Reverse Gradient Distributions of Drug and Polymer Molecules within Electrospun Core–Shell Nanofibers for Sustained Release
Abstract
:1. Introduction
2. Results and Discussion
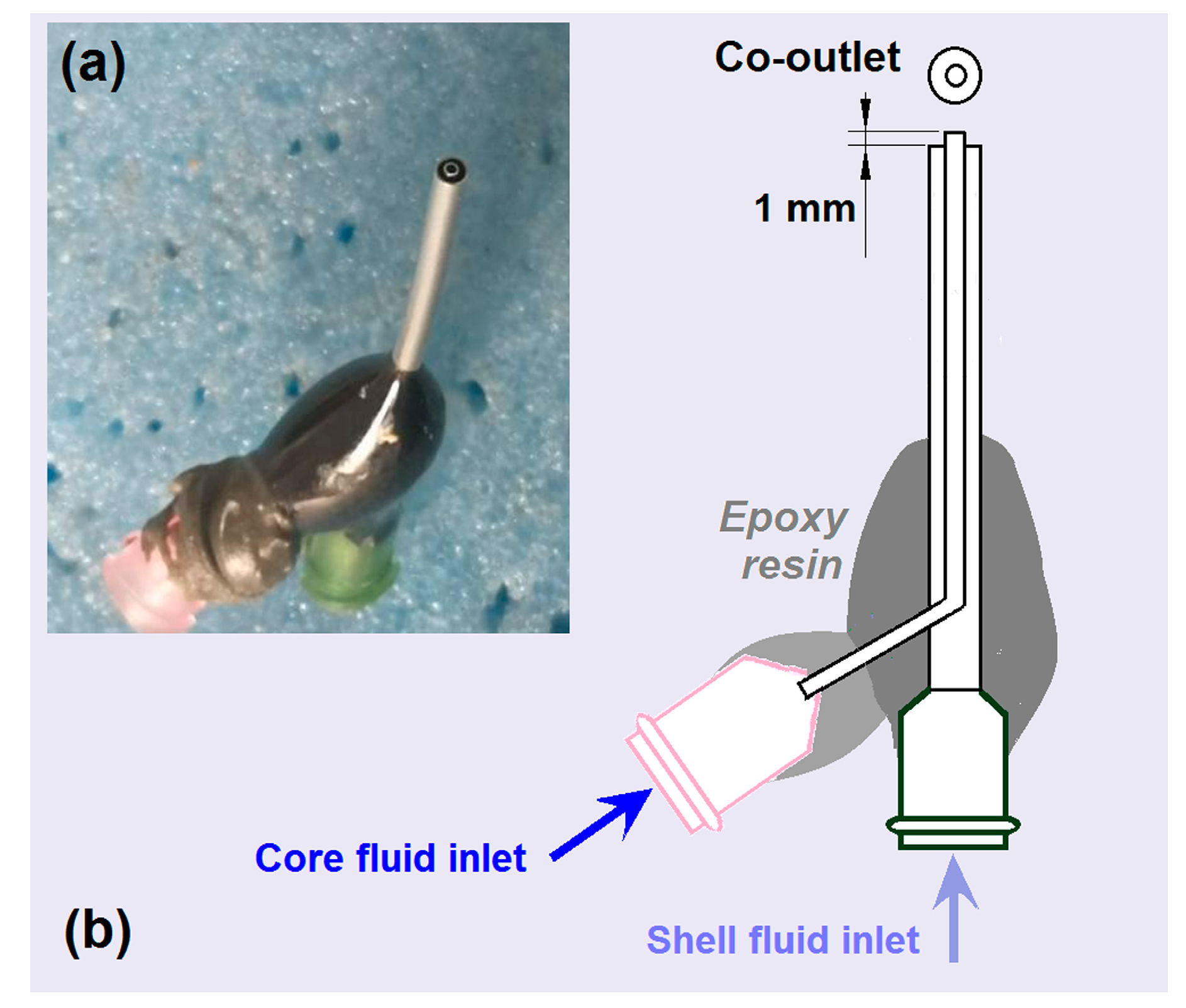
2.1. Key Elements for Implementing Coaxial Electrospinning
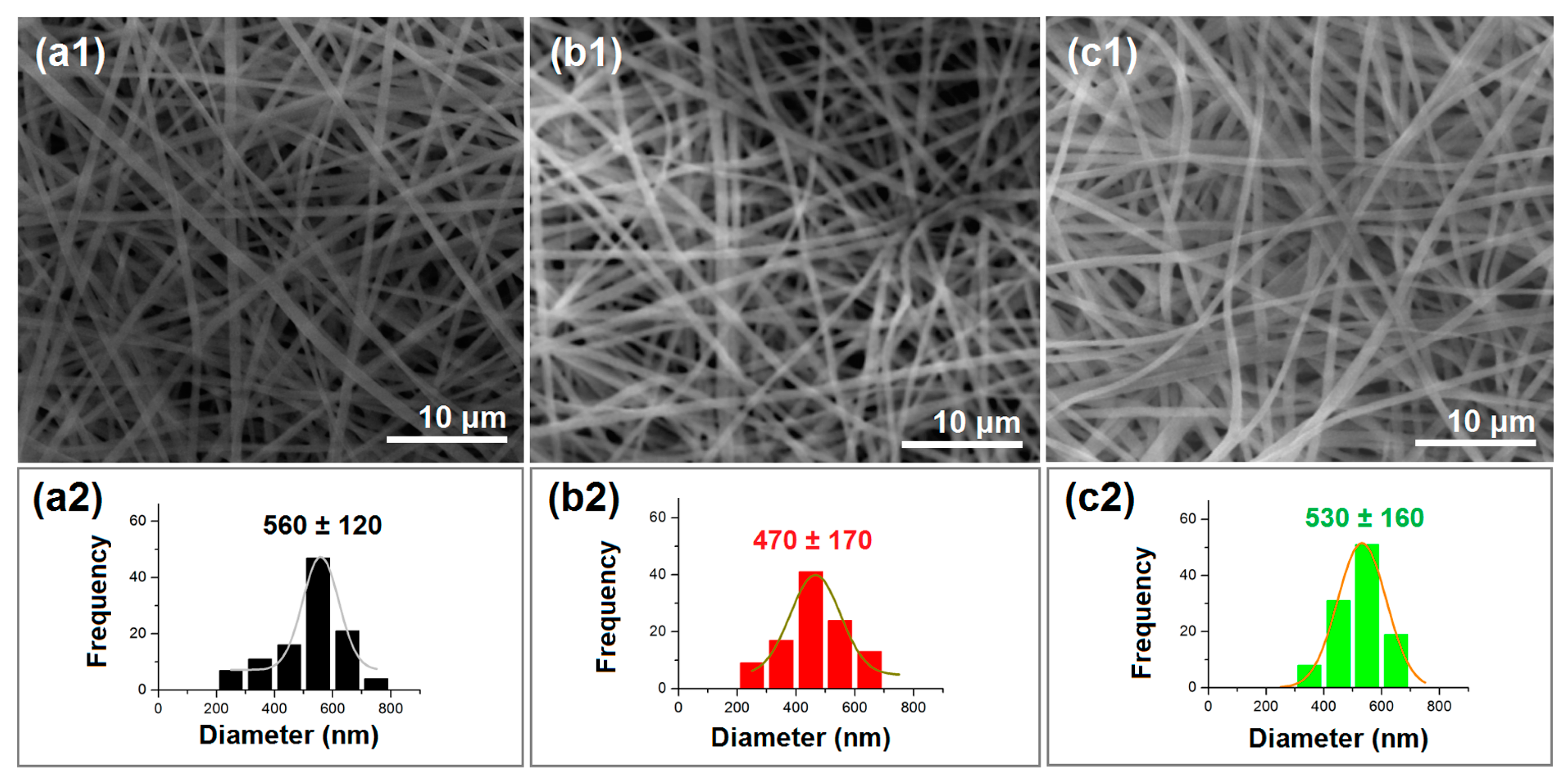
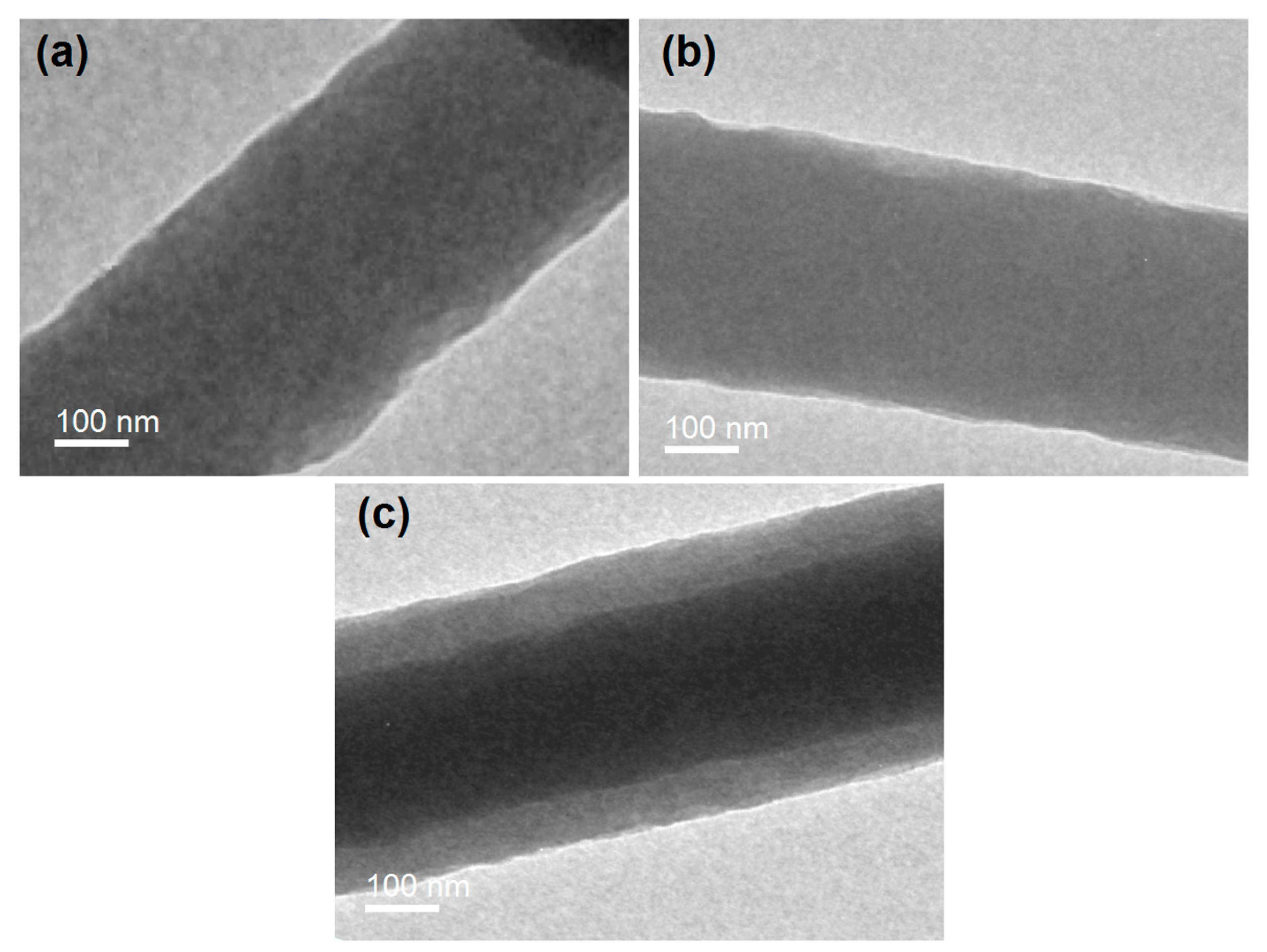
2.2. The Electrospun Nanofibers’ Morphology and Inner Structure
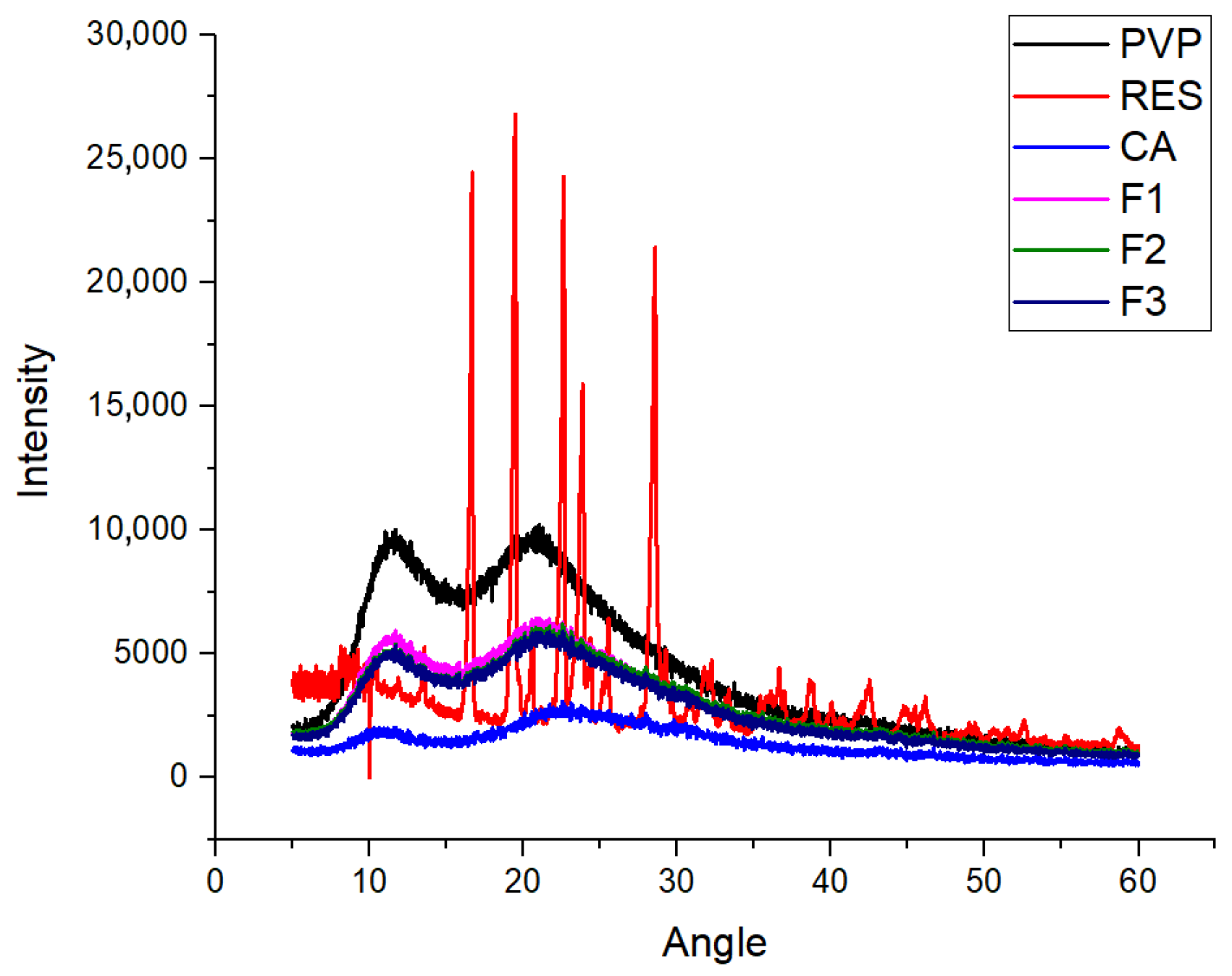
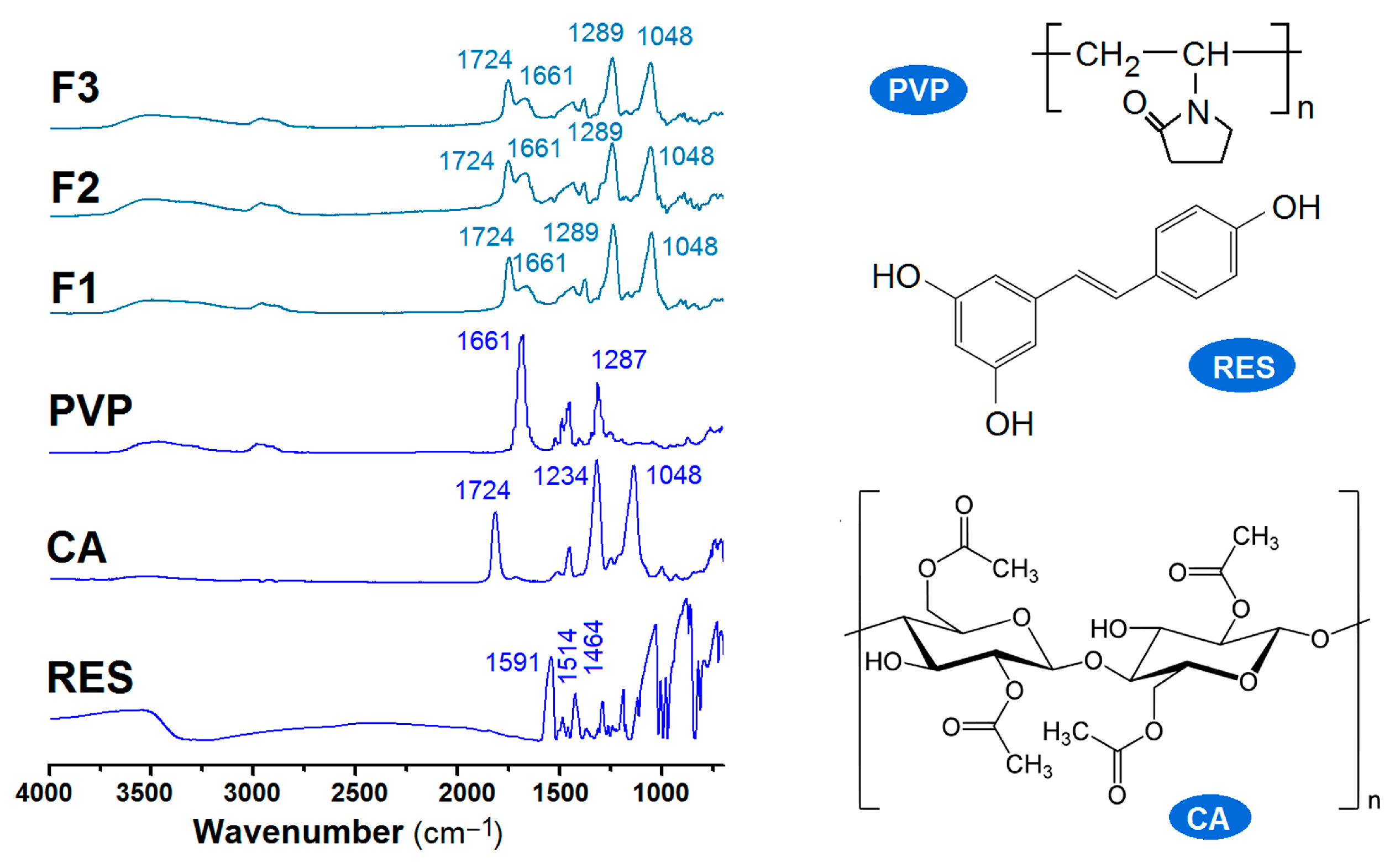
2.3. The Physical State of the Components and Their Compatibility
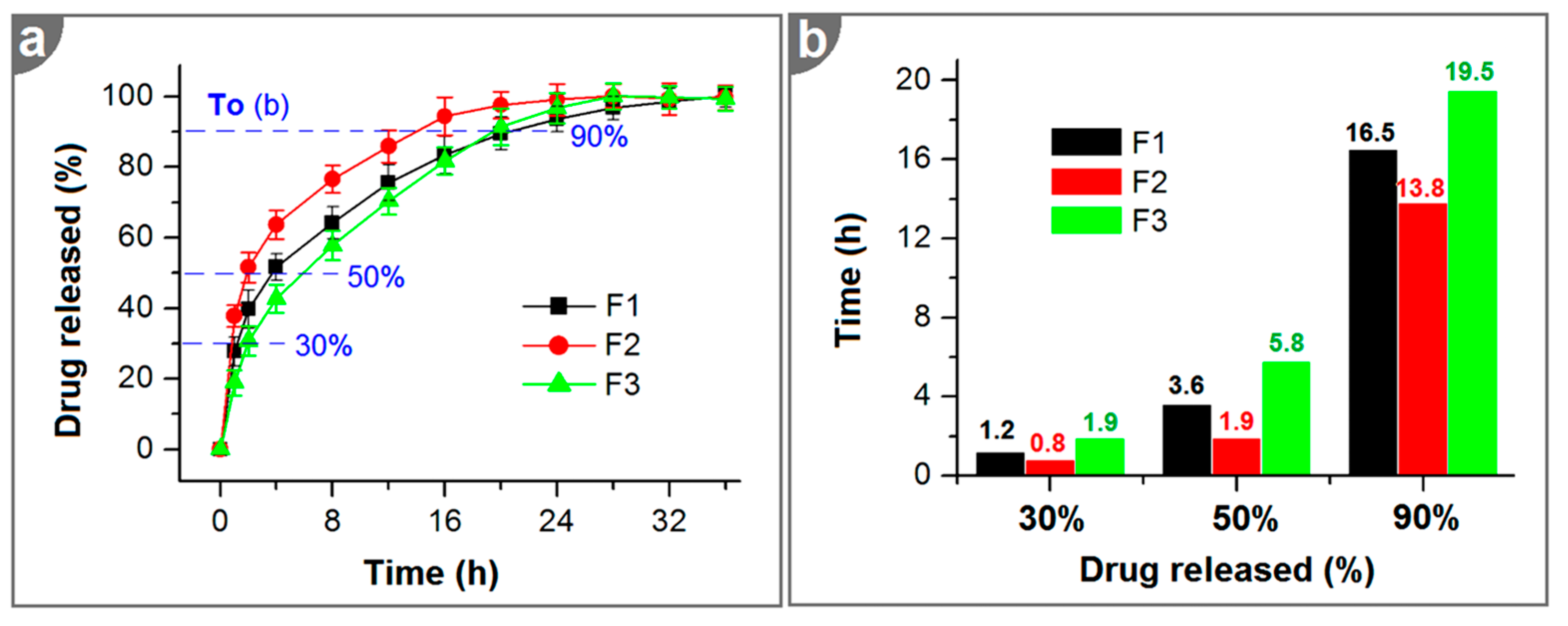
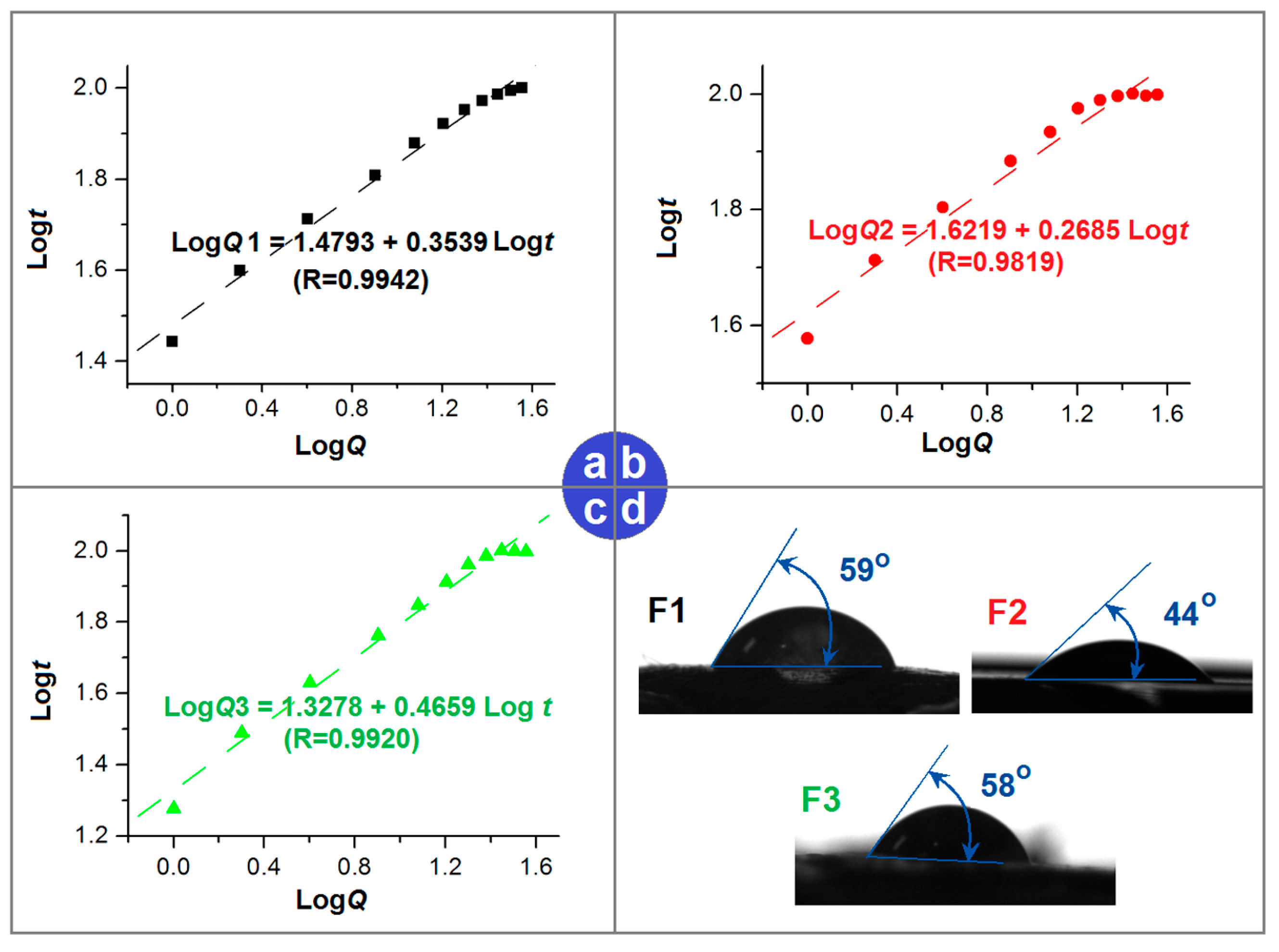
2.4. In Vitro Dissolution Tests
2.5. Mechanisms of the Reserve Gradient Distributions in Sustained Release
3. Materials and Methods
3.1. Materials
3.2. Electrospinning
3.3. Morphology and Inner Structures
3.4. Physical States and Compatibility
3.5. Hydrophilicity
3.6. In Vitro Dissolution Tests
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhan, Y.; Zhang, S. Design of novel PLK4 inhibitors as TRIM37-amplified breast cancer drugs using 3D-QSAR, molecular docking, and molecular dynamics simulation methods. Mol. Simul. 2024, 12, 571–587. [Google Scholar] [CrossRef]
- Râpă, M.; Gaidau, C.; Stefan, L.M.; Lazea-Stoyanova, A.; Berechet, M.D.; Iosageanu, A.; Matei, E.; Jankauskaitė, V.; Predescu, C.; Valeika, V.; et al. Donkey Gelatin and Keratin Nanofibers Loaded with Antioxidant Agents for Wound Healing Dressings. Gels 2024, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Haxhiraj, M.; White, K.; Terry, C. The Role of Fenugreek in the Management of Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 6987. [Google Scholar] [CrossRef] [PubMed]
- Gaidau, C.; Râpă, M.; Ionita, G.; Stanculescu, I.R.; Zaharescu, T.; Constantinescu, R.-R.; Lazea-Stoyanova, A.; Stanca, M. The Influence of Gamma Radiation on Different Gelatin Nanofibers and Gelatins. Gels 2024, 10, 226. [Google Scholar] [CrossRef]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef]
- Gaidău, C.; Râpă, M.; Stefan, L.M.; Matei, E.; Berbecaru, A.C.; Predescu, C.; Mititelu-Tartau, L. Conversion of Animal-Derived Protein By-Products into a New Dual-Layer Nanofiber Biomaterial by Electrospinning Process. Fibers 2023, 11, 87. [Google Scholar] [CrossRef]
- Mao, H.; Zhou, J.; Yan, L.; Zhang, S.; Yu, D.G. Hybrid films loaded with 5-fluorouracil and Reglan for synergistic treatment of colon cancer via asynchronous dual-drug delivery. Front. Bioeng. Biotechnol. 2024, 12, 1398730. [Google Scholar] [CrossRef]
- Madheswaran, D.; Sivan, M.; Hauzerova, S.; Kostakova, E.K.; Jencova, V.; Valtera, J.; Behalek, L.; Mullerova, J.; Nguyen, N.H.A.; Capek, L.; et al. Continuous fabrication of braided composite nanofibrous surgical yarns using advanced AC electrospinning and braiding technology. Compos. Commun. 2024, 48, 101932. [Google Scholar] [CrossRef]
