Lemairamin (Wgx-50) Attenuates DSS-Induced Intestinal Inflammation in Zebrafish
Abstract
1. Introduction
2. Results
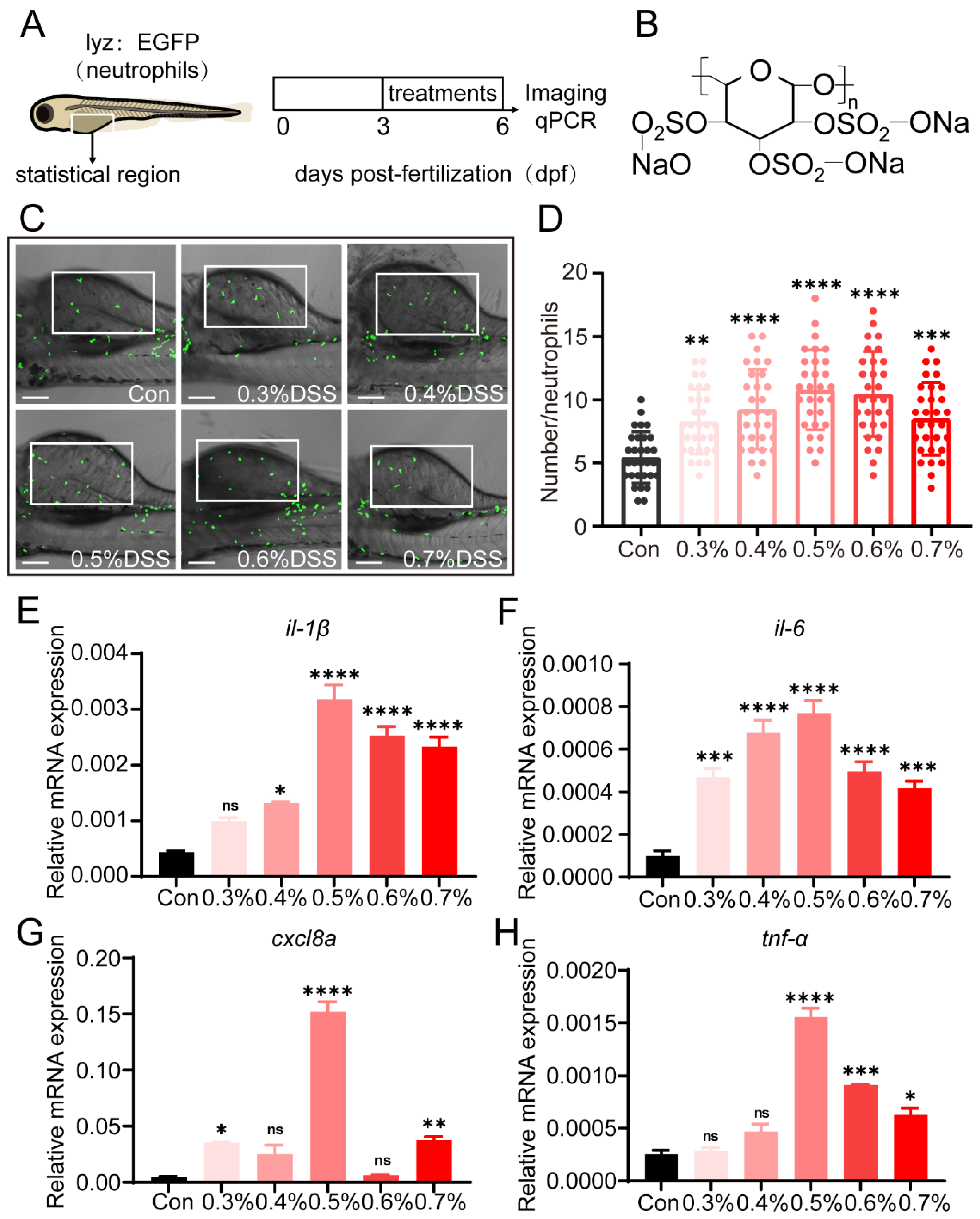
2.1. DSS Promotes Recruitment of Neutrophils and Expression of Pro-Inflammatory Cytokines
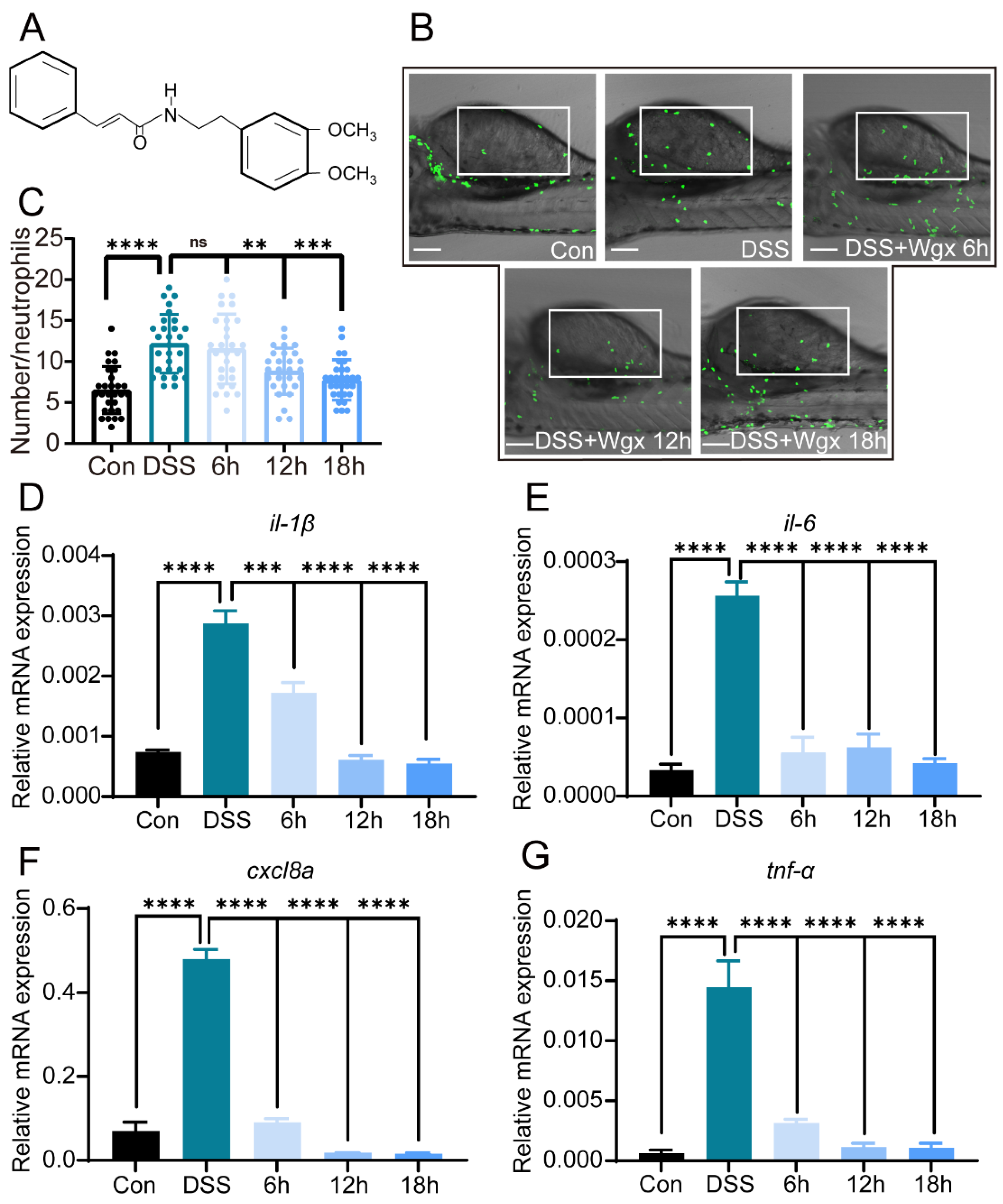
2.2. Wgx-50 Can Effectively Decrease the Inflammatory Levels Induced by DSS in Colitis
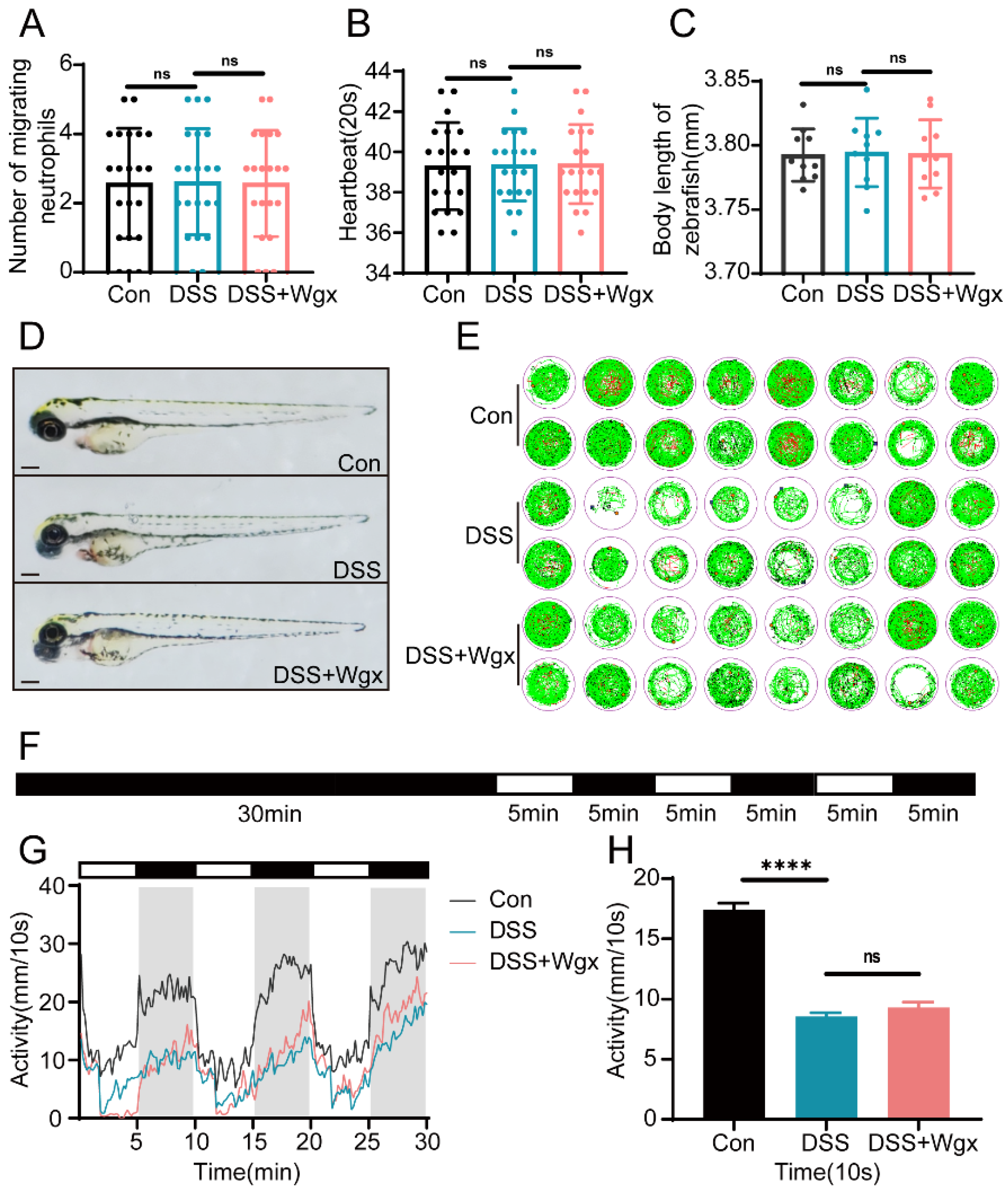
2.3. DSS and Wgx-50 Had No Impact on the Growth and Development of Zebrafish
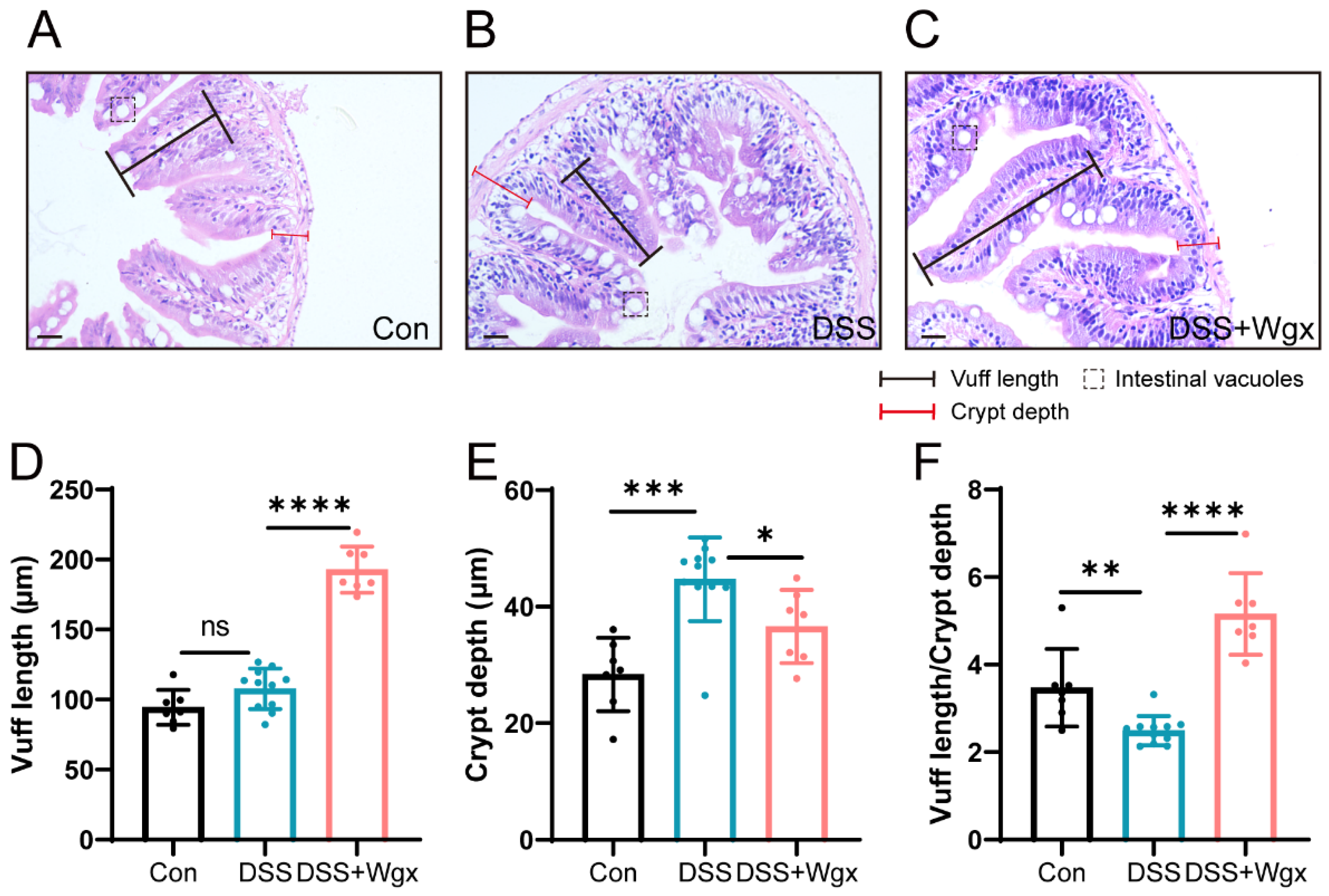
2.4. Wgx-50 Has a Protective Effect on the Morphology of Zebrafish Intestines after DSS Treatment
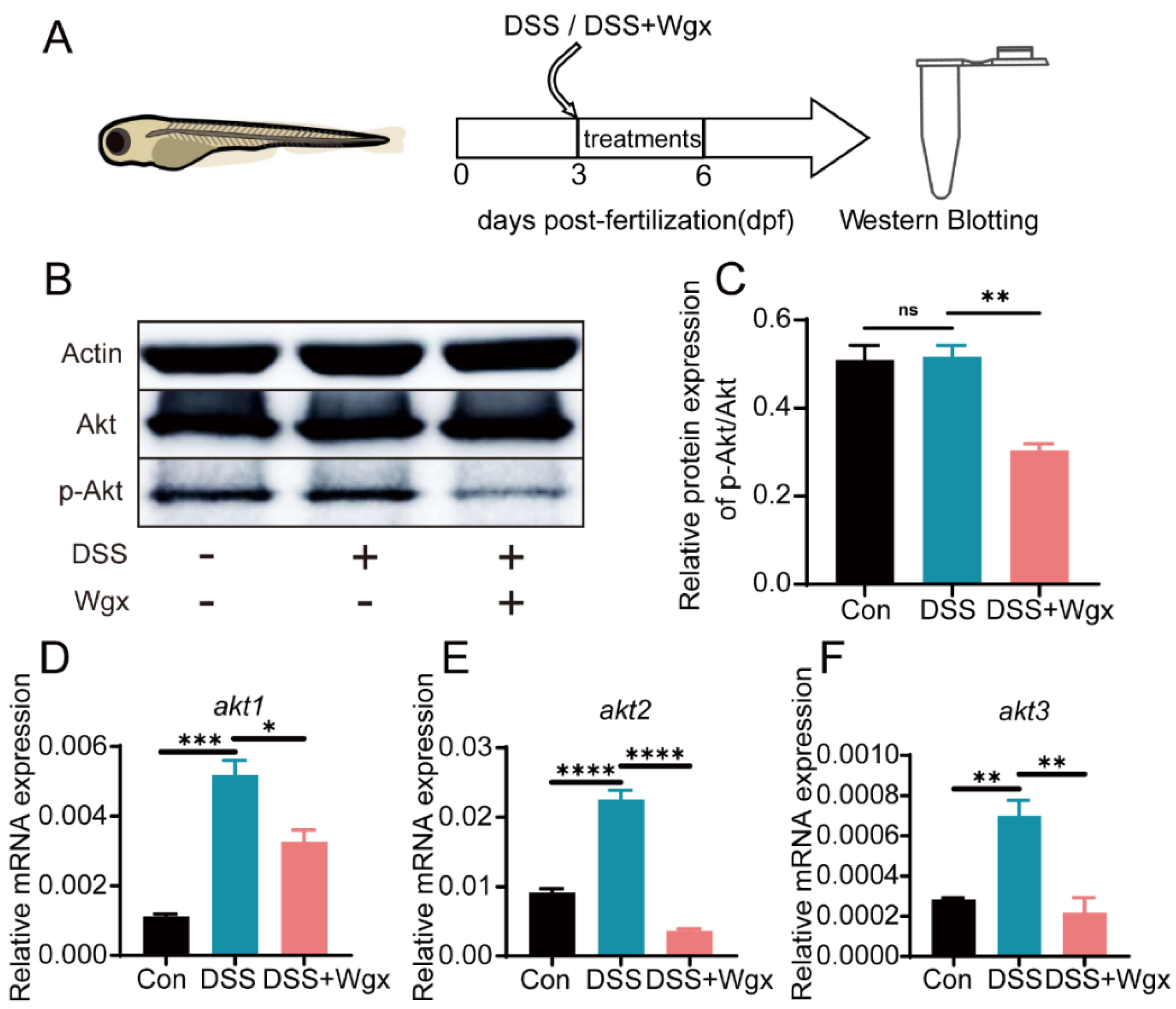
2.5. The Akt Pathway Mediates the Regulation of Inflammation by Wgx-50
3. Discussion
4. Materials and Methods
4.1. Zebrafish Strains and Maintenance
4.2. Drug Treatments
4.3. Behavioral Monitoring
4.4. Histopathological Sections
4.5. Vivo Imaging
4.6. Quantitative Polymerase Chain Reaction
4.7. Western Blotting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower–Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef]
- Khan, K.J.; Dubinsky, M.C.; Ford, A.C.; Ullman, T.A.; Talley, N.J.; Moayyedi, P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: A systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 630–642. [Google Scholar] [CrossRef]
