The Enigmatic Interplay of Interleukin-10 in the Synergy of HIV Infection Comorbid with Preeclampsia
Abstract
1. Introduction
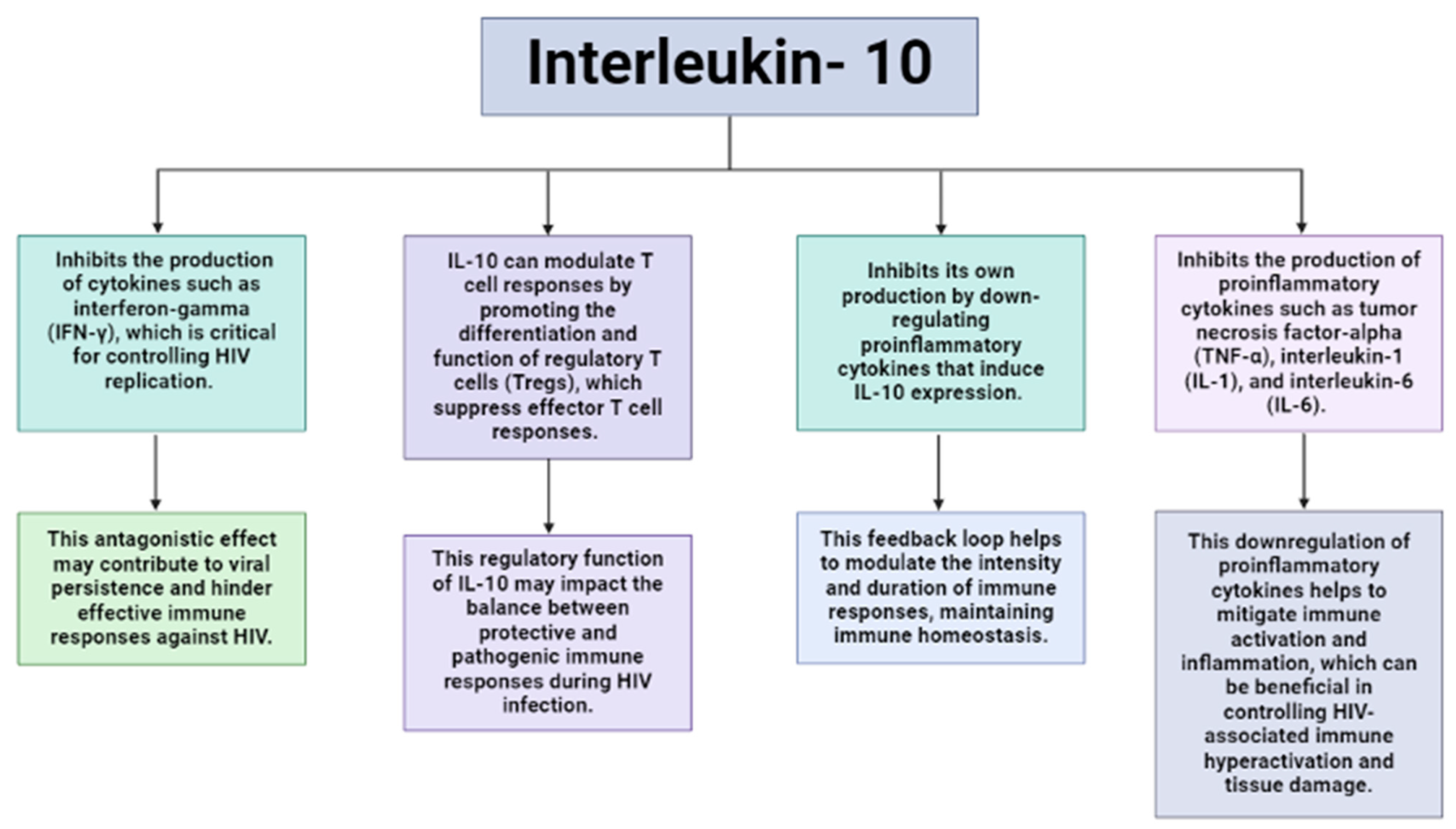
2. Interleukin-10
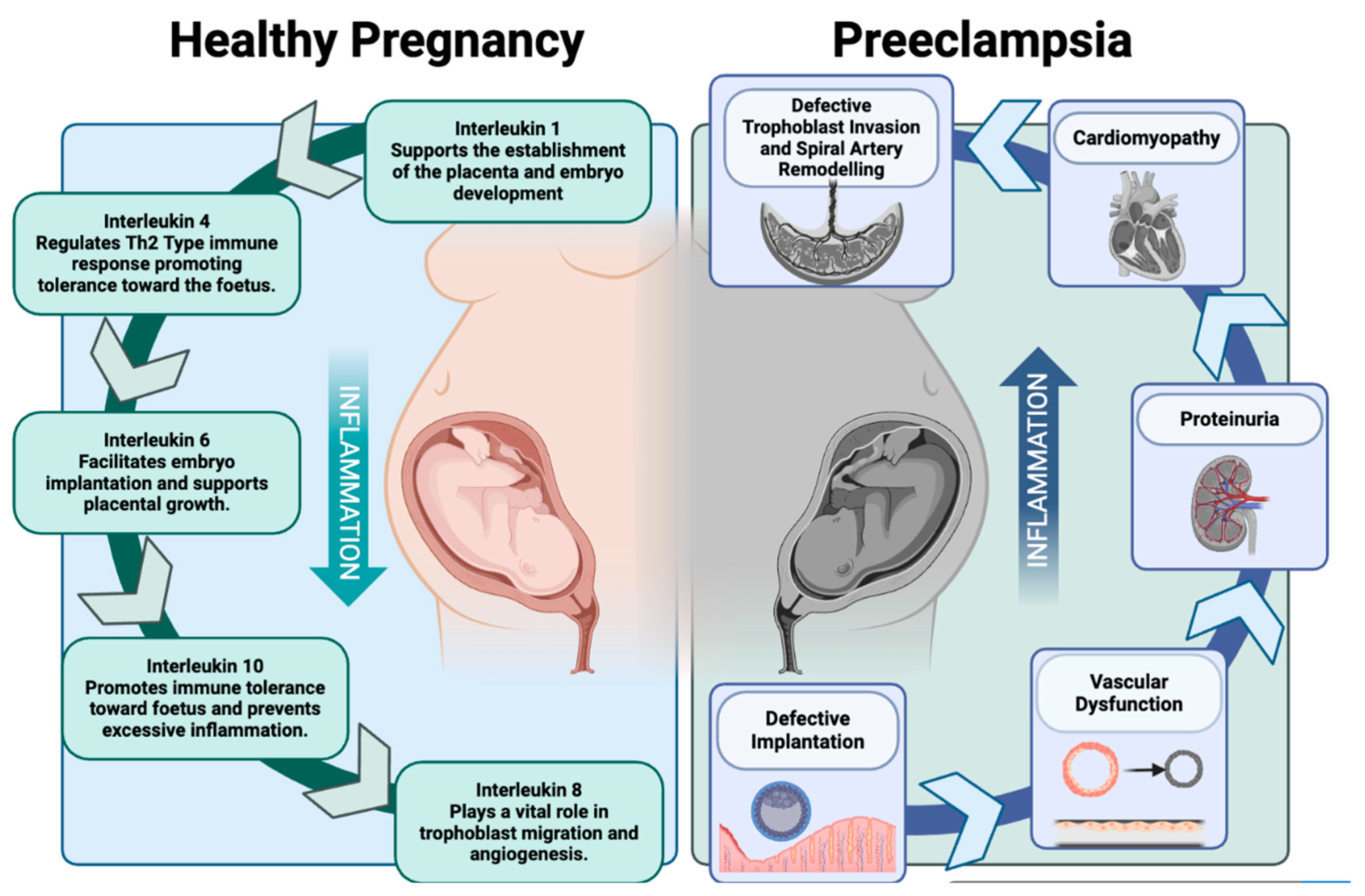
3. The Role of IL-10 in Normal Pregnancy
4. First Trimester
5. Second Trimester
6. Third Trimester
7. Preeclampsia
8. The Influence of IL-10 Promoter Region SNPs on Preeclampsia
9. Human Immunodeficiency Virus
10. IL-10 Gene Polymorphisms and Their Role in HIV Susceptibility
11. Antiretroviral Therapy and Its Effect on Interleukin-10
12. The Synergy of HIV-Infected Preeclamptic Women
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine storm in COVID-19: Immunopathogenesis and therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Paccagnella, M.; Abbona, A.; Michelotti, A.; Geuna, E.; Ruatta, F.; Landucci, E.; Denaro, N.; Vanella, P.; Lo Nigro, C.; Galizia, D.; et al. Circulating cytokines in metastatic breast cancer patients select different prognostic groups and patients who might benefit from treatment beyond progression. Vaccines 2022, 10, 78. [Google Scholar] [CrossRef]
- Wang, J.; Erlacher, M.; Fernandez-Orth, J. The role of inflammation in hematopoiesis and bone marrow failure: What can we learn from mouse models? Front. Immunol. 2022, 13, 951937. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Jan, A.; Sofi, S. Introduction to Cytokine and Chemokine Networks. In Cytokine and Chemokine Networks in Cancer; Springer: Singapore, 2023; pp. 1–31. [Google Scholar]
- Mishra, A.K. Role of cytokines in the control of inflammation: A review. Int. J. Ther. Innov. 2023, 1, 5–8. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Innate immunity. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Druszczyńska, M.; Godkowicz, M.; Kulesza, J.; Wawrocki, S.; Fol, M. Cytokine receptors-regulators of antimycobacterial immune response. Int. J. Mol. Sci. 2022, 23, 1112. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.C.; Sridhar, P.R.; Baldridge, M.T. Differential roles of interferons in innate responses to mucosal viral infections. Trends Immunol. 2021, 42, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.; Huo, J.; Li, X.; Li, W.; Li, X.; Ao, J.; Chen, X. IL-2 signaling couples the MAPK and mTORC1 axes to promote T cell proliferation and differentiation in teleosts. J. Immunol. 2022, 208, 1616–1631. [Google Scholar] [CrossRef]
- Lee, G.R. Molecular mechanisms of T helper cell differentiation and functional specialization. Immune Netw. 2023, 23, e4. [Google Scholar] [CrossRef]
- Bobhate, A.; Viswanathan, V.; Aravindhan, V. Anti-inflammatory cytokines IL-27, IL-10, IL-1Ra and TGF-β in subjects with increasing grades of glucose intolerence (DM-LTB-2). Cytokine 2021, 137, 155333. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morales, P.; Franklin, R.A. Macrophage phenotypes and functions: Resolving inflammation and restoring homeostasis. Trends Immunol. 2023, 44, 986–998. [Google Scholar] [CrossRef]
