Spatial Transcriptomics Analysis: Maternal Obesity Impairs Myogenic Cell Migration and Differentiation during Embryonic Limb Development
Abstract
1. Introduction
2. Results
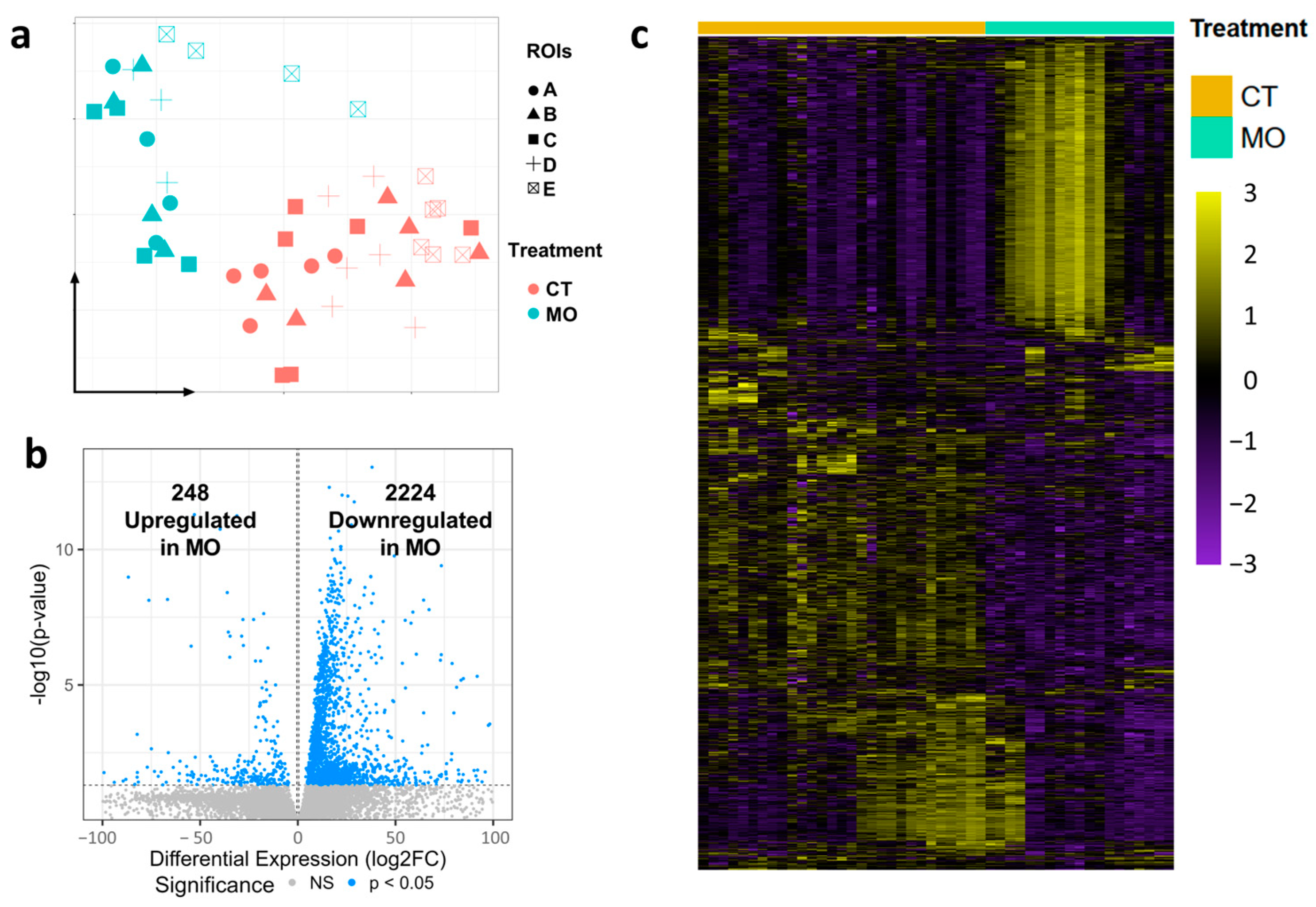
2.1. Maternal HFD Feeding Alters Spatial Transcriptome of E13.5 Embryonic Limb
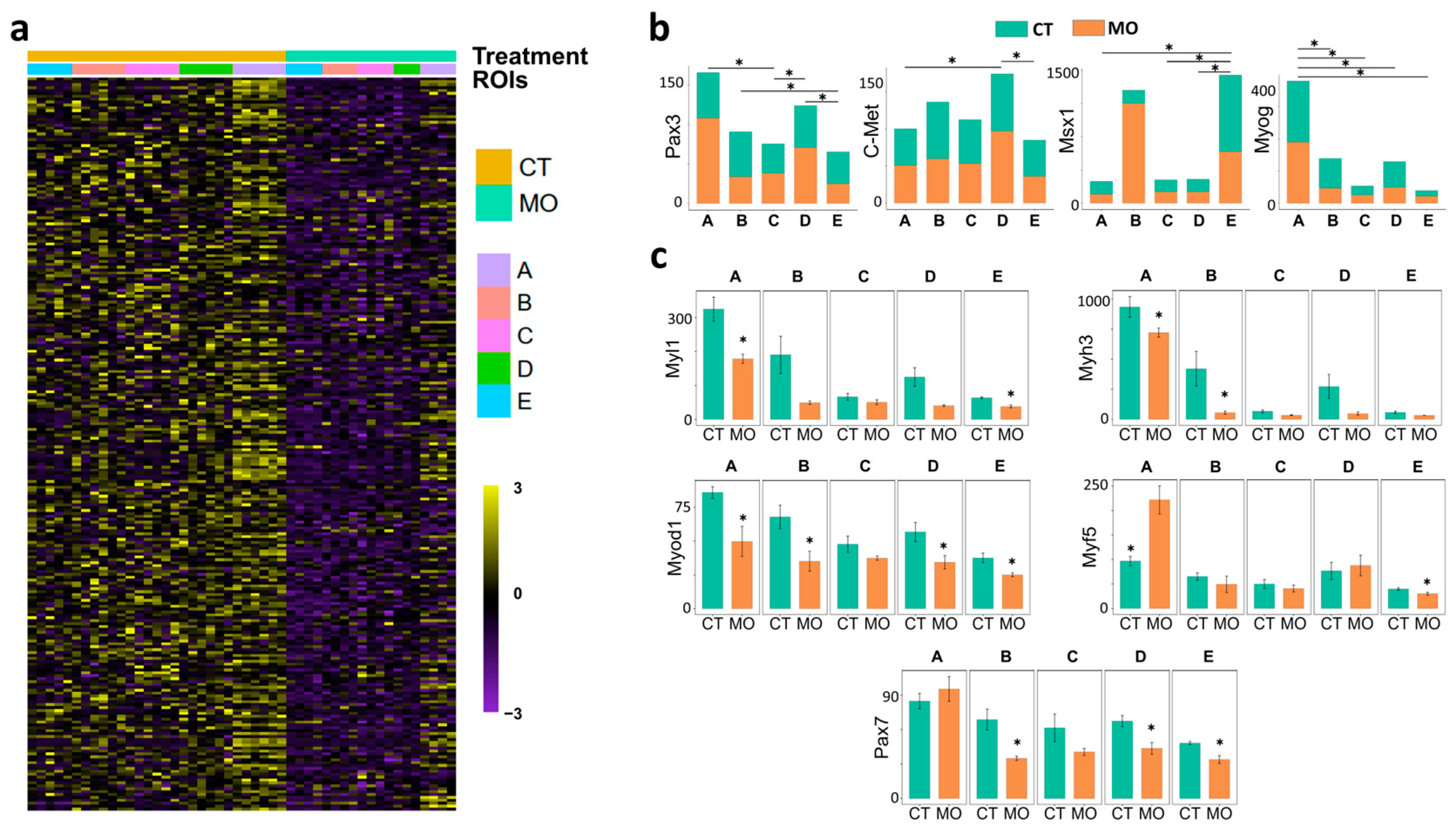
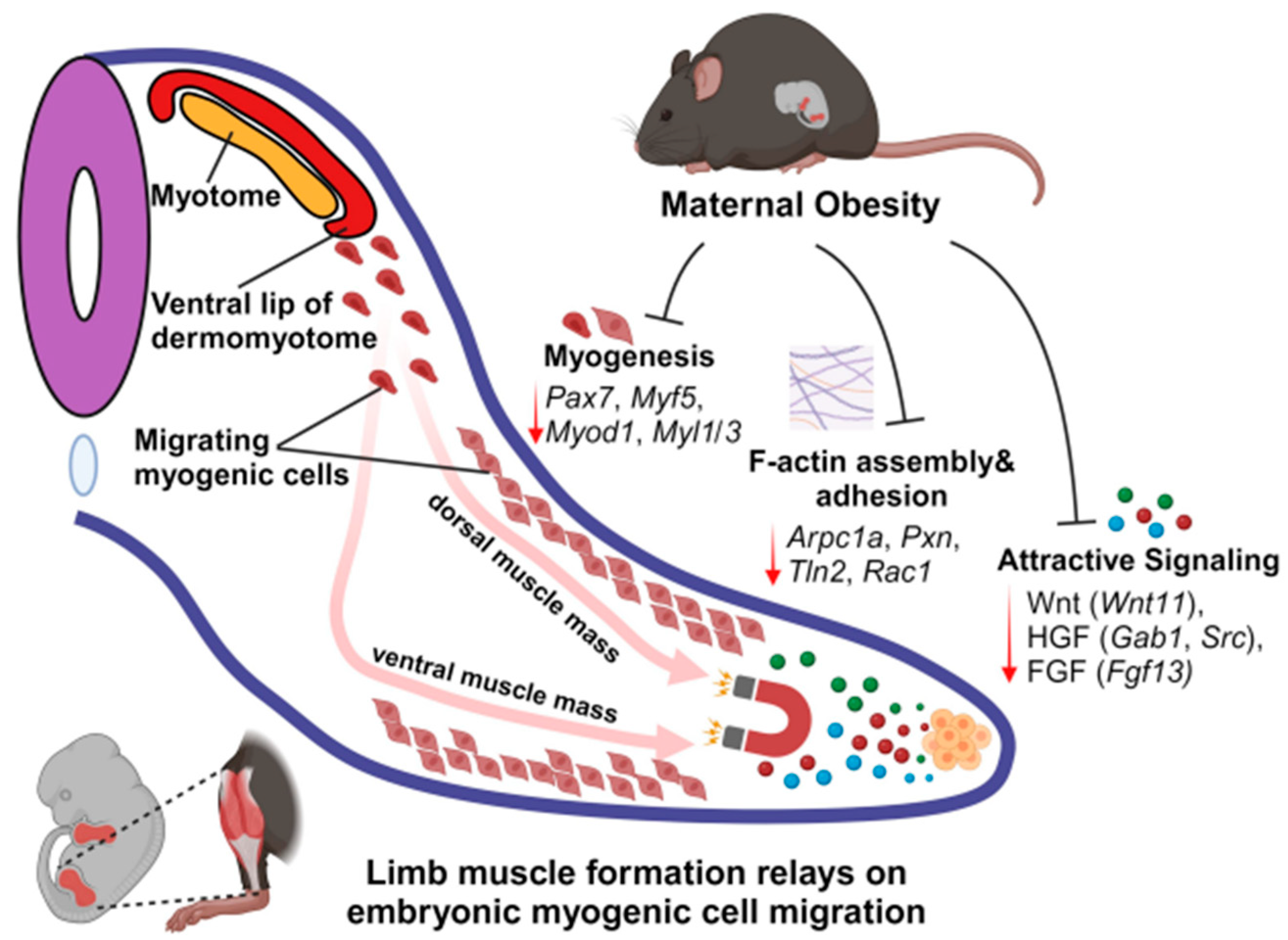
2.2. Maternal HFD Feeding Inhibits Myogenesis and Myogenic Cell Migration in E13.5 Embryonic Limb
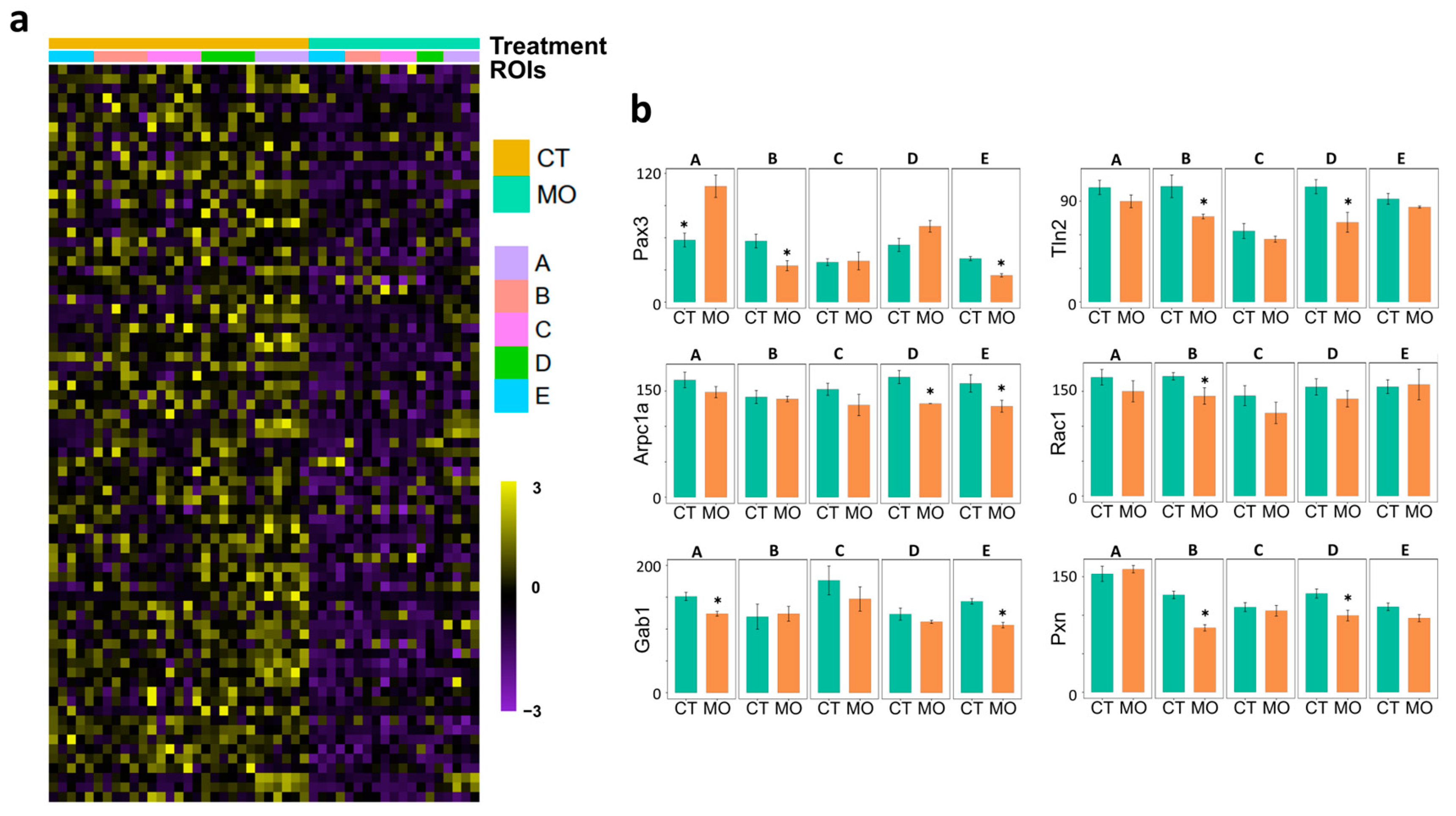
2.3. MO Suppresses Migration Signal Factors Released from the E13.5 Limb Tip
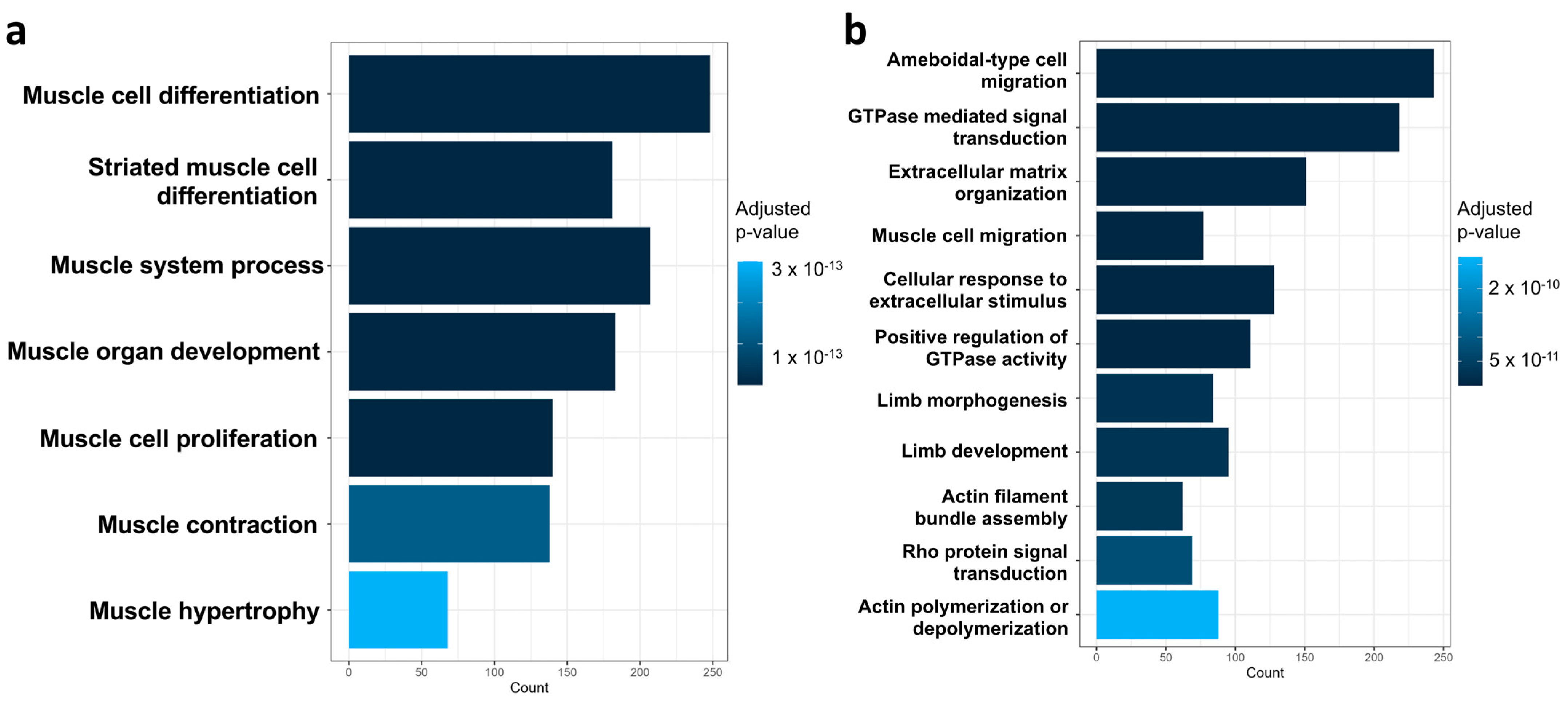
2.4. Integrated Analysis of Transcriptomes Demonstrates the Suppression of Cell Migration and Myogenesis in the MO E13.5 Limb
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Handling and Sample Collection
5.2. Embryo Collection and Spatial RNA Sequencing
5.3. Sequencing Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Souza Lima, B.; Sanches, A.P.V.; Ferreira, M.S.; de Oliveira, J.L.; Cleal, J.K.; Ignacio-Souza, L. Maternal-placental axis and its impact on fetal outcomes, metabolism, and development. BBA-Mol. Basis Dis. 2024, 1870, 166855. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Periasamy, M.; Herrera, J.L.; Reis, F.C. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab. J. 2017, 41, 327. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Chávez, A.L.; Canto, P. Influence of maternal obesity on the skeletal muscle of offspring. BMHIM 2022, 79, 284–292. [Google Scholar] [CrossRef]