- Khan, K.J.; Ullman, T.A.; Ford, A.C.; Abreu, M.T.; Abadir, A.; Marshall, J.K.; Talley, N.J.; Moayyedi, P. Antibiotic therapy in inflammatory bowel disease: A systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Trede, N.S.; Langenau, D.M.; Traver, D.; Look, A.T.; Zon, L.I. The use of zebrafish to understand immunity. Immunity 2004, 20, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Stream, A.; Madigan, C.A. Zebrafish: An underutilized tool for discovery in host–microbe interactions. Trends Immunol. 2022, 43, 426–437. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S.; Liu, K.; Lindenbergh–Kortleve, D.; Samsom, J.N.; Furuta, G.T.; Renshaw, S.A.; Willemsen, R.; Nieuwenhuis, E.E. Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology 2009, 137, 1757–1767.e1. [Google Scholar] [CrossRef]
- Oehlers, S.H.; Flores, M.V.; Okuda, K.S.; Hall, C.J.; Crosier, K.E.; Crosier, P.S. A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Dev. Dyn. 2011, 240, 288–298. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Oehlers, S.H.; Flores, M.V.; Hall, C.J.; Crosier, K.E.; Crosier, P.S. Retinoic acid suppresses intestinal mucus production and exacerbates experimental enterocolitis. Dis. Model. Mech. 2012, 5, 457–467. [Google Scholar] [CrossRef]