- Johnson, J.D.; Barnard, D.F.; Kulp, A.C.; Mehta, D.M. Neuroendocrine regulation of brain cytokines after psychological stress. J. Endocr. Soc. 2019, 3, 1302–1320. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Oh, S.H.; Muhammad, T.; El-Sheikh, M.; Song, S.H.; Lee, K.L.; Kong, I.K. Growth factors, and cytokines; understanding the role of tyrosine phosphatase SHP2 in gametogenesis and early embryo development. Cells 2020, 9, 1798. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Krzyzanski, W.; Wong, R.S.; Yan, X. Fate determination role of erythropoietin and romiplostim in the lineage commitment of hematopoietic progenitors. J. Pharmacol. Exp. Ther. 2022, 382, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Melloni, E.; Zauli, G.; Romani, A.; Secchiero, P.; Maximova, N.; Rimondi, E. Autoinflammatory diseases and cytokine storms-imbalances of innate and adaptative immunity. Int. J. Mol. Sci. 2021, 22, 11241. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. Semin. Immunol. 2019, 44, 101344. [Google Scholar] [CrossRef]
- Oktariana, D.; Saleh, I.; Hafy, Z.; Liberty, I.A.; Salim, E.M.; Legiran, L. The Role of Interleukin-10 in Leprosy: A Review. Indian J. Lepr. 2022, 94, 321–334. [Google Scholar]
- Herawati, S.; Kandarini, Y.; Mulyantari, N.K.; Prabawa, P.Y. Correlation of Neutrophil to Lymphocyte Ratio with Interleukin-10 in Diagnosis and Monitoring of Coronavirus Disease-19 Patients. Open Access Maced. J. Med. Sci. 2022, 10, 63–66. [Google Scholar] [CrossRef]
- Minshawi, F.; Lanvermann, S.; McKenzie, E.; Jeffery, R.; Couper, K.; Papoutsopoulou, S.; Roers, A.; Muller, W. The generation of an engineered interleukin-10 protein with improved stability and biological function. Front. Immunol. 2020, 11, 1794. [Google Scholar] [CrossRef]
- Thaxton, J.E.; Sharma, S. Interleukin-10: A multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 2010, 63, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.P.; Diez, D.; Miranda-Saavedra, D. The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Brief. Funct. Genom. 2013, 12, 489–498. [Google Scholar] [CrossRef]
- Murray, P.J. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 2006, 6, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Sharma, S. Interleukin-10: A pleiotropic regulator in pregnancy. Am. J. Reprod. Immunol. 2015, 73, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef]
- Han, J.; Yoo, I.; Lee, S.; Cheon, Y.; Yun, C.H.; Ka, H. Interleukin-10 and its receptors at the maternal-conceptus interface: Expression, regulation, and implication for T helper 2 cytokine predominance and maternal immune tolerance in the pig, a true epitheliochorial placentation species. Biol. Reprod. 2022, 106, 1159–1174. [Google Scholar] [CrossRef]
