The Many Faces of Cyclodextrins within Self-Assembling Polymer Nanovehicles: From Inclusion Complexes to Valuable Structural and Functional Elements
Abstract
:1. Introduction
2. General Considerations on Amphiphilic Cyclodextrins
3. Drug Delivery
| Carrier | Responsiveness | Drug | Size (PDI)/ ζ Potential | DL% | Cell Line/ Animal Model | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| CD copolymers | |||||||
| βCD-pCL | pH-sensitive ester bonds | Curcumin | 150 nm (0.07) −15.8 mV | 20 | HeLa in vitro and in vivo (heterotopic xenograft) | Significant in vitro cell growth and in vivo tumor inhibition. Improved cancer cell uptake and tumor accumulation via FA cell targeting | [53] |
| βCD-PEG-Chol | - | Curcumin | Empty: 147 nm (<0.25) Loaded: 121 nm (<0.25) | 62 | HepG2 in vitro | Encapsulated curcumin attenuated oxidative stress in vitro. Improved in vitro anticancer activity observed. In LPS-induced inflammation in vitro model, encapsulated curcumin reduced production of pro-inflammatory cytokines (IL-6 and TNα). | [54] |
| (Dex-SS)n-βCD-(PCL)14 (FA-Dex-SS)-βCD-(PCL)14 | GSH-sensitive disulfide (SS) bridge | Doxorubicin and SPIO (diagnostic) | Empty: 66 nm (0.12) Loaded: 127 nm | 10 (Dox) 11 (SPIO) | HepG2 in vitro | (FA-Dex-SS)-βCD-(PCL)14 demonstrated higher antiproliferative activity than (Dex-SS)n-βCD-(PCL)14 or micelles without the SS linker. Therapeutic efficiency of micellar Dox lower than free Dox | [56] |
| βCD-PAMAM 3G | - | NO | - | - | Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) in vitro biofilms. S. aureus in vivo model of chronic rhinosinusitis | Dendron incorporation significantly prolonged NO half-life (from 3 s in small molecule prodrugs to 30 min in dendron). Efficient inhibition and degradation of bacterial biofilms in vitro and in vivo after intranasal spray. Reduced inflammation of nasal cavity. No local or systemic adverse effects observed. | [58] |
| DSPE-mPEG2k-FA/NH2Pr-CD-C12 | pH-sensitive | Ginsenoside Rg3 and quercetin (1:1) * | Co-loaded: 110 nm (0.3) 6 mV | 12 (Rg3) 6 (Querc) | CT26 in vitro and orthotopic CT26-Luc in vivo | ~90% of drugs released after 48 h at acidic pH. Co-loaded formulations stable up to a week. Increased antiproliferative and antimetastatic activity of drug combination. Induced ICD, TME remodeling in vivo. | [62] |
| NH2Pr-CD-C12 | Melarsoprol | Loaded: 110 nm (0.32) 9 mV | 11 | Hepa1–6 (murine hepatocellular) in vitro and orthotopic in vivo | TME remodeling in vivo. Enhanced immunotherapy activity of anti-PD-L1. ~90% of drug released after 24 h at acidic pH (50% at pH 7.4). Increased antiproliferative and apoptotic activity of encapsulated drug. Improved tumor accumulation and antitumor activity when compared with FA-free formulation. | [63] | |
| R7-CD-Man14 | ATP-sensitive | Norfloxacin | Empty: 264 nm Loaded: 302 nm | 11 | E. coli in vitro and in vivo model of abdominal sepsis | Complete release of drug within 11 h upon ATP exposure. Better biocompatibility than PEG-based controls. Drug-loaded nanorods exhibit superior antibacterial activity in tested models than empty rods or free drug. | [64] |
| Host-guest amphiphiles | |||||||
| Sulfonamide-βCD and Mtx-SS-Ad | GSH-sensitive disulfide bridge | Methotrexate | 78 nm (0.245) 11.6 mV | 14.2 | 78 nm (0.245) 11.6 mV | Sulfonamide-based targeting improved cancer cell sensitivity towards Mtx. Cytotoxicity toward normal cells similar to CaCo-2 and A549 cancer cells | [65] |
| [PHEMA-g-(PCL-BM:βCD-star-PMAA-b-PNIPAM)] (w/w 1:3) | pH (PMAA)- and thermo (NIPAM)-sensitive | Doxorubicin | 9.7 | MCF-7 in vitro | Increased efficacy of encapsulated Dox (8.3 vs. 1.75 μg/mL for encapsulated and free Dox, respectively) | [66] | |
| Pc-CD:Fc-PEG | Ad-QRH * | Pc | 141 nm (0.134) | - | EGFR-overexpressed HT29 (human colorectal adenocarcinoma) in vitro and in vivo (heterotopic xenograft) | Significantly higher photodynamic activity in EGFR-sensitive cells pretreated with Ad-QRH *. Anticancer activity in cells without pretreatment indicates micelle degradation followed by Pc activation. Intravenous administration followed by intratumoral Ad-QRH * application increased tumor fluorescence intensity 5-fold, and NIR irradiation completely eradicated tumors. | [68] |
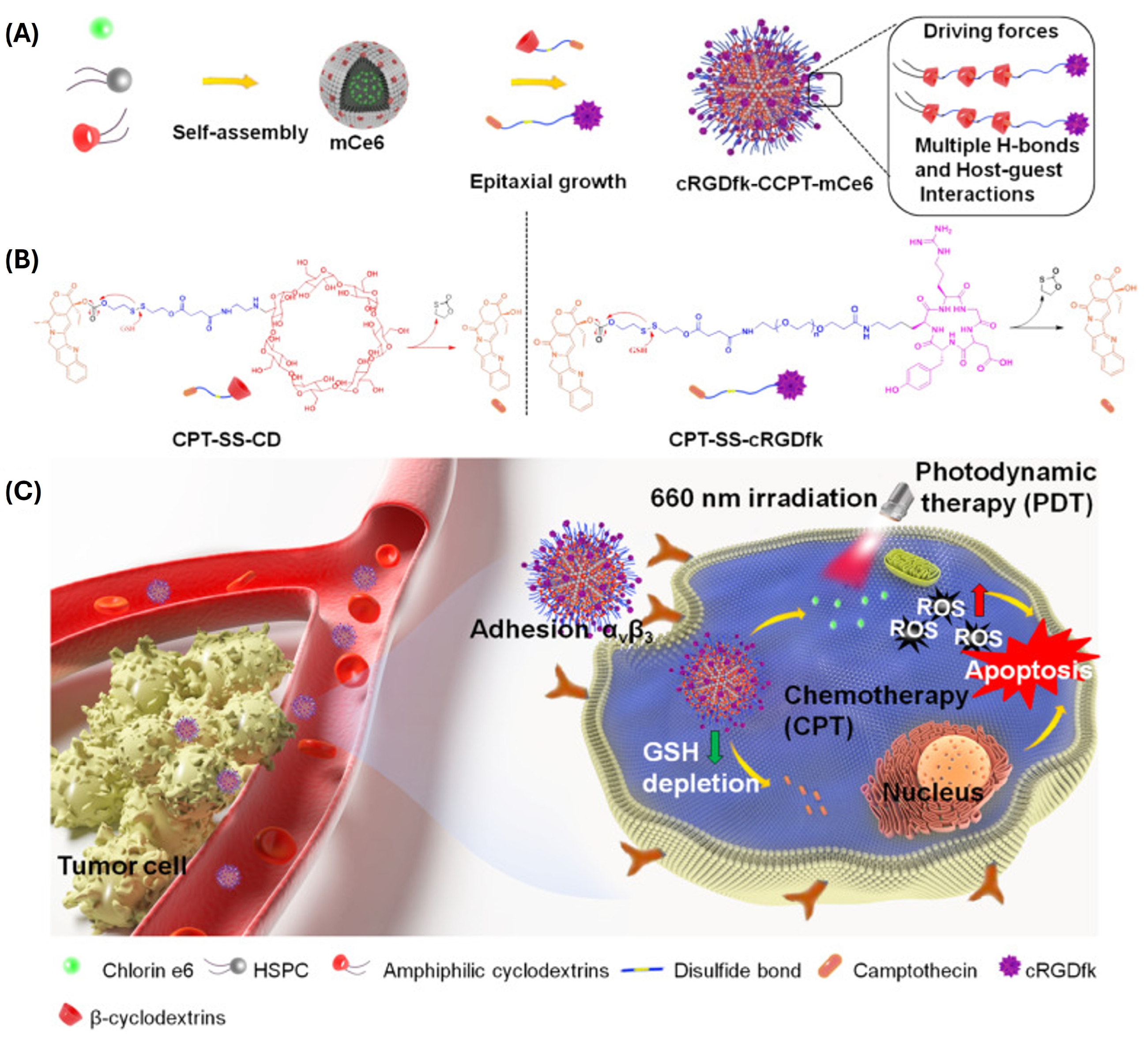
| HSPC/βCD-NHC12H25:Cpt-SS-CD:Cpt-SS-cRGDfk | GSH-sensitive disulfide bridge | Ce6 (PDT) Cpt-SS-CD | Empty: 24 nm Loaded: Ce6 25 nm Ce6 + Cpt 112 nm (Cpt-SS-CD: Cpt-SS-cRGDfk 90:1) 3.9 mV | Ce6 3.2 Cpt 4.4 | U87 in vitro and in vivo (heterotopic xenograft) | GSH-sensitive Cpt release combined with photodynamic therapy resulted in synergistic anticancer activity. Improved biodistribution of cRGDfk-labelled dandelions when compared to free drugs. | [69] |
