A Pro-Inflammatory Stimulus versus Extensive Passaging of DITNC1 Astrocyte Cultures as Models to Study Astrogliosis
Abstract
:1. Introduction
2. Results
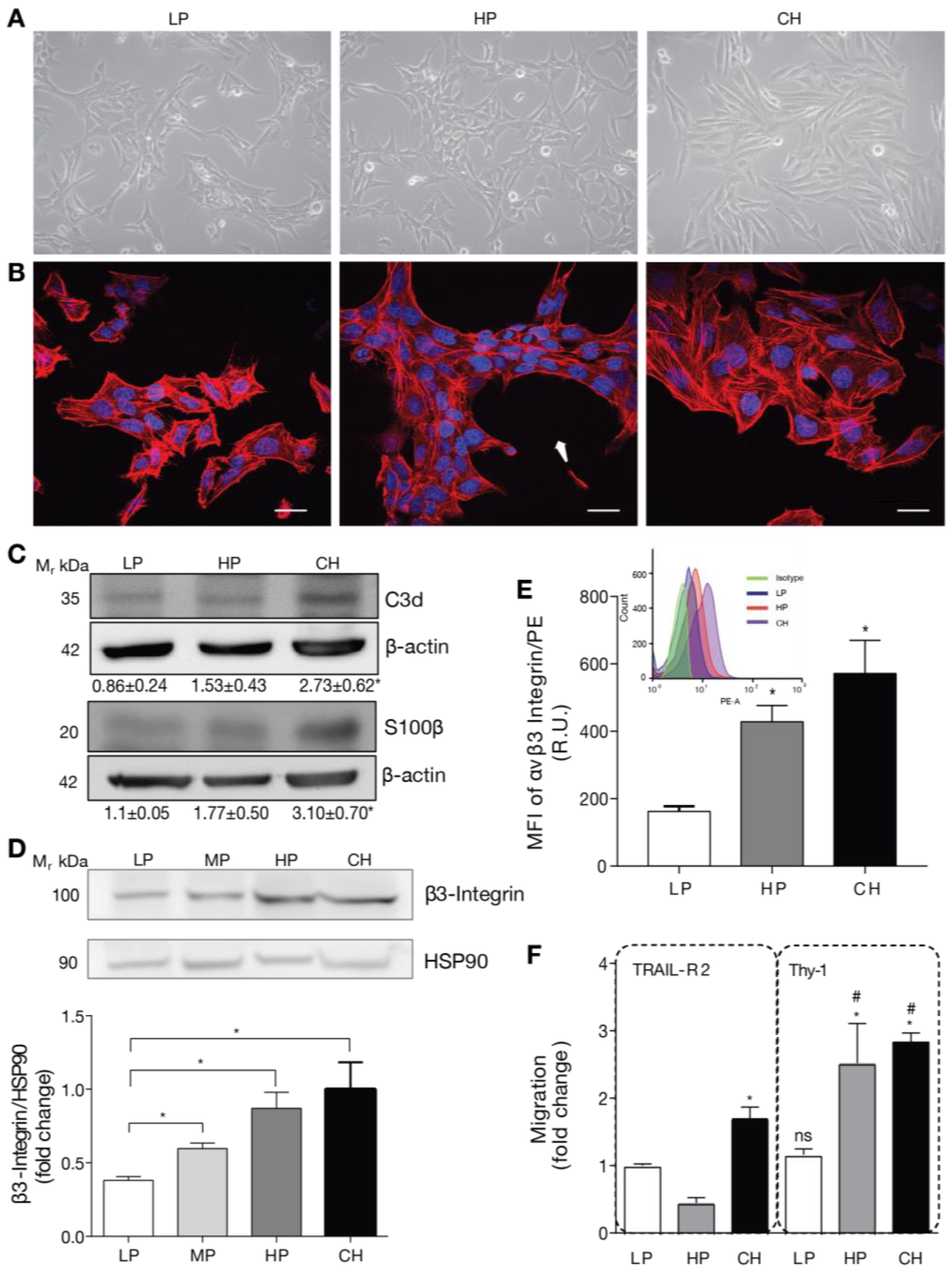
2.1. DITNC1 Astrocytes with Extensive Passaging but Not Low Passage Respond to Neuronal Thy-1 in the Absence of Inflammatory Stimuli
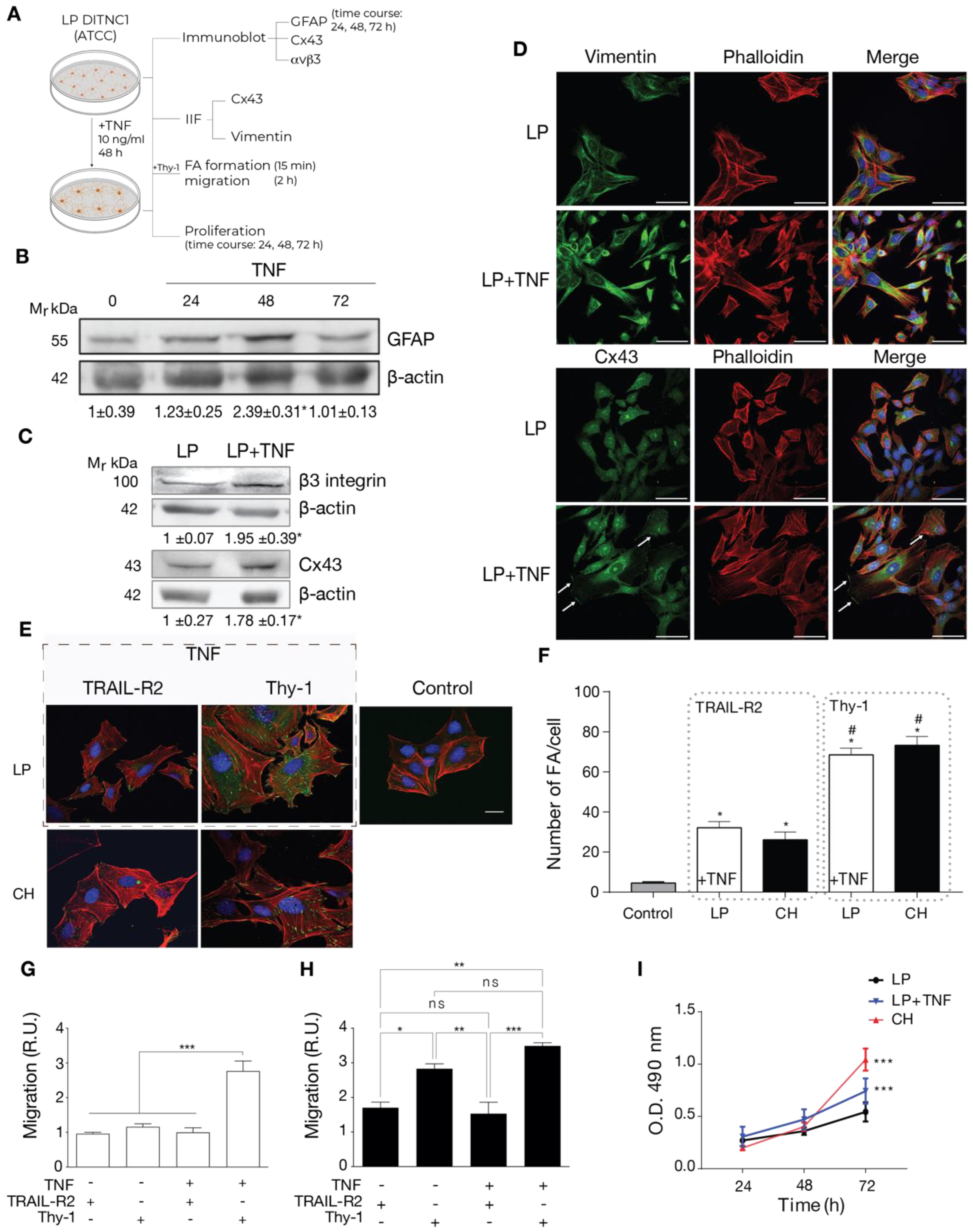
2.2. Cells with a Low Number of Passages Behave as Non-Reactive Astrocytes and Switch to Reactive Astrocytes When Treated with TNF
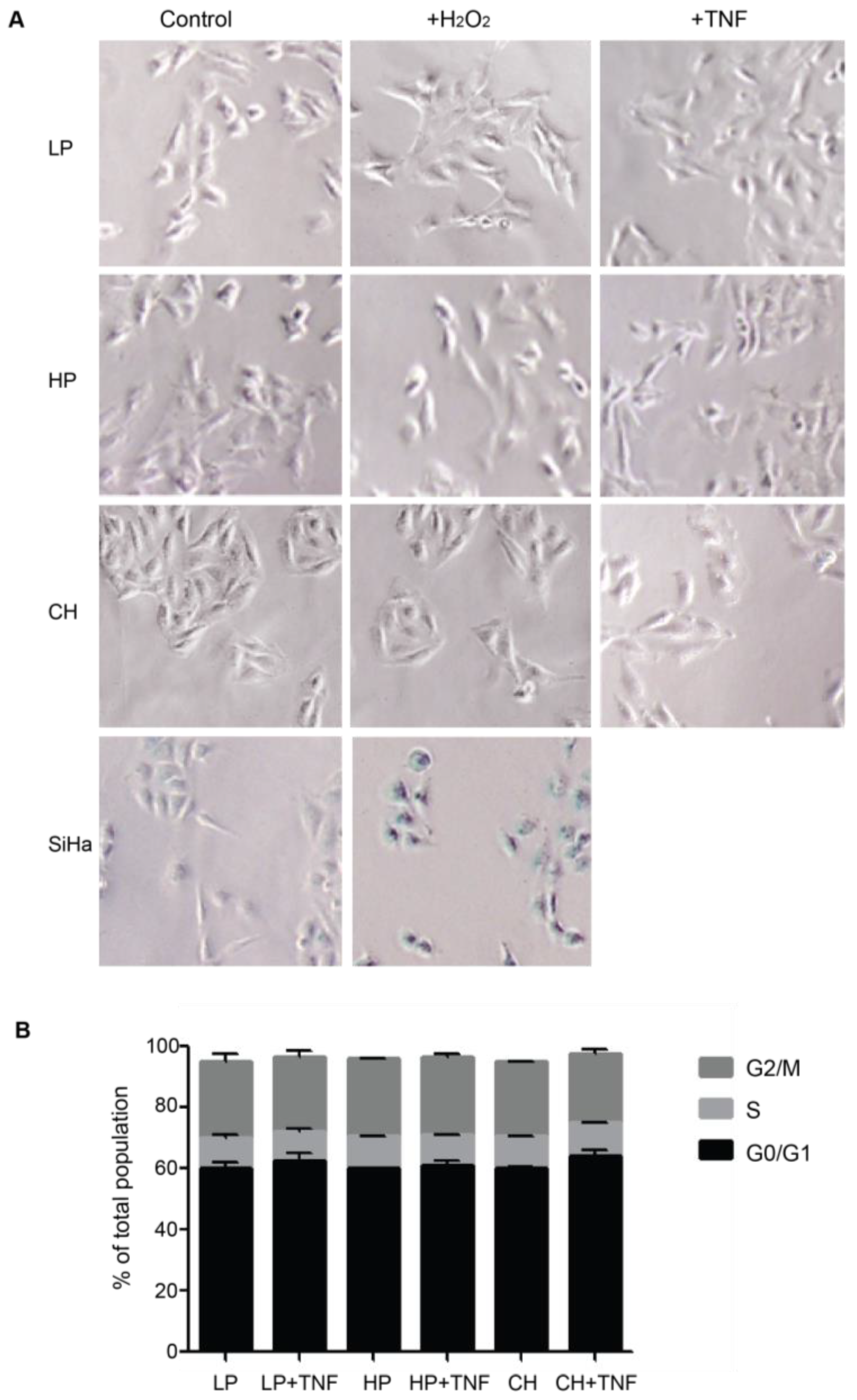
2.3. Reactive Phenotype Traits of DITNC1 Cells with Extensive Passaging or Treated with Pro-Inflammatory Stimuli Are Not Due to Senescence
2.4. DITNC1 Astrocytes with Multiple Passages Inhibit Neurite Outgrowth and Promote Neuronal Death
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents and Antibodies
4.3. Immunoblot Analysis
4.4. Cell Cytometry
4.5. Migration Assay in a Boyden Chamber
4.6. Indirect Immunofluorescence Assay for Visualization of Focal Adhesions
4.7. Proliferation Assays
4.8. Beta-Galactosidase Senescence Assay
4.9. Cell Cycle Assay
4.10. Outgrowth of Neuronal Processes on Fixed Astrocyte Monolayers
4.11. Neuronal Cell Death Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Buffo, A.; Rolando, C.; Ceruti, S. Astrocytes in the Damaged Brain: Molecular and Cellular Insights into Their Reactive Response and Healing Potential. Biochem. Pharmacol. 2010, 79, 77–89. [Google Scholar] [CrossRef]
- Pekny, M.; Nilsson, M. Astrocyte Activation and Reactive Gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef]
- Lagos-Cabré, R.; Burgos-Bravo, F.; Avalos, A.M.; Leyton, L. Connexins in Astrocyte Migration. Front. Pharmacol. 2020, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Krushel, L.A.; Sporns, O.; Cunningham, B.A.; Crossin, K.L.; Edelman, G.M. Neural Cell Adhesion Molecule (N-CAM) Inhibits Astrocyte Proliferation after Injury to Different Regions of the Adult Rat Brain. Proc. Natl. Acad. Sci. USA 1995, 92, 4323–4327. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Lagos-Cabré, R.; Alvarez, A.; Kong, M.; Burgos-Bravo, F.; Cárdenas, A.; Rojas-Mancilla, E.; Pérez-Nuñez, R.; Herrera-Molina, R.; Rojas, F.; Schneider, P.; et al. AVβ3 Integrin Regulates Astrocyte Reactivity. J. Neuroinflamm. 2017, 14, 194. [Google Scholar] [CrossRef]
- Pérez-Núñez, R.; Chamorro, A.; González, M.F.; Contreras, P.; Artigas, R.; Corvalán, A.H.; van Zundert, B.; Reyes, C.; Moya, P.R.; Avalos, A.M.; et al. Protein Kinase B (AKT) Upregulation and Thy-1-Avβ3 Integrin-Induced Phosphorylation of Connexin43 by Activated AKT in Astrogliosis. J. Neuroinflamm. 2023, 20, 5. [Google Scholar] [CrossRef]
- Henriquez, M.; Herrera-Molina, R.; Valdivia, A.; Alvarez, A.; Kong, M.; Munoz, N.; Eisner, V.; Jaimovich, E.; Schneider, P.; Quest, A.F.G.; et al. ATP Release Due to Thy-1-Integrin Binding Induces P2X7-Mediated Calcium Entry Required for Focal Adhesion Formation. J. Cell Sci. 2011, 124, 1581–1588. [Google Scholar] [CrossRef]
- Alvarez, A.; Lagos-Cabré, R.; Kong, M.; Cárdenas, A.; Burgos-Bravo, F.; Schneider, P.; Quest, A.F.G.; Leyton, L. Integrin-Mediated Transactivation of P2X7R via Hemichannel-Dependent ATP Release Stimulates Astrocyte Migration. Biochim. Biophys. Acta 2016, 1863, 2175–2188. [Google Scholar] [CrossRef]
- Brenet, M.; Martínez, S.; Pérez-Nuñez, R.; Pérez, L.A.; Contreras, P.; Díaz, J.; Avalos, A.M.; Schneider, P.; Quest, A.F.G.; Leyton, L. Thy-1 (CD90)-Induced Metastatic Cancer Cell Migration and Invasion Are Β3 Integrin-Dependent and Involve a Ca2+/P2X7 Receptor Signaling Axis. Front. Cell Dev. Biol. 2021, 8, 592442. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, A.; Avalos, A.M.; Leyton, L. Thy-1 (CD90)-Regulated Cell Adhesion and Migration of Mesenchymal Cells: Insights into Adhesomes, Mechanical Forces, and Signaling Pathways. Front. Cell Dev. Biol. 2023, 11, 1221306. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Leyton, L.; Hagood, J.S.; Barker, T.H. Thy-1-Integrin Interactions in Cis and Trans Mediate Distinctive Signaling. Front. Cell Dev. Biol. 2022, 10, 928510. [Google Scholar] [CrossRef]