- Oehlers, S.H.; Flores, M.V.; Hall, C.J.; Okuda, K.S.; Sison, J.O.; Crosier, K.E.; Crosier, P.S. Chemically induced intestinal damage models in zebrafish larvae. Zebrafish 2013, 10, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape exosome like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Cristinziano, L.; Modestino, L.; Antonelli, A.; Marone, G.; Simon, H.-U.; Varricchi, G.; Galdiero, M.R. Neutrophil extracellular traps in cancer. Semin. Cancer Biol. 2022, 79, 91–104. [Google Scholar] [CrossRef]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Muthas, D.; Reznichenko, A.; Balendran, C.A.; Böttcher, G.; Clausen, I.G.; Mårdh, C.K.; Ottosson, T.; Uddin, M.; MacDonald, T.T.; Danese, S.; et al. Neutrophils in ulcerative colitis: A review of selected biomarkers and their potential therapeutic implications. Scand. J. Gastroenterol. 2017, 52, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Ranjan, P.; Dutta, R.K.; Verma, S.K. Management of inflammation in cardiovascular diseases. Pharmacol. Res. 2021, 173, 105912. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Shi, S.; Guo, Y.; Xu, W.; Wang, L.; Chen, Y.; Wang, Z.; Qiao, Z. GSK-3/CREB pathway involved in the gx-50’s effect on Alzheimer’s disease. Neuropharmacology 2014, 81, 256–266. [Google Scholar] [CrossRef]
