Biogenic Zinc Oxide Nanoparticles as a Promising Antibacterial Agent: Synthesis and Characterization
Abstract
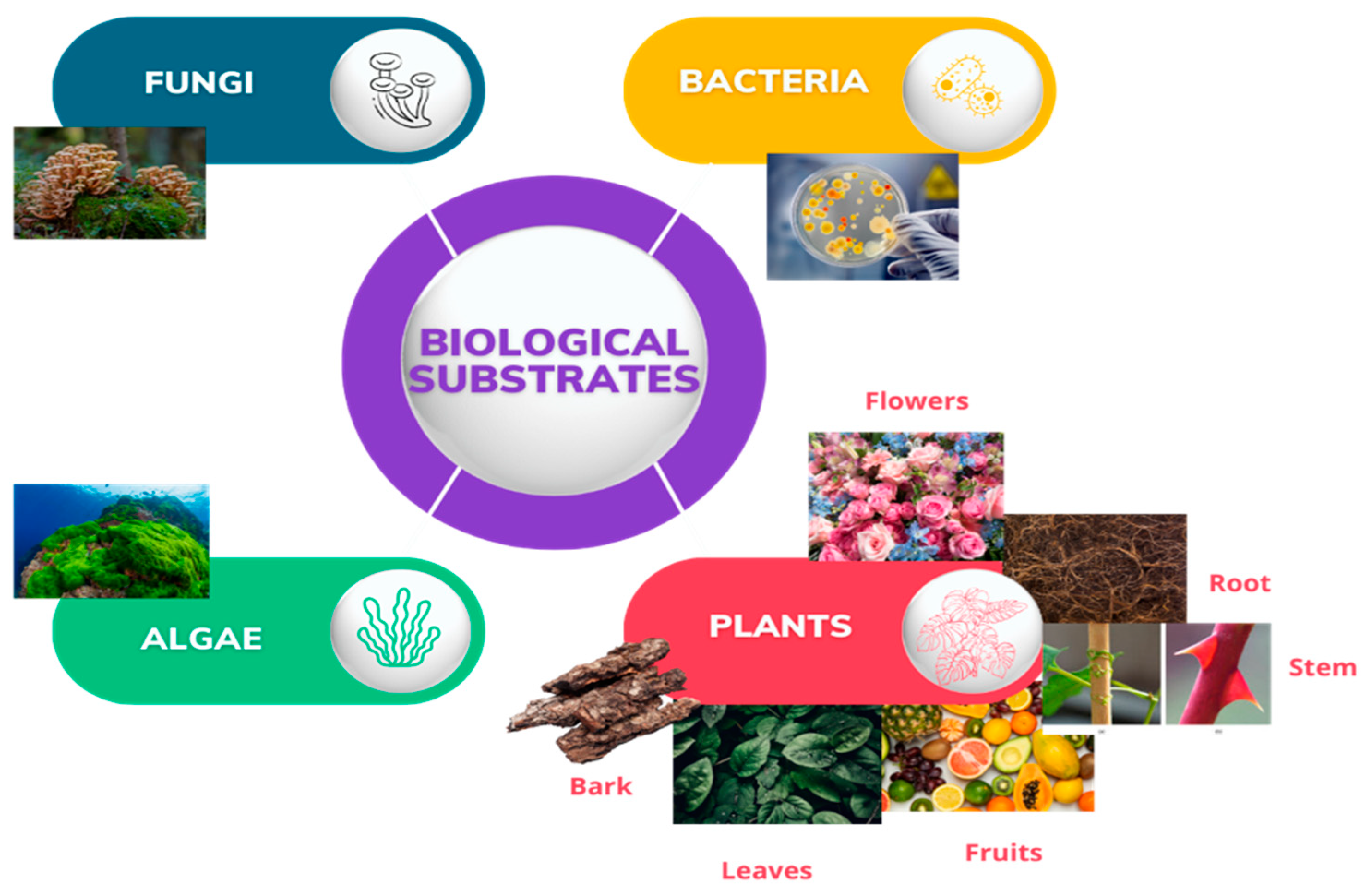
1. Introduction
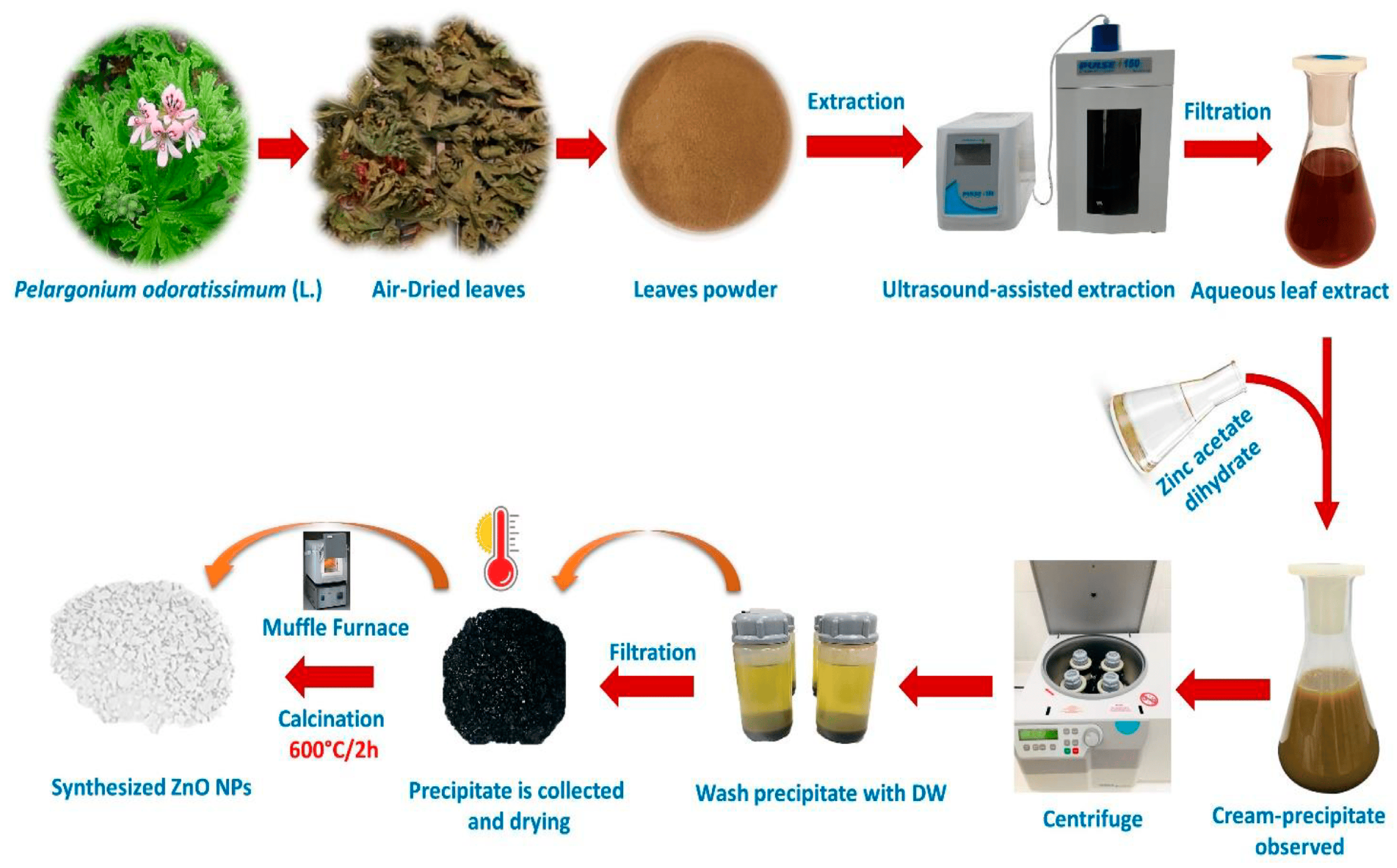
2. Green Synthesis of ZnO NPs
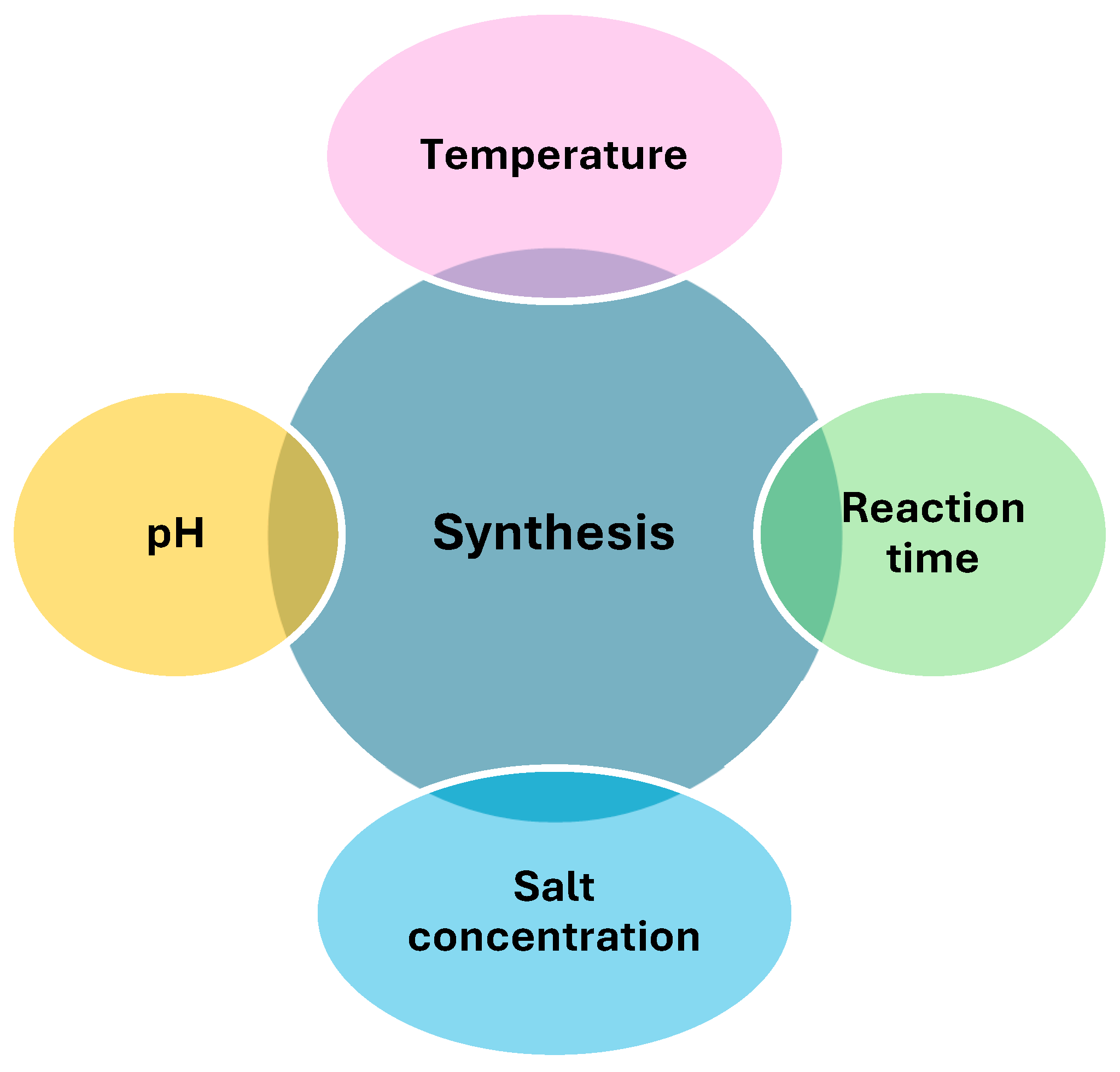
2.1. Optimization of Green Synthesis Conditions
2.1.1. pH
2.1.2. Temperature
2.1.3. Salt Concentration
2.1.4. Reaction Time
2.2. Purification of ZnO NPs
2.3. Calcination
2.4. Phytochemical Screening
2.5. Physicochemical Characterization of Nanoparticles
2.5.1. Ultraviolet–Visible (UV–Vis) Spectrophotometry
2.5.2. Fourier Transform Infrared (FT-IR) Spectroscopy
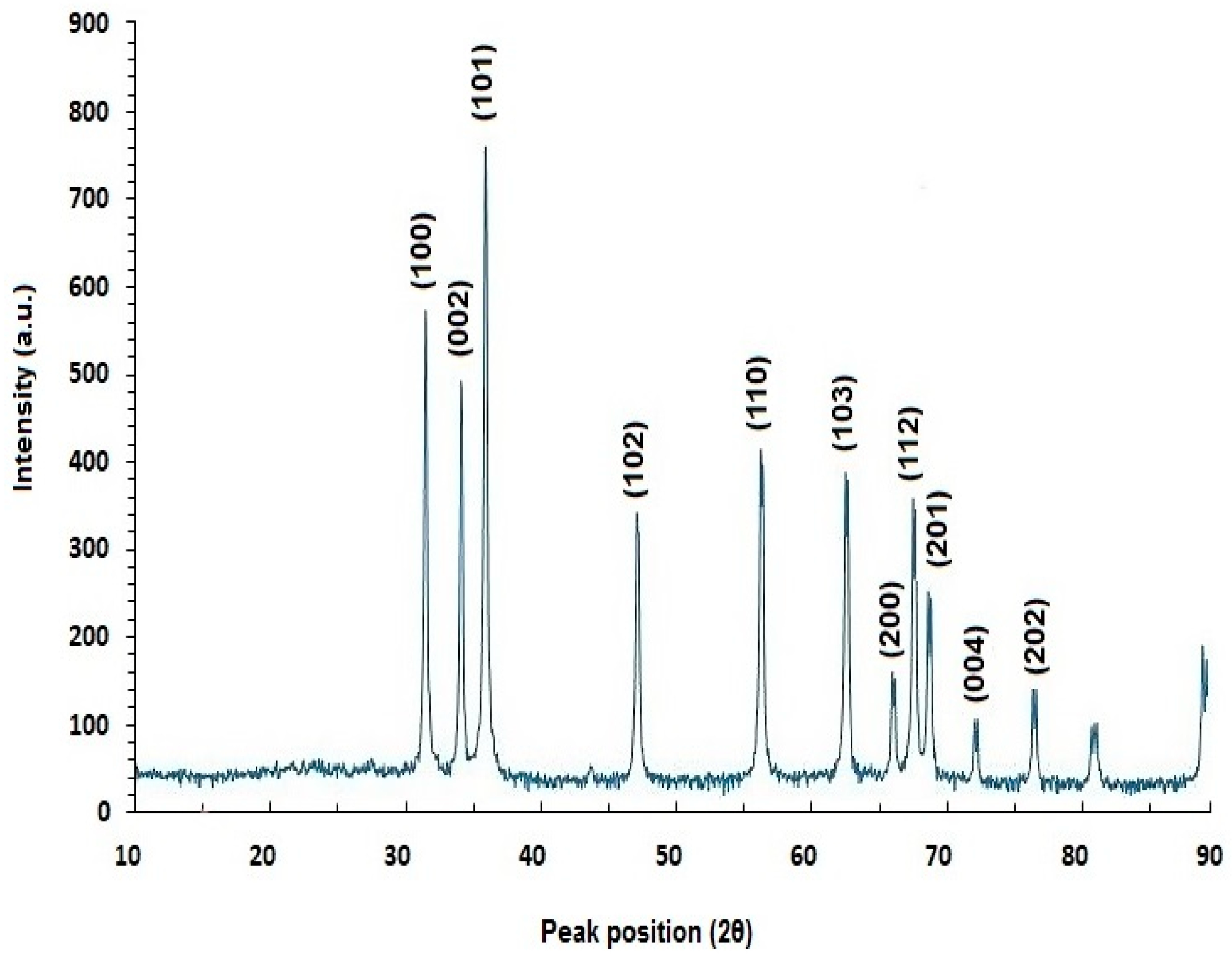
2.5.3. X-ray Diffraction (XRD) Analysis
2.5.4. Scanning Electron Microscopy (SEM)
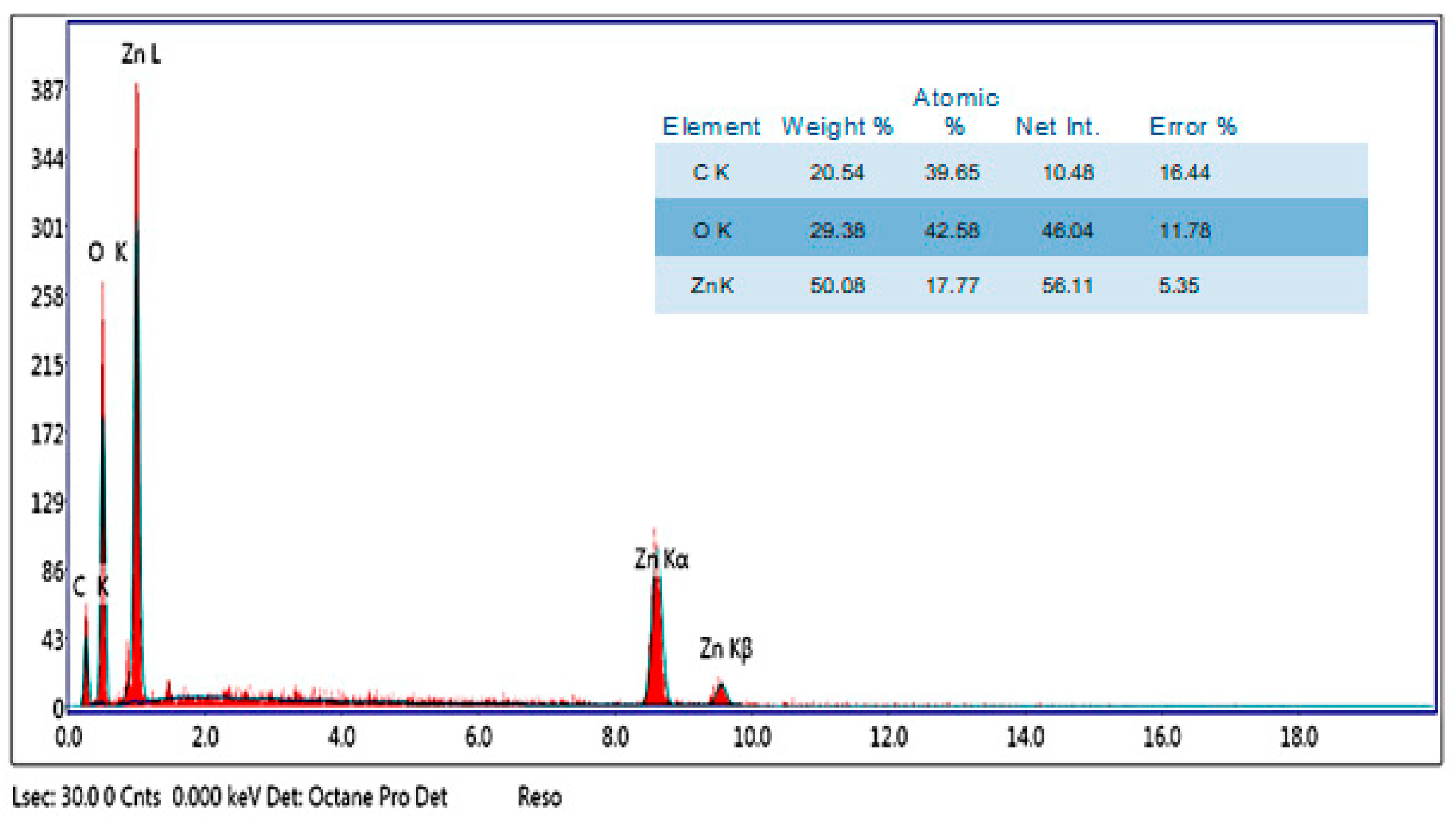
2.5.5. Energy-Dispersive X-ray (EDX)
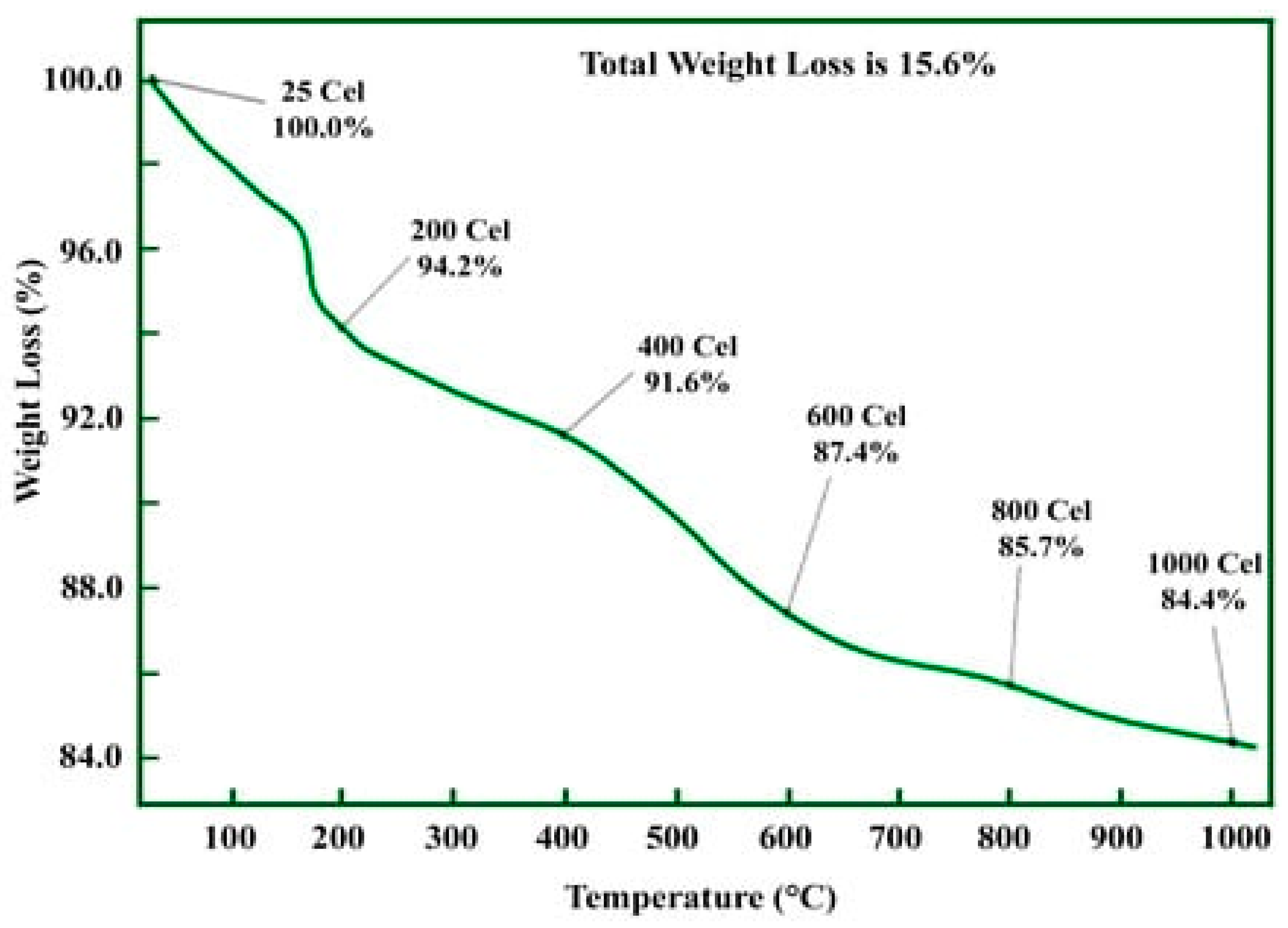
2.5.6. Thermogravimetric Analysis (TGA)
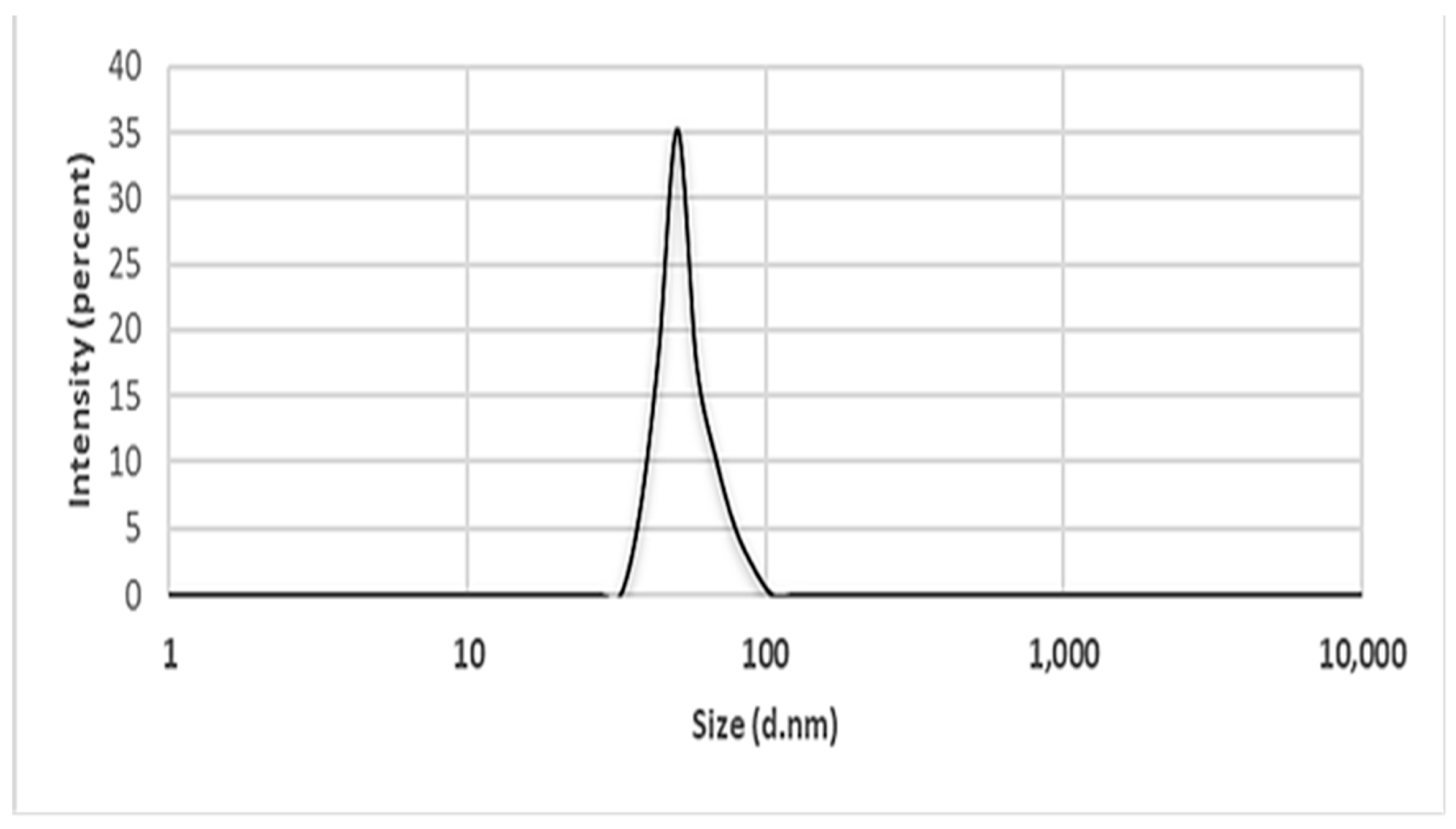
2.5.7. Dynamics Light Scattering (DLS)
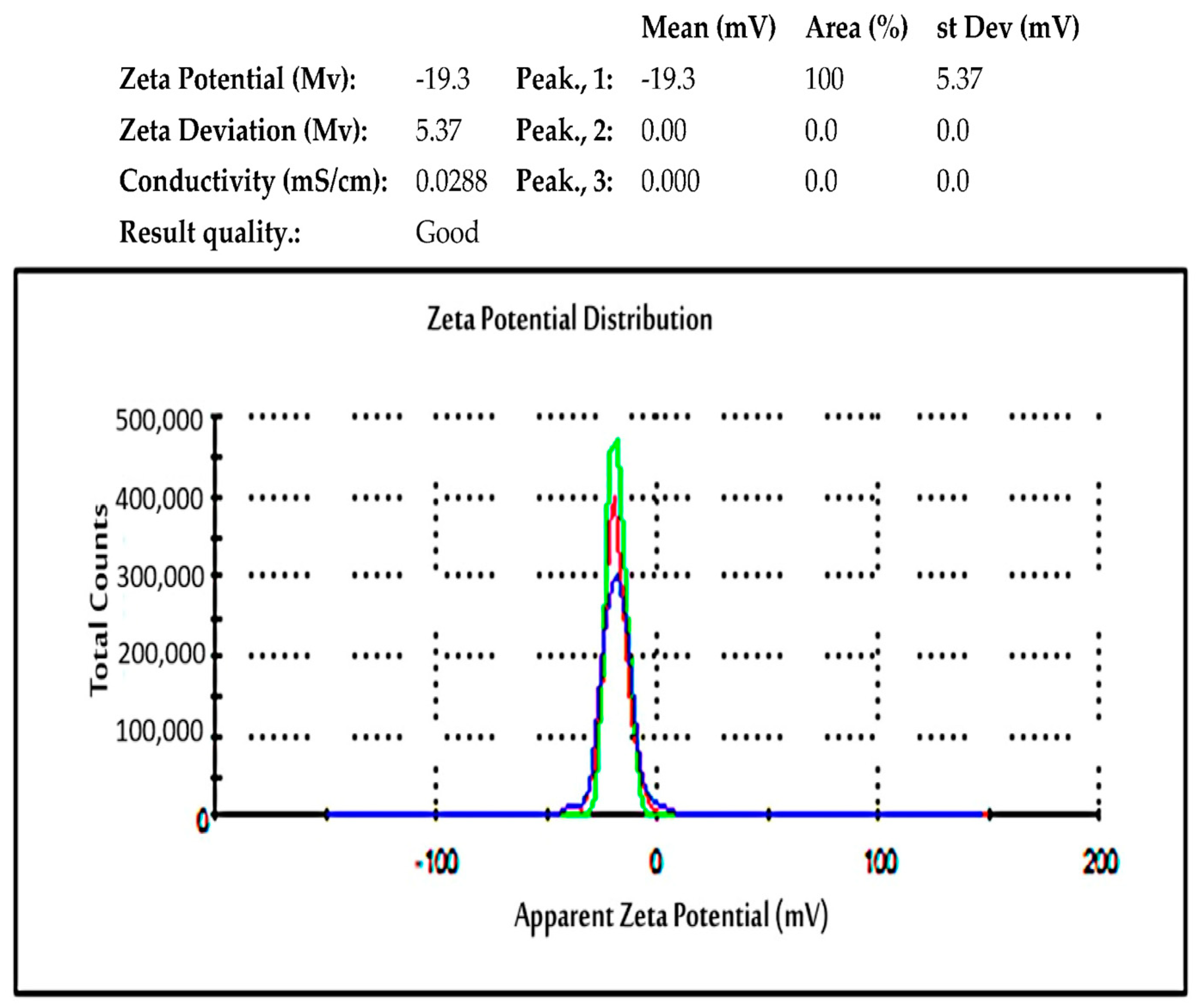
2.5.8. Zeta Potential
2.5.9. Transmission Electron Microscopy (TEM)
3. Biocompatibility and Cytotoxicity Testing
4. Antibacterial Activity of ZnO NPs
5. Antibacterial Assays
6. Possible Mechanism of Action of ZnO NPs
How Biofilms Mediate Antimicrobial Resistance
7. Conclusions, Challenges, and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Karam, S.T.; Abdulrahman, A.F. Green synthesis and characterization of ZnO nanoparticles by using thyme plant leaf extract. Photonics 2022, 9, 594. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Hoppe, H.; Okoh, A.I. Plant-based synthesis of silver nanoparticles using aqueous leaf extract of Salvia officinalis: Characterization and its antiplasmodial activity. J. Clust. Sci. 2021, 32, 101–109. [Google Scholar]
- Maher, S.; Nisar, S.; Aslam, S.M.; Saleem, F.; Behlil, F.; Imran, M.; Assiri, M.A.; Nouroz, A.; Naheed, N.; Khan, Z.A.; et al. Synthesis and characterization of ZnO nanoparticles derived from biomass (Sisymbrium irio) and assessment of potential anticancer activity. ACS Omega 2023, 8, 15920–15931. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal oxides nanoparticles: General structural description, chemical, physical, and biological synthesis methods, role in pesticides and heavy metal removal through wastewater treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef]
- Alghamdi, R.A.; Al-Zahrani, M.H.; Altarjami, L.R.; Al Abdulmonem, W.; Samir, N.; Said, A.; Shami, A.A.; Mohamed, W.S.; Ezzeldien, M. Biogenic Zinc oxide nanoparticles from Celosia argentea: Toward improved antioxidant, antibacterial, and anticancer activities. Front. Bioeng. Biotechnol. 2023, 11, 1283898. [Google Scholar] [CrossRef] [PubMed]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Effect of chemical synthesis compared to biosynthesized ZnO-NPs using Solanum nigrum leaf extract and their photocatalytic antibacterial and in vitro antioxidant activity. J. Environ. Chem. Eng. 2020, 8, 103705. [Google Scholar] [CrossRef]
- Gujel, A.A.; Bandeira, M.; Menti, C.; Perondi, D.; Guégan, R.; Roesch-Ely, M.; Giovanela, M.; Crespo, J.S. Evaluation of vulcanization nanoactivators with low zinc content: Characterization of zinc oxides, cure, physico-mechanical properties, Zn2+ release in water and cytotoxic effect of EPDM compositions. Polym. Eng. Sci. 2018, 58, 1800–1809. [Google Scholar] [CrossRef]
- Samriti, V.; Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2022, 10, 73–95. [Google Scholar] [CrossRef]
- Ahmoum, H.; Boughrara, M.; Su’ait, M.S.; Chopra, S.; Kerouad, M. Impact of position and concentration of sodium on the photovoltaic properties of zinc oxide solar cells. Phys. B Condens. Matter 2019, 560, 28–36. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Hashem, A.H.; Elhussieny, N.I.; Saied, E. Green biosynthesis of zinc oxide nanoparticles using Pluchea indica leaf extract: Antimicrobial and photocatalytic activities. Molecules 2023, 28, 4679. [Google Scholar] [CrossRef]
- Abdulrahman, A.F. The influence of various reactants in the growth solution on the morphological and structural properties of ZnO nanorods. Passer J. Basic Appl. Sci. 2020, 2, 69–75. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Zia, D.; Shinwari, Z.K.; Dhlamini, M.S.; Maaza, M. Structural, morphological and biological features of ZnO nanoparticles using Hyphaene thebaica (L.) Mart. fruits. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3241–3254. [Google Scholar] [CrossRef]
- Khezri, K.; Saeedi, M.; Maleki Dizaj, S. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 25. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Rani, R.; Singh, S. Biogenic zinc oxide nanoparticles and their biomedical applications: A review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1437–1452. [Google Scholar] [CrossRef]
- Amin, Z.S.; Afzal, M.; Ahmad, J.; Ahmed, N.; Zeshan, B.; Hashim, N.H.H.N.; Yean, C.Y. Synthesis, characterization and biological activities of zinc oxide nanoparticles derived from secondary metabolites of Lentinula edodes. Molecules 2023, 28, 3532. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; El-Monaem, A.; Eman, M.; Mohamed, I.; Badr, M.M.; Ihara, I.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Al-darwesh, M.Y.; Ibrahim, S.S.; Mohammed, M.A. A Review on Plant Extract Mediated Green Synthesis of Zinc oxide Nanoparticles and Their biomedical Applications. Results Chem. 2024, 16, 101368. [Google Scholar] [CrossRef]
- Becker, J.; Manske, C.; Randl, S. Green chemistry and sustainability metrics in the pharmaceutical manufacturing sector. Curr. Opin. Green Sustain. Chem. 2022, 33, 100562. [Google Scholar] [CrossRef]
- Xu, J.; Huang, Y.; Zhu, S.; Abbes, N.; Jing, X.; Zhang, L. A review of the green synthesis of ZnO nanoparticles using plant extracts and their prospects for application in antibacterial textiles. J. Eng. Fiber Fabr. 2021, 16, 15589250211046242. [Google Scholar] [CrossRef]
- Ihsan, M.; Din, I.U.; Alam, K.; Munir, I.; Mohamed, H.I.; Khan, F. Green fabrication, characterization of zinc oxide nanoparticles using plant extract of Momordica charantia and Curcuma zedoaria and their antibacterial and antioxidant activities. Appl. Biochem. Biotechnol. 2023, 195, 3546–3565. [Google Scholar] [CrossRef]
- Rahman, F.; Majed Patwary, M.A.; Bakar Siddique, M.A.; Bashar, M.S.; Haque, M.A.; Akter, B.; Rashid, R.; Haque, M.A.; Royhan Uddin, A.K.M. Green synthesis of zinc oxide nanoparticles using cocos nucifera leaf extract: Characterization, antimicrobial, antioxidant and photocatalytic activity. R. Soc. Open Sci. 2022, 9, 220858. [Google Scholar] [CrossRef]