- Peng, W.; Wang, L.; Zhang, M.; Yu, D.G.; Li, X. Biodegradable flexible conductive film based on sliver nanowires and PLA electrospun fibers. J. Appl. Polym. Sci. 2024, 141, e55433. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, C.; Zhai, H.; Peng, C.; Yu, X.; Zheng, X.; Zhang, H.; Wang, X.; Yu, L.; Wang, S.; et al. Electrospun Scaffolds are Not Necessarily Always Made of Nanofibers as Demonstrated by Polymeric Heart Valves for Tissue Engineering. Adv. Healthc. Mater. 2024, 13, 2303395. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhou, J.; Chen, Y.; Yu, D.G.; Liu, P. A combined electrohydrodynamic atomization method for preparing nanofiber/microparticle hybrid medicines. Front. Bioeng. Biotechnol. 2023, 11, 1308004. [Google Scholar] [CrossRef] [PubMed]
- Javadi, B.; Mohsenzadeh, M. Electrospun PEO/WPI Nanofibers with Vanillin for Food Applications. Food Biophys. 2024, 19, 425–438. [Google Scholar] [CrossRef]
- Kan, Y.; Bondareva, J.V.; Statnik, E.S.; Koudan, E.V.; Ippolitov, E.V.; Podporin, M.S.; Kovaleva, P.A.; Kapaev, R.R.; Gordeeva, A.M.; Cvjetinovic, J.; et al. Hydrogel-Inducing Graphene-Oxide-Derived Core–Shell Fiber Composite for Antibacterial Wound Dressing. Int. J. Mol. Sci. 2023, 24, 6255. [Google Scholar] [CrossRef]
- Zheng, W.; Bai, Z.; Huang, S.; Jiang, K.; Liu, L.; Wang, X. The Effect of Angiogenesis-Based Scaffold of MesoporousBioactive Glass Nanofiber on Osteogenesis. Int. J. Mol. Sci. 2022, 23, 12670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Huang, Y.-C.; Kuo, C.-Y.; Kuo, C.-Y.; Chin, C.-Y.; Yip, P.K.; Chen, J.-P. Docosahexaenoic Acid-Loaded Polylactic Acid Core-Shell Nanofiber Membranes for Regenerative Medicine after Spinal Cord Injury: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 7031. [Google Scholar] [CrossRef]
- Başbuğ, B.; Beycan, B.; Erdoğan, M.K.; Karakışla, M.; Saçak, M. Exploring Waste Wool Derived-Keratin Nanofiber Architectures on Cotton Fabrics: Electrospinning Strategies for Enhanced Material Properties. ACS Appl. Polym. Mater. 2024, 6, 7417–7429. [Google Scholar] [CrossRef]
- Qosim, N.; Majd, H.; Huo, S.; Edirisinghe, M.; Williams, G.R. Hydrophilic and hydrophobic drug release from core (polyvinylpyrrolidone)-sheath (ethyl cellulose) pressure-spun fibers. Int. J. Pharm. 2024, 654, 123972. [Google Scholar] [CrossRef]
- Ismaili, D.; Parın, F.N.; Sıcak, Y.; Öztürk, M.; Terzioğlu, P. Electrospun lavender essential oil-loaded polylactic acid nanofibrous mats for antioxidant applications. Polym. Bull. 2024. [Google Scholar] [CrossRef]
- Geng, Y.; Williams, G.R. Developing and scaling up captopril-loaded electrospun ethyl cellulose fibers for sustained-release floating drug delivery. Int. J. Pharm. 2023, 648, 123557. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, V.; Patel, R. A Brief Review of the Essential Role of Nanovehicles for Improving the Therapeutic Efficacy of Pharmacological Agents Against Tumours. Curr. Drug Deliv. 2022, 19, 301–316. [Google Scholar] [CrossRef]
- Murugesan, R.; Raman, S. Recent Trends in Carbon Nanotubes Based Prostate Cancer Therapy: A Biomedical Hybrid for Diagnosis and Treatment. Curr. Drug Deliv. 2022, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Naik, I.; Menon, M.; Ambre, P.; Coutinho, E. A Review on Exploring the Opportunities of Polymer Drug Conjugated Systems for Targeted Cancer Treatment. Curr. Drug Deliv. 2023, 20, 8–30. [Google Scholar] [CrossRef]
- Riaz, Z.; Baddi, S.; Gao, F.; Feng, C.-L. Gallic acid-doped multifunctional hybrid hydrogel for antioxidant and antibacterial studies. Eur. Polym. J. 2024, 206, 112778. [Google Scholar] [CrossRef]