| AM7CD-HAAd | Enzyme-sensitive | Chlorambucil | Empty: 82 nm (0.21); −40.2 mV Loaded: 188 nm (0.27); −19.4 mV | 9.4 | A549 in vitro | Increased efficacy of encapsulated chlorambucil. Upon HAse exposure, 90% of drug released within 12 h. Strong ATP binding of AM7CD. | [70] |
| CD-TPE:Dox | pH-sensitive | Doxorubicin | 165 nm | 67 | A549 in vitro and 4T1 in vivo syngeneic breast cancer model | Under mild acidic conditions (pH 5.4), 70% of Dox was released due to Dox protonation and solubility shift. After 24 h exposure in vitro antiproliferative activity lower than free Dox. CD-TPE:Dox was more efficient than free Dox in reducing tumor size (6.27 vs. 4.15 mm3). | [71] |
| Polyrotaxanes | |||||||
| Cur/βCD | - | Camptothecin | Empty: 27 nm Loaded: 32 nm | 1.2 | Human hepatoblastoma HepG2 in vitro and murine breast 4T1 in vitro and in vivo | Cur-CD PR assembled upon Cur helix renaturation in 28% yield in a CD concentration-dependent manner. PR micelles significantly reduced breast tumors in vivo. | [75] |
| HA/αCD-PEG2k | pH-sensitive | Paclitaxel | Loaded: 395 nm (0.16) −15.2 mV | - | HUVEC human normal endothelial cells (2D in vitro) Human A549 lung cancer cells (2D and 3D in vitro) | Micelles stable over 5 days under physiological conditions (pH 7.4) with pH-dependent drug release (90% at pH and 70% at 7.4 within 52 h). Similar in vitro activity to free drug observed in cancer cells but reduced toxicity in normal cells. Micelles demonstrated better cancer cell spheroid penetrability than free probe. | [76] |
| PLys(BM)/αCD-PEG5k | pH- and redox-sensitive | Chlorin e6 | 120 nm (0.16) −3 mV | - | Human hepatoma LM3 cells (in vitro and in vivo heterotopic xenograft) | Reductive release and activation of Ce6 and led to production of ROS upon laser stimuli. De-capping of protonated BM under reduced pH (6.5) improved cell uptake. Improved in vitro and in vivo antitumor photodynamic effect when compared to free photosensitizer. | [77] |
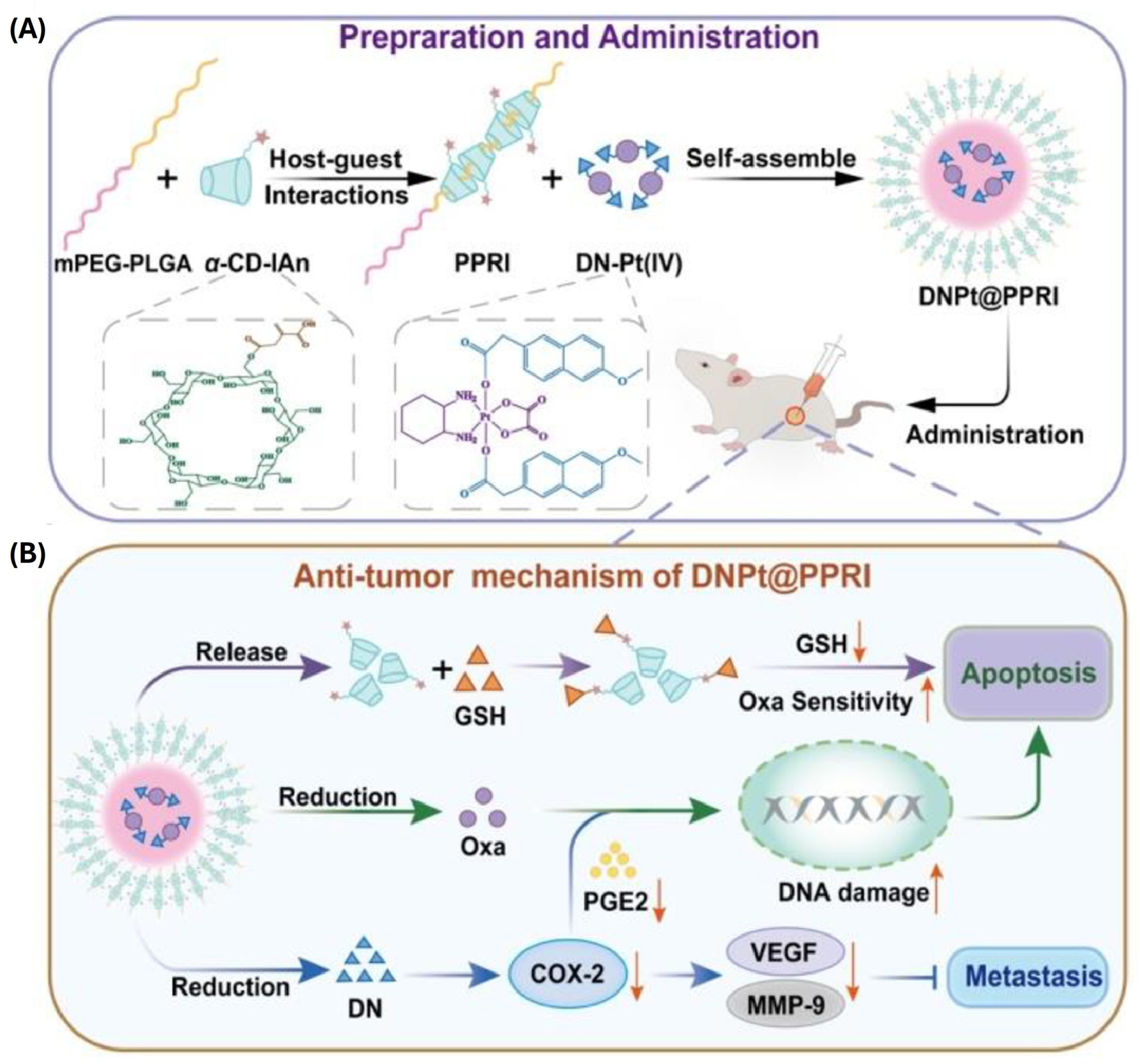
| mPEG2k-PLGA5k/αCD-It PPR | GSH-sensitive | Oxaliplatin-desmethyl naproxen prodrug | 190 nm (0.2) −19.7 mV | - | Murine CT-26 colorectal cancer cells (in vitro and in vivo) HT-29 and HCT-116 human colorectal cancer cells (in vitro) | Improved and cell-dependent in vitro anti-cancer activity when compared with free Oxa or formulation without It. COX2 suppression and GSH sequestration enhanced Oxa efficacy. Unlike Oxa, formulation was well tolerated in animal model. It was accompanied by strong anti-tumor activity. | [52] |
4. Peptide and Protein Delivery
| Carrier | Responsiveness | Protein | Size (PDI)/ ζ Potential | DL% | Cell Line/Animal Model | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| SPSVSS PPSVSS | Enzyme- and GSH-sensitive | Phalloidin (heptapeptide; staining) α-amanitin (octapeptide; anticancer) | ~125 nm/ −14 to −7 mV 145 nm/ −15 mV | - | HeLa (cancer) and HUVEC (endothelial) in vitro | Background cargo leakage observed for the model dye cargo but was not investigated for the biological cargo. Bioactive peptide cargo colocalized into the cytoplasm and inhibited cell proliferation (cancer and endothelial). | [81] |
| βCD-C8 βCD-C12 | - | SFL; SFL-c | C8-SFL: 178 nm (0.08) C12-SFL: 129 nm (0.1) C8-SFLc: 358 nm (0.22); C12-SFLc:: 287 nm (0.17) (w/w βCD/peptide 1:10) ~−35 mV all | - | IC-22 macrophages and BMDC in vitro | Encapsulated peptides induced DC maturation and were presented on surface MHC I. Lower DC maturation by SFL-c might indicate the need for intracellular decapping for MHC I presentation. In vivo stimulation with βCD-C87:SFL-c induced a faster and stronger response of CD8+ T cells than βCD-C87:SFL or control poly(I:C)-adjuvanted SFL. | [82] |
| βCD-(DIBO-Lys)7 | - | BSA DNase I Nrf2 | ~150 nm - 80 nm/−15 mV | 14 20 - | HeLa in vitro (DNase I) Hepatocytes in vitro (Nrf2) APAP-induced in vivo hepatic injury murine model | βCD-(DIBO-Lys)7 colocalized proteins in nucleus. Therapeutic DNase I caused HeLa cell apoptosis. Therapeutic Nrf2 triggered antioxidative response in hepatocytes exposed to H2O2 and in in vivo model of hepatic toxicity after intravenous administration. | [83] |
| βCD-(DIBO- Lys)7 | Sod Cat | 156 nm (βCD-(DIBO-Lys)7/Sod/Cat w/w/w 8:1:1) 3.5 mV | - | RAW264.7 in vitro DSS-induced in vivo murine colitis model | Synergistic anti-inflammatory effect of encapsulated Sod and Cat on secretion of pro-inflammatory factors. After oral administration Sod/Cat/βCD-(DIBO-Lys)7 accumulated in inflamed colon and attenuated colitis symptoms. | [84] | |
5. Nucleic Acid Delivery
| Carrier | Functionalization | NA | Size (PDI)/ ζ Potential | Cell Line/Animal Model | Remarks | Ref. |
|---|---|---|---|---|---|---|
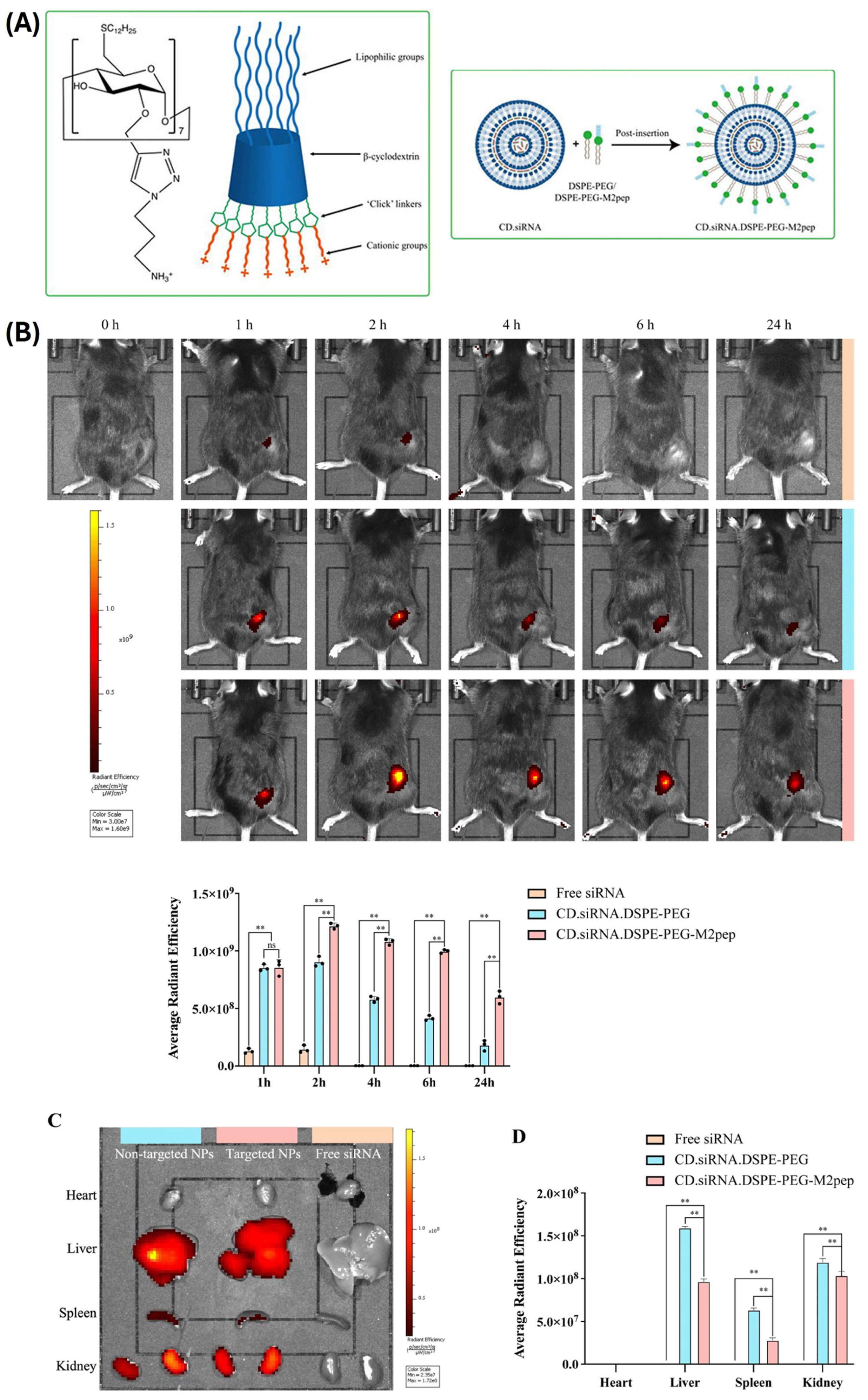