- Haseeb, Q.; Hamdani, S.D.A.; Akram, A.; Khan, D.A.; Rajput, T.A.; Babar, M.M. Nanobiotechnology: Paving the way to personalized medicine. NanoBioMedicine 2020, 2020, 17–32. [Google Scholar]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and microbial approaches as green methods for the synthesis of nanomaterials: Synthesis, applications, and future perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, X.; Wang, X.; Shao, P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ. Pollut. 2011, 159, 1783–1788. [Google Scholar] [CrossRef]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the green synthesis of nanoparticles: Uncovering influences of plant extracts as reducing agents for enhanced synthesis efficiency and its biomedical applications. Int. J. Nanomed. 2023, 31, 4727–4750. [Google Scholar] [CrossRef]
- Hamed, R.; Obeid, R.Z.; Abu-Huwaij, R. Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications. Nanotechnol. Rev. 2023, 12, 20230112. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.H.A. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-infammatory activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; El-Maghrabi, N.; Hosny, M.; Fawzy, M. Biogenic synthesis of reduced graphene oxide from Ziziphus spinachristi (Christ’s thorn jujube) extracts for catalytic, antimicrobial, and antioxidant potentialities. Environ. Sci. Pollut. Res. 2022, 20, 89772–89787. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. (Англoязычная Версия) 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Sadiq, H.M.; Shah, N.S.; Khan, A.U.; Muhammad, N.; Hassan, S.U.; Tahir, K.; Khan, F.U.; Imran, M.; Ahmad, N.; et al. Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. J. Photochem Photobiol. B Biol. 2019, 192, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cao, X.; Chen, C.; Liao, L.; Yuan, S.; Huang, S. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extracts of Hibiscus cannabinus L.: Wastewater Purification and Antibacterial Activity. Separations 2023, 10, 466. [Google Scholar] [CrossRef]
- Chabattula, S.C.; Gupta, P.K.; Tripathi, S.K.; Gahtori, R.; Padhi, P.; Mahapatra, S.; Biswal, B.K.; Singh, S.K.; Dua, K.; Ruokolainen, J.; et al. Anticancer therapeutic efficacy of biogenic Am-ZnO nanoparticles on 2D and 3D tumor models. Mater. Today Chem. 2021, 22, 25. [Google Scholar] [CrossRef]
- El-Fallal, A.A.; Elfayoumy, R.A.; El-Zahed, M.M. Antibacterial activity of biosynthesized zinc oxide nanoparticles using Kombucha extract. SN Appl. Sci. 2023, 5, 332. [Google Scholar] [CrossRef]
- Hamdy, E.; Al-Askar, A.A.; El-Gendi, H.; Khamis, W.M.; Behiry, S.I.; Valentini, F.; Abd-Elsalam, K.A.; Abdelkhalek, A. Zinc oxide nanoparticles biosynthesized by eriobotrya japonica leaf extract: Characterization, insecticidal and antibacterial properties. Plants 2023, 12, 2826. [Google Scholar] [CrossRef]
- Al-Khaial, M.Q.; Chan, S.Y.; Abu-Zurayk, R.A.; Alnairat, N. Biosynthesis and Characterization of Zinc Oxide Nanoparticles (ZnO-NPs) Utilizing Banana Peel Extract. Inorganics 2024, 12, 121. [Google Scholar] [CrossRef]
- Kanimozhi, V.M.; Sridhar, S. Optimization of process parameters using Box-Benkhen design towards uptake of acid orange 10 using Casuarina charcoal. Adv. Biotechnol. 2013, 12, 5–9. [Google Scholar]
- Hormozi-Nezhad, M.R.; Jalali-Heravi, M.; Robatjazi, H.; Ebrahimi-Najafabadi, H. Controlling aspect ratio of colloidal silver nanorods using response surface methodology. Colloid. Surf. A 2012, 393, 46–52. [Google Scholar] [CrossRef]
- Radha, K.V.; Thamilselvi, V. Synthesis of silver nanoparticles from Pseudomonas putida NCIM 2650 in silver nitrate supplemented growth medium and optimization using response surface methodology. Digest J. Nanomater. Biostructures 2013, 8, 1101–1111. [Google Scholar]
- Krupa, A.N.D.; Abigail, M.E.A.; Santhosh, C.; Grace, A.N.; Vimala, R. Optimization of process parameters for the microbial synthesis of silver nanoparticles using 3-level Box-Behnken design. Ecol. Eng. 2016, 87, 168–174. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.R. Plant mediated fabrication of silver nanoparticles, process optimization, and impact on tomato plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Arif, H.; Qayyum, S.; Akhtar, W.; Fatima, I.; Kayani, W.K.; Rahman, K.U.; Al-Onazi, W.A.; Al-Mohaimeed, A.M.; Bangash, N.K.; Ashraf, N.; et al. Synthesis and Characterization of Zinc Oxide Nanoparticles at Different pH Values from Clinopodium vulgare L. and Their Assessment as an Antimicrobial Agent and Biomedical Application. Micromachines 2023, 14, 1285. [Google Scholar] [CrossRef]
- Fernandes, C.A.; Jesudoss, M.N.; Nizam, A.; Krishna, S.B.N.; Lakshmaiah, V.V. Biogenic Synthesis of Zinc Oxide Nanoparticles Mediated by the Extract of Terminalia catappa Fruit Pericarp and Its Multifaceted Applications. ACS Omega 2023, 8, 39315–39328. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: Characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective antimicrobial activity of green ZnO nanoparticles of Catharanthus roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Umamaheswari, A.; Prabu, S.L.; John, S.A.; Puratchikody, A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. 2021, 29, e00595. [Google Scholar] [CrossRef]
- Singh, B.N.; Rawat, A.K.S.; Khan, W.; Naqvi, A.H.; Singh, B.R. Biosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa Rhamnolipids. PLoS ONE 2014, 9, 106937. [Google Scholar] [CrossRef]
- Bekele, S.G.; Ganta, D.D.; Endashaw, M. Green synthesis and characterization of zinc oxide nanoparticles using Monoon longifolium leave extract for biological applications. Discover Chem. 2024, 1, 1–17. [Google Scholar] [CrossRef]
- Divya, B.; Karthikeyan, C.H.; Rajasimman, M. Chemical synthesis of zinc oxide nanoparticles and its application of dye decolourization. Int. J. Nanosci. Nanotechnol. 2018, 14, 267–275. [Google Scholar]
- Kahsay, M.H. Synthesis and characterization of ZnO nanoparticles using aqueous extract of Becium Grandiflorum for antimicrobial activity and adsorption of Methylene Blue. Appl. Water Sci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Haddi, R.; El Kharraz, A.M.; Kerroumi, M.I. Green Synthesis of Zinc Oxide Nanoparticles Using Pistacia lentiscus L. Leaf Extact and Evaluating their Antioxydant and Antibacterial Properties. Nano Biomed. Eng. 2024, 1, 16. [Google Scholar] [CrossRef]