- Kong, M.; Muñoz, N.; Valdivia, A.; Alvarez, A.; Herrera-Molina, R.; Cárdenas, A.; Schneider, P.; Burridge, K.; Quest, A.F.G.; Leyton, L. Thy-1-Mediated Cell–Cell Contact Induces Astrocyte Migration through the Engagement of AVβ3 Integrin and Syndecan-4. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Rahemtulla, N.; Deschepper, C.F.; Maurice, J.; Mittal, B.; David, S. Immunocytochemical and Functional Characterization of an Immortalized Type 1 Astrocytic Cell Line. Brain Res. 1994, 642, 221–227. [Google Scholar] [CrossRef]
- Herrera-Molina, R.; Valdivia, A.; Kong, M.; Alvarez, A.; Cárdenas, A.; Quest, A.F.G.; Leyton, L. Thy-1-Interacting Molecules and Cellular Signaling in Cis and Trans. Int. Rev. Cell Mol. Biol. 2013, 305, 163–216. [Google Scholar]
- Maurer, L.L.; Latham, J.D.; Landis, R.W.; Song, D.H.; Epstein, T.; Philbert, M.A. 1,3-Dinitrobenzene Neurotoxicity—Passage Effect in Immortalized Astrocytes. Neurotoxicology 2016, 53, 74–84. [Google Scholar] [CrossRef]
- Bang, M.; Gonzales, E.L.; Shin, C.Y.; Kwon, K.J. Late Passage Cultivation Induces Aged Astrocyte Phenotypes in Rat Primary Cultured Cells. Biomol. Ther. 2021, 29, 144–153. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal Aging Induces A1-like Astrocyte Reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef]
- Rapisarda, V.; Borghesan, M.; Miguela, V.; Encheva, V.; Snijders, A.P.; Lujambio, A.; O’Loghlen, A. Integrin Beta 3 Regulates Cellular Senescence by Activating the TGF-β Pathway. Cell Rep. 2017, 18, 2480–2493. [Google Scholar] [CrossRef]
- Filardo, E.J.; Brooks, P.C.; Deming, S.L.; Damsky, C.; Cheresh, D.A. Requirement of the NPXY Motif in the Integrin Β3 Subunit Cytoplasmic Tail for Melanoma Cell Migration in Vitro and in Vivo. J. Cell Biol. 1995, 130, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, A.; Cárdenas, A.; Brenet, M.; Maldonado, H.; Kong, M.; Díaz, J.; Burridge, K.; Schneider, P.; San Martín, A.; García-Mata, R.; et al. Syndecan-4/PAR-3 Signaling Regulates Focal Adhesion Dynamics in Mesenchymal Cells. Cell Commun. Signal. 