- Roberts, L.; Passmore, J.A.S.; Williamson, C.; Little, F.; Bebell, L.M.; Mlisana, K.; Burgers, W.A.; van Loggerenberg, F.; Walzl, G.; Siawaya, J.F.D.; et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. Aids 2010, 24, 819–831. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsueh, Y.W.; Chang, C.W.; Hsu, H.C.; Yang, T.C.; Lin, W.C.; Chang, H.M. Establishment of the fetal-maternal interface: Developmental events in human implantation and placentation. Front. Cell Dev. Biol. 2023, 11, 1200330. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Guo, X.; Wong, C.K.; Chen, X.; Chan, Y.L.; Wang, C.C.; Laird, S.; Li, T.C. Successful implantation is associated with a transient increase in serum pro-inflammatory cytokine profile followed by a switch to anti-inflammatory cytokine profile prior to confirmation of pregnancy. Fertil. Steril. 2021, 115, 1044–1053. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Kakar, S. Cytokines evolution: Role in various diseases. Curr. Med. Res. Pract. 2015, 5, 176–182. [Google Scholar] [CrossRef]
- Hemalatha, V.T.; Manigandan, T.; Sarumathi, T.; Amudhan, A. Dental considerations in pregnancy—A critical review on the oral care. J. Clin. Diagn. Res. JCDR 2013, 7, 948. [Google Scholar]
- Weng, J.; Couture, C.; Girard, S. Innate and adaptive immune systems in physiological and pathological pregnancy. Biology 2023, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.L.; Zhao, S.J.; Lin, X.X.; Liao, A.H. IL-10: A bridge between immune cells and metabolism during pregnancy. J. Reprod. Immunol. 2022, 154, 103750. [Google Scholar] [CrossRef] [PubMed]
- Spence, T.; Allsopp, P.J.; Yeates, A.J.; Mulhern, M.S.; Strain, J.J.; McSorley, E.M. Maternal serum cytokine concentrations in healthy pregnancy and preeclampsia. J. Pregnancy 2021, 2021, 6649608. [Google Scholar] [CrossRef] [PubMed]
- Raguema, N.; Gannoun, M.B.A.; Zitouni, H.; Meddeb, S.; Benletaifa, D.; Lavoie, J.L.; Almawi, W.Y.; Mahjoub, T. Interleukin-10-819C>T (-819C/T) and ATA haplotype are associated with preeclampsia in a Tunisian population. Pregnancy Hypertens. 2018, 11, 105–110. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Defining pregnancy phases with cytokine shift. J. Pregnancy Reprod. 2017, 1, 1–3. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Panda, B.S.; Verma, A.K.; Kapila, R.; Dang, A.K. Implantation associated changes in expression profile of indoleamine-2, 3-dioxygenase 1, Th1-Th2 cytokines and interferon-stimulated genes on neutrophils and peripheral blood mononuclear cells of crossbred cows. J. Reprod. Immunol. 2020, 142, 103188. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar]
- Psilopatis, I.; Vrettou, K.; Fleckenstein, F.N.; Theocharis, S. The role of peroxisome proliferator-activated receptors in preeclampsia. Cells 2023, 12, 647. [Google Scholar] [CrossRef]