- Osaili, T.M.; Albiss, B.A.; Al–Nabulsi, A.A.; Alromi, R.F.; Olaimat, A.; Al-Holy, M.; Savvaidis, I.; Holley, R. Effects of metal oxide nanoparticles with plant extract on viability of foodborne pathogens. J. Food Saf. 2019, 39, 12681. [Google Scholar] [CrossRef]
- Konan, F.K.; N’cho, J.S.; Nkuissi, H.T.; Hartiti, B.; Boko, A. Influence of the precursor concentration on the morphological and structural properties of zinc oxide (ZnO). Mater. Chem. Phys. 2019, 229, 330–333. [Google Scholar] [CrossRef]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent advances in green synthesized nanoparticles: From production to application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A Review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Surdu, V.A.; Ficai, A.; Ficai, D.; Grumezescu, A.M.; Andronescu, E. Green synthesis of metal and metal oxide nanoparticles: A review of the principles and biomedical applications. Int. J. Mol. Sci. 2023, 24, 15397. [Google Scholar] [CrossRef]
- Thatyana, M.; Dube, N.P.; Kemboi, D.; Manicum, A.L.E.; Mokgalaka-Fleischmann, N.S.; Tembu, J.V. Advances in phytonanotechnology: A plant-mediated green synthesis of metal nanoparticles using phyllanthus plant extracts and their antimicrobial and anticancer applications. Nanomaterials 2023, 13, 2616. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant secondary metabolites: The weapons for biotic stress management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Berehu, H.M.; Khan, M.I.; Chakraborty, R.; Lavudi, K.; Penchalaneni, J.; Mohapatra, B.; Mishra, A.; Patnaik, S. Cytotoxic potential of biogenic zinc oxide nanoparticles synthesized from swertia chirayita leaf extract on colorectal cancer cells. Front. Bioeng. Biotechnol. 2021, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-Extract-Mediated Synthesis of Metal Nanoparticles. J. Chem. 2021, 2021, 6562687. [Google Scholar] [CrossRef]
- Chauhan, C.C.; Gupta, T.; Meena, S.S.; Desimone, M.F.; Das, A.; Sandhu, C.S.; Jotania, K.R.; Jotania, R.B. Tailoring magnetic and dielectric properties of SrFe12O19/NiFe2O4 ferrite nanocomposites synthesized in presence of Calotropis gigantea (crown) flower extract. J. Alloys Compd. 2022, 900, 163415. [Google Scholar] [CrossRef]
- Mbenga, Y.; Mthiyane, M.N.; Botha, T.L.; Horn, S.; Pieters, R.; Wepener, V.; Onwudiwe, D.C. Nanoarchitectonics of ZnO nanoparticles mediated by extract of tulbaghia violacea and their cytotoxicity evaluation. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3249–3259. [Google Scholar] [CrossRef]
- Gamedze, N.P.; Mthiyane, D.M.; Mavengahama, S.; Singh, M.; Onwudiwe, D.C. Biosynthesis of ZnO nanoparticles using the aqueous extract of Mucuna pruriens (utilis): Structural characterization, and the anticancer and antioxidant activities. Chem. Afr. 2024, 7, 219–228. [Google Scholar] [CrossRef]
- Sánchez-Pérez, D.M.; Flores-Loyola, E.; Márquez-Guerrero, S.Y.; Galindo-Guzman, M.; Marszalek, J.E. Green synthesis and characterization of zinc oxide nanoparticles using Larrea tridentata extract and their impact on the in-vitro germination and seedling growth of Capsicum annuum. Sustainability 2023, 15, 3080. [Google Scholar] [CrossRef]
- Perrotta, A.; Pilz, J.; Milella, A.; Coclite, A.M. Opto-chemical control through thermal treatment of plasma enhanced atomic layer deposited ZnO: An in situ study. Appl. Surf. Sci. 2019, 483, 10–18. [Google Scholar] [CrossRef]
- Abdulhafiz, F.; Reduan, M.F.H.; Hisam, A.H.; Mohammad, I.; Abdul Wahab, I.R.; Abdul Hamid, F.F.; Mohammed, A.; Nordin, M.L.; Shaari, R.; Bakar, L.A.; et al. LC–TOF-MS/MS and GC-MS based phytochemical profiling and evaluation of wound healing activity of Oroxylum Indicum (L.) Kurz (Beka). Front. Pharmacol. 2022, 13, 1050453. [Google Scholar] [CrossRef]
- Hasan, I.M.; Tawfik, A.R.; Assaf, F.H. GC/MS screening of buckthorn phytochemicals and their use to synthesize ZnO nanoparticles for photocatalytic degradation of malachite green dye in water. Water Sci. Technol. 2022, 85, 664–684. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Sarmin, M.; Gurung, S.; Sarkar, S.; Das, S.; Hoda, M. Photocatalysis-enhanced synthesis and stabilization of silver nanoparticles by methanol-based phytochemicals extract of Trigonella foenum-graecum seeds. JCIS Open 2024, 12, 100116. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.; Rahimi, R.; Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V.K. Synthesis and optimization of zinc oxide nanoparticles using Kalanchoe pinnata towards the evaluation of its anti-inflammatory activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101291. [Google Scholar] [CrossRef]
- Krajczewski, J.; Kołątaj, K.; Kudelski, A. Plasmonic nanoparticles in chemical analysis. RSC Adv. 2017, 7, 17559–17576. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen Vacancy Induced Band-Gap Narrowing and Enhanced Visible Light Photocatalytic Activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [PubMed]
- Roza, L.; Rahman, M.Y.A.; Umar, A.A.; Salleh, M.M. Direct growth of oriented ZnO nanotubes by self-selective etching at lower temperature for photo-electrochemical (PEC) solar cell application. J. Alloys Compd. 2015, 618, 153. [Google Scholar]
- Ahmed, N.A.; Othman, A.S. Green fabrication of ZnO nanoparticles via spirulina platensis and its efficiency against biofilm-forming pathogens. Microb. Cell Fact. 2024, 23, 92. [Google Scholar] [CrossRef]
- Alhujaily, M.; Albukhaty, S.; Yusuf, M.; Mohammed, M.K.; Sulaiman, G.M.; Al-Karagoly, H.; Alyamani, A.A.; Albaqami, J.; AlMalki, F.A. Recent advances in plant-mediated zinc oxide nanoparticles with their significant biomedical properties. Bioengineering 2022, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Karagoly, H.; Rhyaf, A.; Naji, H.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A.; Albaqami, J.; Aloufi, S. Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract. Green Proc. Synth. 2022, 11, 254–265. [Google Scholar] [CrossRef]
- Shafey, A.M.E. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Proc. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Faye, G.; Jebessa, T.; Wubalem, T. Biosynthesis, characterization and antimicrobial activity of zinc oxide and nickel doped zinc oxide nanoparticles using Euphorbia abyssinica bark extract. IET Nanobiotechnol. 2021, 16, 25–32. [Google Scholar] [CrossRef]
- Dias, C.; Ayyanar, M.; Amalraj, S.; Khanal, P.; Subramaniyan, V.; Das, S.; Gandhale, P.; Biswa, V.; Ali, R.; Gurav, N.; et al. Biogenic synthesis of zinc oxide nanoparticles using mushroom fungus Cordyceps militaris: Characterization and mechanistic insights of therapeutic investigation. J. Drug Deliv. Sci. Technol. 2022, 73, 103444. [Google Scholar] [CrossRef]