- Son, J.S.; Liu, X.; Tian, Q.; Zhao, L.; Chen, Y.; Hu, Y.; Chae, S.A.; de Avila, J.M.; Zhu, M.J.; Du, M. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J. Physiol. 2019, 597, 3333–3347. [Google Scholar] [CrossRef]
- Zhao, L.; Law, N.C.; Gomez, N.A.; Son, J.; Gao, Y.; Liu, X.; de Avila, J.M.; Zhu, M.J.; Du, M. Obesity impairs embryonic myogenesis by enhancing BMP signaling within the dermomyotome. Adv. Sci. 2021, 8, 2102157. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Brand-Saberi, B. Limb muscle development. Int. J. Dev. Biol. 2004, 46, 905–914. [Google Scholar]
- Buckingham, M.; Bajard, L.; Chang, T.; Daubas, P.; Hadchouel, J.; Meilhac, S.; Montarras, D.; Rocancourt, D.; Relaix, F. The formation of skeletal muscle: From somite to limb. J. Anat. 2003, 202, 59–68. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Yokoyama, S.; Asahara, H. The myogenic transcriptional network. CMLS 2011, 68, 1843–1849. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Cramer, L.P. Mechanism of cell rear retraction in migrating cells. COCEBI 2013, 25, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Ferrari, G.; Tedesco, F.S. Cellular dynamics of myogenic cell migration: Molecular mechanisms and implications for skeletal muscle cell therapies. EMBO Mol. Med. 2020, 12, e12357. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, A.; Zollinger, A.; Merritt, C.; Nguyen, K.; McKay-Fleisch, J.; Anderson, C.; Ma, X.-J. Molecularly guided highly multiplexed digital spatial analysis reveals differential gene expression profiles in the WNT-β-catenin pathway between melanoma and prostate tumors. Cancer Res. 2020, 80 (Suppl. S16), 2707. [Google Scholar] [CrossRef]
- Zollinger, D.R.; Lingle, S.E.; Sorg, K.; Beechem, J.M.; Merritt, C.R. GeoMx™ RNA assay: High multiplex, digital, spatial analysis of RNA in FFPE tissue. Situ Hybrid. Protoc. 2020, 2148, 331–345. [Google Scholar]
- Shrestha, A.; Prowak, M.; Berlandi-Short, V.-M.; Garay, J.; Ramalingam, L. Maternal obesity: A focus on maternal interventions to improve health of offspring. Front. Cardiovasc. Med. 2021, 8, 696812. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, L.; Son, J.S.; Liu, X.; Chen, Y.; Deavila, J.M.; Zhu, M.-J.; Murdoch, G.K.; Du, M. Maternal exercise before and during pregnancy facilitates embryonic myogenesis by enhancing thyroid hormone signaling. Thyroid 2022, 32, 581–593. [Google Scholar] [CrossRef]
- Rafipay, A.; Berg, A.L.; Erskine, L.; Vargesson, N. Expression analysis of limb element markers during mouse embryonic development. Dev. Dyn. 2018, 247, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Mok, G.F.; Sweetman, D. Many routes to the same destination: Lessons from skeletal muscle development. Reproduction 2011, 141, 301. [Google Scholar] [CrossRef]