- Cornelius, D.C. Preeclampsia: From inflammation to immunoregulation. Clin. Med. Insights Blood Disord. 2018, 11, 1179545X17752325. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Gumilar, K.E.; Priangga, B.; Lu, C.H.; Dachlan, E.G.; Tan, M. Iron metabolism and ferroptosis: A pathway for understanding preeclampsia. Biomed. Pharmacother. 2023, 167, 115565. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, P.; Zhao, S.; Liu, H.; Liu, L.; Mor, G.; Liu, C.; Liao, A. The dynamic profile and potential function of B-cell subsets during pregnancy. Cell. Mol. Immunol. 2021, 18, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, I.; Singh, S.; Karmakar, A.; Kashyap, N.; Mridha, A.R.; Sharma, J.B.; Luthra, K.; Sharma, R.S.; Biswas, S.; Dhar, R.; et al. New immune horizons in therapeutics and diagnostic approaches to Preeclampsia. Am. J. Reprod. Immunol. 2023, 89, e13670. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Sun, H. Fetal-maternal interactions during pregnancy: A ‘three-in-one’perspective. Front. Immunol. 2023, 14, 1198430. [Google Scholar]
- Nath, M.C.; Cubro, H.; McCormick, D.J.; Milic, N.M.; Garovic, V.D. Preeclamptic women have decreased circulating IL-10 (Interleukin-10) values at the time of preeclampsia diagnosis: Systematic review and meta-analysis. Hypertension 2020, 76, 1817–1827. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Meeker, J.D.; McElrath, T.F.; Mukherjee, B.; Cantonwine, D.E. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am. J. Obstet. Gynecol. 2017, 216, 527-e1. [Google Scholar] [CrossRef]
- Mora, C.E.; Aguilar, E.M.; Avendaño, M.M.; Payán, R.R.; Cortez, M.B.; Lara, L.; Herrera, A.; Murillo, J.; Sanchez, G.; Romero, J.G. IL-6, IL-10 and TNF-Œ± gene polymorphisms in preeclampsia: A case-control study in a Mexican population. Ginekol. Pol. 2023, 95, 108–113. [Google Scholar]
- Herrock, O.; Deer, E.; LaMarca, B. Setting a stage: Inflammation during preeclampsia and postpartum. Front. Physiol. 2023, 14, 1130116. [Google Scholar] [CrossRef]
- Aneman, I.; Pienaar, D.; Suvakov, S.; Simic, T.P.; Garovic, V.D.; McClements, L. Mechanisms of key innate immune cells in early-and late-onset preeclampsia. Front. Immunol. 2020, 11, 1864. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Galindo, A.; Hund, M.; Schlembach, D.; Sillman, J.; Surbek, D.; Vatish, M. Clinical utility of sFlt-1 and PlGF in screening, prediction, diagnosis and monitoring of pre-eclampsia and fetal growth restriction. Ultrasound Obstet. Gynecol. 2023, 61, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Moumad, K.; Khaali, W.; Benider, A.; Ayoub, W.; Cherif, M.; Boualga, K. The Involvement of Interleukin-10 Promoter Genetic Polymorphism in Epstein-Barr Virus-Associated Nasopharyngeal Carcinoma from North Africa. Eurasian J. Med. Oncol. 2022, 6, 232–240. [Google Scholar]
- Salman, O.; Merdaw, M.A.Z.; Almaliky, A.A. A Novel Single Nucleotide Polymorphism of Interleukin-10 Gene is Linked to Type 2 Diabetes Mellitus in Iraqi Patients with Toxoplasmosis (Conference Paper). Iraqi J. Pharm. Sci. 2022, 31, 1–8. [Google Scholar] [CrossRef]
