Anti-Warburg Mechanism of Ginsenoside F2 in Human Cervical Cancer Cells via Activation of miR193a-5p and Inhibition of β-Catenin/c-Myc/Hexokinase 2 Signaling Axis
Abstract
:1. Introduction
2. Results
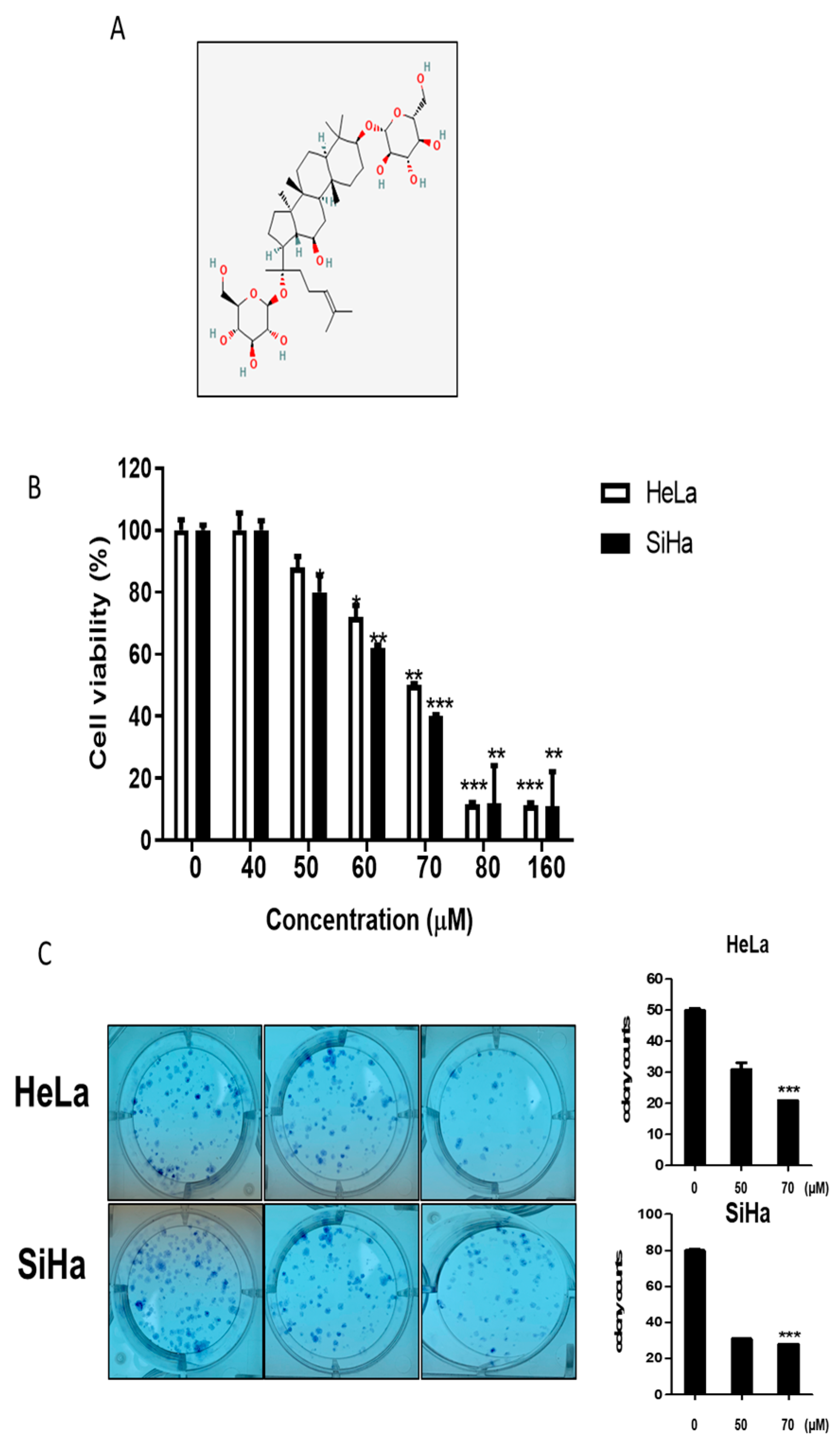
2.1. Cytotoxic and Antiproliferative Effects of GF2 in Human Cervical Cancer Cells
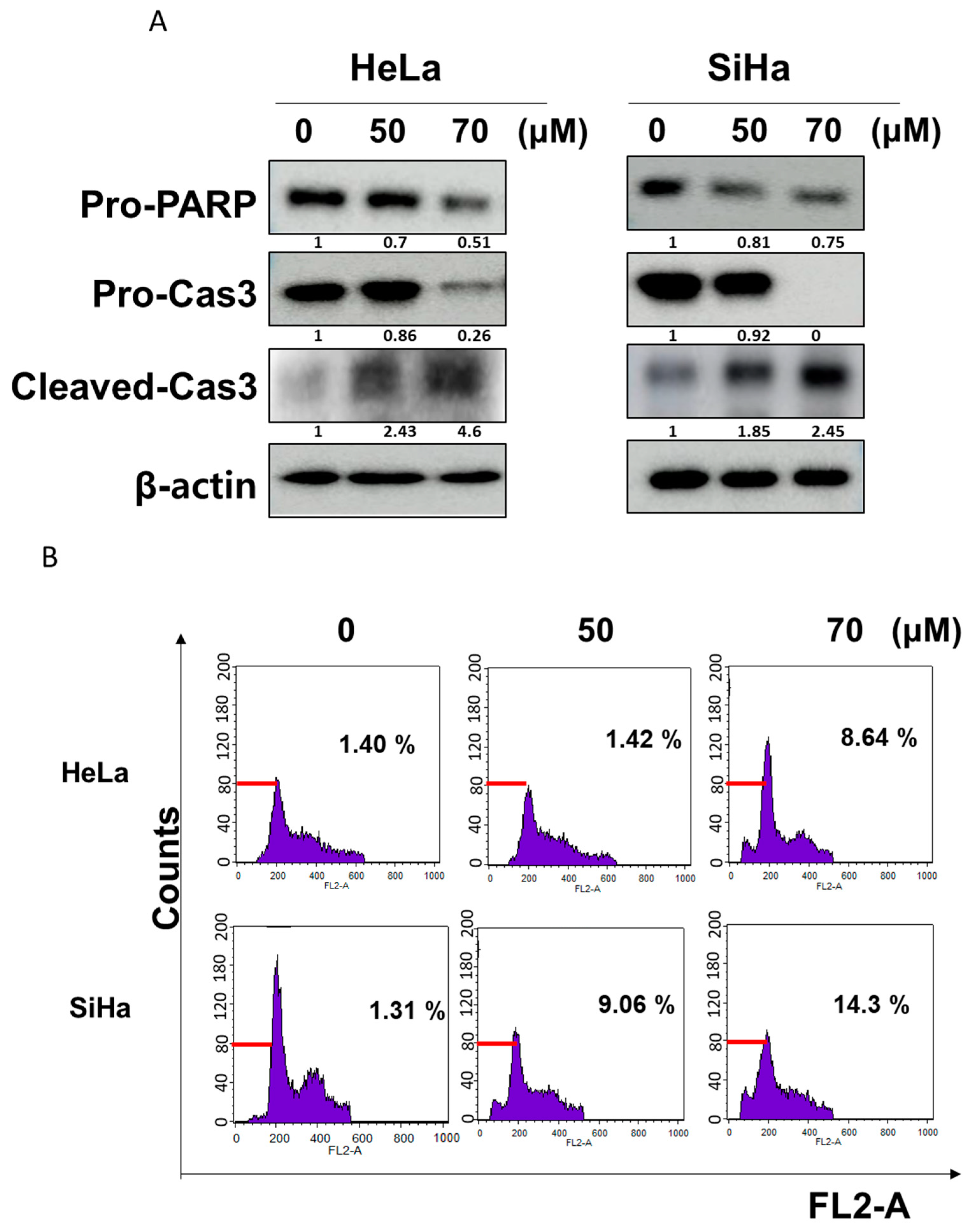
2.2. GF2 Increased Sub-G1 Population and Induced Apoptosis in HeLa and SiHa Cells
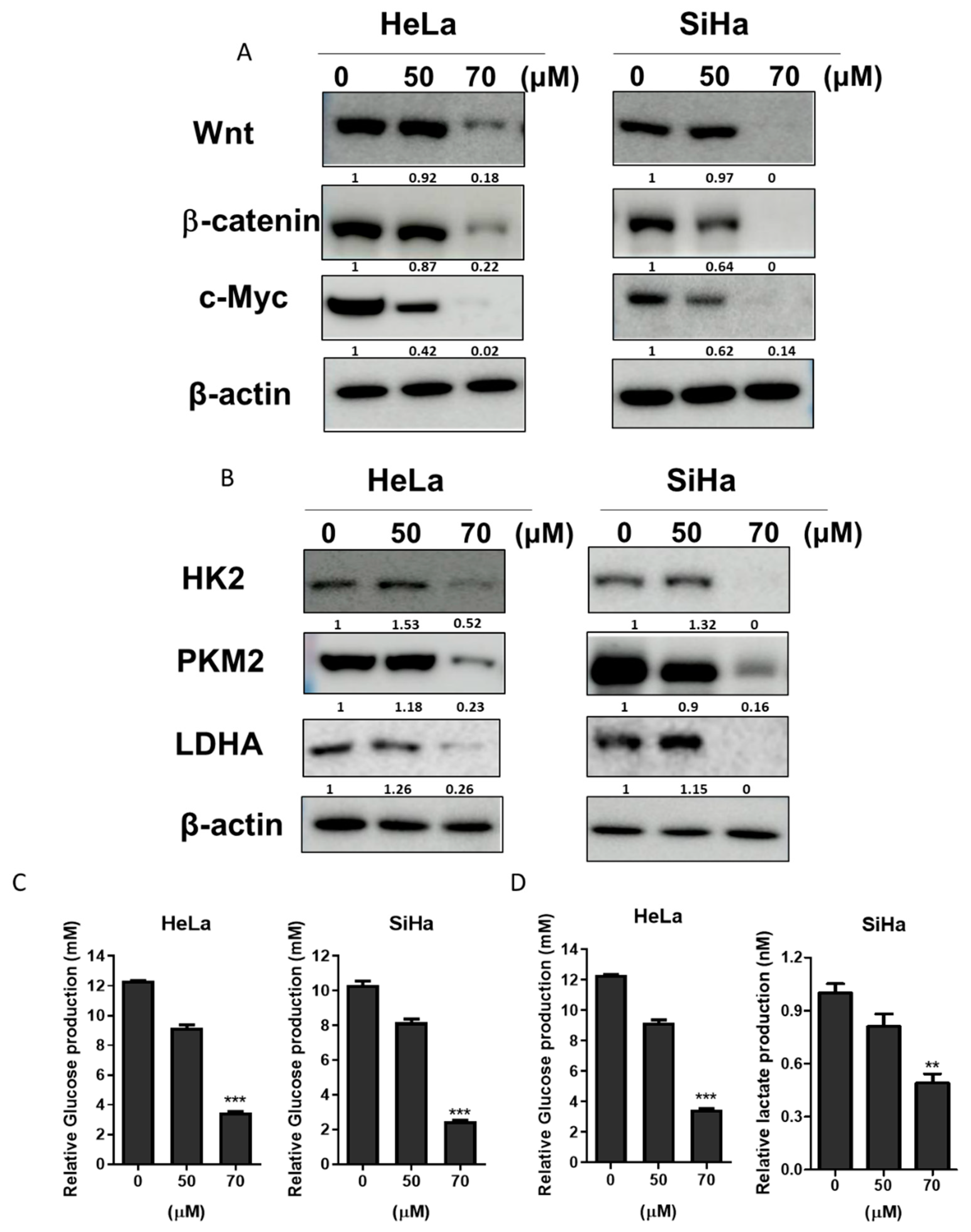
2.3. GF2 Diminished the Expression Level of Glycolysis Proteins as Well as the Production of Lactate in HeLa and SiHa Cells
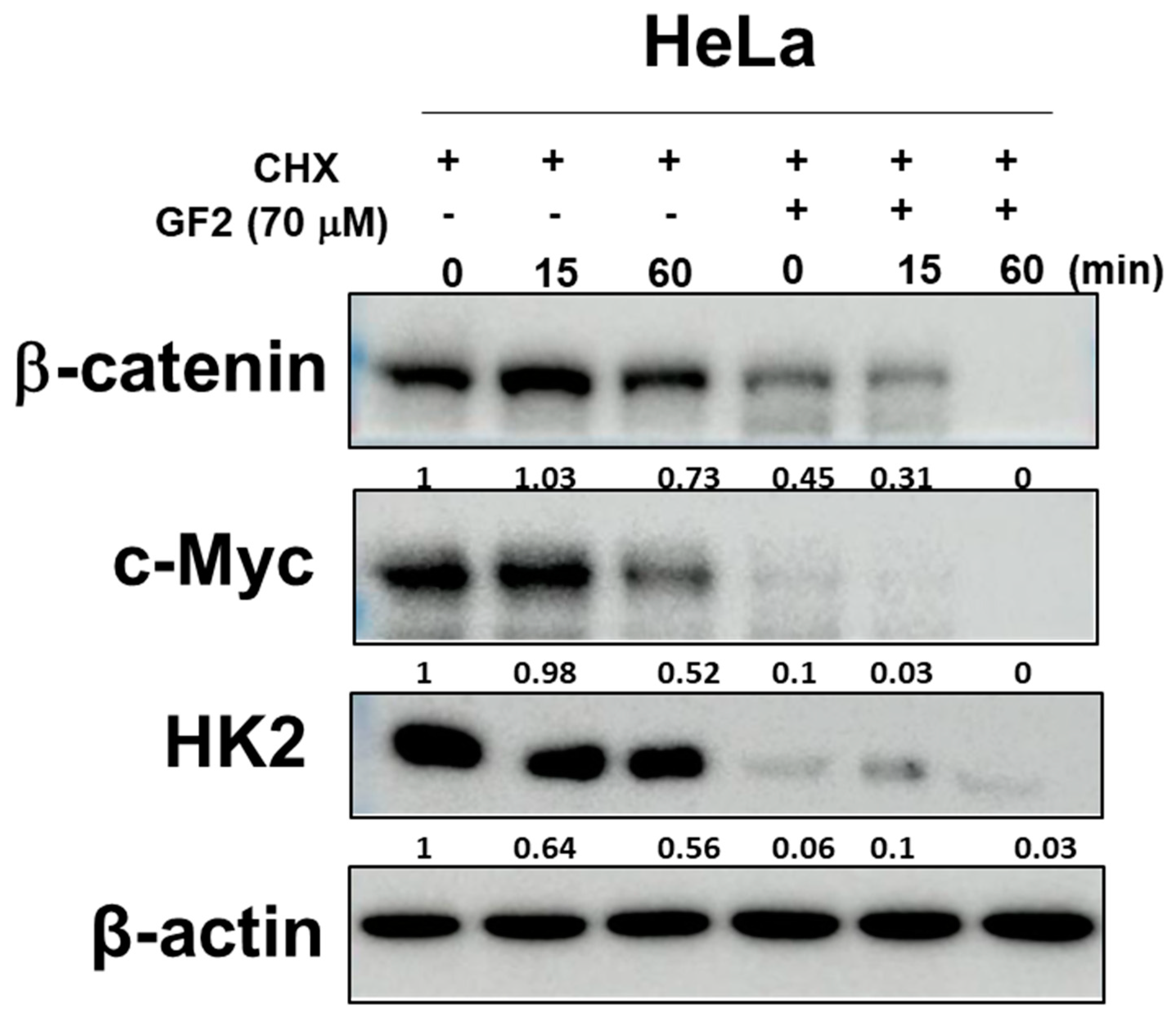
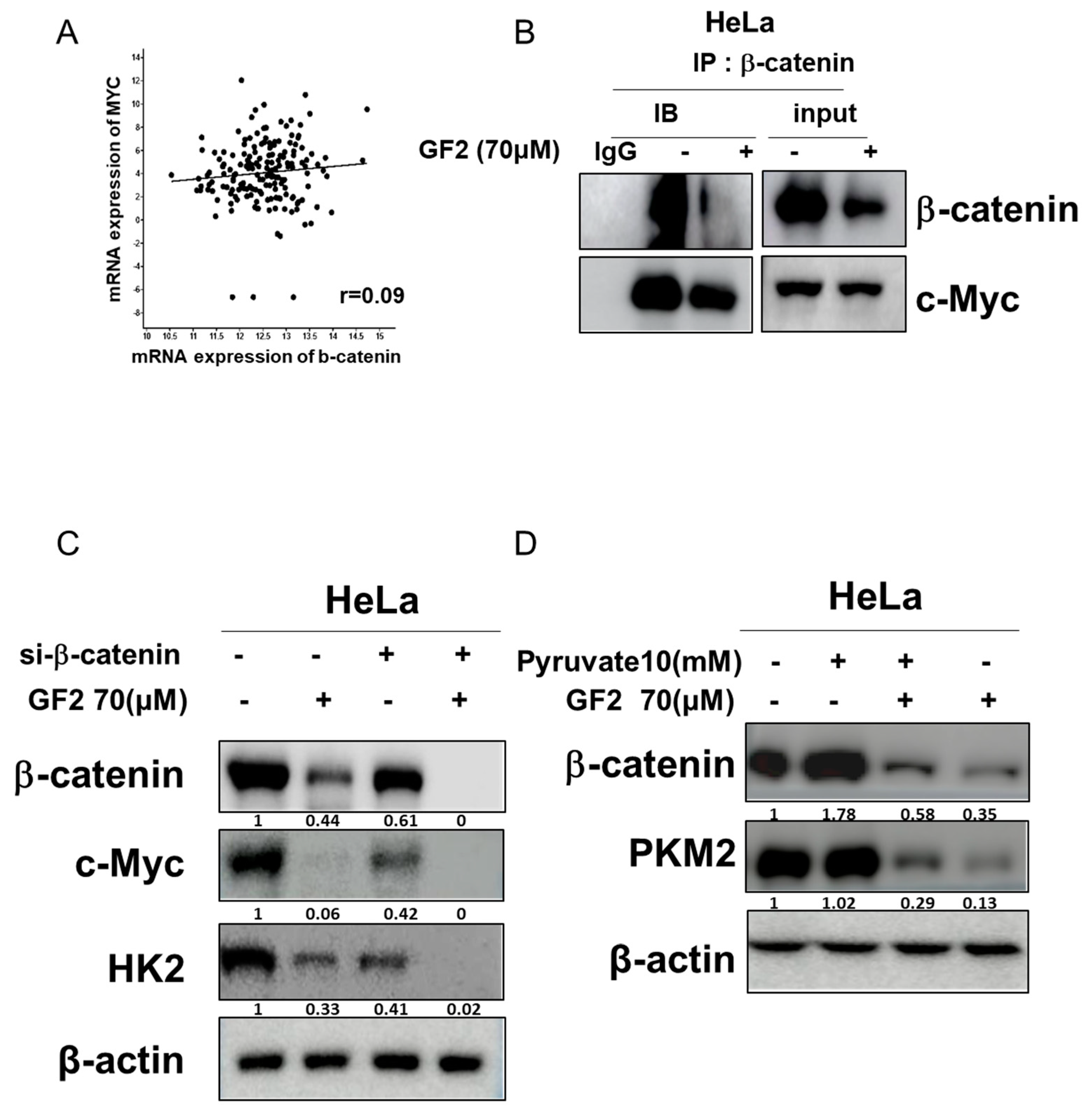
2.4. GF2 Reduced β-Catenin, Glucose-Related Proteins, and c-Myc Stability in HeLa Cells
2.5. GF2 Disrupted the Interaction between β-Catenin and c-Myc, While β-Catenin siRNA Reduced the Band Level of Glycolysis-Associated Proteins and GF2-Induced ATP Depletion in HeLa Cells
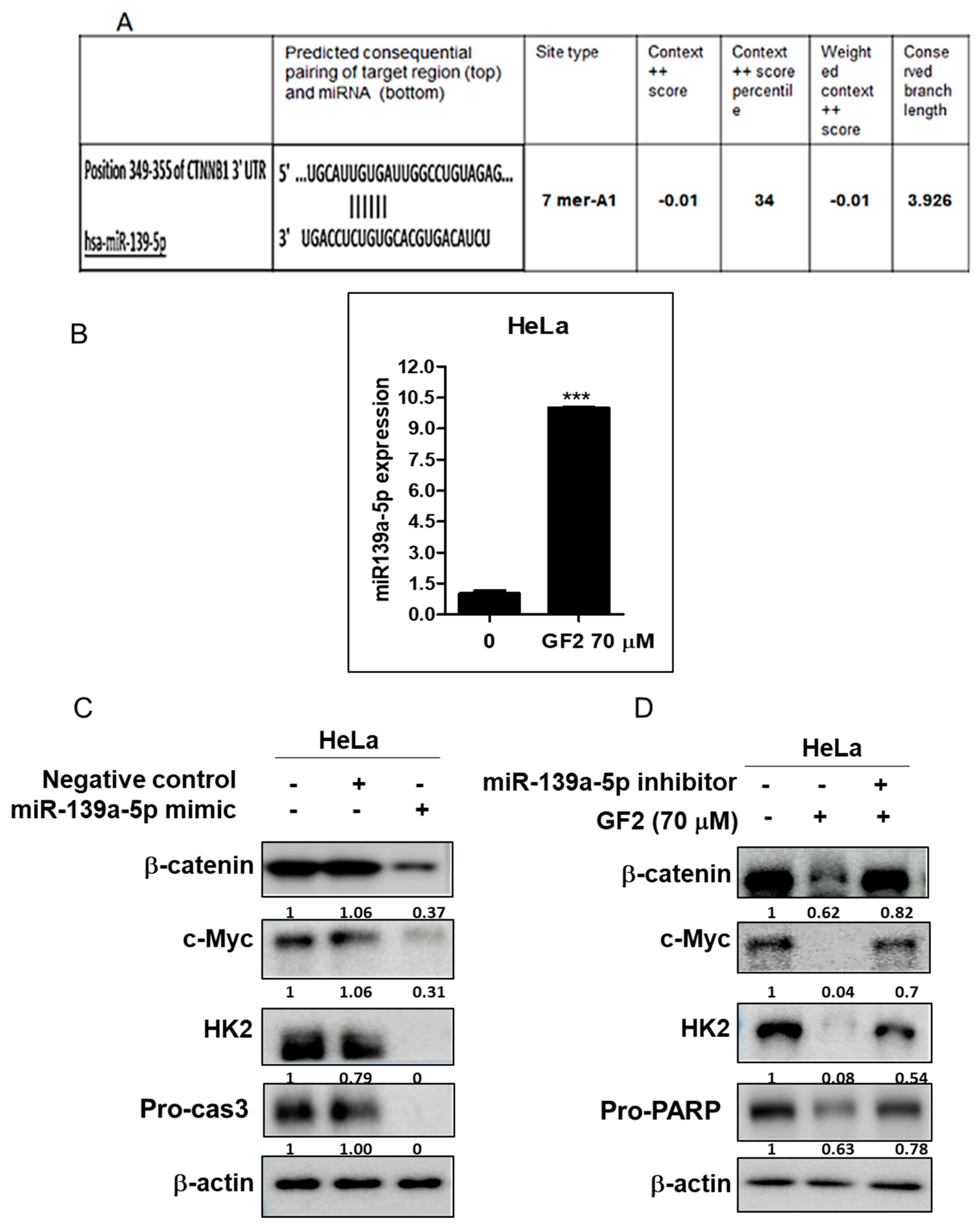
2.6. miR139a-5p Plays a Critical Role in GF2-Induced Apoptosis in HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Ginsenoside F2 (GF2)
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell Cycle Analysis
4.5. RT-qPCR Analysis
4.6. Western Blotting