- Șerban, A.-M.; Nacu, I.; Rosca, I.; Ghilan, A.; Rusu, A.G.; Niță, L.E.; Darie-Niță, R.N.; Chiriac, A.P. Preparation and Characterization of Polymeric Microparticles Based on Poly(ethylene brassylate-co-squaric Acid) Loaded with Norfloxacin. Pharmaceutics 2024, 16, 550. [Google Scholar] [CrossRef] [PubMed]
- Darie-Nita, R.N.; Rapa, M. Micro- and macrostructure of polyimide blends and composites: Methods of investigations. In Polyimides; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Râpă, M.; Darie-Niță, R.N. Polymers in wound dressing. In Polymeric Materials for Biomedical Implants; Woodhead Publishing: Cambridge, UK, 2024. [Google Scholar] [CrossRef]
- Ajalli, N.; Pourmadadi, M.; Yazdian, F.; Abdouss, M.; Rashedi, H.; Rahdar, A. PVA Based Nanofiber Containing GO Modified with Cu Nanoparticles and Loaded Curcumin; High Antibacterial Activity with Acceleration Wound Healing. Curr. Drug Deliv. 2023, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cheng, Y.; Luo, Y.; Teng, Y.; Liu, Y.; Feng, L.; Wang, N.; Zhao, Y. Electrospun Nanofiber Materials for Photothermal Interfacial Evaporation. Materials 2023, 16, 5676. [Google Scholar] [CrossRef]
- González López, E.J.; Palacios, Y.B.; Martinez, S.R.; Durantini, A.M.; Durantini, E.N.; Abraham, G.A.; Bongiovanni Abel, S.; Heredia, D.A. Light-Activated Antibacterial Ethylcellulose Electrospun Nanofibrous Mats Containing Fluorinated Zn(II) Porphyrin. ACS Appl. Polym. Mater. 2024, 6, 7691–7704. [Google Scholar] [CrossRef]
- Yan, S.; Qian, Y.; Haghayegh, M.; Xia, Y.; Yang, S.; Cao, R.; Zhu, M. Electrospun organic/inorganic hybrid nanofibers for accelerating wound healing: A review. J. Mater. Chem. B 2024, 12, 3171–3190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, N.; Ren, Q.; Niu, S.; Zhu, L.; Hong, L.; Cui, K.; Wang, X.; Jiang, W.; Wen, M.; et al. Janus Nanofiber Membranes with Photothermal-Enhanced Biofluid Drainage and Sterilization for Diabetic Wounds. Adv. Funct. Mater. 2024, 34, 2315020. [Google Scholar] [CrossRef]
- Nazarkina, Z.K.; Stepanova, A.O.; Chelobanov, B.P.; Kvon, R.I.; Simonov, P.A.; Karpenko, A.A.; Laktionov, P.P. Activated Carbon-Enriched Electrospun-Produced Scaffolds for Drug Delivery/Release in Biological Systems. Int. J. Mol. Sci. 2023, 24, 6713. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Zhang, C.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Designer fibers from 2D to 3D—Simultaneous and controlled engineering of morphology, shape and size. Chem. Eng. J. 2018, 334, 89–98. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Zhang, C.; Xing, Z.; Ahmad, Z.; Ding, Q.; Chang, M.-W. Controlled engineering of multifunctional porous structures using tri-needle co-axial electrohydrodynamic flow and sacrificial media. Chem. Eng. J. 2022, 429, 132221. [Google Scholar] [CrossRef]
- Wang, L.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Multi-compartment centrifugal electrospinning based composite fibers. Chem. Eng. J. 2017, 330, 541–549. [Google Scholar] [CrossRef]