| NH2Pr-CD-C12/DSPE-mPEG2kPEG/DSPE-mPEG2kM2pep | M2pep | CSF-1R siRNA | 252 nm (0.25) 10.8 mV (w/w NH2Pr-CD-C12/siRNA 10:1) | Human THP1 and RAW 264.7 murine monocytes Human PC-3 and murine TRAMP-C1 prostate cancer cells in vitro and in vivo (heterotopic xenograft) | Complexed siRNA stable up to 24 h in 50% serum. M2pep significantly increased M2 uptake, reduction of CSF-1R mRNA (~50%), and reprogramming to M1 (~50%). Production of M1 factors was accompanied by cancer cell apoptosis. Increased tumor accumulation was observed 12 h after administration (3.5-fold) when compared with non-targeted formulation. Reduction of tumor was accompanied by immune remodeling of TME, and redistribution of immune cells and factors was observed. | [93] |
| NH2Pr-CD-C12/ DSPE-mPEG2kPEG/ C18-PEG-SA | SA | CSF-1R siRNA | 246 nm 29 mV | Human THP1 and RAW 264.7 murine monocytes Human PC-3 and murine TRAMP-C1 prostate cancer cells in vitro | Insertion of PEG and SA had no impact on siRNA complexation. PEGylation prevented CDplex aggregation under physiological conditions. SA ligand improved macrophage uptake and reduced targeted mRNA expression, resulting in reprograming M2 (50%). M1 cytokines increased apoptosis of prostate cancer cells. | [94] |
| NH2Pr-CD-C12/ C12-CD-SO3 | - | KAT2 siRNA | 161 nm (0.16) and 24 mV (vs. 164 nm (0.46) and 34 mV for NH2Pr-CD-C12) | Human HL-60 myeloid leukemia in vitro | Coated polyplex possesses bilayer structure with hydrophobic C12 interactions contributing to polyplex uniformity. Maximal internalization at 6 h (decreased by half at 24 h) | [95] |
| TEI7-CD-C1614 | - | p42-MAPK and Rheb siRNA | 170 nm/58 mV 100 nm/25 mV (CDplex) | Human LNCaP and PC3 prostate cancer cells (in vitro). Human U87 MG and rat C6 glioblastoma cells, GL-261 mouse glioma cells (in vitro) | Transfection and mRNA knockdown observed in various cell types. | [101] |
| Spermidine-CD:Ad-PVA-PEG | pH sensitive acetal linker | siRNA | 130–260 nm (0.08–0.18) −23–−61 mV | A549 in vitro | Formation of polyplex followed by hydrophilic polymer insertion resulted in more stable CDplexes, while the length of PEG-directed size and ζ. siRNA complexation led to size contraction. | [34] |
| Drug-NA co-delivery | ||||||
| NH2Pr-CD-C12 NH2Pr-CD-C12/PEG NH2Pr-CD-C12/PEG/FA | FA | Docetaxel RelA siRNA | 100 nm (0.27)/10 mV 122 nm (0.24)/40 mV (PEG) 125 nm (0.26)/9 Mv (PEG/FA) | Murine CT26 CRC line | CD derivatization significantly increased the size of loaded CDplex. pH-dependent drug release observed. Synergistic anticancer activity was observed and was especially pronounced for targeting CDplex. | [98] |
| CatCD:Fc-prodrug | H2O2 | MTH1 siRNA Ferrocene | 80 nm (polydisperse) 4.9 mV | HeLa in vitro MDA-MB-23 3D speroid (in vitro) | Oxidation products of Fc-prodrug include ROS and p-quinone methide (GSH scavenger). NPs are taken up by cholesterol-dependent endocytosis and are colocalized within cytoplasm. Fe-siRNA co-delivery improves Fe chemodynamic activity in 2D and 3D in vitro models. | [102] |
| βCD-PCL-Ad:βCD-PCL-PDMAEMA | - | Doxorubicin Nur77DDBD pDNA | ~200 nm ~20 mV | Human HepG2/MDR1-Bcl2 hepatoma cells (in vitro) | Complexation improves drug loading and release and is efficient in transfecting MDR cancer cells. Suppression of gene related to drug resistance improved Dox activity. | [103] |
6. Final Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ghitman, J.; Voicu, S.I. Controlled drug delivery mediated by cyclodextrin-based supramolecular self-assembled carriers: From design to clinical performances. Carbohydr. Polym. Technol. Appl. 2023, 5, 100266. [Google Scholar] [CrossRef]
- Dummert, S.V.; Saini, H.; Hussain, M.Z.; Yadava, K.; Jayaramulu, K.; Casini, A.; Fischer, R. Cyclodextrin metal–organic frameworks and derivatives: Recent developments and applications. Chem. Soc. Rev. 2022, 51, 5175–5213. [Google Scholar]
- Cengiz, B.; Gevrek, T.N.; Chambre, L.; Sanyal, A. Self-Assembly of Cyclodextrin-Coated Nanoparticles: Fabrication of Functional Nanostructures for Sensing and Delivery. Molecules 2023, 28, 1076. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef] [PubMed]
- Rimsha, Y.; Razzaq, F.A.; Asghar, S.; Irfan, M.; Khan, I.U.; Khalid, S.H. Cyclodextrins: An Overview of Fundamentals, Types, and Applications. In Cyclodextrins; Rashid, A., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 2. [Google Scholar]
- Rasheed, A.; Ashok Kumar, C.K.; Sravanthi, V.V.N.S.S. Cyclodextrins as Drug Carrier Molecule: A Review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef]
- Murjan, S.; Saeedi, S.; Nabid, M.R. Comparison between novel star-like redox-sensitive amphiphilic block copolymer and its linear counterpart copolymer as nanocarriers for doxorubicin. Drug Dev. Ind. Pharm. 2020, 46, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Sun, P.; Tong, G.; Zhu, X. Star polymer-based unimolecular micelles and their application in bio-imaging and diagnosis. Biomaterials 2018, 178, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, C.; Li, S.; Chen, X.; Fan, X.; Hu, Z.; Li, Z. Cyclodextrin based unimolecular micelles with targeting and biocleavable abilities as chemotherapeutic carrier to overcome drug resistance. Mater. Sci. Eng. C 2019, 105, 110047. [Google Scholar] [CrossRef] [PubMed]