- Searcy, M.B.; Larsen IV, R.K.; Stevens, B.T.; Zhang, Y.; Jin, H.; Drummond, C.J.; Langdon, C.G.; Gadek, K.E.; Vuong, K.; Reed, K.B. PAX3-FOXO1 dictates myogenic reprogramming and rhabdomyosarcoma identity in endothelial progenitors. Nat. Commun. 2023, 14, 7291. [Google Scholar] [CrossRef] [PubMed]
- Seki, R.; Kamiyama, N.; Tadokoro, A.; Nomura, N.; Tsuihiji, T.; Manabe, M.; Tamura, K. Evolutionary and developmental aspects of avian-specific traits in limb skeletal pattern. Zool. Sci. 2012, 29, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, T.; Kuratani, S. Evolution of the muscular system in tetrapod limbs. Zool. Lett. 2018, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Sharma, A.; Kumar, P.; Kumar, A.; Bharadwaj, A.; Saini, M.; Kardon, G.; Mathew, S.J. Myosin heavy chain-embryonic regulates skeletal muscle differentiation during mammalian development. Development 2020, 147, dev184507. [Google Scholar] [CrossRef]
- Shellard, A.; Mayor, R. All roads lead to directional cell migration. Trends Cell Biol. 2020, 30, 852–868. [Google Scholar] [CrossRef]
- Adachi, N.; Pascual-Anaya, J.; Hirai, T.; Higuchi, S.; Kuroda, S.; Kuratani, S. Stepwise participation of HGF/MET signaling in the development of migratory muscle precursors during vertebrate evolution. Zool. Lett. 2018, 4, 18. [Google Scholar] [CrossRef]