4.7. Co-Immunoprecipitation
4.8. RNA Interference
4.9. Statistical Analysis
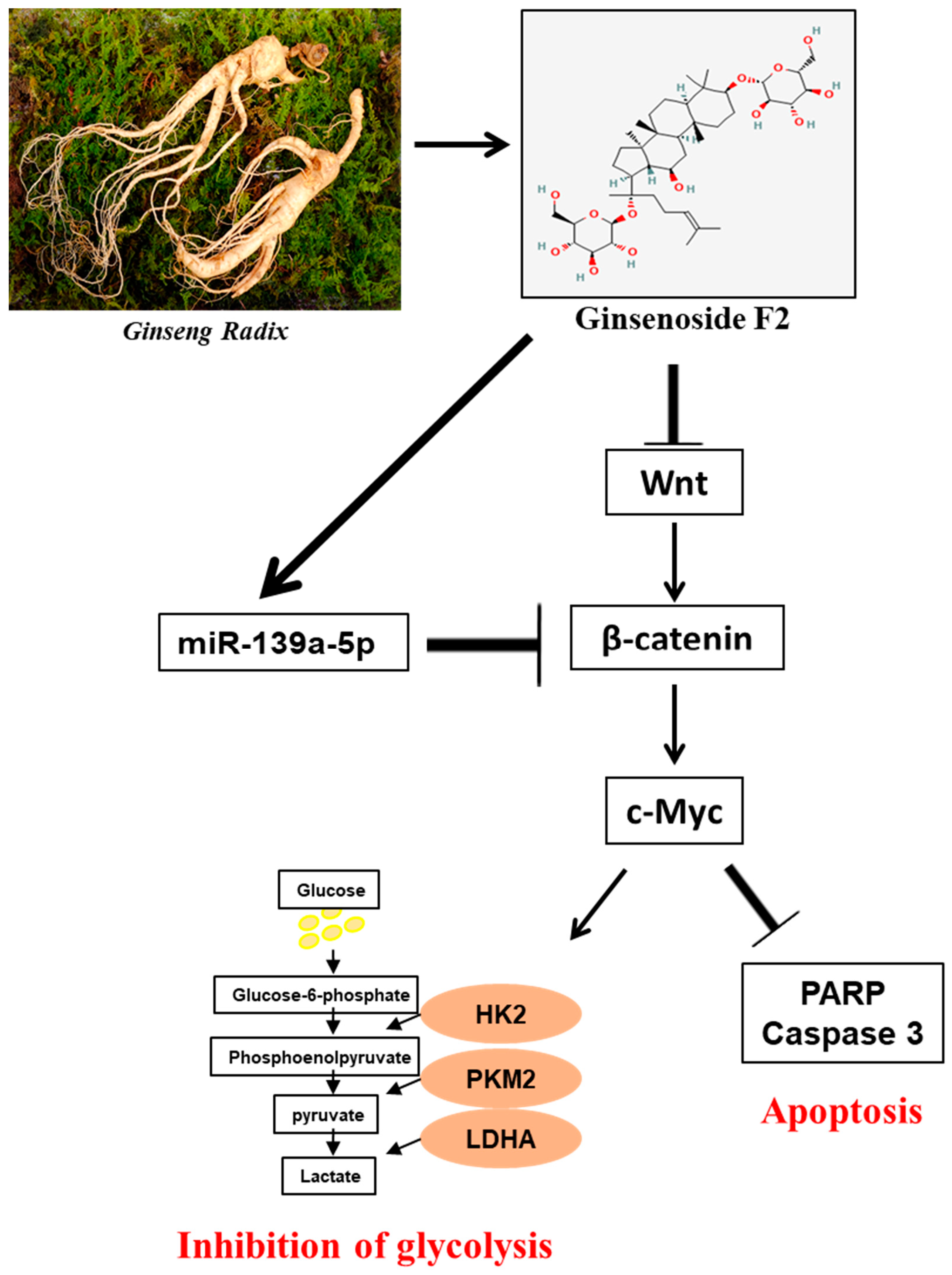
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buskwofie, A.; David-West, G.; Clare, C.A. A Review of Cervical Cancer: Incidence and Disparities. J. Natl. Med. Assoc. 2020, 112, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Franconi, R.; Massa, S.; Paolini, F.; Vici, P.; Venuti, A. Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers. Cancers 2020, 12, 3101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017, 4, 25–27. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Huang, P.; Zhu, S.; Liang, X.; Zhang, Q.; Luo, X.; Liu, C.; Song, L. Regulatory Mechanisms of LncRNAs in Cancer Glycolysis: Facts and Perspectives. Cancer Manag. Res. 2021, 13, 5317–5336. [Google Scholar] [CrossRef]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef]
- Song, M.Y.; Lee, D.Y.; Chun, K.S.; Kim, E.H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y.; Vallee, J.N. The Key Role of the WNT/beta-Catenin Pathway in Metabolic Reprogramming in Cancers under Normoxic Conditions. Cancers 2021, 13, 5557. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Zhang, L.; Yang, L.; Zhou, M.; Li, X.; Li, Y.; Li, G.; Zeng, Z.; Xiong, W.; et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J. Cancer 2019, 10, 3789–3797. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y.; Guillevin, R.; Vallee, J.N. Aerobic Glycolysis Hypothesis Through WNT/Beta-Catenin Pathway in Exudative Age-Related Macular Degeneration. J. Mol. Neurosci. 2017, 62, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, Y.; Zhang, P.; Li, J.; Lu, X. Solasonine Inhibits Cancer Stemness and Metastasis by Modulating Glucose Metabolism via Wnt/beta-Catenin/Snail Pathway in Osteosarcoma. Am. J. Chin. Med. 2023, 51, 1293–1308. [Google Scholar] [CrossRef]
- Zhou, J.; Su, C.M.; Chen, H.A.; Du, S.; Li, C.W.; Wu, H.; Tsai, S.H.; Yeh, Y.T. Cryptanshinone Inhibits the Glycolysis and Inhibits Cell Migration Through PKM2/beta-Catenin Axis in Breast Cancer. OncoTargets Ther. 2020, 13, 8629–8639. [Google Scholar] [CrossRef]
- Christensen, L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2009, 55, 1–99. [Google Scholar] [CrossRef]
- Park, S.H.; Seo, W.; Eun, H.S.; Kim, S.Y.; Jo, E.; Kim, M.H.; Choi, W.M.; Lee, J.H.; Shim, Y.R.; Cui, C.H.; et al. Protective effects of ginsenoside F2 on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Biochem. Biophys. Res. Commun. 