rep. 2019, 6, 1176–1181. [Google Scholar] [CrossRef]
- Jia, Y.Y.; Wang, Q.; Liu, T. Toxicity Research of PM(2.5) Compositions In Vitro. Int. J. Environ. Res. Public. Health 2017, 14, 232. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Gołębiewska, E.; Świderski, G.; Lewandowska, H.; Pruszyński, M.; Zawadzka, M.; Kozłowski, M.; Sienkiewicz-Gromiuk, J.; Lewandowski, W. Fe(III) and Cu(II) Complexes of Chlorogenic Acid: Spectroscopic, Thermal, Anti-/Pro-Oxidant, and Cytotoxic Studies. Materials 2022, 15, 6832. [Google Scholar] [CrossRef]
- Jiang, Y.; Kusama, K.; Satoh, K.; Takayama, E.; Watanabe, S.; Sakagami, H. Induction of cytotoxicity by chlorogenic acid in human oral tumor cell lines. Phytomedicine 2000, 7, 483–491. [Google Scholar] [CrossRef]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Mantecca, P.; Corvaja, V.; Longhin, E.; Perrone, M.G.; Bolzacchini, E.; Camatini, M. Winter fine particulate matter from Milan induces morphological and functional alterations in human pulmonary epithelial cells (A549). Toxicol. Lett. 2009, 188, 52–62. [Google Scholar] [CrossRef]
- Puri, P.; Nandar, S.K.; Kathuria, S.; Ramesh, V. Effects of air pollution on the skin: A review. Indian. J. Dermatol. Venereol. Leprol. 2017, 83, 415–423. [Google Scholar] [CrossRef]
- Devasagayam, T.P.; Kamat, J.P.; Mohan, H.; Kesavan, P.C. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. Biophys. Acta 1996, 1282, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Boo, Y.C. Siegesbeckiae herba extract and chlorogenic acid ameliorate the death of HaCaT keratinocytes exposed to airborne particulate matter by mitigating oxidative stress. Antioxidants 2021, 10, 1762–1767. [Google Scholar] [CrossRef]
- Shirai, T.; Hilhorst, M.; Harrison, D.G.; Goronzy, J.J.; Weyand, C.M. Macrophages in vascular inflammation--From atherosclerosis to vasculitis. Autoimmunity 2015, 48, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, S.Y.; Park, Y.L.; Myung, D.S.; Rew, J.S.; Joo, Y.E. Chlorogenic acid suppresses lipopolysaccharide-induced nitric oxide and interleukin-1β expression by inhibiting JAK2/STAT3 activation in RAW264.7 cells. Mol. Med. Rep. 2017, 16, 9224–9232. [Google Scholar] [CrossRef] [PubMed]
- Sansone, G.R.; Matin, A.; Wang, S.F.; Bouboulis, D.; Frieri, M. Theophylline inhibits the production of nitric oxide by peripheral blood mononuclear cells from patients with asthma. Ann. Allergy Asthma Immunol. 1998, 81, 90–95. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.-H.; Lee, C.-K.; Ha, N.-J.; Yim, D.; et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef]
- Xiao, T.; Yan, Z.; Xiao, S.; Xia, Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef]
- Sugaya, M. Macrophages and fibroblasts underpin skin immune responses. Explor. Immunol. 2021, 1, 226–242. [Google Scholar] [CrossRef]
- Mizutani, H.; Ohmoto, Y.; Mizutani, T.; Murata, M.; Shimizu, M. Role of increased production of monocytes TNF-α, IL-1β and IL-6 in psoriasis: Relation to focal infection, disease activity and responses to treatments. J. Dermatol. Sci. 1997, 14, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, A.; Chabros, P.; Grywalska, E.; Pietrzak, D.; Kandzierski, G.; Wawrzycki, B.O.; Roliñski, J.; Gawêda, K.; Krasowska, D. Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Postep. Dermatol. Alergol. 2020, 37, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, C.; Otsuka, A.; Kitoh, A.; Honda, T.; Egawa, G.; Nakajima, S.; Nakamizo, S.; Arita, M.; Kubo, M.; Miyachi, Y.; et al. Basophils regulate the recruitment of eosinophils in a murine model of irritant contact dermatitis. J. Allergy Clin. Immunol. 2014, 134, 100–107. [Google Scholar] [CrossRef]
- Korn, T.; Hiltensperger, M. Role of IL-6 in the commitment of T cell subsets. Cytokine 2021, 146, 155654. [Google Scholar] [CrossRef]