2020, 18, 129. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Cabré, R.; Brenet, M.; Díaz, J.; Pérez, R.; Pérez, L.; Herrera-Molina, R.; Quest, A.; Leyton, L. Intracellular Ca2+ Increases and Connexin 43 Hemichannel Opening Are Necessary but Not Sufficient for Thy-1-Induced Astrocyte Migration. Int. J. Mol. Sci. 2018, 19, 2179. [Google Scholar] [CrossRef]
- Cohen, J.; Torres, C. Astrocyte Senescence: Evidence and Significance. Aging Cell 2019, 18, e12937. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yin, H.; Wang, S.; Wei, Y.; Peng, N.; Bi, W.; Wang, X. Different Effects of H 2 O 2 Treatment on Cervical Squamous Carcinoma Cells and Adenocarcinoma Cells. Arch. Med. Sci. 2015, 6, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Bravo, F.; Martínez-Meza, S.; Quest, A.F.G.; Wilson, C.A.M.; Leyton, L. Application of Force to a Syndecan-4 Containing Complex With Thy-1–AVβ3 Integrin Accelerates Neurite Retraction. Front. Mol. Biosci. 2020, 7, 582257. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Molina, R.; Frischknecht, R.; Maldonado, H.; Seidenbecher, C.I.; Gundelfinger, E.D.; Hetz, C.; de la Luz Aylwin, M.; Schneider, P.; Quest, A.F.G.; Leyton, L. Astrocytic Avβ3 Integrin Inhibits Neurite Outgrowth and Promotes Retraction of Neuronal Processes by Clustering Thy-1. PLoS ONE 2012, 7, e34295. [Google Scholar] [CrossRef]
- Maldonado, H.; Calderon, C.; Burgos-Bravo, F.; Kobler, O.; Zuschratter, W.; Ramirez, O.; Härtel, S.; Schneider, P.; Quest, A.F.G.; Herrera-Molina, R.; et al. Astrocyte-to-Neuron Communication through Integrin-Engaged Thy-1/CBP/Csk/Src Complex Triggers Neurite Retraction via the RhoA/ROCK Pathway. Biochim. Biophys. Acta—Mol. Cell Res. 2017, 1864, 243–254. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Palacios, E.; Lobos-González, L.; Guerrero, S.; Kogan, M.J.; Shao, B.; Heinecke, J.W.; Quest, A.F.G.; Leyton, L.; Valenzuela-Valderrama, M. Helicobacter Pylori Outer Membrane Vesicles Induce Astrocyte Reactivity through Nuclear Factor-Κappa B Activation and Cause Neuronal Damage in Vivo in a Murine Model. J. Neuroinflamm. 2023, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Orellana, J.A.; Shoji, K.F.; Abudara, V.; Ezan, P.; Amigou, E.; Saez, P.J.; Jiang, J.X.; Naus, C.C.; Saez, J.C.; Giaume, C. Amyloid β-Induced Death in Neurons Involves Glial and Neuronal Hemichannels. J. Neurosci. 2011, 31, 4962–4977. [Google Scholar] [CrossRef]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic Reactive Astrocytes Induce Cell Death via Saturated Lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef]
- Arredondo, C.; Cefaliello, C.; Dyrda, A.; Jury, N.; Martinez, P.; Díaz, I.; Amaro, A.; Tran, H.; Morales, D.; Pertusa, M.; et al. Excessive Release of Inorganic Polyphosphate by ALS/FTD Astrocytes Causes Non-Cell-Autonomous Toxicity to Motoneurons. Neuron 2022, 110, 1656–1670.e12. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Ben Haim, L.; Carrillo-de Sauvage, M.-A.; Ceyzériat, K.; Escartin, C. Elusive Roles for Reactive Astrocytes in Neurodegenerative Diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J. Thy-1, a Pathfinder Protein for the Post-Genomic Era. Front. Cell Dev. Biol. 2018, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Yang, L.; Cole, A.; Sun, L.; Chiang, A.C.-A.; Fowler, S.W.; Shim, D.J.; Rodriguez-Rivera, J.; Taglialatela, G.; Jankowsky, J.L.; et al. NFκB-Activated Astroglial Release of Complement C3 Compromises Neuronal Morphology and Function Associated with Alzheimer’s Disease. Neuron 2015, 85, 101–115. [Google Scholar] [CrossRef]
- Gandelman, M.; Peluffo, H.; Beckman, J.S.; Cassina, P.; Barbeito, L. Extracellular ATP and the P2X7 Receptor in Astrocyte-Mediated Motor Neuron Death: Implications for Amyotrophic Lateral Sclerosis. J. Neuroinflamm. 2010, 7, 33. [Google Scholar] [CrossRef]