2016, 478, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Kabadayı, S.N.; Sadiq, N.B.; Hamayun, M.; Park, N.I.; Kim, H.Y. Impact of Sodium Silicate Supplemented, IR-Treated Panax Ginseng on Extraction Optimization for Enhanced Anti-Tyrosinase and Antioxidant Activity: A Response Surface Methodology (RSM) Approach. Antioxidants 2023, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Li, J.; Guan, Y.; Shen, T.; Li, F.; Li, X.; Yang, X.; Hu, W. Ginsenoside F2 Suppresses Adipogenesis in 3T3-L1 Cells and Obesity in Mice via the AMPK Pathway. J. Agric. Food Chem. 2021, 69, 9299–9312. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, P.H.; Wang, Q.; Li, S.L. Ginsenoside F(2) induces apoptosis in humor gastric carcinoma cells through reactive oxygen species-mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine 2014, 21, 515–522. [Google Scholar] [CrossRef]
- Mai, T.T.; Moon, J.; Song, Y.; Viet, P.Q.; Phuc, P.V.; Lee, J.M.; Yi, T.H.; Cho, M.; Cho, S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012, 321, 144–153. [Google Scholar] [CrossRef]
- Tan, X.; Ma, X.; Dai, Y.; An, J.; Yu, X.; Li, S.; Liao, Y.; Pei, T.; Tang, Y.; Gui, Y.; et al. A large-scale transcriptional analysis reveals herb-derived ginsenoside F2 suppressing hepatocellular carcinoma via inhibiting STAT3. Phytomedicine 2023, 120, 155031. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, H.J.; Kang, M.; Cho, J.H.; Kim, Y.G.; Lee, S.M.; Byun, J.S.; Kim, D.Y. Ginsenoside F2 induces cellular toxicity to glioblastoma through the impairment of mitochondrial function. Phytomedicine 2021, 83, 153483. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Shen, Z.Y.; Zhan, Y.Z.; Feng, X.C.; Chen, K.L.; Li, Y.S.; Deng, H.J.; Pan, S.M.; Wu, D.H.; Ding, Y. CD36 inhibits beta-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat. Commun. 2019, 10, 3981. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of cancer cell metabolism: Oncogenic MYC in the driver’s seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Gu, Z.; Xia, J.; Xu, H.; Frech, I.; Tricot, G.; Zhan, F. NEK2 Promotes Aerobic Glycolysis in Multiple Myeloma Through Regulating Splicing of Pyruvate Kinase. J. Hematol. Oncol. 2017, 10, 17. [Google Scholar] [CrossRef]
- Shin, N.; Lee, H.J.; Sim, D.Y.; Im, E.; Park, J.E.; Park, W.Y.; Cho, A.R.; Shim, B.S.; Kim, S.H. Apoptotic effect of compound K in hepatocellular carcinoma cells via inhibition of glycolysis and Akt/mTOR/c-Myc signaling. Phytother. Res. 2021, 35, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef]