- Li, D.; Yue, G.; Li, S.; Liu, J.; Li, H.; Gao, Y.; Liu, J.; Hou, L.; Liu, X.; Cui, Z.; et al. Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers. Engineering 2022, 13, 116–127. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Zhu, L.; Miao, Y.; Zhu, Y.; Yue, B. Tailored Drug Delivery Platforms: Stimulus-Responsive Core–Shell Structured Nanocarriers. Adv. Healthc. Mater. 2024, 13, 2301726. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, H.; Liu, H.; Zhao, P.; Yu, D.G. Electrosprayed Stearic-Acid-Coated Ethylcellulose Microparticles for an Improved Sustained Release of Anticancer Drug. Gels 2023, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.G. Processes of Electrospun Polyvinylidene Fluoride-Based Nanofibers, Their Piezoelectric Properties, and Several Fantastic Applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef] [PubMed]
- Jianxin, L.; Hengzhe, Y.; Xuedi, C.; Xiaolei, Z.; Junlin, F. Review of electrospinning technology of photocatalysis, electrocatalysis and magnetic response. J. Mater. Sci. 2024, 59, 10623–10649. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, Y.-E.; Li, C.; Wang, K.; Yu, D.G. Enhancing diabetic wound healing: Advances in electrospun scaffolds from pathogenesis to therapeutic applications. Front. Bioeng. Biotechnol. 2024, 12, 1354286. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, R.; Liang, Z.; Guo, J.; Chen, B.; Zhou, S.; Yu, D. Application of Electrospun Drug-Loaded Nanofibers in Cancer Therapy. Polymers 2024, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Liu, Y.; Chen, S.; Zhang, C.; Chen, X.; Liu, Y.; Liu, P. Electrospun core–sheath PCL nanofibers loaded with nHA and simvastatin and their potential bone regeneration applications. Front. Bioeng. Biotechnol. 2023, 11, 1205252. [Google Scholar] [CrossRef]
- Huang, C.; Wang, M.; Yu, S.; Yu, D.G.; Bligh, S.W.A. Electrospun Fenoprofen/Polycaprolactone @ Tranexamic Acid/Hydroxyapatite Nanofibers as Orthopedic Hemostasis Dressings. Nanomaterials 2024, 14, 646. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Liu, P. Electrospun Core–Sheath Nanofibers with a Cellulose Acetate Coating for the Synergistic Release of Zinc Ion and Drugs. Mol. Pharm. 2024, 21, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Y.; Liao, Q.; Qian, Y.; Zhu, L.; Yu, D.G.; Xu, Y.; Lu, X.; Kim, I.; Song, W. Silver oxide decorated urchin-like microporous organic polymer composites as versatile antibacterial organic coating materials. J. Mater. Chem. B 2024, 12, 2054–2069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Li, Y.; Xu, Y.; Song, W. Poly(Glutamic Acid)-Engineered Nanoplatforms for Enhanced Cancer Phototherapy. Curr. Drug Deliv. 2024, 21, 326–338. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.G. Engineered Spindles of Little Molecules Around Electrospun Nanofibers for Biphasic Drug Release. Adv. Fiber Mater. 2022, 4, 305–317. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Alizadeh, A.M.; Mousavi, M.; Hashempour-baltork, F.; Kooki, S.; Shadan, M.R.; Hosseini, S.M.; McClements, D.J. Advances and applications of crosslinked electrospun biomacromolecular nanofibers. Int. J. Biol. Macromol. 2024, 271, 132743. [Google Scholar] [CrossRef]