- Benkovics, G.; Balint, M.; Fenyvesi, E.; Varga, E.; Beni, S.; Yannakopoulou, K.; Malanga, M. Homo- and hetero-difunctionalized β-cyclodextrins: Short direct synthesis in gram scale and analysis of regiochemistry. Beilstein J. Org. Chem. 2019, 15, 710–720. [Google Scholar] [CrossRef]
- Řezanka, M. Synthesis of substituted cyclodextrins. Environ. Chem. Lett. 2019, 17, 49–63. [Google Scholar] [CrossRef]
- Mendez-Ardoy, A.; Gómez-García, M.; Gèze, A.; Putaux, J.L.; Wouessidjewe, D.; Ortiz Mellet, C.; Defaye, J.; García Fernández, J.M.; Benito, J.M. Monodisperse nanoparticles from self-assembling amphiphilic cyclodextrins: Modulable tools for the encapsulation and controlled release of pharmaceuticals. Med. Chem. 2012, 8, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Gèze, A.; Aous, S.; Baussanne, I.; Putaux, J.; Defaye, J.; Wouessidjewe, D. Influence of chemical structure of amphiphilic β-cyclodextrins on their ability to form stable nanoparticles. Int. J. Pharm. 2002, 242, 301–305. [Google Scholar] [CrossRef]
- Choisnard, L.; Gèze, A.; Putaux, J.L.; Wong, Y.S.; Wouessidjewe, D. Nanoparticles of β-Cyclodextrin Esters Obtained by Self-Assembling of Biotransesterified β-Cyclodextrins. Biomacromolecules 2006, 7, 515–520. [Google Scholar] [CrossRef]
- Putaux, J.-L.; Lancelon-Pin, C.; Legrand, F.X.; Pastrello, M.; Choisnard, L.; Gèze, A.; Rochas, C.; Wouessidjewe, D. Self-Assembly of Amphiphilic Biotransesterified β-Cyclodextrins: Supramolecular Structure of Nanoparticles and Surface Properties. Langmuir 2017, 33, 7917–7928. [Google Scholar] [CrossRef]
- Augis, L.; Nerbø Reiten, I.; Førde, J.L.; Casas-Solvas, J.M.; Sizun, C.; Bizien, T.; Rajkovic, I.; Larquet, E.; Michelet, A.; Collot, M.; et al. Development of nanoparticles based on amphiphilic cyclodextrins for the delivery of active substances. Int. J. Pharm. 2024, 651, 123723. [Google Scholar] [CrossRef]
- Yin, X.; Wang, L.; Zhang, X.; Zhao, H.; Cui, Z.; Fu, P.; Liu, M.; Pang, X.; Qiao, X. Synthesis of amphiphilic star-shaped block copolymers through photo-induced metal free atom transfer radical polymerization. Eur. Polym. J. 2020, 126, 109557. [Google Scholar] [CrossRef]
- Harn, Y.-W.; He, Y.; Wang, Z.; Chen, Y.; Liang, S.; Li, Z.; Li, Q.; Zhu, L.; Lin, Z. Synthesis of Amphiphilic and Double Hydrophilic Star-like Block Copolymers and the Dual pH-Responsiveness of Unimolecular Micelle. Macromolecules 2020, 53, 8286–8295. [Google Scholar] [CrossRef]
- Koyanagi, K.; Takashima, Y.; Nakamura, T.; Yamaguchi, H.; Harada, A. Radical polymerization by a supramolecular catalyst: Cyclodextrin with a RAFT reagent. Beilstein J. Org. Chem. 2016, 12, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Faye, I.; Huin, C.; Illy, N.; Bennevault, V.; Guégan, P. β-Cyclodextrin-Based Star Amphiphilic Copolymers: Synthesis, Characterization, and Evaluation as Artificial Channels. Macromol. Chem. Phys. 2019, 220, 1800308. [Google Scholar] [CrossRef]
- Rojas-Aguirre, Y.; Torres-Mena, M.A.; López-Méndez, L.J.; Alcaraz-Estrada, S.L.; Guadarrama, P.; Urucha-Ortíz, J.M. PEGylated β-cyclodextrins: Click synthesis and in vitro biological insights. Carbohydr. Polym. 2019, 223, 115113. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Qi, C.; Sun, C.; Huo, F.; Jiang, X. Poly(ethylene glycol) alternatives in biomedical applications. Nano Today 2023, 48, 101738. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, H.; Wang, Y.; Feng, Y.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Tang, X. The interplay between PEGylated nanoparticles and blood immune system. Adv. Drug Deliv. Rev. 2023, 200, 115044. [Google Scholar] [CrossRef]
- Fan, W.; Peng, H.; Yu, Z.; Wang, L.; He, H.; Ma, Y.; Qi, J.; Lu, Y.; Wu, W. The long-circulating effect of pegylated nanoparticles revisited via simultaneous monitoring of both the drug payloads and nanocarriers. Acta Pharm. Sin. B 2022, 12, 2479–2493. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Ogier, J.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. Cationic and PEGylated Amphiphilic Cyclodextrins: Co-Formulation Opportunities for Neuronal Sirna Delivery. PLoS ONE 2013, 8, e66413. [Google Scholar] [CrossRef]
- Shi, L.; Zhamg, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Ge, X.; Chen, L.; Zhao, B.; Yuan, W. Rationale and Application of PEGylated Lipid-Based System for Advanced Target Delivery of siRNA. Front. Pharmacol. 2021, 11, 598175. [Google Scholar] [CrossRef]
- Godinho, B.M.D.C.; Ogier, J.R.; Quinlan, A.; Darcy, R.; Griffin, B.T.; Cryan, J.F.; O’Driscoll, C.M. PEGylated cyclodextrins as novel siRNA nanosystems: Correlations between polyethylene glycol length and nanoparticle stability. Int. J. Pharm. 2014, 473, 105–112. [Google Scholar] [CrossRef]