- Ko, S.R.; Suzuki, Y.; Suzuki, K.; Choi, K.J.; Cho, B.G. Marked production of ginsenosides Rd, F2, Rg3, and compound K by enzymatic method. Chem. Pharm. Bull. 2007, 55, 1522–1527. [Google Scholar] [CrossRef]
- Cai, C.F.; Ye, G.D.; Shen, D.Y.; Zhang, W.; Chen, M.L.; Chen, X.X.; Han, D.X.; Mi, Y.J.; Luo, Q.C.; Cai, W.Y.; et al. Chibby suppresses aerobic glycolysis and proliferation of nasopharyngeal carcinoma via the Wnt/beta-catenin-Lin28/let7-PDK1 cascade. J. Exp. Clin. Cancer Res. 2018, 37, 104. [Google Scholar] [CrossRef]
- Akiyama, T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000, 11, 273–282. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of beta-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Cha, P.H.; Hwang, J.H.; Kwak, D.K.; Koh, E.; Kim, K.S.; Choi, K.Y. APC loss induces Warburg effect via increased PKM2 transcription in colorectal cancer. Br. J. Cancer 2021, 124, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; Liu, L.; Wang, M. beta-Catenin: Oncogenic role and therapeutic target in cervical cancer. Biol. Res. 2020, 53, 33. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, P.; Li, H.; Liu, Y.; Wang, T.; Cheng, Y. Ginsenoside Rh2 Inhibits Glycolysis through the STAT3/c-MYC Axis in Non-Small-Cell Lung Cancer. J. Oncol. 2021, 2021, 9715154. [Google Scholar] [CrossRef]