- Song, W.; Wen, Y.; Wang, Z.; Xu, H.; Liao, Q.; Tang, Y.; Yu, D.G.; Kim, I. Versatile Hyper-Cross-Linked Polymers Derived from Waste Polystyrene: Synthesis, Properties, and Intentional Recycling. Langmuir 2024, 40, 16670–16689. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Yu, D.G.; Bligh, S.-W.A. Alginate-Based Electrospun Nanofibers and the Enabled Drug Controlled Release Profiles: A Review. Biomolecules 2024, 14, 789. [Google Scholar] [CrossRef]
- Yu, D.G.; Gong, W.; Zhou, J.; Liu, Y.; Zhu, Y.; Lu, X. Engineered shapes using electrohydrodynamic atomization for an improved drug delivery. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1964. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Lin, X.; Yan, J.; Yu, D.G.; Liu, P.; Yang, H. Electrospun multi-chamber core–shell nanofibers and their controlled release behaviors: A review. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1954. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Q.; Wang, H.; Liu, H.; Yu, D.G.; Bligh, S.-W.A.; Lu, X. Electrospun multi-functional medicated tri-section Janus nanofibers for an improved anti-adhesion tendon repair. Chem. Eng. J. 2024, 492, 152359. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, K.; Xia, Y.; Qian, C.; Yu, D.G.; Xie, Y.; Liao, Y. Electrospun trilayer eccentric Janus nanofibers for a combined treatment of periodontitis. Adv. Fiber Mater. 2024, 6, 1053–1073. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, L.; Chen, Z.; Yu, Y. Engineering multi-level structured electrospun nanofiber Janus membrane for direct contact membrane distillation. Colloids Surf. C Environ. Asp. 2024, 2, 100041. [Google Scholar] [CrossRef]
- Türkoğlu, G.C.; Khomarloo, N.; Mohsenzadeh, E.; Gospodinova, D.N.; Neznakomova, M.; Salaün, F. PVA-Based Electrospun Materials—A Promising Route to Designing Nanofiber Mats with Desired Morphological Shape—A Review. Int. J. Mol. Sci. 2024, 25, 1668. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, T.; Cheng, Y.; Zhao, K.; Meng, Z.; Huang, J.; Cai, W.; Lai, Y. Recent Advances in Functional Cellulose-Based Materials: Classification, Properties, and Applications. Adv. Fiber Mater. 2024. [Google Scholar] [CrossRef]
- do Nascimento Soares, G.O.; Ribeiro Lima Machado, R.; Mendonça Diniz, M.; da Silva, A.B. Electrospun progesterone-loaded cellulose acetate nanofibers and their drug sustained-release profiles. Polym. Eng. Sci. 2020, 60, 3231–3243. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Shahir, S.; Mohd Bohari, S.P.; Nayan, N.H.M.; Razak, S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef]
- Arslan, O.; Aytac, Z.; Uyar, T. Superhydrophobic, Hybrid, Electrospun Cellulose Acetate Nanofibrous Mats for Oil/Water Separation by Tailored Surface Modification. ACS Appl. Mater. Interfaces 2016, 8, 19747–19754. [Google Scholar] [CrossRef]
- Singh, G. Resveratrol: Nanocarrier-Based Delivery Systems to Enhance its Therapeutic Potential. Nanomedicine 2020, 15, 2081–2817. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Albanawany, N.M.; Samy, D.M.; Zahran, N.; El-Moslemany, R.M.; Elsawy, S.M.; Nazel, M.W.A. Histopathological, physiological and biochemical assessment of resveratrol nanocapsules efficacy in bleomycin-induced acute and chronic lung injury in rats. Drug Deliv. 2022, 29, 2592–2608. [Google Scholar] [CrossRef] [PubMed]