- Tang, M.; Wang, Z.; Zhou, Y.; Xu, W.; Li, S.; Wang, L.; Wei, D.; Qiao, Z. A novel drug candidate for Alzheimer’s disease treatment: Gx-50 derived from Zanthoxylum bungeanum. J. Alzheimers Dis. 2013, 34, 203–213. [Google Scholar] [CrossRef]
- Dwivedi, A.P.; Kumar, S.; Varshney, V.; Singh, A.B.; Srivastava, A.K.; Sahu, D.P. Synthesis and antihyperglycemic activity of novel N-acyl-2-arylethylamines and N-acyl-3-coumarylamines. Bioorganic Med. Chem. Lett. 2008, 18, 2301–2305. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Ru-taceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef]
- Shi, S.; Liang, D.; Chen, Y.; Xie, Y.; Wang, Y.; Wang, L.; Wang, Z.; Qiao, Z. Gx-50 reduces β-amyloid-induced TNF-α, IL-1β, NO, and PGE2 expression and inhibits NF-κB signaling in a mouse model of Alzheimer’s disease. Eur. J. Immunol. 2016, 46, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, J.T.; Wang, Y.L.; Seidelin, J.B.; Nielsen, O.H. IBD metabonomics predicts phenotype, disease course, and treatment response. EBioMedicine 2021, 71, 103551. [Google Scholar] [CrossRef] [PubMed]
- Massimino, L.; Lamparelli, L.A.; Houshyar, Y.; D’alessio, S.; Peyrin-Biroulet, L.; Vetrano, S.; Danese, S.; Ungaro, F. The Inflammatory Bowel Disease Transcriptome and Metatranscriptome Meta-Analysis (IBD TaMMA) framework. Nat. Comput. Sci. 2021, 1, 511–515. [Google Scholar] [CrossRef]