- Morosan-Puopolo, G.; Balakrishnan-Renuka, A.; Yusuf, F.; Chen, J.; Dai, F.; Zoidl, G.; Lüdtke, T.H.-W.; Kispert, A.; Theiss, C.; Abdelsabour-Khalaf, M. Wnt11 is required for oriented migration of dermogenic progenitor cells from the dorsomedial lip of the avian dermomyotome. PLoS ONE 2014, 9, e92679. [Google Scholar] [CrossRef]
- Wu, Q.F.; Yang, L.; Li, S.; Wang, Q.; Yuan, X.B.; Gao, X.; Bao, L.; Zhang, X. Fibroblast growth factor 13 is a microtubule-stabilizing protein regulating neuronal polarization and migration. Cell 2012, 149, 1549–1564. [Google Scholar] [CrossRef]
- Flanagan-Steet, H.; Hannon, K.; McAvoy, M.J.; Hullinger, R.; Olwin, B.B. Loss of FGF receptor 1 signaling reduces skeletal muscle mass and disrupts myofiber organization in the developing limb. Dev. Biol. 2000, 218, 21–37. [Google Scholar] [CrossRef]
- Vasyutina, E.; Stebler, J.; Brand-Saberi, B.; Schulz, S.; Raz, E.; Birchmeier, C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev. 2005, 19, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Parsons, S.J. Src family protein tyrosine kinases: Cooperating with growth factor and adhesion signaling pathways. COCEBI 1997, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hollern, D.P.; Swiatnicki, M.R.; Rennhack, J.P.; Misek, S.A.; Matson, B.C.; McAuliff, A.; Gallo, K.A.; Caron, K.M.; Andrechek, E.R. E2F1 drives breast cancer metastasis by regulating the target gene FGF13 and altering cell migration. Sci. Rep. 2019, 9, 10718. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef]