- Ioniţă, S.; Lincu, D.; Mitran, R.-A.; Ziko, L.; Sedky, N.K.; Deaconu, M.; Brezoiu, A.-M.; Matei, C.; Berger, D. Resveratrol Encapsulation and Release from Pristine and Functionalized Mesoporous Silica Carriers. Pharmaceutics 2022, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Dzenis, Y. Spinning Continuous Fibers for Nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, W.; Gong, W.; Lu, Y.; Yu, D.G.; Liu, P. Recent progress of electrospun nanofibers as burning dressings. RSC Adv. 2024, 14, 14374–14391. [Google Scholar] [CrossRef]
- Zhou, J.; Pan, H.; Gong, W.; Yu, D.G.; Sun, Y. Electrosprayed Eudragit RL100 nanoparticles with Janus polyvinylpyrrolidone patches for multiphase release of paracetamol. Nanoscale 2024, 16, 8573–8582. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.; Zhou, J.; Bai, Y.; Shi, H.; Yue, B.; Shen, S.; Yu, D.G. Efficient piezophotocatalysis of ZnO@PVDF coaxial nanofibers modified with BiVO4 and Ag for the simultaneous generation of H2O2 and removal of pefloxacin and Cr(VI) in water. Chem. Eng. J. 2024, 484, 149514. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Liu, Y.; Huang, T.; Xing, J.; Ge, R.; Yu, D.G. Electrospun medicated gelatin/polycaprolactone Janus fibers for photothermal-chem combined therapy of liver cancer. Int. J. Biol. Macromol. 2024, 269, 132113. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Zhang, Z.; Yu, D.G.; Bligh, S.W.A. Integrated Janus nanofibers enabled by a co-shell solvent for enhancing icariin delivery efficiency. Int. J. Pharm. 2024, 658, 124180. [Google Scholar] [CrossRef]
- Abid, S.; Wang, L.; Haider, M.K.; Mayakrishnan, G.; Lakshminarayanan, R.; Kim, K.O.; Ullah, A.; Kim, I.S. Investigating alginate and chitosan electrospun nanofibers as a potential wound dressing: An in vitro study. Nanocomposites 2024, 10, 254–267. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Qosim, N.; Majd, H.; Ahmed, J.; Williams, G.; Edirisinghe, M. Making fibers from cellulose derivatives by pressurized gyration and electrospinning. Cellulose 2024, 31, 2815–2832. [Google Scholar] [CrossRef]
- Alzahrani, D.A.; Alsulami, K.A.; Alsulaihem, F.M.; Bakr, A.A.; Booq, R.Y.; Alfahad, A.J.; Aodah, A.H.; Alsudir, S.A.; Fathaddin, A.A.; Alyamani, E.J.; et al. Dual Drug-Loaded Coaxial Nanofiber Dressings for the Treatment of Diabetic Foot Ulcer. Int. J. Nanomed. 2024, 19, 5681–5703. [Google Scholar] [CrossRef]
- Gong, W.; Yang, W.; Zhou, J.; Zhang, S.; Yu, D.G.; Liu, P. Engineered beads-on-a-string nanocomposites for an improved drug fast-sustained bi-stage release. Nanocomposites 2024, 10, 240–253. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, J.; Fang, B.; Ying, Y.; Yu, D.G.; He, H. Three EHDA Processes from a Detachable Spinneret for Fabricating Drug Fast Dissolution Composites. Macromol. Mater. Eng. 2024, 309, 2300361. [Google Scholar] [CrossRef]
- Brimo, N.; Serdaroğlu, D.Ç.; Uyar, T.; Uysal, B.; Çakıcı, E.B.; Dikmen, M.; Canturk, Z. Novel Electrospun Polymeric Nanofibers Loaded Different Medicaments as Drug Delivery Systems for Regenerative Endodontics. Curr. Drug Deliv. 2023, 20, 992–1014. [Google Scholar] [CrossRef] [PubMed]