- Shi, S.; Liang, D.; Bao, M.; Xie, Y.; Xu, W.; Wang, L.; Wang, Z.; Qiao, Z. Gx-50 Inhibits Neuroinflammation via α7 nAChR Activation of the JAK2/STAT3 and PI3K/AKT Pathways. J. Alzheimers Dis. 2016, 50, 859–871. [Google Scholar] [CrossRef]
- Wu, D.; Chen, S.; Ye, X.; Ahmadi, S.; Hu, W.; Yu, C.; Zhu, K.; Cheng, H.; Linhardt, R.J.; He, Q. Protective effects of six different pectic polysaccharides on DSS-induced IBD in mice. Food Hydrocoll. 2022, 127, 107209. [Google Scholar] [CrossRef]
- Langheinrich, U. Zebrafish: A new model on the pharmaceutical catwalk. BioEssays 2003, 25, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Oehlers, S.H.; Flores, M.V.; Chen, T.; Hall, C.J.; Crosier, K.E.; Crosier, P.S. Topographical distribution of antimicrobial genes in the zebrafish intestine. Dev. Comp. Immunol. 2011, 35, 385–391. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767.e1. [Google Scholar] [CrossRef]
- Ronchetti, L.; Boubaker, N.S.; Barba, M.; Vici, P.; Gurtner, A.; Piaggio, G. Neutrophil extracellular traps in cancer: Not only catching microbes. J. Exp. Clin. Cancer Res. 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Yilmaz, B. Microbial drivers of DSS variability. Nat. Microbiol. 2022, 7, 478–479. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, H.; Hay, N.; Xu, J.; Ye, R.D. Akt isoforms differentially regulate neutrophil functions. Blood 2010, 115, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.-L.; Sun, A.-A.; Li, Y.-J.; Chen, M.; Ge, S.-C.; Hu, B. Exogenous melatonin inhibits neutrophil migration through suppression of ERK activation. J. Endocrinol. 2015, 227, 49–60. [Google Scholar] [CrossRef]
- Yang, L.-Q.; Chen, M.; Ren, D.-L.; Hu, B. Dual Oxidase Mutant Retards Mauthner-Cell Axon Regeneration at an Early Stage via Modulating Mitochondrial Dynamics in Zebrafish. Neurosci. Bull. 2020, 36, 1500–1512. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Sun, S.; Tang, L.-S.; He, S.-M.; Chen, A.-Q.; Yao, L.-N.; Ren, D.-L. Cordycepin inhibits inflammatory responses through suppression of ERK activation in zebrafish. Dev. Comp. Immunol. 2021, 124, 104178. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
| Gene | Gene ID | Note | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|---|
| tnf-α | NM_212859.2 | qRT-PCR | GCGCTTTTCTGAATCCTACG | TGCCCAGTCTGTCTCCTTCT |
| il-1β | NM_212844.2 | qRT-PCR | GTACTCAAGGAGATCAGCGG | CTCGGTGTCTTTCCTGTCCA |
| il-6 | NM_001261449.1 | qRT-PCR | GCTATTCCTGTCTGCTACACTGG | TGAGGAGAGGAGTGCTGATCC |
| cxcl8a | XM_009306855.3 | qRT-PCR | CCACACACACTCCACACACA | CCACTGAATTGTCCTTTCATCA |