- Seripracharat, C.; Sinthuvanich, C.; Karpkird, T. Cationic cyclodextrin-adamantane poly(vinyl alcohol)-poly(ethylene glycol) assembly for siRNA delivery. J. Drug Deliv. Sci. Technol. 2022, 68, 103052. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.; Lim, D.; Joo, M.K.; Kim, K. Perspectives for Improving the Tumor Targeting of Nanomedicine via the EPR Effect in Clinical Tumors. Int. J. Mol. Sci. 2023, 24, 10082. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xu, L.; Liu, Y.; Li, Q.; Zhao, D.; Li, Z.; Zhang, H.; Zhang, H.; Kan, Q.; Wang, Y.; et al. Transformative hyaluronic acid-based active targeting supramolecular nanoplatform improves long circulation and enhances cellular uptake in cancer therapy. Acta Pharm. Sin. B 2019, 9, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Russell, E.G.; Darcy, R.; Cotter, T.G.; McKenna, S.L.; Cahill, M.R.; O’Driscoll, C.M. Antibody-Targeted Cyclodextrin-Based Nanoparticles for siRNA Delivery in the Treatment of Acute Myeloid Leukemia: Physicochemical Characteristics, in Vitro Mechanistic Studies, and ex Vivo Patient Derived Therapeutic Efficacy. Mol. Pharm. 2017, 14, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Tonegawa, A.; Tamura, A.; Zhang, S.; Yui, N. Hydrophobicity of acyl groups in α-cyclodextrin-threaded polyrotaxanes dominates the formation and stability of self-assembled nanoparticles. Polymer 2020, 200, 122537. [Google Scholar] [CrossRef]
- Zhang, S.; Tamura, A.; Yui, N. Supramolecular nanoarchitectonics of propionylated polyrotaxanes with bulky nitrobenzyl stoppers for light-triggered drug release. RSC Adv. 2024, 14, 3798–3806. [Google Scholar] [CrossRef]
- Ghodke, S.B.; Parkar, J.N.; Deshpande, A.R.; Dandekar, P.P.; Jain, R.D. Structure–Activity Relationship of Polyester-Based Cationic Polyrotaxane Vector-Mediated In Vitro siRNA Delivery: Effect on Gene Silencing Efficiency. ACS Appl. Bio Mater. 2020, 3, 7500–7514. [Google Scholar] [CrossRef] [PubMed]
- Taharabaru, T.; Kihara, K.; Onodera, R.; Kogo, T.; Higashi, K.; Moribe, K.; Nakamura, T.; Motoyama, K.; Higashi, T. Polyrotaxane-based multi-step transformable materials for the delivery of Cas9 ribonucleoprotein. Appl. Mater. Today 2022, 27, 101488. [Google Scholar] [CrossRef]
- Taharabaru, T.; Kihara, T.; Onodera, R.; Kogo, T.; Wen, Y.; Li, J.; Motoyama, K.; Higashi, T. Versatile delivery platform for nucleic acids, negatively charged protein drugs, and genome-editing ribonucleoproteins using a multi-step transformable polyrotaxane. Mater. Today Bio 2023, 20, 100690. [Google Scholar] [CrossRef]
- Taharabaru, T.; Kihara, T.; Obata, A.; Onodera, R.; Wen, Y.; Li, J.; Motoyama, K.; Higashi, T. Cyclodextrin-based tailored polyrotaxanes for highly efficient delivery of the genome-editing molecule. Carbohydr. Polym. 2024, 323, 121443. [Google Scholar] [CrossRef]
- Zhang, S.; Tamura, A.; Yui, N. Weakly acidic carboxy group-grafted β-cyclodextrin-threaded acid-degradable polyrotaxanes for modulating protein interaction and cellular internalization. Sci. Technol. Adv. Mater. 2021, 22, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Osawa, M.; Yui, N. Supermolecule—Drug Conjugates Based on Acid-Degradable Polyrotaxanes for pH-Dependent Intracellular Release of Doxorubicin. Molecules 2023, 28, 2517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tamura, A.; Yui, N. Cothreading of Unmodified and Monoazidated β-Cyclodextrins in Polyrotaxanes for Orthogonal Modification of Cell-Penetrating Peptides via Click Chemistry. ACS Appl. Polym. Mater. 2022, 4, 3866–3876. [Google Scholar] [CrossRef]
- Ohashi, M.; Tamura, A.; Yui, N. Exploring Receptor Binding Affinities and Hepatic Cell Association of N-Acetyl-d-Galactosamine-Modified β-Cyclodextrin-Based Polyrotaxanes for Liver-Targeted Therapies. Biomacromolecules 2023, 24, 2327–2341. [Google Scholar] [CrossRef]
- Dal Poggetto, G.; Troisse, S.S.; Conte, C.; Marchetti, R.; Moret, F.; Iadonisi, A.; Silipo, A.; Lanzetta, R.; Malinconico, M.; Quaglia, F.; et al. Nanoparticles decorated with folate based on a site-selective αCD-rotaxanated PEG-b-PCL copolymer for targeted cancer therapy. Polym. Chem. 2020, 11, 3892–3903. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin–Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials 2019, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, N.; Motoyama, K.; Iohara, D.; Hirayama, F.; Taharabaru, T.; Watabe, N.; Kawabata, Y.; Onodera, R.; Higashi, T. Polypseudorotaxane-based supramolecular hydrogels consisting of cyclodextrins and Pluronics as stabilizing agents for antibody drugs. Carbohydr. Polym. 2021, 256, 117419. [Google Scholar] [CrossRef]
- Wang, W.; He, W.; Wang, X.; Zhao, T.; Muraoka, O.; Tanabe, G.; Xie, W.; Zhou, T.; Xing, L.; Jin, Q.; et al. Glutathione-depleted cyclodextrin pseudo-polyrotaxane nanoparticles for anti-inflammatory oxaliplatin (IV) prodrug delivery and enhanced colorectal cancer therapy. Chin. Chem. Lett. 2024, 35, 108656. [Google Scholar] [CrossRef]