- Tayebi-Khorrami, V.; Rahmanian-Devin, P.; Fadaei, M.R.; Movaffagh, J.; Askari, V.R. Advanced applications of smart electrospun nanofibers in cancer therapy: With insight into material capabilities and electrospinning parameters. Int. J. Pharm. X 2024, 8, 100265. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Abu-Serie, M.M. Preparation of poly(vinyl alcohol) nanofibers containing disulfiram-copper complex by electrospinning: A potential delivery system against melanoma. DARU J. Pharm. Sci. 2024. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Rangappa, S.M.; Siengchin, S. Advanced characterization techniques for nanostructured materials in biomedical applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 122–143. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, B.; Meng, X.; Ye, X.; Luo, T.; Xin, X.; Wen, Z. Effect of NiO Addition on the Sintering and Electrochemical Properties of BaCe0.55Zr0.35Y0.1O3-δ Proton-Conducting Ceramic Electrolyte. Membranes 2024, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ma, J.; Zhu, L.; Wang, H.; Hao, W.; Min, Y.; Bi, Q.; Li, G. Silver Nanowires Cascaded Layered Double Hydroxides Nanocages with Enhanced Directional Electron Transport for Efficient Electrocatalytic Oxygen Evolution. Small 2024, 20, 2309859. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.S.; Khanfar, M.S. Enhancement of the Solubility of Asenapine Maleate Through the Preparation of Co-Crystals. Curr. Drug Deliv. 2022, 19, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Teixeira, N.; Sarmento, B.; Vasconcelos, T. Freeze-Drying Cycle Optimization of An Amorphous Solid Dispersion of Resveratrol. Eur. J. Pharm. Sci. 2024, 200, 106855. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics 2023, 15, 1364. [Google Scholar] [CrossRef] [PubMed]
- Rusdin, A.; Mohd Gazzali, A.; Ain Thomas, N.; Megantara, S.; Aulifa, D.L.; Budiman, A.; Muchtaridi, M. Advancing Drug Delivery Paradigms: Polyvinyl Pyrolidone (PVP)-Based Amorphous Solid Dispersion for Enhanced Physicochemical Properties and Therapeutic Efficacy. Polymers 2024, 16, 286. [Google Scholar] [CrossRef]
- Morillo-Bargues, M.J.; Osorno, A.O.; Guerri, C.; Pradas, M.M.; Martínez-Ramos, C. Characterization of Electrospun BDMC-Loaded PLA Nanofibers with Drug Delivery Function and Anti-Inflammatory Activity. Int. J. Mol. Sci. 2023, 24, 10340. [Google Scholar] [CrossRef]
- Mottola, S.; Viscusi, G.; Iannone, G.; Belvedere, R.; Petrella, A.; De Marco, I.; Gorrasi, G. Supercritical Impregnation of Mesoglycan and Lactoferrin on Polyurethane Electrospun Fibers for Wound Healing Applications. Int. J. Mol. Sci. 2023, 24, 9269. [Google Scholar] [CrossRef] [PubMed]
- Boda, R.; Lázár, I.; Keczánné-Üveges, A.; Bakó, J.; Tóth, F.; Trencsényi, G.; Kálmán-Szabó, I.; Béresová, M.; Sajtos, Z.; Tóth, E.D.; et al. β-Tricalcium Phosphate-Modified Aerogel Containing PVA/Chitosan Hybrid Nanospun Scaffolds for Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 7562. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Kim, E.J.; Tang, Y.; Xu, H.; Yu, D.-G.; Song, W.; Kim, B.J. Rational design of hyper-crosslinked polymers for biomedical applications. J. Polym. Sci. 2024, 62, 1517–1535. [Google Scholar] [CrossRef]