- Ding, B.; Narvaez-Ortiz, H.Y.; Singh, Y.; Hocky, G.M.; Chowdhury, S.; Nolen, B.J. Structure of Arp2/3 complex at a branched actin filament junction resolved by single-particle cryo-electron microscopy. Proc. Natl. Acad. Sci. USA 2022, 119, e2202723119. [Google Scholar] [CrossRef]
- von Loeffelholz, O.; Purkiss, A.; Cao, L.; Kjaer, S.; Kogata, N.; Romet-Lemonne, G.; Way, M.; Moores, C.A. Cryo-EM of human Arp2/3 complexes provides structural insights into actin nucleation modulation by ARPC5 isoforms. Biol. Open 2020, 9, bio054304. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.; Zhou, Y.; Ostrowski, P.; Mylvaganam, S.; Boroumand, P.; Mulder, D.J.; Guo, C.; Muise, A.M.; Freeman, S.A. ARPC1B binds WASP to control actin polymerization and curtail tonic signaling in B cells. JCI Insight 2021, 6, e149376. [Google Scholar] [CrossRef]
- Mishra, Y.G.; Manavathi, B. Focal adhesion dynamics in cellular function and disease. Cell. Signal. 2021, 85, 110046. [Google Scholar] [CrossRef] [PubMed]
- Tapial Martínez, P.; López Navajas, P.; Lietha, D. FAK structure and regulation by membrane interactions and force in focal adhesions. Biomolecules 2020, 10, 179. [Google Scholar] [CrossRef]
- Alpha, K.M.; Xu, W.; Turner, C.E. Paxillin family of focal adhesion adaptor proteins and regulation of cancer cell invasion. Int. Rev. Cell Mol. Biol. 2020, 355, 1–52. [Google Scholar]