- Patel, A.B.; Tsilioni, I.; Weng, Z.; Theoharides, T.C. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp. Dermatol. 2018, 27, 135–143. [Google Scholar] [CrossRef]
- Campbell, I.K.; O’Donnell, K.; Lawlor, K.E.; Wicks, I.P. Severe inflammatory arthritis and lymphadenopathy in the absence of TNF. J. Clin. Investig. 2001, 107, 1519–1527. [Google Scholar] [CrossRef]
- Lemos, M.F.; de Andrade Salustriano, N.; de Souza Costa, M.M.; Lirio, K.; da Fonseca, A.F.A.; Pacheco, H.P.; Endringer, D.C.; Fronza, M.; Scherer, R. Chlorogenic acid and caffeine contents and anti-inflammatory and antioxidant activities of green beans of conilon and arabica coffees harvested with different degrees of maturation. J. Saudi Chem. Soc. 2022, 26, 101467. [Google Scholar] [CrossRef]
- Zheng, Z.; Sheng, Y.; Lu, B.; Ji, L. The therapeutic detoxification of chlorogenic acid against acetaminophen-induced liver injury by ameliorating hepatic inflammation. Chem. Biol. Interact. 2015, 238, 93–101. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Miranda-Cortés, A.; Ibarra-Ríos, D.; Casas-Alvarado, A.; Mora-Medina, P.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Avalos, I. Caffeine: Cardiorespiratory effects and tissue protection in animal models. Exp. Anim. 2021, 70, 431–439. [Google Scholar] [CrossRef]
- Ezeamuzie, C.I.; Shihab, P.K. Interactions between theophylline and salbutamol on cytokine release in human monocytes. J. Pharmacol. Exp. Ther. 2010, 334, 302–309. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, H.; Wei, X.; Shi, M.; Fan, P.; Xie, W.; Zhang, Y.; Xu, N. Aloin Suppresses Lipopolysaccharide-Induced Inflammatory Response and Apoptosis by Inhibiting the Activation of NF-κB. Molecules 2018, 23, 517. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001, 480-481, 243–268. [Google Scholar] [CrossRef] [PubMed]
- Kapp, A. The role of cytokines in the psoriatic inflammation. J. Dermatol. Sci. 1993, 5, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Han, W.; Lee, S.G.; Shin, J.H. Anti-Inflammatory Activity of Chlorogenic Acid on Macrophages: A Simplified Simulation of Pharmacokinetics Following Ingestion Using a Windup Syringe Pump. Appl. Sci. 2023, 13, 627. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline in chronic obstructive pulmonary disease: New horizons. Proc. Am. Thorac. Soc. 2005, 2, 334–339; discussion 340–341. [Google Scholar] [CrossRef]
- Ősz, B.-E.; Jîtcă, G.; Ștefănescu, R.-E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.-E. Caffeine and Its Antioxidant Properties—It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, K.J.; Ryu, S.J.; Lee, B.Y. Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem. Biol. Interact. 2016, 248, 1–7. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Vaddhanaphuti, C.S.; Amornlerdpison, D.; Pengnet, S.; Kamkaew, N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon 2023, 9, e13917. [Google Scholar] [CrossRef]
- Qu, R.; Chen, X.; Hu, J.; Fu, Y.; Peng, J.; Li, Y.; Chen, J.; Li, P.; Liu, L.; Cao, J.; et al. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci. Rep. 2019, 9, 1348. [Google Scholar] [CrossRef]
- Scheel, J.; Kleber, M.; Kreutz, J.; Lehringer, E.; Mehling, A.; Reisinger, K.; Steiling, W. Eye irritation potential: Usefulness of the HET-CAM under the Globally Harmonized System of classification and labeling of chemicals (GHS). Regul. Toxicol. Pharmacol. 2011, 59, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Ruscinc, N.; Massarico Serafim, R.A.; Almeida, C.; Rosado, C.; Baby, A.R. Challenging the safety and efficacy of topically applied chlorogenic acid, apigenin, kaempferol, and naringenin by HET-CAM, HPLC-TBARS-EVSC, and laser Doppler flowmetry. Front. Chem. 2024, 12, 1400881. [Google Scholar] [CrossRef] [PubMed]