- Khan, A.U.; Malik, N.; Singh, B. Biosynthesis, and characterization of zinc oxide nanoparticles (ZnONPs) obtained from the extract of waste of strawberry. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 268–275. [Google Scholar] [CrossRef]
- Niskanen, M.; Kuisma, M.; Cramariuc, O.; Golovanov, V.; Hukka, T.I.; Tkachenko, N.; Rantala, T.T. Porphyrin adsorbed on the (101 [combining macron] 0) surface of the wurtzite structure of ZnO–conformation induced effects on the electron transfer characteristics. Phys. Chem. Chem. Phys. 2013, 15, 17408–17418. [Google Scholar] [CrossRef]
- Alyamani, A.A.; Albukhaty, S.; Aloufi, S.; AlMalki, F.A.; Al-Karagoly, H.; Sulaiman, G.M. Green fabrication of zinc oxide nanoparticles using Phlomis leaf extract: Characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules 2021, 26, 6140. [Google Scholar] [CrossRef] [PubMed]
- Erazo, A.; Mosquera, S.A.; Rodríguez-Paéz, J.E. Synthesis of ZnO nanoparticles with different morphology: Study of their antifungal effect on strains of Aspergillus niger and Botrytis cinerea. Mater. Chem. Phys. 2019, 234, 172–184. [Google Scholar] [CrossRef]
- Hamelian, M.; Hemmati, S.; Varmira, K.; Veisi, H. Green synthesis, antibacterial, antioxidant and cytotoxic effect of gold nanoparticles using Pistacia Atlantica extract. J. Taiwan Inst. Chem. Eng. 2018, 93, 21–30. [Google Scholar] [CrossRef]
- Velsankar, K.; Sudhahar, S.; Parvathy, G.; Kaliammal, R. Effect of cytotoxicity and Antibacterial activity of biosynthesis of ZnO hexagonal shaped nanoparticles by Echinochloa frumentacea grains extract as a reducing agent. Mater. Chem. Phys. 2020, 239, 121976. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Shobiya, M. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother. 2016, 84, 1213–1222. [Google Scholar]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Pomastowski, P.; Król-Górniak, A.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanocomposites—Extracellular synthesis, physicochemical characterization and antibacterial potential. Materials 2020, 13, 4347. [Google Scholar] [CrossRef] [PubMed]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Optimization of biosynthesis zinc oxide nanoparticles: Desirability-function based response surface methodology, physicochemical characteristics, and its antioxidant properties. OpenNano 2022, 8, 100106. [Google Scholar] [CrossRef]
- Sana, S.S.; Kumbhakar, D.V.; Pasha, A.; Pawar, S.C.; Grace, A.N.; Singh, R.P.; Nguyen, V.H.; Le, Q.V.; Peng, W. Crotalaria verrucosa leaf extract mediated synthesis of zinc oxide nanoparticles: Assessment of antimicrobial and anticancer activity. Molecules 2020, 25, 4896. [Google Scholar] [CrossRef]
- De Assis, D.N.; Mosqueira, V.C.; Vilela, J.M.; Andrade, M.S.; Cardoso, V.N. Release profiles and morphological characterization by atomic force microscopy and photon correlation spectroscopy of 99m Technetium fluconazole nanocapsules. Int. J. Pharm. X 2008, 349, 152–160. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Abdelaal, N.; Saeed Al-Zuhairy, S.A.K.; Elrefai, M.F.M.; Elsaid Hamdan, A.M.; Khalifa, M.M.; Hababeh, S.; Khasawneh, M.A.; Khamis, G.M.; Nelson, J.; et al. Green Synthesis of Zinc Oxide Nanoparticles from Althaea officinalis Flower Extract Coated with Chitosan for Potential Healing Effects on Diabetic Wounds by Inhibiting TNF-α and IL-6/IL-1β Signaling Pathways. Int. J. Nanomed. 2024, 31, 3045–3070. [Google Scholar] [CrossRef]
- Kim, K.M.; Choi, M.H.; Lee, J.K.; Jeong, J.; Kim, Y.R.; Kim, M.K.; Paek, S.M.; Oh, J.M. Physicochemical properties of surface charge-modified ZnO nanoparticles with different particle sizes. Int. J. Nanomed. 2014, 9, 41–56. [Google Scholar]
- Abbas, Z.; Labbez, C.; Nordholm, S.; Ahlberg, E. Size-dependent surface charging of nanoparticles. J. Phys. Chem. C 2008, 112, 5715–5723. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of zinc oxide nanoparticles using ixora coccinea leaf extract—A green approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Nandhakumar, E.; Priya, P.; Soni, D.; Vimalan, M.; Potheher, I.V. Synthesis of ZnO nanoparticles using leaf extract of Tectona grandis (L.) and their anti-bacterial, anti-arthritic, anti-oxidant and in vitro cytotoxicity activities. New J. Chem. 2017, 41, 10347–10356. [Google Scholar] [CrossRef]
- Baek, M.; Chung, H.E.; Yu, J.; Lee, J.A.; Kim, T.H.; Oh, J.M.; Lee, W.J.; Paek, S.M.; Lee, J.K.; Jeong, J.; et al. Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int. J. Nanomed. 2012, 26, 3081–3097. [Google Scholar]
- Sadhasivam, S.; Shanmugam, M.; Umamaheswaran, P.D.; Venkattappan, A. Zinc oxide nanoparticles: Green synthesis and biomedical applications. J. Clust. Sci. 2021, 32, 1441–1455. [Google Scholar] [CrossRef]
- Ahmad, N.; Ali, S.; Abbas, M.; Fazal, H.; Saqib, S.; Ali, A.; Ullah, Z.; Zaman, S.; Sawati, L.; Zada, A.; et al. Antimicrobial efficacy of Mentha piperata-derived biogenic zinc oxide nanoparticles against UTI-resistant pathogens. Sci. Rep. 2023, 13, 14972. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.B.; Aminuzzaman, M.; Rahman, M.K.; Win, Y.F.; Sultana, S.; Cheah, S.Y.; Watanabe, A.; Wong, L.S.; Guha, S.K.; Djearamane, S.; et al. Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study. Green Process. Synth. 2024, 13, 20230251. [Google Scholar] [CrossRef]
- Abduljabbar, B.; El-Zayat, M.; El-Amier, Y.; El-Halawany, E.S. Biosynthesis, characterization, and cytotoxic activities of zinc nanoparticles (ZnNPs) using Euphorbia retusa extract. Kuwait J Sci. 2024, 51, 100108. [Google Scholar] [CrossRef]
- Kokhdan, E. Cytotoxic effect of methanolic extract, alkaloid and terpenoid fractions of Stachys pilifera against HT-29 cell line. Res. Pharm. Sci. 2018, 13, 404−412. [Google Scholar]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Neamah, S.A.; Albukhaty, S.; Falih, I.Q.; Dewir, Y.H.; Mahood, H.B. Biosynthesis of zinc oxide nanoparticles using Capparis spinosa L. fruit extract: Characterization, biocompatibility, and antioxidant activity. Appl. Sci. 2023, 13, 6604. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Majeed, S.; Danish, M.; Ismail, M.H.B.; Ansari, M.T.; Ibrahim, M.N.M. Anticancer and apoptotic activity of biologically synthesized zinc oxide nanoparticles against human colon cancer HCT-116 cell line- in vitro study. Sustain. Chem. Pharm. 2019, 14, 100179. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.C.; Bou, G.; Blackburn, J.M. Proteomics of microbial human pathogens. Front. Microbiol. 2016, 7, 1742. [Google Scholar] [CrossRef]
