Transient Increases in Neural Oscillations and Motor Deficits in a Mouse Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
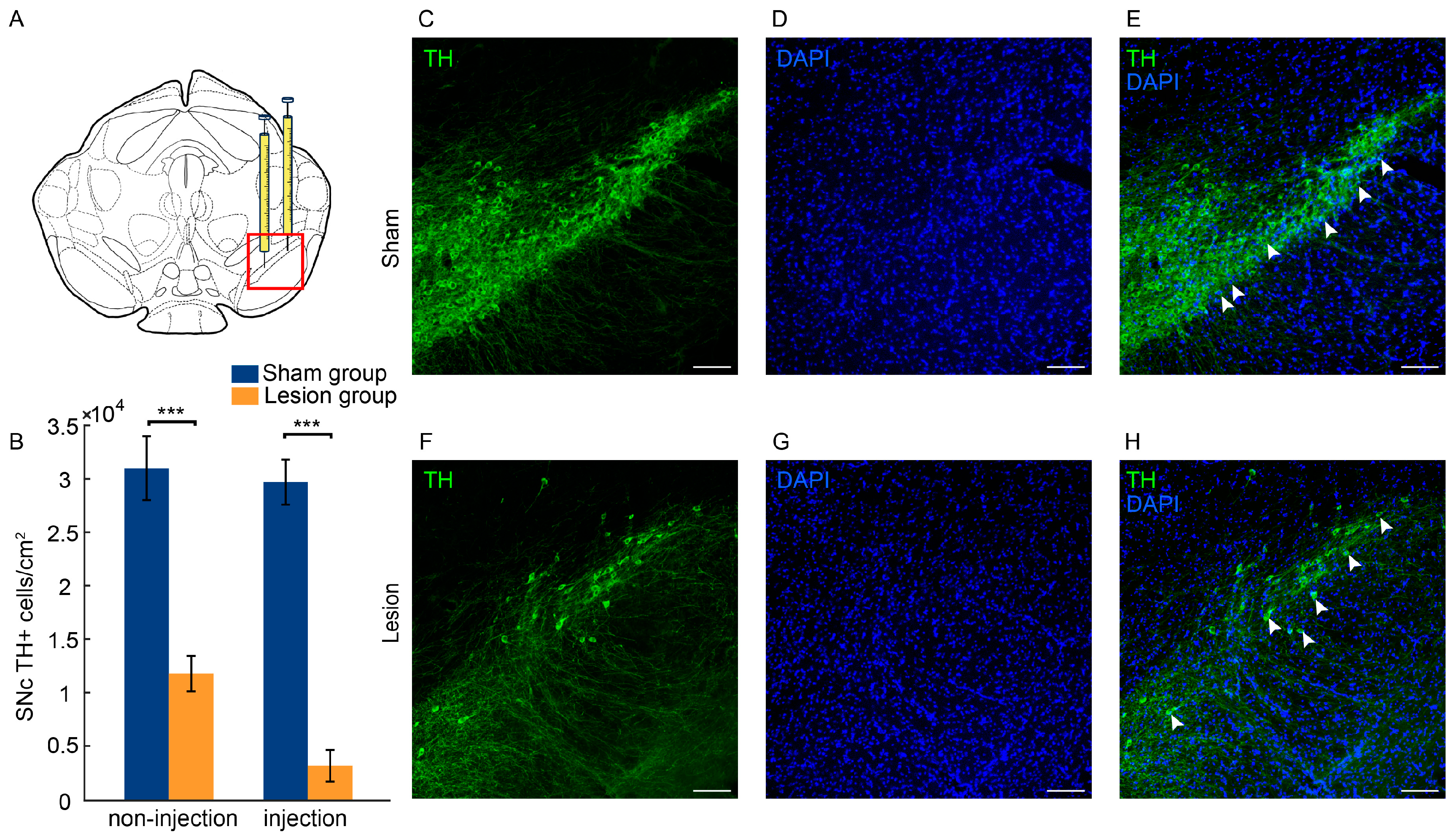
2.1. Histological Evaluation