| β-actin | NM_131031.2 | qRT-PCR | ACGAACGACCAACCTAAACTCT | TTAGACAACTACCTCCCTTTGC |
| akt1 | NC_007128.7 | qRT-PCR | GTTTTCTGCGGGATTTCAGCG | GTCTTCACACGGGTCACCAGG |
| akt2 | NC_007129.7 | qRT-PCR | AAAGCTGCTGGGTAAAGGCA | TGCAACTTCGTCCTTAGCGA |
| akt3 | NC_007124.7 | qRT-PCR | AATAACCCCTCCTGAAAAATTTGAT | CGAGTAGGAGAACTGGGGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, H.; Zhang, H.; Yuan, H.; Ren, D. Lemairamin (Wgx-50) Attenuates DSS-Induced Intestinal Inflammation in Zebrafish. Int. J. Mol. Sci. 2024, 25, 9510. https://doi.org/10.3390/ijms25179510
Zhang L, Liu H, Zhang H, Yuan H, Ren D. Lemairamin (Wgx-50) Attenuates DSS-Induced Intestinal Inflammation in Zebrafish. International Journal of Molecular Sciences. 2024; 25(17):9510. https://doi.org/10.3390/ijms25179510
Chicago/Turabian StyleZhang, Ling, Huiru Liu, Haoyi Zhang, Hao Yuan, and Dalong Ren. 2024. "Lemairamin (Wgx-50) Attenuates DSS-Induced Intestinal Inflammation in Zebrafish" International Journal of Molecular Sciences 25, no. 17: 9510. https://doi.org/10.3390/ijms25179510
APA StyleZhang, L., Liu, H., Zhang, H., Yuan, H., & Ren, D. (2024). Lemairamin (Wgx-50) Attenuates DSS-Induced Intestinal Inflammation in Zebrafish. International Journal of Molecular Sciences, 25(17), 9510. https://doi.org/10.3390/ijms25179510