- Hong, W.; Guo, F.; Yu, N.; Ying, S.; Lou, B.; Wu, J.; Gao, Y.; Ji, X.; Wang, H.; Li, A.; et al. A Novel Folic Acid Receptor-Targeted Drug Delivery System Based on Curcumin-Loaded β-Cyclodextrin Nanoparticles for Cancer Treatment. Drug Des. Dev. Ther. 2021, 15, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, S.; Mu, M.; Liu, X.; Zhao, L.; Xu, Z.; Mu, C.; Li, D.; Ge, L. Multifunctional β-Cyclodextrin-Poly(ethylene glycol)-Cholesterol Nanomicelle for Anticancer Drug Delivery. ACS Appl. Bio Mater. 2022, 5, 5418–5431. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liu, Y.; Wang, D.; Wu, C.; Xia, C.; Gong, Q.; Song, B.; Ai, H. Amphiphilic starlike dextran wrapped superparamagnetic iron oxide nanoparticle clsuters as effective magnetic resonance imaging probes. Biomaterials 2013, 34, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, N.; Yang, R.; Zhang, L.; Jiang, X. Folic Acid-Decorated β-Cyclodextrin-Based Poly(ε-caprolactone)-dextran Star Polymer with Disulfide Bond-Linker as Theranostic Nanoparticle for Tumor-Targeted MRI and Chemotherapy. Pharmaceutics 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
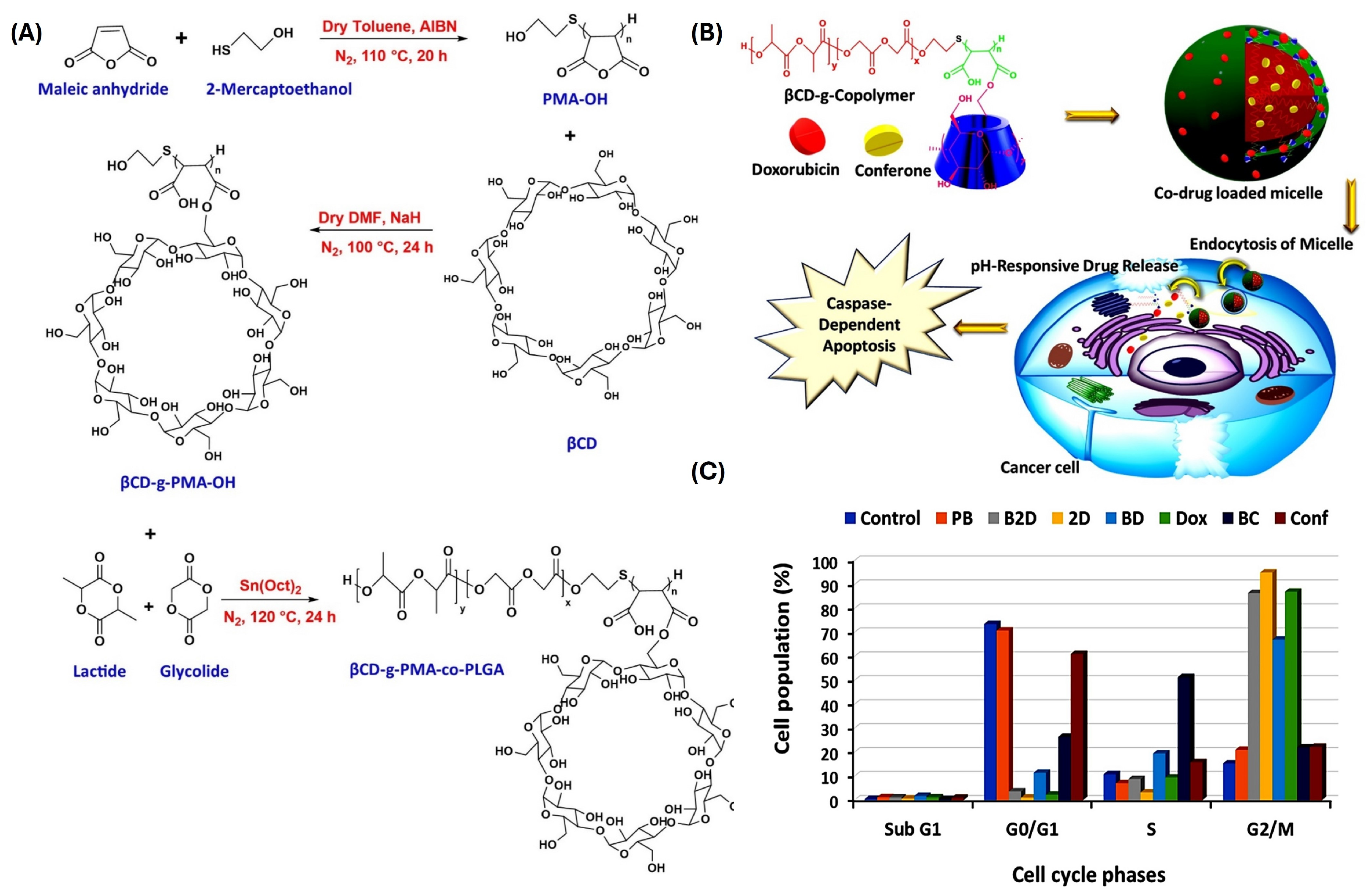
- Rahmani, A.; Rahimi, F.; Iranshahi, M.; Kahroba, H.; Zarebkohan, A.; Talebi, M.; Salehi, R.; Mousavi, H.Z. Co-delivery of doxorubicin and conferone by novel pH-responsive β-cyclodextrin grafted micelles triggers apoptosis of metastatic human breast cancer cells. Sci. Rep. 2021, 11, 21425. [Google Scholar] [CrossRef]
- Liu, T.; Li, G.; Wu, X.; Chen, S.; Zhang, S.; Han, H.; Zhang, H.; Luo, X.; Cai, X.; Ma, D. Β-Cyclodextrin-graft-poly(amidoamine) dendrons as the nitric oxide deliver system for the chronic rhinosinusitis therapy. Drug Deliv. 2021, 28, 306–318. [Google Scholar] [CrossRef]
- Shi, E.; Bai, L.; Mao, L.; Wang, H.; Yang, X.; Wang, Y.; Zhang, M.; Li, C.; Wang, Y. Self-assembled nanoparticles containing photosensitizer and polycationic brush for synergistic photothermal and photodynamic therapy against periodontitis. J. Nanobiotechnol. 2021, 19, 413. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Godinho, B.M.; Ogier, J.; Devocelle, M.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. Click-Modified Cyclodextrins as Nonviral Vectors for Neuronal siRNA Delivery. ACS Chem. Neurosci. 2012, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Ogier, J.; Desgranges, S.; Cryan, J.F.; Darcy, R.; O’Driscol, C.M. A click chemistry route to 2-functionalised PEGylated and cationic β-cyclodextrins: Co-formulation opportunities for siRNA delivery. Org. Biomol. Chem. 2012, 10, 4954–4960. [Google Scholar] [CrossRef]
- Sun, D.; Zou, Y.; Song, L.; Han, S.; Yang, H.; Chu, D.; Dai, Y.; Ma, J.; O’Driscoll, C.M.; Yu, Z.; et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm. Sin. B 2022, 12, 378–393. [Google Scholar] [CrossRef]
- Li, Y.-N.; Shi, X.; Sun, D.; Han, S.; Zou, Y.; Wang, L.; Yang, L.; Li, Y.; Shi, Y.; Guo, J.; et al. Delivery of melarsoprol using folate-targeted PEGylated cyclodextrin-based nanoparticles for hepatocellular carcinoma. Int. J. Pharm. 2023, 636, 122791. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Zhang, J.; Xie, R.; Yu, D.; Wei, H.; Wang, Y.; Hua, Z.; Qi, X.; Huang, B.; Yang, G. Adenosine Triphosphate-Responsive Glyconanorods through Self-Assembly of β-Cyclodextrin-Based Glycoconjugates for Targeted and Effective Bacterial Sensing and Killing. Biomacromolecules 2023, 24, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.; Wang, L.; Ly, P.; Chen, M.; Xiao, F.; Si, T.; Tao, J.; Yang, B. Bifunctional drug delivery system with carbonic anhydrase IX targeting and glutathione-responsivity driven by host-guest amphiphiles for effective tumor therapy. Carbohydr. Polym. 2024, 326, 121577. [Google Scholar] [CrossRef] [PubMed]
- Adeli, F.; Abbasi, F.; Babazadeh, M.; Davaran, S. Thermo/pH dual-responsive micelles based on the host–guest interaction between benzimidazole-terminated graft copolymer and β-cyclodextrin-functionalized star block copolymer for smart drug delivery. J. Nanobiotechnol. 2022, 20, 91. [Google Scholar] [CrossRef]
- Xue, E.Y.; Yang, C.; Fong, W.P.; Ng, D.K.P. Site-Specific Displacement-Driven Activation of Supramolecular Photosensitizing Nanoassemblies for Antitumoral Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 14903–14915. [Google Scholar] [CrossRef]
- Yin-Ku, L.; Shiu-Wei, W.; Ren-Shen, L. Photo and redox dual-stimuli-responsive β-cyclodextrin-ferrocene supramolecules for drug delivery. J. Macromol. Sci. Part A 2020, 58, 8–21. [Google Scholar] [CrossRef]