- Zhu, L.; Plow, E.F.; Qin, J. Initiation of focal adhesion assembly by talin and kindlin: A dynamic view. Prot. Sci. 2021, 30, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Z.S.; Witt, H.; Hazlett, L.; Harman, M.; Neumann, B.M.; Whitman, A.; Patel, M.; Ross, R.S.; Franck, C.; Reichner, J.S. Context-dependent role of vinculin in neutrophil adhesion, motility and trafficking. Sci. Rep. 2020, 10, 2142. [Google Scholar] [CrossRef] [PubMed]
- Pongchairerk, U.; Guan, J.-L.; Leardkamolkarn, V. Focal adhesion kinase and Src phosphorylations in HGF-induced proliferation and invasion of human cholangiocarcinoma cell line, HuCCA-1. WJG 2005, 11, 5845. [Google Scholar] [CrossRef]
- Chan, Z.C.-K.; Oentaryo, M.J.; Lee, C.W. MMP-mediated modulation of ECM environment during axonal growth and NMJ development. Neurosci. Lett. 2020, 724, 134822. [Google Scholar] [CrossRef]
- Hildyard, J.C.; Wells, D.J.; Piercy, R.J. Identification of qPCR reference genes suitable for normalising gene expression in the developing mouse embryo. Wellcome Open Res. 2021, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Enninful, A.; Deng, Y.; Fan, R. Spatial transcriptome sequencing of FFPE tissues at the cellular level. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kruse, A.R.; Malek, M.C.; Allen, J.; Farrow, M.; Spraggins, J. GeoMx-NGS Manual RNA Slide Preparation Protocol. 2023. [Google Scholar] [CrossRef]