- Sowmya, S.; Sri Manjari, K.; Ramaiah, A.; Sunitha, T.; Nallari, P.; Jyothy, A.; Venkateshwari, A. Interleukin 10 gene promoter polymorphisms in women with early-onset pre-eclampsia. Clin. Exp. Immunol. 2014, 178, 334–341. [Google Scholar] [CrossRef]
- Song, L.; Zhong, M. Association between Interleukin-10 gene polymorphisms and risk of early-onset preeclampsia. Int. J. Clin. Exp. Pathol. 2015, 8, 11659. [Google Scholar]
- Liu, Q.Y.; Gao, F.Y.; Liu, X.R.; Li, J.; Ji, M.; Dong, J.; Wang, X.T. Investigations into the association between polymorphisms in the interleukin-10 gene and risk of early-onset preeclampsia. Genet. Mol. Res. 2015, 14, 19323–19328. [Google Scholar] [CrossRef]
- Zubor, P.; Lasabova, Z.; Jezkova, E.; Mendelova, A.; Svecova, I.; Danko, J. P41. The role of IL-10 polymorphism in pathology of hypertensive disorders in pregnancy. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2015, 5, 245–246. [Google Scholar] [CrossRef]
- Andraweera, P.H.; Dekker, G.A.; Jayasekara, R.W.; Dissanayake, V.H.; Roberts, C.T. Polymorphisms in the inflammatory pathway genes and the risk of preeclampsia in Sinhalese women. J. Matern.-Fetal Neonatal Med. 2016, 29, 1072–1076. [Google Scholar] [CrossRef]
- Zhou, L.; Hui, X.; Yuan, H.; Liu, Y.; Wang, Y. Combination of genetic markers and age effectively facilitates the identification of people with high risk of preeclampsia in the Han Chinese population. BioMed Res. Int. 2018, 1, 4808046. [Google Scholar] [CrossRef]
- Vural, P.; Degirmencioglu, S.; Saral, N.Y.; Demirkan, A.; Akgul, C.; Yildirim, G.; Issever, H.; Eroglu, H. Tumor necrosis factor Œ±, interleukin-6 and interleukin-10 polymorphisms in preeclampsia. J. Obstet. Gynaecol. Res. 2010, 36, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Pissetti, C.W.; Bianco, T.M.; Tanaka, S.C.S.V.; Nascentes, G.A.N.; Grecco, R.L.D.S.; Silva, S.R.D.; Balarin, M.A.S. Protective role of the G allele of the polymorphism in the Interleukin 10 gene (-1082G/A) against the development of preeclampsia. Rev. Bras. Ginecol. E Obs. 2014, 36, 456–460. [Google Scholar] [CrossRef]
- Akhmedov, F.K.; Negmatullaeva, M.N. Comparative Analysis of Rs1800896 Polymorphism of IL-10 Gene (G1082A) in Pregnant Women at Risk of Preeclampsia and Complications of Preeclampsia. J. Pharm. Negat. Results 2022, 13, 696–700. [Google Scholar]
- Sowmya, S.; Ramaiah, A.; Sunitha, T.; Nallari, P.; Jyothy, A.; Venkateshwari, A. Evaluation of interleukin-10 (G-1082A) promoter polymorphism in preeclampsia. J. Reprod. Infertil. 2013, 14, 62. [Google Scholar] [PubMed]
- Kamali-Sarvestani, E.; Kiany, S.; Gharesi-Fard, B.; Robati, M. Association study of IL-10 and IFN-Œ ≥ gene polymorphisms in Iranian women with preeclampsia. J. Reprod. Immunol. 2006, 72, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, J.; Yi, J.; Luan, Y.; Wang, Q. Association between gene polymorphisms on chromosome 1 and susceptibility to pre-eclampsia: An updated meta-analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2202. [Google Scholar] [CrossRef]