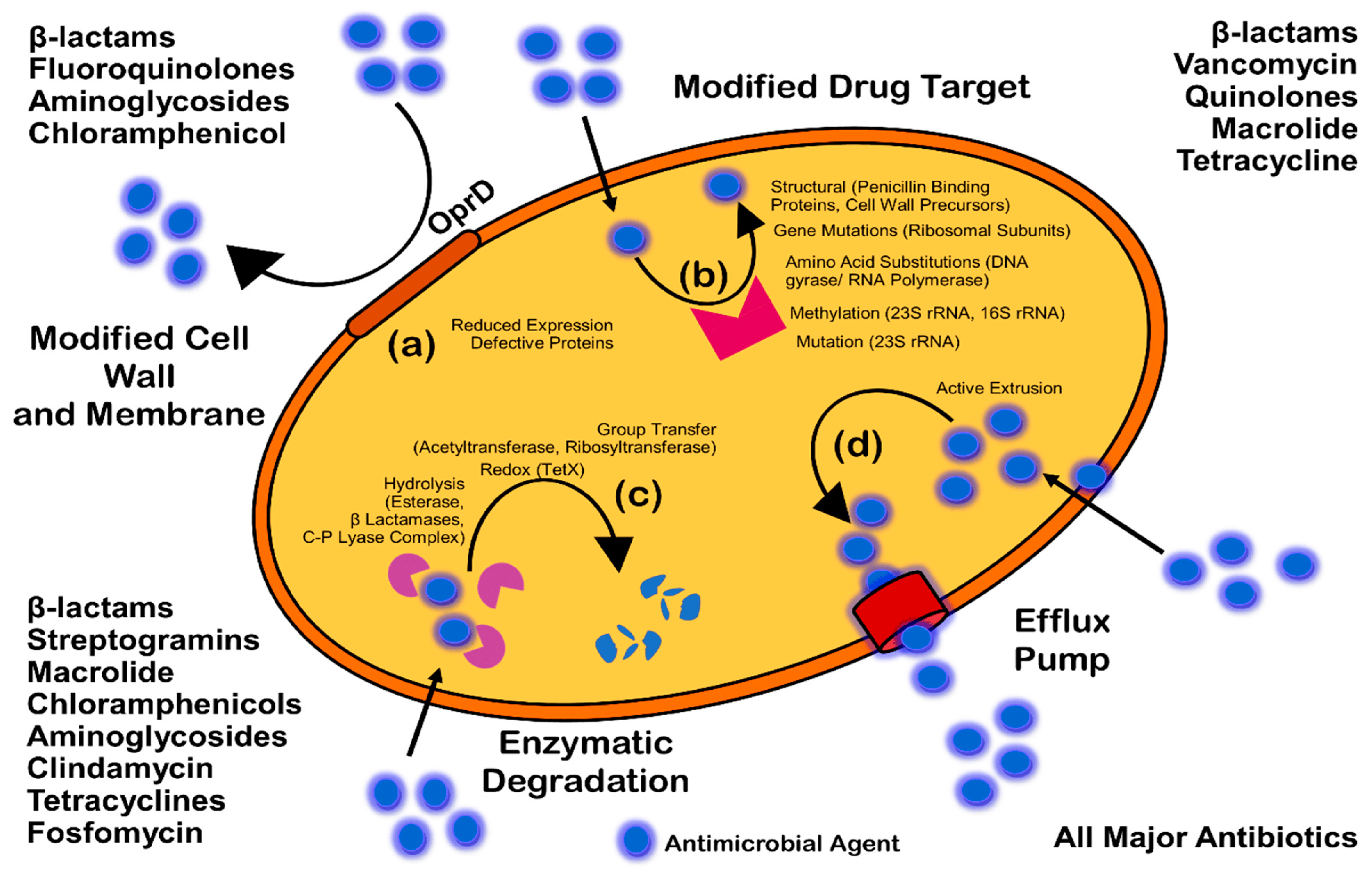
- Reygaert, W. An overview of the antimicrobial resistance mechanisms of bacteria. J. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Reygaert, W. Methicillin-resistant Staphylococcus aureus (MRSA): Molecular aspects of antimicrobial resistance and virulence. Clin. Lab. Sci. 2009, 22, 115–119. [Google Scholar] [PubMed]
- Randall, C.; Mariner, K.; Mariner, K.R.; Chopra, I.; O’Neill, A.J. The target of daptomycin is absent from Escherichia coli and other gram-negative pathogens. Antimicrob. Agents Chemother. 2013, 57, 637–639. [Google Scholar] [CrossRef]
- Kumar, A.; Schweizer, H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005, 57, 1486–1513. [Google Scholar] [CrossRef]
- Blair, J.M.; Richmond, G.E.; Piddock, L.J. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014, 9, 1165–1677. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Komerik, N.; MacRobert, A.J. Photodynamic therapy as an alternative antimicrobial modality for oral infections. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 487–504. [Google Scholar]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, M.; Gurkok, S. Recent advances in nanoparticles as antibacterial agent. ADMET DMPK 2022, 10, 115–129. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Ahmad, N.; Khan, M.M. Antioxidant and antibacterial studies of phytogenic fabricated ZnO using aqueous leaf extract of Ziziphus mauritiana Lam. Chem. Pap. 2021, 75, 3295–3308. [Google Scholar] [CrossRef]
- Mushtaq, W.; Ishtiaq, M.; Maqbool, M.; Mazhar, M.W.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Green Synthesis of Zinc Oxide Nanoparticles Using Viscum album Extracts: Unveiling Bioactive Compounds, Antibacterial Potential, and Antioxidant Activities. Plants 2023, 12, 2130. [Google Scholar] [CrossRef]
- Mohammed, Y.H.I.; Alghamdi, S.; Jabbar, B.; Marghani, D.; Beigh, S.; Abouzied, A.S.; Khalifa, N.E.; Khojali, W.M.; Huwaimel, B.; Alkhalifah, D.H.M.; et al. Green synthesis of zinc oxide nanoparticles using Cymbopogon citratus extract and its antibacterial activity. ACS Omega 2023, 8, 32027–32042. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Thangadurai, T.D.; Bharathy, P.V. Greenbased Biosynthesis of Zinc Oxide Nanoparticles Using Clitoria ternatea Flower Extract and Its Antibacterial Activity. Nano Biomed. Eng. 2021, 1, 394–400. [Google Scholar]
- Nezamabadi, V.; Akhgar, M.R.; Tahamipour, B.; Rajaei, P. Biosynthesis and antibacterial activity of ZnO nanoparticles by Artemisia aucheri extract. Iranian J. Biotechnol. 2020, 18, 2426. [Google Scholar]
- Yagoub, A.E.A.; Al-Shammari, G.M.; Al-Harbi, L.N.; Subash-Babu, P.; Elsayim, R.; Mohammed, M.A.; Yahya, M.A.; Fattiny, S.Z. Antimicrobial properties of zinc oxide nanoparticles synthesized from lavandula pubescens shoot methanol extract. Appl. Sci. 2022, 12, 11613. [Google Scholar] [CrossRef]
- Umavathi, S.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Virik, P.; Al-Mulhm, N.; Subash, M.; Gopinath, K.; Kavitha, C. Green synthesis of ZnO nanoparticles for antimicrobial and vegetative growth applications: A novel approach for advancing efficient high quality health care to human wellbeing. Saudi J. Biol.Sci. 2021, 28, 1808–1815. [Google Scholar] [CrossRef]
- Ghdeeb, N.J.; Hussain, N.A. Antimicrobial activity of ZnO nanoparticles prepared using a green synthesis approach. Nano Biomed. Eng. 2023, 15, 14–20. [Google Scholar] [CrossRef]
- Alnehia, A.; Al-Sharabi, A.; Al-Odayni, A.B.; Al-Hammadi, A.H.; Al-Ostoot, F.H.; Saeed, W.S.; Abduh, N.A.; Alrahlah, A. Lepidium sativum Seed Extract-Mediated Synthesis of Zinc Oxide Nanoparticles: Structural, Morphological, Optical, Hemolysis, and Antibacterial Studies. Bioinorg. Chem. Appl. 2023, 4166128. [Google Scholar] [CrossRef]
- Rahayu, E.; Wonoputri, V.; Samadhi, T.W. Plant extract-assisted biosynthesis of zinc oxide nanoparticles and their antibacterial application. IOP Conf. Ser. Mater. Sci. Eng. 2020, 823, 012036. [Google Scholar] [CrossRef]
- Chaudhary, A.; Kumar, N.; Kumar, R.; Salar, R.K. Antimicrobial activity of zinc oxide nanoparticles synthesized from Aloe vera peel extract. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Tilahun, E.; Adimasu, Y.; Dessie, Y. Biosynthesis and optimization of ZnO nanoparticles using Ocimum lamifolium leaf extract for electrochemical sensor and antibacterial activity. ACS Omega 2023, 8, 27344–27354. [Google Scholar] [CrossRef]
- Varghese, R.M.; Kumar, A.; Shanmugam, R. Antimicrobial Activity of Zinc Oxide Nanoparticles Synthesized Using Ocimum Tenuiflorum and Ocimum Gratissimum Herbal Formulation Against Oral Pathogens. Cureus 2024, 16, 2. [Google Scholar] [CrossRef]
- Thi, T.U.D.; Nguyen, T.T.; Thi, Y.D.; Thi, K.H.T.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar]
- Dangana, R.S.; George, R.C.; Agboola, F.K. The biosynthesis of zinc oxide nanoparticles using aqueous leaf extracts of Cnidoscolus aconitifolius and their biological activities. Green Chem. Lett. Rev. 2023, 16, 2169591. [Google Scholar] [CrossRef]
- Tamanna, S.; Pitchiah, S.; Suresh, V.; Ramasamy, P. Synthesis of zinc oxide nanoparticles from aqueous extract of Avicennia marina mangrove leaves and their antibacterial activities against oral pathogens. Cureus 2023, 15, e47627. [Google Scholar]
- Upadhyaya, H.; Shome, S.; Sarma, R.; Tewari, S.; Bhattacharya, M.K.; Panda, S.K. Green synthesis, characterization and antibacterial activity of ZnO nanoparticles. Am. J. Plant Sci. 2018, 9, 1279–1291. [Google Scholar] [CrossRef]
- Mohammadi, C.; Mahmud, S.; Abdullah, S.M.; Mirzaei, Y. Green synthesis of ZnO nanoparticles using the aqueous extract of Euphorbia petiolata and study of its stability and antibacterial properties. Mor. J. Chem. 2017, 5. [Google Scholar] [CrossRef]
- Awwad, A.M.; Amer, M.W.; Salem, N.M.; Abdeen, A.O. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Ailanthus altissima fruit extracts and antibacterial activity. Chem. Int. 2020, 6, 151–159. [Google Scholar]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef]
- Demissie, M.G.; Sabir, F.K.; Edossa, G.D.; Gonfa, B.A. Synthesis of zinc oxide nanoparticles using leaf extract of lippia adoensis (koseret) and evaluation of its antibacterial activity. J. Chem. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Mirza, A.U.; Kareem, A.; Nami, S.A.; Bhat, S.A.; Mohammad, A.; Nishat, N. Malus pumila and Juglen regia plant species mediated zinc oxide nanoparticles: Synthesis, spectral characterization, antioxidant and antibacterial studies. Microb. Pathog. 2019, 129, 233–241. [Google Scholar] [CrossRef]