- Wu, J.; Holstein, J.D.; Upadhyay, G.; Lin, D.T.; Conway, S.; Muller, E.; Lechleiter, J.D. Purinergic Receptor-Stimulated IP3-Mediated Ca2+ Release Enhances Neuroprotection by Increasing Astrocyte Mitochondrial Metabolism during Aging. J. Neurosci. 2007, 27, 6510–6520. [Google Scholar] [CrossRef]
- Lin, D.-T.; Wu, J.; Holstein, D.; Upadhyay, G.; Rourk, W.; Muller, E.; Lechleiter, J.D. Ca2+ Signaling, Mitochondria and Sensitivity to Oxidative Stress in Aging Astrocytes. Neurobiol. Aging 2007, 28, 99–111. [Google Scholar] [CrossRef]
- Spina Purrello, V.; Cormaci, G.; Denaro, L.; Reale, S.; Costa, A.; Lalicata, C.; Sabbatini, M.; Marchetti, B.; Avola, R. Effect of Growth Factors on Nuclear and Mitochondrial ADP-Ribosylation Processes during Astroglial Cell Development and Aging in Culture. Mech. Ageing Dev. 2002, 123, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Klamt, F.; Gottfried, C.; Tramontina, F.; Dal-Pizzol, F.; da Frota, M.L.C.; Fonseca Moreira, J.C.; Dias, R.D.; Moriguchi, E.; Wofchuk, S.; Souza, D.O. Time-Related Increase in Mitochondrial Superoxide Production, Biomolecule Damage and Antioxidant Enzyme Activities in Cortical Astrocyte Cultures. Neuroreport 2002, 13, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Bang, M.; Kim, D.G.; Gonzales, E.L.; Kwon, K.J.; Shin, C.Y. Etoposide Induces Mitochondrial Dysfunction and Cellular Senescence in Primary Cultured Rat Astrocytes. Biomol. Ther. 2019, 27, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, J.K.T.; McMillian, M.; Chikaraishi, D.M. Characterization of a CNS Cell Line, CAD, in Which Morphological Differentiation Is Initiated by Serum Deprivation. J. Neurosci. 1997, 17, 1217–1225. [Google Scholar] [CrossRef]
- Schneider, P. Production of Recombinant TRAIL and TRAIL Receptor: Fc Chimeric Proteins; Academic Press: Cambridge, MA, USA, 2000; Volume 322, pp. 325–345. [Google Scholar] [CrossRef]