- Che, G.; Liu, F.; Chang, L.; Jiang, Y. Association of IL-10-819C/T,-592A/C polymorphisms with the risk of preeclampsia: An updated meta-analysis. Medicine 2021, 100, e27437. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.; Ebeling, M.D.; Baatz, J.E.; Shary, J.R.; Mulligan, J.R.; Wagner, C.L. Sociodemographic factors affecting perceived stress during pregnancy and the association with immune-mediator concentrations. J. Perinat. Med. 2022, 50, 192–199. [Google Scholar] [CrossRef]
- Steckle, V.; Shynlova, O.; Lye, S.; Bocking, A. Low-intensity physical activity may protect pregnant women against spontaneous preterm labour: A prospective case-control study. Appl. Physiol. Nutr. Metab. 2021, 46, 337–345. [Google Scholar] [CrossRef]
- Oleksyk, T.K.; Shrestha, S.; Truelove, A.L.; Goedert, J.J.; Donfield, S.M.; Phair, J.; Mehta, S.; O’Brien, S.J.; Smith, M.W. Extended IL10 haplotypes and their association with HIV progression to AIDS. Genes Immun. 2009, 10, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 1–22. [Google Scholar] [CrossRef]
- Decrion, A.Z.; Dichamp, I.; Varin, A.; Herbein, G. HIV and inflammation. Curr. HIV Res. 2005, 3, 243–259. [Google Scholar] [CrossRef]
- Vinhaes, C.L.; Araujo-Pereira, M.; Tibúrcio, R.; Cubillos-Angulo, J.M.; Demitto, F.O.; Akrami, K.M.; Andrade, B.B. Systemic inflammation associated with immune reconstitution inflammatory syndrome in persons living with HIV. Life 2021, 11, 65. [Google Scholar] [CrossRef]
- Lu, W.; Feng, Y.; Jing, F.; Han, Y.; Lyu, N.; Liu, F.; Li, J.; Song, X.; Xie, J.; Qiu, Z.; et al. Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front. Microbiol. 2018, 9, 1451. [Google Scholar] [CrossRef]
- Nasi, M.; De Biasi, S.; Gibellini, L.; Bianchini, E.; Pecorini, S.; Bacca, V.; Guaraldi, G.; Mussini, C.; Pinti, M.; Cossarizza, A. Ageing and inflammation in patients with HIV infection. Clin. Exp. Immunol. 2017, 187, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Fourman, L.T.; Saylor, C.F.; Cheru, L.; Fitch, K.; Looby, S.; Keller, K.; Robinson, J.; Hoffmann, U.; Lu, M.; Burdo, T. Anti-inflammatory interleukin-10 inversely relates to coronary atherosclerosis in people with HIV. J. Infect. Dis. 2020, 4, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.H.; Deng, W.J.; Yang, Z.; Hong, S.; Ding, Q.L.; Zhao, Y.; Chen, J.; Su, D.K. Association between polymorphisms in the interleukin-10 gene and susceptibility to human immunodeficiency virus-1 infection: A systematic review and meta-analysis. Medicine 2020, 99, e23069. [Google Scholar] [CrossRef]