- Kavitha, M.; Shenbagam, K.; Kanmani, R.; Mary, S.P. Biogenic Synthesis of Copper oxide and zinc oxide Nanoparticles using Catharanthus roseus L. flower extract and Evaluation of Its Antioxidant and Antibacterial Properties. Orient. J. Chem. 2022, 38, 1320. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.S.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Santhiya, S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostructure Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Happy, A.; Soumya, M.; Kumar, S.V.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Nallaswamy, V.D. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, K.V.; Anbumani, D.; Gandhi, A.D.; Annamalai, P.; Muthuvenkatachalam, B.S.; Kavitha, P.; Ranganathan, B. Green route for the synthesis of zinc oxide nanoparticles from Melia azedarach leaf extract and evaluation of their antioxidant and antibacterial activities. Biocatal. Agric. Biotechnol. 2020, 24, 101517. [Google Scholar] [CrossRef]
- Bozer, B.D.; Dede, A.; Güven, K. Green synthesized zinc oxide nanoparticles with Salvadora persica L. root extract and their antagonistic activity against oral and health-threatening Pathogens. Indian J. Microbiol. 2024, 22, 1–9. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Prasad, K.R.S.; Krishna, P.M.; Supraja, N. Saussurea lappa plant rhizome extract-based zinc oxide nanoparticles: Synthesis, characterization and its antibacterial, antifungal activities and cytotoxic studies against Chinese Hamster Ovary (CHO) cell lines. Heliyon 2021, 7, 6. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Sharma, A.; Nagraik, R.; Venkidasamy, B.; Khan, A.; Dulta, K.; Kumar, C.P.; Kumar, D.; Shin, D.S. In vitro antidiabetic, antioxidant, antimicrobial, and cytotoxic activity of Murraya koenigii leaf extract intercedes ZnO nanoparticles. Luminescence 2023, 38, 1139–1148. [Google Scholar] [CrossRef]
- Khara, G.; Padalia, H.; Moteriya, P.; Chanda, S. Peltophorum pterocarpum flower-mediated synthesis, characterization, antimicrobial and cytotoxic activities of ZnO nanoparticles. Arab. J. Sci. Eng. 2018, 43, 3393–3401. [Google Scholar] [CrossRef]
- Ansari, M.A.; Murali, M.; Prasad, D.; Alzohairy, M.A.; Almatroudi, A.; Alomary, M.N.; Udayashankar, A.C.; Singh, S.B.; Asiri, S.M.M.; Ashwini, B.S.; et al. Cinnamomum verum bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules 2020, 10, 336. [Google Scholar] [CrossRef]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- El-mesallamy, A.M.A.N.I.; Alahwany, A.K.; El-Zaidy, M.I.; Hussein, S.A.A. Eco-friendly Synthesis of Zinc Oxide Nanoparticles by Garcinia cambogia and Evaluation of Their Obesity and Antimicrobial Activities. Egypt. J. Chem. 2024, 67, 17–27. [Google Scholar] [CrossRef]
- Rao, S.M.; Kotteeswaran, S.; Visagamani, A.M. Green synthesis of zinc oxide nanoparticles from camellia sinensis: Organic dye degradation and antibacterial activity. Inorg. Chem. Commun. 2021, 134, 108956. [Google Scholar]
- Manojkumar, U.; Kaliannan, D.; Srinivasan, V.; Balasubramanian, B.; Kamyab, H.; Mussa, Z.H.; Palaniyappan, J.; Mesbah, M.; Chelliapan, S.; Palaninaicker, S. Green synthesis of zinc oxide nanoparticles using Brassica oleracea var. botrytis leaf extract: Photocatalytic, antimicrobial and larvicidal activity. Chemosphere 2023, 323, 138263. [Google Scholar] [CrossRef] [PubMed]
- Sivasankarapillai, V.S.; Krishnamoorthy, N.; Eldesoky, G.E.; Wabaidur, S.M.; Islam, M.A.; Dhanusuraman, R.; Ponnusamy, V.K. One-pot green synthesis of ZnO nanoparticles using Scoparia Dulcis plant extract for antimicrobial and antioxidant activities. Appl. Nanosci. 2023, 13, 6093–6103. [Google Scholar] [CrossRef]
- Takcı, D.K.; Ozdenefe, M.S.; Huner, T.; Takcı, H.A.M. Plant-mediated green route to the synthesis of zinc oxide nanoparticles: In vitro antibacterial potential. J. Aust. Ceram. Soc. 2024, 30, 1–9. [Google Scholar] [CrossRef]
- Mirza, S.; Hussaini, A.A.; Öztürk, G.; Turgut, M.; Öztürk, T.; Tugay, O.; Ulukuş, D.; Yıldırım, M. Photocatalytic and antibacterial activities of ZnO nanoparticles synthesized from Lupinus albus and Lupinus pilosus plant extracts via green synthesis approach. Inorg. Chem. Commun. 2023, 155, 111124. [Google Scholar] [CrossRef]
- Karmous, I.; Taheur, F.B.; Zuverza-Mena, N.; Jebahi, S.; Vaidya, S.; Tlahig, S.; Mhadhbi, M.; Gorai, M.; Raouafi, A.; Debara, M.; et al. Phytosynthesis of zinc oxide nanoparticles using Ceratonia siliqua L. and evidence of antimicrobial activity. Plants 2022, 11, 3079. [Google Scholar] [CrossRef]
- Agila, A.; Jeyaleela, G.D.; Vimala, J.D.R.; Bharathy, M.S.; Sheela, S.A.M. Inhibitory effect of Basella alba-mediated zinc oxide nanoparticles against the infection-causing bacteria. Biomed. Biotechnol. Res. J. 2022, 6, 353–359. [Google Scholar] [CrossRef]
- Dulta, K.; Koşarsoy Ağçeli, G.; Chauhan, P.; Jasrotia, R.; Chauhan, P.K. A novel approach of synthesis zinc oxide nanoparticles by bergenia ciliata rhizome extract: Antibacterial and anticancer potential. J. Inorg. Organomet. Polym. Mater. 2021, 31, 180–190. [Google Scholar] [CrossRef]
- Ghosh, S.; Kaushik, R.; Nagalakshmi, K.; Hoti, S.L.; Menezes, G.A.; Harish, B.N.; Vasan, H.N. Antimicrobial Activity of Highly Stable Silver Nanoparticles Embedded in Agar–Agar Matrix as a Thin Film. Carbohydr. Res. 2010, 345, 2220–2227. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Cui, J. Graphene Oxide Loaded with Copper Oxide Nanoparticles as an Antibacterial Agent against Pseudomonas Syringae Pv. Tomato. RSC Adv. 2017, 7, 38853–38860. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Soren, S.; Kumar, S.; Mishra, S.; Jena, P.K.; Verma, S.K.; Parhi, P. Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb. Pathog. 2018, 119, 145–151. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper nanoparticles with high antimicrobial activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Allayeith, H.K. Zinc-Based Nanoparticles Prepared by a Top-Down Method Exhibit Extraordinary Antibacterial Activity Against Both Pseudomonas aeruginosa and Staphylococcus aureus. Doctoral Dissertation, Kent State University, Kent, CO, USA, 2020. [Google Scholar]
- Raja, S.; Ramesh, V.; Thivaharan, V. Green Biosynthesis of Silver Nanoparticles Using Calliandra Haematocephala Leaf Extract, Their Antibacterial Activity and Hydrogen Peroxide Sensing Capability. Arab. J. Chem. 2017, 10, 253–261. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.l.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta A Mol Biomol Spectrosc 2012, 90, 78–84. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar]