- Chiangnoon, R.; Samee, W.; Uttayarat, P.; Jittachai, W.; Ruksiriwanich, W.; Sommano, S.R.; Athikomkulchai, S.; Chittasupho, C. Phytochemical Analysis, Antioxidant, and Wound Healing Activity of Pluchea indica L. (Less) Branch Extract Nanoparticles. Molecules 2022, 27, 635. [Google Scholar] [CrossRef]
- Assiry, A.A.; Bhavikatti, S.K.; Althobaiti, F.A.; Mohamed, R.N.; Karobari, M.I. Evaluation of In Vitro Antiprotease Activity of Selected Traditional Medicinal Herbs in Dentistry and Its In Silico PASS Prediction. Biomed. Res. Int. 2022, 2022, 5870443. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.Y.; Soo, W.K.; Chan, K.Y.; Mustafa, M.R.; Goh, S.H.; Imiyabir, Z. Lipoxygenase inhibiting activity of some Malaysian plants. Pharm. Biol. 2009, 47, 1142–1148. [Google Scholar] [CrossRef]
- Chittasupho, C.; Ditsri, S.; Singh, S.; Kanlayavattanakul, M.; Duangnin, N.; Ruksiriwanich, W.; Athikomkulchai, S. Ultraviolet Radiation Protective and Anti-Inflammatory Effects of Kaempferia galanga L. Rhizome Oil and Microemulsion: Formulation, Characterization, and Hydrogel Preparation. Gels 2022, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Lowry, M.A.; Goldberg, J.I.; Belosevic, M. Induction of nitric oxide (NO) synthesis in murine macrophages requires potassium channel activity. Clin. Exp. Immunol. 1998, 111, 597–603. [Google Scholar] [CrossRef]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R.; et al. Inhibition of 5α-Reductase, IL-6 Secretion, and Oxidation Process of Equisetum debile Roxb. ex Vaucher Extract as Functional Food and Nutraceuticals Ingredients. Nutrients 2017, 9, 105. [Google Scholar] [CrossRef]


| Tested Compound | Antioxidant Activity | |
|---|---|---|
| ABTS TEAC (µM Trolox/g) | FRAP (mM FeSO4) | |
| CCS extract | 193.6 ± 3.7 a | 6.52 ± 0.7 a |
| CGA | 310.3 ± 2.5 d | 18.71 ± 1.3 d |
| CAF | 213.8 ± 1.0 c | 10.05 ± 0.9 c |
| THP | 204.6 ± 1.4 b | 8.30 ± 1.0 b |
| Gene | Primer | Sequence: (5′-3′) |
|---|---|---|
| β–actin | Forward | TCATGAAGTGTGACGTTGACATCCGT |
| Reverse | CCTAGAAGCATTTGCGGTGCACGATG | |
| IL-6 | Forward | CATCCAGTTGCCTTCTTGGGA |
| Reverse | GCATTGGAAATTGGGGTAGGAAG | |
| TNF-α | Forward | ATGAGCACAGAAAGCATGATC |
| Reverse | TACAGGCTTGTCACTCGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Duangnin, N.; Kiattisin, K. Potential of Coffee Cherry Pulp Extract against Polycyclic Aromatic Hydrocarbons in Air Pollution Induced Inflammation and Oxidative Stress for Topical Applications. Int. J. Mol. Sci. 2024, 25, 9416. https://doi.org/10.3390/ijms25179416
Preedalikit W, Chittasupho C, Leelapornpisid P, Duangnin N, Kiattisin K. Potential of Coffee Cherry Pulp Extract against Polycyclic Aromatic Hydrocarbons in Air Pollution Induced Inflammation and Oxidative Stress for Topical Applications. International Journal of Molecular Sciences. 2024; 25(17):9416. https://doi.org/10.3390/ijms25179416
Chicago/Turabian StylePreedalikit, Weeraya, Chuda Chittasupho, Pimporn Leelapornpisid, Natthachai Duangnin, and Kanokwan Kiattisin. 2024. "Potential of Coffee Cherry Pulp Extract against Polycyclic Aromatic Hydrocarbons in Air Pollution Induced Inflammation and Oxidative Stress for Topical Applications" International Journal of Molecular Sciences 25, no. 17: 9416. https://doi.org/10.3390/ijms25179416
APA StylePreedalikit, W., Chittasupho, C., Leelapornpisid, P., Duangnin, N., & Kiattisin, K. (2024). Potential of Coffee Cherry Pulp Extract against Polycyclic Aromatic Hydrocarbons in Air Pollution Induced Inflammation and Oxidative Stress for Topical Applications. International Journal of Molecular Sciences, 25(17), 9416. https://doi.org/10.3390/ijms25179416