- González, M.F.; Burgos-Ravanal, R.; Shao, B.; Heinecke, J.; Valenzuela-Valderrama, M.; Corvalán, A.H.; Quest, A.F.G. Extracellular Vesicles from Gastric Epithelial GES-1 Cells Infected with Helicobacter Pylori Promote Changes in Recipient Cells Associated with Malignancy. Front. Oncol. 2022, 12, 962920. [Google Scholar] [CrossRef]
- Hidalgo, A.; Bravo, D.; Soto, C.; Maturana, G.; Cordero-Machuca, J.; Zúñiga-López, M.C.; Oyarzun-Ampuero, F.; Quest, A.F.G. The Anti-Oxidant Curcumin Solubilized as Oil-in-Water Nanoemulsions or Chitosan Nanocapsules Effectively Reduces Helicobacter Pylori Growth, Bacterial Biofilm Formation, Gastric Cell Adhesion and Internalization. Antioxidants 2023, 12, 1866. [Google Scholar] [CrossRef]
- Villota, C.; Varas-Godoy, M.; Jeldes, E.; Campos, A.; Villegas, J.; Borgna, V.; Burzio, L.O.; Burzio, V.A. HPV-18 E2 Protein Downregulates Antisense Noncoding Mitochondrial RNA-2, Delaying Replicative Senescence of Human Keratinocytes. Aging 2018, 11, 33–47. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, L.A.; Palacios, E.; González, M.F.; Leyton-Rivera, I.; Martínez-Meza, S.; Pérez-Núñez, R.; Jeldes, E.; Avalos, A.M.; Díaz, J.; Leyton, L. A Pro-Inflammatory Stimulus versus Extensive Passaging of DITNC1 Astrocyte Cultures as Models to Study Astrogliosis. Int. J. Mol. Sci. 2024, 25, 9454. https://doi.org/10.3390/ijms25179454
Pérez LA, Palacios E, González MF, Leyton-Rivera I, Martínez-Meza S, Pérez-Núñez R, Jeldes E, Avalos AM, Díaz J, Leyton L. A Pro-Inflammatory Stimulus versus Extensive Passaging of DITNC1 Astrocyte Cultures as Models to Study Astrogliosis. International Journal of Molecular Sciences. 2024; 25(17):9454. https://doi.org/10.3390/ijms25179454
Chicago/Turabian StylePérez, Leonardo A., Esteban Palacios, María Fernanda González, Ignacio Leyton-Rivera, Samuel Martínez-Meza, Ramón Pérez-Núñez, Emanuel Jeldes, Ana María Avalos, Jorge Díaz, and Lisette Leyton. 2024. "A Pro-Inflammatory Stimulus versus Extensive Passaging of DITNC1 Astrocyte Cultures as Models to Study Astrogliosis" International Journal of Molecular Sciences 25, no. 17: 9454. https://doi.org/10.3390/ijms25179454