- Brusini, R.; Varna, M.; Couvreur, P. Advanced nanomedicines for the treatment of inflammatory diseases. Adv. Drug Deliv. Rev. 2020, 157, 161. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, M.L. Nanotechnology in Cancer Drug Delivery and Selective Targeting. ISRN Nanotechnol. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Lamprecht, A. Nanomedicines in Gastroenterology and Hepatology. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Penesyan, A.; Nagy, S.S.; Kjelleberg, S.; Gillings, M.R.; Paulsen, I.T. Rapid microevolution of biofilm cells in response to antibiotics. NPJ Biofilms Microbiomes 2019, 5, 34. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, S.; Silva, A.F.; Madsen, J.S. Cooperative antibiotic resistance facilitates horizontal gene transfer. ISME J. 2023, 17, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020, 96, 031. [Google Scholar] [CrossRef]
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm formation, and related impacts on healthcare, food processing and packaging, industrial manufacturing, marine industries, and sanitation–A review. Appl. Microbiol. 2023, 3, 629–665. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Dutt, Y.; Dhiman, R.; Singh, T.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Raj, V.S.; Chang, C.M.; Priyadarshini, A. The association between biofilm formation and antimicrobial resistance with possible ingenious bio-remedial approaches. Antibiotics 2022, 11, 930. [Google Scholar] [CrossRef]





| Plant | Part Used | Concentration of Salt | Average Size (nm) | Shape | Reaction Time | Reaction Temperature and pH | Test Bacteria | Reference |
|---|---|---|---|---|---|---|---|---|
| Limonium pruinosum | Whole plant | 0.5 M zinc acetate dihydrate | ~41 nm | Hexagonal/cubic | 30 min | 70 °C pH 8 | Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 6538), Gram negative bacteria (Escherichia coli (ATCC 8739), and Enterobacter aeruginosa | [44] |
| Cassia fistula and Melia azadarach | Leaves | 0.01 M zinc acetate dihydrate | 68.1 nm and 3.62 nm | Spherical | 60 min | 70 °C | Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) | [127] |
| Cocos nucifera | Leaves | 0.05 M aqueous solution of Zn(NO3)2·6H2O | 16.6 nm | Spherical/hexagonal | 3 h | 12 | Staphylococcus aureus (cars-2), Bacillus megaterium (BTCC-18), and Bacillus cereus (carsgp-1) | [21] |
| Myristica fragrans (Jaiphal) | Fruit | zinc acetate dihydrate | 41.23 nm | Flower-shaped | 2 h | 60 °C | Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus | [94] |
| Ziziphus mauritiana Lam | Leaves | (Zn(NO3)2·6H2O) | 63–83 nm | Spherical | - | 60 °C | Staphylococcus aureus and Escherichia coli | [128] |
| Viscum album | Leaves | 0.01 M zinc acetate dihydrate | 13.5 nm | Quasi-spherical | 30 min | 70 °C | Pseudomonas aeruginosa (ATCC 10145), Escherichia coli (ATCC 10799), Staphylococcus aureus (ATCC 29213), and Bacillus subtilis (ATCC 11774) | [129] |
| Pistacia lentiscus | Leaves | 0.1 mol/L (Zn(CH3COO)2 and 2H2O) | 33.90 nm | Dried cotton-like appearance | 30 min | 78 °C | Staphylococcus aureus, Bacillus cereus, Escherichia coli, and Pseudomonas aeruginosa | [51] |
| Cymbopogon citratus | Leaves | (Zn(NO3)2 6H2O) | 20–24 nm | Hexagonal rod-like shape | 5 min | Room temperature | S. aureus (MTCC 9760) and E. coli (MTCC 443) | [130] |
| Clitoria ternatea | Flower | Zn (NO3)2·6H2O | 41 nm | Partially/roughly spherical | 2 h | 60 °C | Escherichia coli and Staphylococcus aureus | [131] |
| Artemisia aucheri | Leaves | 1g in 15 mL of Zn(NO3)2 | 76 nm | Spherical and granular | 20 min | Room temperature | Escherichia coli and Staphylococcus aureus | [132] |
| Lavandula pubescens | Shoots | 2.5 M ZnCl2 | 10.76–20.42 nm | Rod-shaped | 30 min | 60 °C | Pseudomonas aeruginosa (ATCC 27853) and Staphylococcus aureus (ATCC 29213) | [133] |
| Parthenium hysterophorus | Leaves | 1 mM zinc nitrate | 10 nm | Spherical | 8 h | 90 °C | Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, and Klebsiella pneumoniae | [134] |
| Laurus nobilis | Leaves | 0.1 mol/L zinc acetate | 29.983 nm | Spherical | 1 h | Room temperature | Staphylococcus aureus, S. epidermidis, Escherichia coli, and Klebsiella spp. | [135] |
| Lepidium sativum | Seed | zinc nitrate | 24.2 nm | Hexagonal | 75 min | - | Escherichia coli and Staphylococcus aureus | [136] |
| Averrhoa bilimbi | Fruit | Zn(NO3)2·4H2O | 35.4 to 59.5 nm | Round shape | 5 h | 70 °C | Escherichia coli | [137] |
| Aloe vera | leaves | 10 mM ZnSO4·7H2O | 50 to 220 nm | Hexagonal | 24 h | Room temperature | Staphylococcus epidermidis, Staphylococcus aureus, K. pneumoniae, and Escherichia coli | [138] |
| Ocimum lamifolium | Leaves | 0.06 M zinc acetate | 22.8 nm | Spherical | 2 h | pH 12 30 °C | E. coli, S. aureus, P. aeruginosa, and S. pyogen | [139] |
| Ocimum tenuiflorum and Ocimum sanctum | Leaves | 30 mM zinc nitrate | - | - | 10 min | Room temperature | Streptococcus mutans, Enterococcus faecalis, Staphylococcus aureus, and Lactobacillus | [140] |
| Orange peel | Peel | 2 g of zinc nitrate with 42.5 mL of Zn(NO3)2·6H2O | 10–20 nm | Spherical | 60 min | 60 °C | Escherichia coli and Staphylococcus aureus | [141] |
| Cinnamomum verum | Fruit | zinc acetate dehydrate (0.1 M) | 56–71 nm | Spherical | 60 min | 50 °C | E. coli O157:H7 (02–0628) and L. monocytogenes (ATCC 7644) | [52] |
| Myristica fragrans | Fruit | 6.0 g in 100 mL of (Zn(NO3)2·2H2O) | 41.23 nm | Spherical or elliptical | 2 h | 60 °C | K. pneumoniae, E. coli, P. aeruginosa, and S. aureus | [94] |
| Pistacia lentiscus L. | Leaf | 0.1 mol/L zinc acetate dihydrate (Zn(CH3COO)2 and 2H2O) | 33.90 nm | Dried cotton shape | 30 min | pH 12 78 °C | Staphylococcus aureus (ATCC 6538), Bacillus cereus (ATCC 10876), Escherichia coli (ATCC 8739), and Pseudomonas aeuroginosa | [51] |
| Cnidoscolus aconitifolius | Leaves | 0.5 M Zn(CH3COO)2 | 100 nm | Spherical | 9 h | 50 °C | Escherichia coli ATCC 35218 and ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumonia ATCC 700603 and Chromobacterium violaceum ATCC 12472, Staphylococcus aureus ATCC 43300 and ATCC 29213, Enterococcus faecalis ATCC 51299, and Bacillus cereus ATCC 29212 | [142] |
| Avicennia marina | Leaves | 10 mM ZnS | - | - | 24 h | Room temperature | Klebsiella sp., Staphylococcus aureus, and Streptococcus mutans | [143] |
| Lawsonia inermis | Leaves | Zn(NO3)2 | 100 nm | Hexagonal | 2 h | Room temperature | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis | [144] |