- Liu, X.; Zuo, X.; Sun, X.; Tian, X.; Teng, Y. Hexokinase 2 Promotes Cell Proliferation and Tumor Formation through the Wnt/beta-catenin Pathway-mediated Cyclin D1/c-myc Upregulation in Epithelial Ovarian Cancer. J. Cancer 2022, 13, 2559–2569. [Google Scholar] [CrossRef]
- Ji, X.; Guo, H.; Yin, S.; Du, H. miR-139-5p functions as a tumor suppressor in cervical cancer by targeting TCF4 and inhibiting Wnt/beta-catenin signaling. OncoTargets Ther. 2019, 12, 7739–7748. [Google Scholar] [CrossRef]
- Cheng, C.W.; Liao, W.L.; Chen, P.M.; Yu, J.C.; Shiau, H.P.; Hsieh, Y.H.; Lee, H.J.; Cheng, Y.C.; Wu, P.E.; Shen, C.Y. MiR-139 Modulates Cancer Stem Cell Function of Human Breast Cancer through Targeting CXCR4. Cancers 2021, 13, 2582. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.I.; Sim, D.Y.; Lee, H.J.; Ahn, C.H.; Park, J.; Park, S.Y.; Lee, D.; Shim, B.S.; Kim, B.; Kim, S.H. Apoptotic and anti-Warburg effect of Morusin via ROS mediated inhibition of FOXM1/c-Myc signaling in prostate cancer cells. Phytother. Res. 2023, 37, 4473–4487. [Google Scholar] [CrossRef]