- Liang, B.; Chen, Y.; Dai, X.; Li, J.; Jia, S.; Wang, S.; Liu, Y. A dandelion-like nanomedicine via hierarchical self-assembly for synergistic chemotherapy and photo-dynamic cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2023, 49, 102660. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Dai, X.; Li, J.; Jia, S.; Wang, S.; Liu, Y. Multicharge β-cyclodextrin supramolecular assembly for ATP capture and drug release. Chem. Commun. 2021, 57, 2812–2815. [Google Scholar] [CrossRef]
- Guan, L.; Zeng, Z.; Liu, W.; Wang, T.; Tian, S.; Hu, S.; Tian, D. Aggregation-induced emission (AIE) nanoparticles based on γ-cyclodextrin and their applications in biomedicine. Carbohydr. Polym. 2022, 298, 120130. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, S.; Yuan, J.; Zhu, P.; Gan, Y.; Fan, G.; Yu, S.; Shi, J. Porphyrin-terminated nanoscale fluorescent polyrotaxane as a biodegradable drug carrier for anticancer research. Nanotechnology 2020, 31, 255101. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Jia, S.; Lin, K.; Fan, G.; Yuan, J.; Yu, S.; Shi, J. Specific Modification with TPGS and Drug Loading of Cyclodextrin Polyrotaxanes and the Enhanced Antitumor Activity Study in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 46427–46436. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Huang, K.; Jiang, W.; Fu, L.; Zhang, R.; Shen, L.; Ou, Z.; Huang, Y.; Zhang, Z. Exploration of the inhibition action of TPGS on tumor cells and its combined use with chemotherapy drugs. Drug Deliv. 2023, 30, 2183830. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, D.; Shang, T.; Guo, L.; Yang, B.; Xu, X. Chain conformation transition induced host–guest assembly between triple helical curdlan and β-CD for drug delivery. Biomater. Sci. 2020, 8, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, C.; Yu, S.; Shafiq, F.; Qiao, W. Amphiphilic conjugates for solubilization of paclitaxel as efficient drug carriers based on hyaluronic acid and cyclodextrins. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133026. [Google Scholar] [CrossRef]
- Hu, H.; Dai, W.; Zhang, Y.; Huang, Y.; Qian, J.; Jin, Q.; Ji, J.; Tang, Z. Fabrication of programmed photosensitizer-conjugated nanoassemblies by dual supramolecular self-assembly for photodynamic therapy of orthotopic hepatoma. Chem. Eng. J. 2022, 435, 134930. [Google Scholar] [CrossRef]
- Chan, A.; Tsourkas, A. Intracellular Protein Delivery: Approaches, Challenges, and Clinical Applications. BME Front. 2024, 5, 0035. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Kanjilal, P.; Das, R.; Thayumanavan, S. Synergistic Interplay of Covalent and Non-Covalent Interactions in Reactive Polymer Nanoassembly Facilitates Intracellular Delivery of Antibodies. Angew. Chem. 2021, 60, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
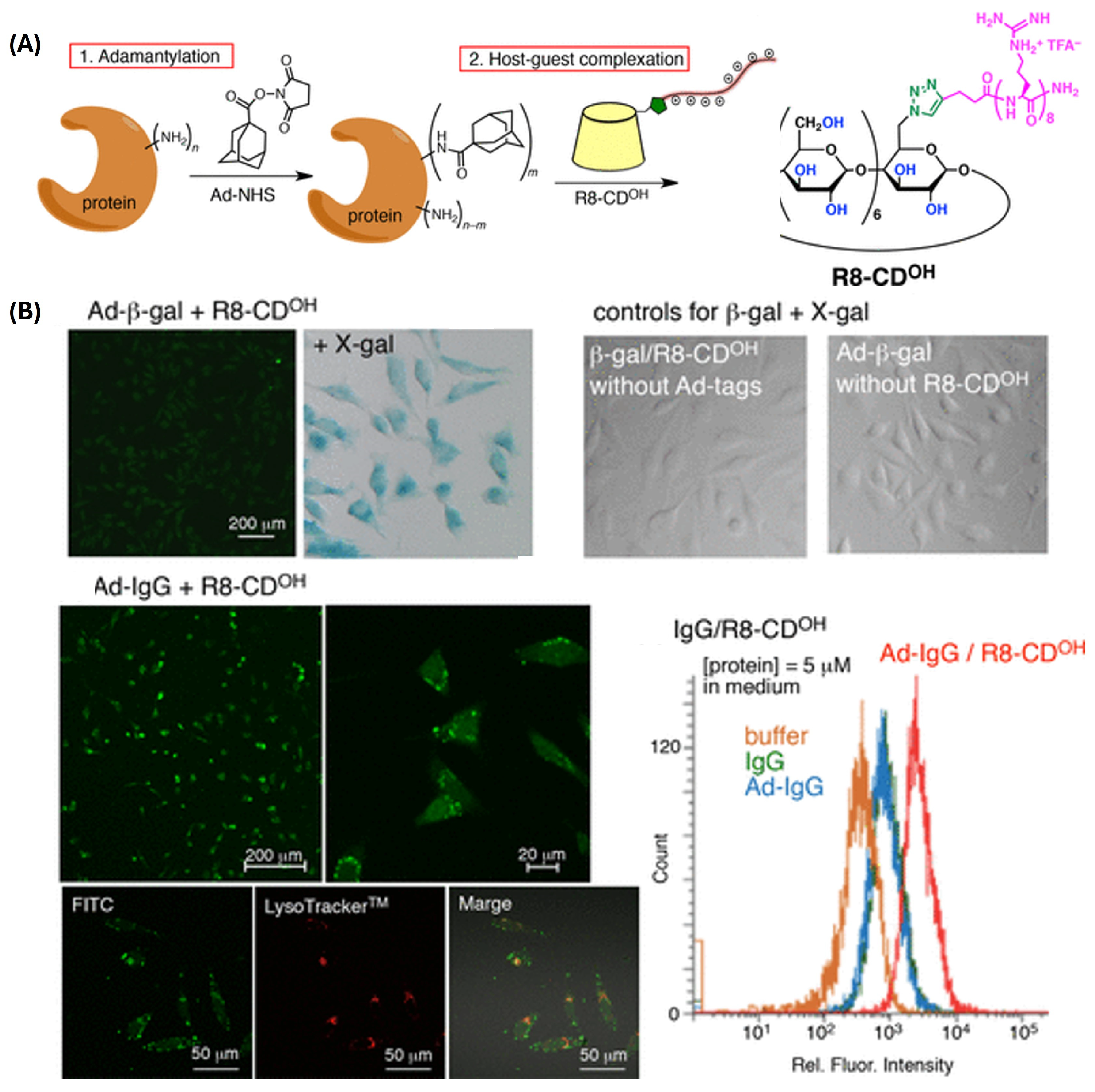
- Kitagishi, H.; Jiromaru, M.; Hasegawa, N. Intracellular Delivery of Adamantane-Tagged Small Molecule, Proteins, and Liposomes Using an Octaarginine-Conjugated β-Cyclodextrin. ACS Appl. Bio Mater. 2020, 3, 4902–4911. [Google Scholar] [CrossRef]
- Kudruk, S.; Chali, S.P.; Matos, A.L.L.; Bourque, C.; Dunker, C.; Gatsogiannis, C.; Ravoo, B.J.; Gerke, V. Biodegradable and Dual-Responsive Polypeptide-Shelled Cyclodextrin-Containers for Intracellular Delivery of Membrane-Impermeable Cargo. Adv. Sci. 2021, 8, 2100694. [Google Scholar] [CrossRef]
- Geisshüsler, S.; Schineis, P.; Langer, L.; Wäckerle-Men, Y.; Leroux, J.-C.; Halin, C.; Vogel-Kindgen, S.; Johansen, P.; Gander, B. Amphiphilic Cyclodextrin-Based Nanoparticulate Vaccines Can Trigger T-Cell Immune Responses. Adv. NanoBiomed Res. 2022, 2, 2100082. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, C.; Zhu, Y.; Yang, L.; Shi, S.; Yang, F.; Feng, X. Dibenzocyclooctyne linked lysine-cyclodextrin for efficient intranucleus delivery of proteins. J. Control. Release 2022, 352, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; He, X.; Li, C.; Lin, S.; Chen, H.; Liu, L.; Feng, X. Oral delivery of antioxidant enzymes for effective treatment of inflammatory disease. Biomaterials 2021, 271, 120753. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, Y.; Qi, D.; Yang, L.; Chen, H.; Wang, C.; Feng, X. Nucleus-Targeted Delivery of Multi-Protein Self-Assembly for Combined Anticancer Therapy. Small 2021, 17, e2101219. [Google Scholar] [CrossRef]
- Nappi, F. Non-Coding RNA-Targeted Therapy: A State-of-the-Art Review. Int. J. Mol. Sci. 2024, 25, 3630. [Google Scholar] [CrossRef]
- Jarak, I.; Pereira-Silva, M.; Santos, A.C.; Veiga, F.; Cabral, H.; Figueiras, A. Multifunctional polymeric micelle-based nucleic acid delivery: Current advances and future perspectives. Appl. Mater. Today 2021, 25, 101217. [Google Scholar] [CrossRef]
- Sun, Z.; Ren, Y.; Zhu, W.; Xiao, Y.; Wu, H. DNA nanotechnology-based nucleic acid delivery systems for bioimaging and disease treatment. Analyst 2024, 149, 599–613. [Google Scholar] [CrossRef]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef]