- Harper, J.; Ribeiro, S.P.; Chan, C.N.; Aid, M.; Deleage, C.; Micci, L.; Pino, M.; Cervasi, B.; Raghunathan, G.; Rimmer, E.; et al. Interleukin-10 contributes to reservoir establishment and persistence in SIV-infected macaques treated with antiretroviral therapy. J. Clin. Investig. 2022, 132, e155251. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, M.; Sun, X.; Chen, X.; Ma, M.; Yin, X.; Qian, S.; Zhang, Z.; Fu, Y.; Liu, J.; et al. IL-10+ NK and TGF-Œ≤+ NK cells play negative regulatory roles in HIV infection. BMC Infect. Dis. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Ngobeni, R.; Ramalivhana, J.N.; Traore, A.N.; Samie, A. Interleukin 10 (IL-10) Production and Seroprevalence of Entamoeba histolytica Infection among HIV-Infected Patients in South Africa. Pathogens 2022, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Samani, D.; Nain, S.; Dhole, T.N. Interleukin-10 polymorphisms and susceptibility to ARV associated hepatotoxicity. Microb. Pathog. 2019, 133, 103544. [Google Scholar] [CrossRef]
- Shrestha, S.; Wiener, H.W.; Aissani, B.; Song, W.; Shendre, A.; Wilson, C.M.; Kaslow, R.A.; Tang, J. Interleukin-10 (IL-10) pathway: Genetic variants and outcomes of HIV-1 infection in African American adolescents. PLoS ONE 2010, 5, e13384. [Google Scholar] [CrossRef]
- Naicker, D.D.; Werner, L.; Kormuth, E.; Passmore, J.A.; Mlisana, K.; Karim, S.A.; Ndung’u, T.; CAPRISA Acute Infection Study Team. Interleukin-10 promoter polymorphisms influence HIV-1 susceptibility and primary HIV-1 pathogenesis. J. Infect. Dis. 2009, 200, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.D.; Winkler, C.; Stephens, J.C.; Bream, J.; Young, H.; Goedert, J.J.; O’Brien, T.R.; Vlahov, D.; Buchbinder, S.; Giorgi, J.; et al. Genetic restriction of HIV-1 pathogenesis to AIDS by promoter alleles of IL10. Proc. Natl. Acad. Sci. USA 2000, 97, 14467–14472. [Google Scholar] [CrossRef]
- Erikstrup, C.; Kallestrup, P.; Zinyama-Gutsire, R.B.; Gomo, E.; Butterworth, A.E.; Pedersen, B.K.; Ostrowski, S.R.; Gerstoft, J.; Ullum, H. Reduced mortality and CD4 cell loss among carriers of the interleukin-10-1082G allele in a Zimbabwean cohort of HIV-1-infected adults. Aids 2007, 21, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, J.S.; Nagalli, S. Highly Active Antiretroviral Therapy (HAART). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Holec, A.D.; Mandal, S.; Prathipati, P.K.; Destache, C.J. Nucleotide Reverse Transcriptase Inhibitors: A Thorough Review, Present Status and Future Perspective as HIV Therapeutics. Curr HIV Res. 2017, 15, 411–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 5172–5208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, T.E.; Mohamed, A.; Kalabalik, J.; Sharma, R. Review of integrase strand transfer inhibitors for the treatment of human immunodeficiency virus infection. Expert Rev. Anti Infect. Ther. 2015, 13, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, G.P.; Chakraborty, H. Entry Inhibitors: Efficient Means to Block Viral Infection. J. Membr. Biol. 2020, 253, 425–444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stylianou, E.; Aukrust, P.; Kvale, D.; Müller, F.; Frøland, S.S. IL-10 in HIV infection: Increasing serum IL-10 levels with disease progression-down-regulatory effect of potent anti-retroviral therapy. Clin. Exp. Immunol. 