- Kim, R.; Kim, J.W.; Choi, H.; Oh, J.E.; Kim, T.H.; Go, G.Y.; Lee, S.J.; Bae, G.U. Ginsenoside Rg5 promotes muscle regeneration via p38MAPK and Akt/mTOR signaling. J. Ginseng Res. 2023, 47, 726–734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, N.; Lee, H.-J.; Sim, D.Y.; Ahn, C.-H.; Park, S.-Y.; Koh, W.; Khil, J.; Shim, B.-S.; Kim, B.; Kim, S.-H. Anti-Warburg Mechanism of Ginsenoside F2 in Human Cervical Cancer Cells via Activation of miR193a-5p and Inhibition of β-Catenin/c-Myc/Hexokinase 2 Signaling Axis. Int. J. Mol. Sci. 2024, 25, 9418. https://doi.org/10.3390/ijms25179418
Shin N, Lee H-J, Sim DY, Ahn C-H, Park S-Y, Koh W, Khil J, Shim B-S, Kim B, Kim S-H. Anti-Warburg Mechanism of Ginsenoside F2 in Human Cervical Cancer Cells via Activation of miR193a-5p and Inhibition of β-Catenin/c-Myc/Hexokinase 2 Signaling Axis. International Journal of Molecular Sciences. 2024; 25(17):9418. https://doi.org/10.3390/ijms25179418
Chicago/Turabian StyleShin, Nari, Hyo-Jung Lee, Deok Yong Sim, Chi-Hoon Ahn, Su-Yeon Park, Wonil Koh, Jaeho Khil, Bum-Sang Shim, Bonglee Kim, and Sung-Hoon Kim. 2024. "Anti-Warburg Mechanism of Ginsenoside F2 in Human Cervical Cancer Cells via Activation of miR193a-5p and Inhibition of β-Catenin/c-Myc/Hexokinase 2 Signaling Axis" International Journal of Molecular Sciences 25, no. 17: 9418. https://doi.org/10.3390/ijms25179418