- Lu, Q. Bioresponsive and multifunctional cyclodextrin-based non-viral nanocomplexes in cancer therapy: Building foundations for gene and drug delivery, immunotherapy and bioimaging. Environ. Res. 2023, 234, 116507. [Google Scholar] [CrossRef]
- Haley, R.M.; Gottardi, R.; Langer, R.; Mitchell, M.J. Cyclodextrins in drug delivery: Applications in gene and combination therapy. Drug Deliv. Transl. Res. 2020, 10, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cronin, M.F.; Mendonça, M.C.P.; Guo, J.; O’Driscoll, C.M. M2pep-Modified Cyclodextrin-siRNA Nanoparticles Modulate the Immunosuppressive Tumor Microenvironment for Prostate Cancer Therapy. Mol. Pharm. 2023, 20, 5921–5936. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cronin, M.F.; Mendonça, M.C.P.; Guo, J.; O’Driscoll, C.M. Sialic acid-targeted cyclodextrin-based nanoparticles deliver CSF-1R siRNA and reprogram tumour-associated macrophages for immunotherapy of prostate cancer. Eur. J. Pharm. Sci. 2023, 185, 106427. [Google Scholar] [CrossRef]
- Kont, A.; Mendonça, M.C.P.; Cronin, M.F.; Cahill, M.R.; O’Driscoll, C.M. Co-Formulation of Amphiphilic Cationic and Anionic Cyclodextrins Forming Nanoparticles for siRNA Delivery in the Treatment of Acute Myeloid Leukaemia. Int. J. Mol. Sci. 2022, 23, 9791. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, L.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Wue, W. Construction of a High-Efficiency Drug and Gene Co-Delivery System for Cancer Therapy from a pH-Sensitive Supramolecular Inclusion between Oligoethylenimine-graft-β-cyclodextrin and Hyperbranched Polyglycerol Derivative. ACS Appl. Mater. Interfaces 2018, 10, 35812–35829. [Google Scholar] [CrossRef]
- Li, J.M.; Zhang, W.; Su, H.; Wang, Y.Y.; Tan, C.P.; Ji, L.N.; Mao, Z.W. Reversal of multidrug resistance in MCF-7/Adr cells by codelivery of doxorubicin and BCL2 siRNA using a folic acid-conjugated polyethylenimine hydroxypropyl-β-cyclodextrin nanocarrier. Int. J. Nanomed. 2015, 10, 3147–3162. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, F.; Song, L.; Sun, B.; Sun, D.; Chu, D.; Wang, L.; Han, S.; Yu, Z.; O’Driscoll, C.M.; et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int. J. Pharm. 2021, 606, 120888. [Google Scholar] [CrossRef]
- Villari, V.; Mazzaglia, A.; Darcy, R.; O’Driscoll, C.M.; Micali, N. Nanostructures of Cationic Amphiphilic Cyclodextrin Complexes with DNA. Biomacromolecules 2013, 14, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Negro, M.; Caracciolo, G.; Palchetti, S.; Pozzi, D.; Capriotti, A.L.; Cavaliere, C.; Laganà, A.; Ortiz Mellet, C.; Benito, J.M.; Fernández, G.; et al. Biophysics and protein corona analysis of Janus cyclodextrin-DNA nanocomplexes. Efficient cellular transfection on cancer cells. Biochim. et Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, C.; Játiva, P.; Posadas, I.; Manzanares, D.; Blanco, J.L.J.; Mellet, C.O.; Fernández, J.M.G.; Ceña, V. A β-Cyclodextrin-Based Nanoparticle with Very High Transfection Efficiency Unveils siRNA-Activated TLR3 Responses in Human Prostate Cancer Cells. Pharmaceutics 2022, 14, 2424. [Google Scholar] [CrossRef]
- Raj, G.; Vasudev, D.S.; Christopher, S.; Babulal, A.; Harsha, P.; Ram, S.; Tiwari, M.; Sauer, M.; Varghese, R. Multifunctional siRNA/ferrocene/cyclodextrin nanoparticles for enhanced chemodynamic cancer therapy. Nanoscale 2024, 16, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Li, Z.; Fan, X.; Loh, X.J.; Cheng, H.; Wu, Y.L.; Li, Z. Cyclodextrin-Based Hybrid Polymeric Complex to Overcome Dual Drug Resistance Mechanisms for Cancer Therapy. Polymers 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarak, I.; Ramos, S.; Caldeira, B.; Domingues, C.; Veiga, F.; Figueiras, A. The Many Faces of Cyclodextrins within Self-Assembling Polymer Nanovehicles: From Inclusion Complexes to Valuable Structural and Functional Elements. Int. J. Mol. Sci. 2024, 25, 9516. https://doi.org/10.3390/ijms25179516
Jarak I, Ramos S, Caldeira B, Domingues C, Veiga F, Figueiras A. The Many Faces of Cyclodextrins within Self-Assembling Polymer Nanovehicles: From Inclusion Complexes to Valuable Structural and Functional Elements. International Journal of Molecular Sciences. 2024; 25(17):9516. https://doi.org/10.3390/ijms25179516
Chicago/Turabian StyleJarak, Ivana, Sara Ramos, Beatriz Caldeira, Cátia Domingues, Francisco Veiga, and Ana Figueiras. 2024. "The Many Faces of Cyclodextrins within Self-Assembling Polymer Nanovehicles: From Inclusion Complexes to Valuable Structural and Functional Elements" International Journal of Molecular Sciences 25, no. 17: 9516. https://doi.org/10.3390/ijms25179516
APA StyleJarak, I., Ramos, S., Caldeira, B., Domingues, C., Veiga, F., & Figueiras, A. (2024). The Many Faces of Cyclodextrins within Self-Assembling Polymer Nanovehicles: From Inclusion Complexes to Valuable Structural and Functional Elements. International Journal of Molecular Sciences, 25(17), 9516. https://doi.org/10.3390/ijms25179516