- Han, X.; Zhao, M.; Xu, R.; Zou, Y.; Wang, Y.; Liang, J.; Jiang, Q.; Sun, Y.; Fan, Y.; Zhang, X. Electrospun Hyaluronan Nanofiber Membrane Immobilizing Aromatic Doxorubicin as Therapeutic and Regenerative Biomaterial. Int. J. Mol. Sci. 2023, 24, 7023. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Sun, M.; Jia, W.; Liu, Y.; Wang, S.; Yang, Y.; Dai, Z.; Liu, L.; Cheng, Z.; Liu, G.; et al. The polycaprolactone and silk fibroin nanofibers with Janus-structured sheaths for antibacterial and antioxidant by loading Taxifolin. Heliyon 2024, 10, e33770. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Liu, Y.; Gao, J.; Wang, D.; Xia, D.; Liang, C.; Li, N.; Xu, R. Synergistic Effect of Co-Delivering Ciprofloxacin and Tetracycline Hydrochloride for Promoted Wound Healing by Utilizing Coaxial PCL/Gelatin Nanofiber Membrane. Int. J. Mol. Sci. 2022, 23, 1895. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Liu, Y.; Cao, W.; Gao, J.; Wang, D.; Lin, M.; Liang, C.; Li, N.; Xu, R. Dual-Functional Nanofibrous Patches for Accelerating Wound Healing. Int. J. Mol. Sci. 2022, 23, 10983. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Y.; Zhang, J.; Zhang, H.; Guo, B. Structural and Functional Design of Electrospun Nanofibers for Hemostasis and Wound Healing. Adv. Fiber Mater. 2022, 4, 1027–1057. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Irimia, A.; Doroftei, F.; Stefan, L.M.; Iwanczuk, A.; Trusz, A. Bioactive and Physico-Chemical Assessment of Innovative Poly(lactic acid)-Based Biocomposites Containing Sage, Coconut Oil, and Modified Nanoclay. Int. J. Mol. Sci. 2023, 24, 3646. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M.V. Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. Int. J. Mol. Sci. 2023, 24, 4962. [Google Scholar] [CrossRef]
- Huang, Y.; Zhan, M.; Shen, M.; Zhang, L.; Shi, X. Electrospun Short Fibers: A New Platform for Cancer Nanomedicine Applications. Explor. Drug Sci. 2023, 1, 454–467. [Google Scholar] [CrossRef]



| Sample No. | Electro- Spinning | Operational Parameters a | Drug Content (%) | CA Content (%) | |||
|---|---|---|---|---|---|---|---|
| V (kV) | F (mL/h) | L (cm) | |||||
| Core | Shell | ||||||
| F1 | Blending | 11 | 0.0 | 2.0 | 20 | 25% | 50% |
| F2 | Blending | 11 | 2.0 | 0.0 | 20 | 50% | 25% |
| F3 | Coaxial | 11 | 1.0 | 1.0 | 20 | 37.5% b | 37.5% b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Gong, W.; Zhang, Z.; Zhou, J.; Yu, D.-G.; Yi, T. Reverse Gradient Distributions of Drug and Polymer Molecules within Electrospun Core–Shell Nanofibers for Sustained Release. Int. J. Mol. Sci. 2024, 25, 9524. https://doi.org/10.3390/ijms25179524
Chen Y, Gong W, Zhang Z, Zhou J, Yu D-G, Yi T. Reverse Gradient Distributions of Drug and Polymer Molecules within Electrospun Core–Shell Nanofibers for Sustained Release. International Journal of Molecular Sciences. 2024; 25(17):9524. https://doi.org/10.3390/ijms25179524
Chicago/Turabian StyleChen, Yaoning, Wenjian Gong, Zhiyuan Zhang, Jianfeng Zhou, Deng-Guang Yu, and Tao Yi. 2024. "Reverse Gradient Distributions of Drug and Polymer Molecules within Electrospun Core–Shell Nanofibers for Sustained Release" International Journal of Molecular Sciences 25, no. 17: 9524. https://doi.org/10.3390/ijms25179524