- Reeves, J.; Divakar, P.; Ortogero, N.; Griswold, M.; Yang, Z.; Zimmerman, S.; Vitancol, R.; Henderson, D. Analyzing GeoMx-NGS RNA Expression Data with GeomxTools; NanoString Technologies, Inc.: Seattle, WA, USA, 2021. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Hossain, M.N.; Zhao, L.; Deavila, J.M.; Law, N.C.; Zhu, M.-J.; Murdoch, G.K.; Du, M. Spatial Transcriptomics Analysis: Maternal Obesity Impairs Myogenic Cell Migration and Differentiation during Embryonic Limb Development. Int. J. Mol. Sci. 2024, 25, 9488. https://doi.org/10.3390/ijms25179488
Gao Y, Hossain MN, Zhao L, Deavila JM, Law NC, Zhu M-J, Murdoch GK, Du M. Spatial Transcriptomics Analysis: Maternal Obesity Impairs Myogenic Cell Migration and Differentiation during Embryonic Limb Development. International Journal of Molecular Sciences. 2024; 25(17):9488. https://doi.org/10.3390/ijms25179488
Chicago/Turabian StyleGao, Yao, Md Nazmul Hossain, Liang Zhao, Jeanene Marie Deavila, Nathan C. Law, Mei-Jun Zhu, Gordon K. Murdoch, and Min Du. 2024. "Spatial Transcriptomics Analysis: Maternal Obesity Impairs Myogenic Cell Migration and Differentiation during Embryonic Limb Development" International Journal of Molecular Sciences 25, no. 17: 9488. https://doi.org/10.3390/ijms25179488
APA StyleGao, Y., Hossain, M. N., Zhao, L., Deavila, J. M., Law, N. C., Zhu, M.-J., Murdoch, G. K., & Du, M. (2024). Spatial Transcriptomics Analysis: Maternal Obesity Impairs Myogenic Cell Migration and Differentiation during Embryonic Limb Development. International Journal of Molecular Sciences, 25(17), 9488. https://doi.org/10.3390/ijms25179488