| Euphorbia petiolata | Leaves | 1 M zinc nitrate | - | Spongy shape | 2 h | 80 °C | Escherichia coli | [145] |
| Ailanthus altissima | Fruit | 10 g in 200 mL of [(ZnNO3)2·6H2O] | 5 to 18 nm | Spherical | Not specified | 27 °C | Escherichia coli and Staphylococcus aureus | [146] |
| Matricaria chamomilla L. | Flower | 1 M zinc oxide | 48.2 nm | Cubic structures | 4 h | Room temp | Xanthomonas oryzae pv. Oryzae | [147] |
| Lippia adoensis (Koseret) | Leaves | 0.45 M zinc acetate dihydrate | 19.78 nm | Spherical | 2 h | Room temp | Staphylococcus aureus and Enterococcus faecalis and Gram-negative (Escherichia coli and Klebsiella pneumonia) | [148] |
| Malus pumila | Fruit | 0.2 M zinc acetate dihydrate | 12 nm | Spherical/grain rice-like (ellipsoidal)/cylindrical, dumbbell and needle-like shape | 2 h | pH 6 70 °C | E. coli, K. pneumoniae and P. aeruginosa | [149] |
| Catharanthus roseus L. | Flower | 0.1 g in 10 mL of ZnCl2 | - | Spherical | 3 | Room temp | Staphylococcus aureus B23 and Pseudomonas aeruginosa 424 | [150] |
| Berberis aristata | Leaves | 0.1 M zinc acetate dihydrate | Needle like | Not specified | 70 °C | Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Bacillus subtilis, Bacillus cereus, and Serratia marcescens | [151] | |
| Bauhinia tomentosa | Leaves | 2 mM ZnSO4 | 22–94 nm | Hexagonal | 4 days | - | Escherichia coli and Pseudomonas aeruginosa | [152] |
| Trifolium pratense | Flower | 0.5 M ZnO | 60–70 nm | - | 24 h | 90 °C | S. aureus ATCC 4163, E. coli ATCC 25922, and P. aeruginosa ATCC 6749 | [153] |
| Cassia alata | Leaves | 0.01 M Zinc acetate | 60–80 nm | Spherical | 3 h | 80 °C | Escherichia coli | [154] |
| Melia azedarach | Leaves | 0.006 M zinc nitrate hexahydrate (Zn(NO3)·6H2O) | 33–96 nm | Hexagonal and spherical | 24 h | 50 °C | Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853), Sphingobacterium thalpophilum (NCTC 11429), Bacillus subtilis (ATCC 6051), and Klebsella pneumonia (ATCC 13883) | [155] |
| Salvadora persica L. | Root | 0.1 M zinc acetate dihydrate (Zn(CH3COO)2·2H2O) | 165–287 nm | Spherical | 4.5 h | pH 8 70 °C | Staphylococcus aureus NRRL B-767, Acinetobacter baumannii 2.3, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, Klebsiella pneumoniae NRRL B-4420, Proteus vulgaris NRRL B-123, and Streptococcus mutans (wild type) | [156] |
| Saussurea lappa | Rhizome, root | 1 M hexahydrate zinc nitrate | 123.5 nm | Hexagonal | 2 h | 70–80 °C | Streptococcus aureus, Bacillus subtilis, Sphingobacterium thalpophilum, Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, Sphingobacterium sp., Acinetobacter sp., and Ochrobactrum sp. | [157] |
| Pongamia pinnata | Leaves | 0.1 M zinc nitrate hexahydrate | 10 to 120 nm | Spherical | 24 h | Room temp | S. aureus and E. coli | [158] |
| Murraya koenigii | Leaves | 1 M zinc acetate | 15 nm | Spherical | 2 h | pH 12 35–40 °C | Staphylococcus aureus, Bacillus subtilis, and Salmonella typhii; Escherichia coli, and Klebsiella pneumoniae | [159] |
| Peltophorum pterocarpum | Flower | zinc nitrate | 69.45 nm | Spherical and irregular shaped | - | 80 °C | (Bacillus cereus (BC) ATCC 11778, Bacillus subtilis (BS) ATCC 6633, Staphylococcus aureus (SA) ATCC 29737, Corynebacterium rubrum (CR) ATCC 14898), (Escherichia coli (EC) NCIM2931, Pseudomonas aeruginosa (PA) ATCC 9027, Klebsiella pneumoniae (KP) NCIM2719, and Salmonella typhimurium (ST) ATCC 23564) | [160] |
| Cinnamomum verum | Bark | zinc nitrate hexahydrate | ~45 nm | Spherical | - | 45–60 °C | Staphylococcus aureus (MTCC 7443) and Escherichia coli (MTCC 7410) | [161] |
| Tecoma castanifolia | Leaves | 1 mM zinc sulphate | 70–75 nm | Spherical | 4 days | Room temp | Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa | [162] |
| Garcinia cambogia | Leaves | 0.01 M Zn (NO3)2·6H2O | 131.5 nm | Rod-like/hexagonal | 12 h | 60 °C | Escherichia coli (E. coli) ATCC 10536 and Staphylococcus aureus (S. aureus) ATCC 6538 | [163] |
| Camellia sinensis | Leaves | 1.5 g of zinc acetate dihydrate in 20 mL | 10–20 nm | Spherical rods/needle and particle-like | 1 h | Room temp | E. coli and S. aureus | [164] |
| Brassica oleracea var. botrytis | Leaves | 5 g of Zn(NO3)2·6H2O in 50 ml | 52 nm | Flower like | 24 h | 70 °C | K. pneumonia and E. coli | [165] |
| Scoparia Dulcis | Leaves | 2 g of Zn(NO3)2·6H2O in 60 ml | ~20 nm | Pebble-like | - | 60 °C | Staphylococcus aureus-902 and E. coli-443 | [166] |
| Piper guineense | seeds | 0.25 g of Zn(CH3COO)2·2H2O was dissolved in 25 mL | 7.39 nm | Hexagonal/spherical | 2 h | 60 °C | E. coli (ATCC 25922) | [167] |
| Lupinus albus and Lupinus pilosus | Leaves | 40 mM zinc nitrate | 19.70 nm and 28.13 nm | Rod-like | 30 min | 90 °C | E. coli, P. aeruginosa and S. aureus | [168] |
| Ceratonia siliqua L. | Pods | 0.1 M zinc acetate dehydrate | - | Spherical/hexagonal | 2 h | 80 °C | Staphylococcus aureus ATCC 25923, Micrococcus luteus NCIMB8166, Salmonella enterica, Typhimurium ATCC 1408, and Escherichia coli ATCC 35218 | [169] |
| Basella alba | Leaves | 0.1 M zinc acetate | 28.64 nm | Spherical | 24 h | 60 °C | Pseudomonas aeruginosa, Escherichia coli, Enterobacter aerogenes, Staphylococcus aureus, and Proteus vulgaris | [170] |
| Bergenia ciliata | Rhizome | 1 M zinc acetate dihydrate | 30 nm | Flower shape | 2 h | pH 12 60 °C | Yersenia enterocolitica, Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli, Staphylococcus aureus, and Bacillus subtilis | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okaiyeto, K.; Gigliobianco, M.R.; Di Martino, P. Biogenic Zinc Oxide Nanoparticles as a Promising Antibacterial Agent: Synthesis and Characterization. Int. J. Mol. Sci. 2024, 25, 9500. https://doi.org/10.3390/ijms25179500
Okaiyeto K, Gigliobianco MR, Di Martino P. Biogenic Zinc Oxide Nanoparticles as a Promising Antibacterial Agent: Synthesis and Characterization. International Journal of Molecular Sciences. 2024; 25(17):9500. https://doi.org/10.3390/ijms25179500
Chicago/Turabian StyleOkaiyeto, Kunle, Maria Rosa Gigliobianco, and Piera Di Martino. 2024. "Biogenic Zinc Oxide Nanoparticles as a Promising Antibacterial Agent: Synthesis and Characterization" International Journal of Molecular Sciences 25, no. 17: 9500. https://doi.org/10.3390/ijms25179500
APA StyleOkaiyeto, K., Gigliobianco, M. R., & Di Martino, P. (2024). Biogenic Zinc Oxide Nanoparticles as a Promising Antibacterial Agent: Synthesis and Characterization. International Journal of Molecular Sciences, 25(17), 9500. https://doi.org/10.3390/ijms25179500