1999, 116, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Trabattoni, D.; Schenal, M.; Cesari, M.; Castelletti, E.; Pacei, M.; Goldberg, B.; Gori, A.; Clerici, M. Low interleukin-10 production is associated with diabetes in HIV-infected patients undergoing antiviral therapy. Med. Microbiol. Immunol. 2006, 195, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Osuji, F.N.; Onyenekwe, C.C.; Ahaneku, J.E.; Ukibe, N.R. The effects of highly active antiretroviral therapy on the serum levels of pro-inflammatory and anti-inflammatory cytokines in HIV infected subjects. J. Biomed. Sci. 2018, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barqasho, B.; Nowak, P.; Tjernlund, A.; Kinloch, S.; Goh, L.E.; Lampe, F.; Fisher, M.; Andersson, J.; Sönnerborg, A.; QUEST Study Group. Kinetics of plasma cytokines and chemokines during primary HIV-1 infection and after analytical treatment interruption. HIV Med. 2009, 10, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Twizerimana, A.P.; Mwatha, J.; Musabyimana, J.P.; Kayigi, E.; Harelimana, J.D.D.; Karanja, S.M.; Mutesa, L. Immunological profiles in HIV positive patients following HAART initiation in Kigali, Rwanda. East Afr. Med. J. 2014, 91, 261–266. [Google Scholar]
- Gao, X.; Zhong, Y.; Liu, Y.; Ding, R.; Chen, J. The role and function of regulatory T cells in toxoplasma gondii-induced adverse pregnancy outcomes. J. Immunol. Res. 2021, 2021, 8782672. [Google Scholar] [CrossRef]
- Norris, P.J.; Pappalardo, B.L.; Custer, B.; Spotts, G.; Hecht, F.M.; Busch, M.P. Elevations in IL-10, TNF-Œ±, and IFN-Œ≥ from the earliest point of HIV type 1 infection. AIDS Res. Hum. Retroviruses 2006, 22, 757–762. [Google Scholar] [CrossRef]
- Brockman, M.A.; Kwon, D.S.; Tighe, D.P.; Pavlik, D.F.; Rosato, P.C.; Sela, J.; Porichis, F.; Le Gall, S.; Waring, M.T.; Moss, K.; et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood J. Am. Soc. Hematol. 2009, 114, 346–356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidoo, S.J.; Naicker, T. The Enigmatic Interplay of Interleukin-10 in the Synergy of HIV Infection Comorbid with Preeclampsia. Int. J. Mol. Sci. 2024, 25, 9434. https://doi.org/10.3390/ijms25179434
Naidoo SJ, Naicker T. The Enigmatic Interplay of Interleukin-10 in the Synergy of HIV Infection Comorbid with Preeclampsia. International Journal of Molecular Sciences. 2024; 25(17):9434. https://doi.org/10.3390/ijms25179434
Chicago/Turabian StyleNaidoo, Shirelle Janine, and Thajasvarie Naicker. 2024. "The Enigmatic Interplay of Interleukin-10 in the Synergy of HIV Infection Comorbid with Preeclampsia" International Journal of Molecular Sciences 25, no. 17: 9434. https://doi.org/10.3390/ijms25179434
APA StyleNaidoo, S. J., & Naicker, T. (2024). The Enigmatic Interplay of Interleukin-10 in the Synergy of HIV Infection Comorbid with Preeclampsia. International Journal of Molecular Sciences, 25(17), 9434. https://doi.org/10.3390/ijms25179434