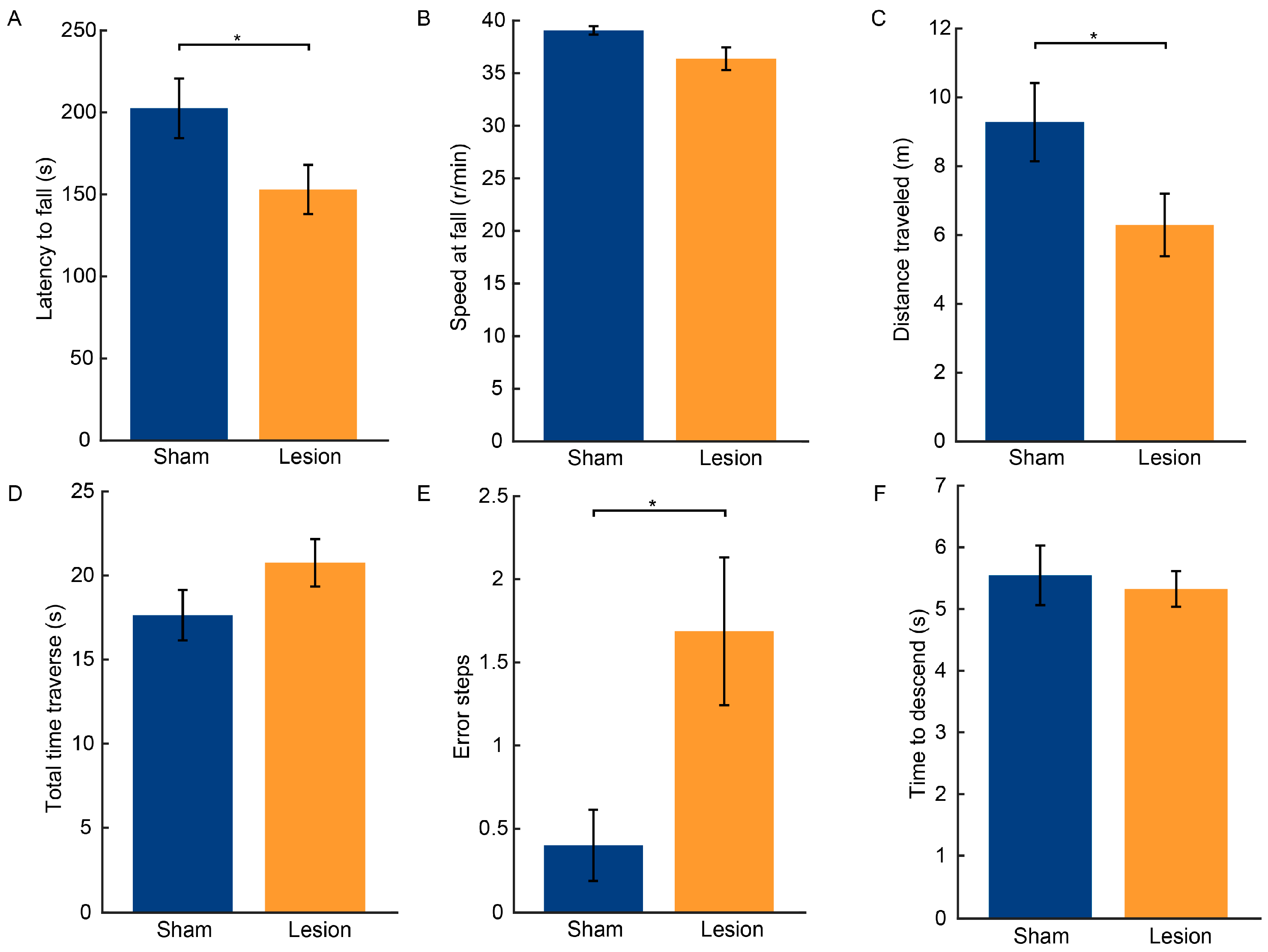
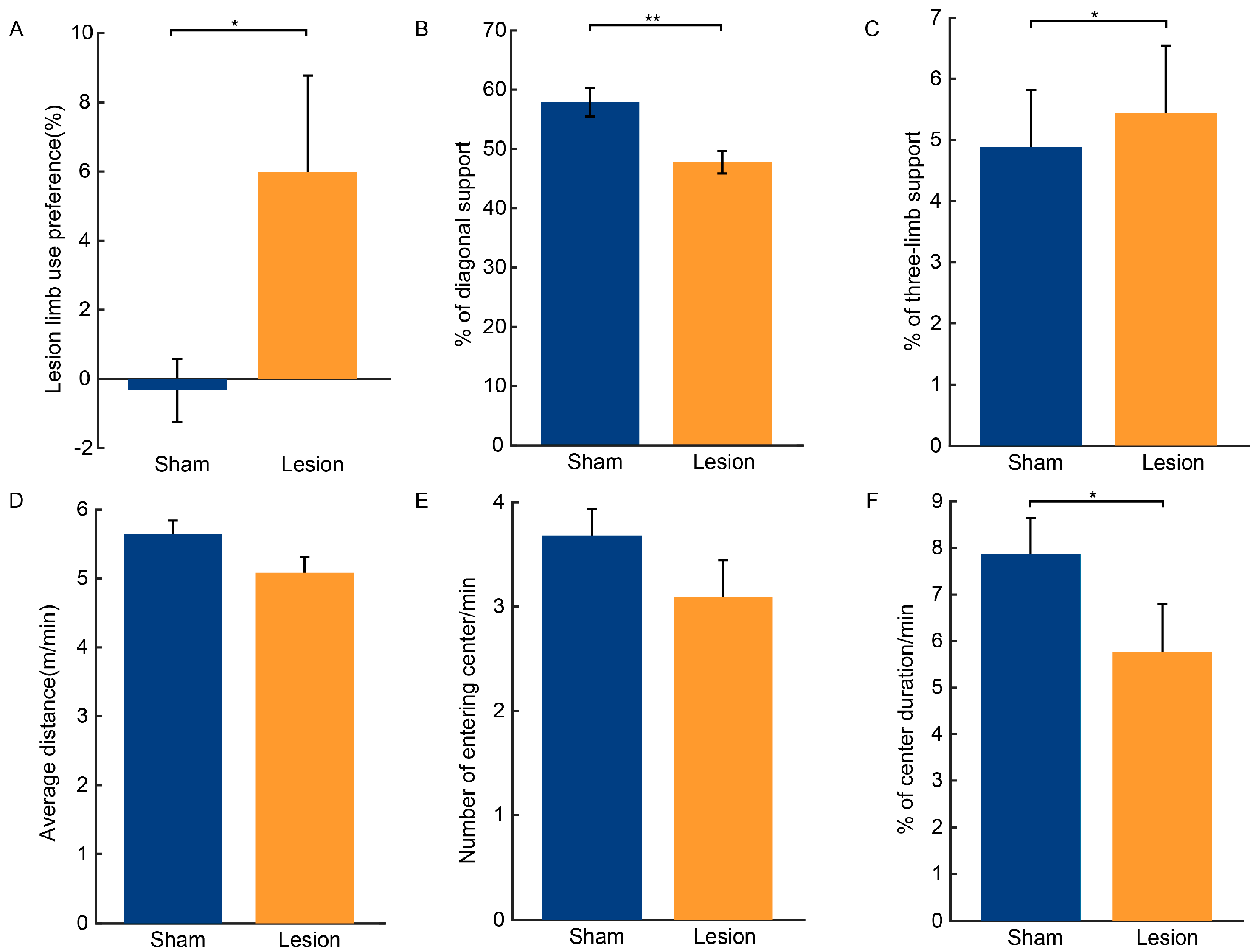
2.2. Results of Behavioral Tests
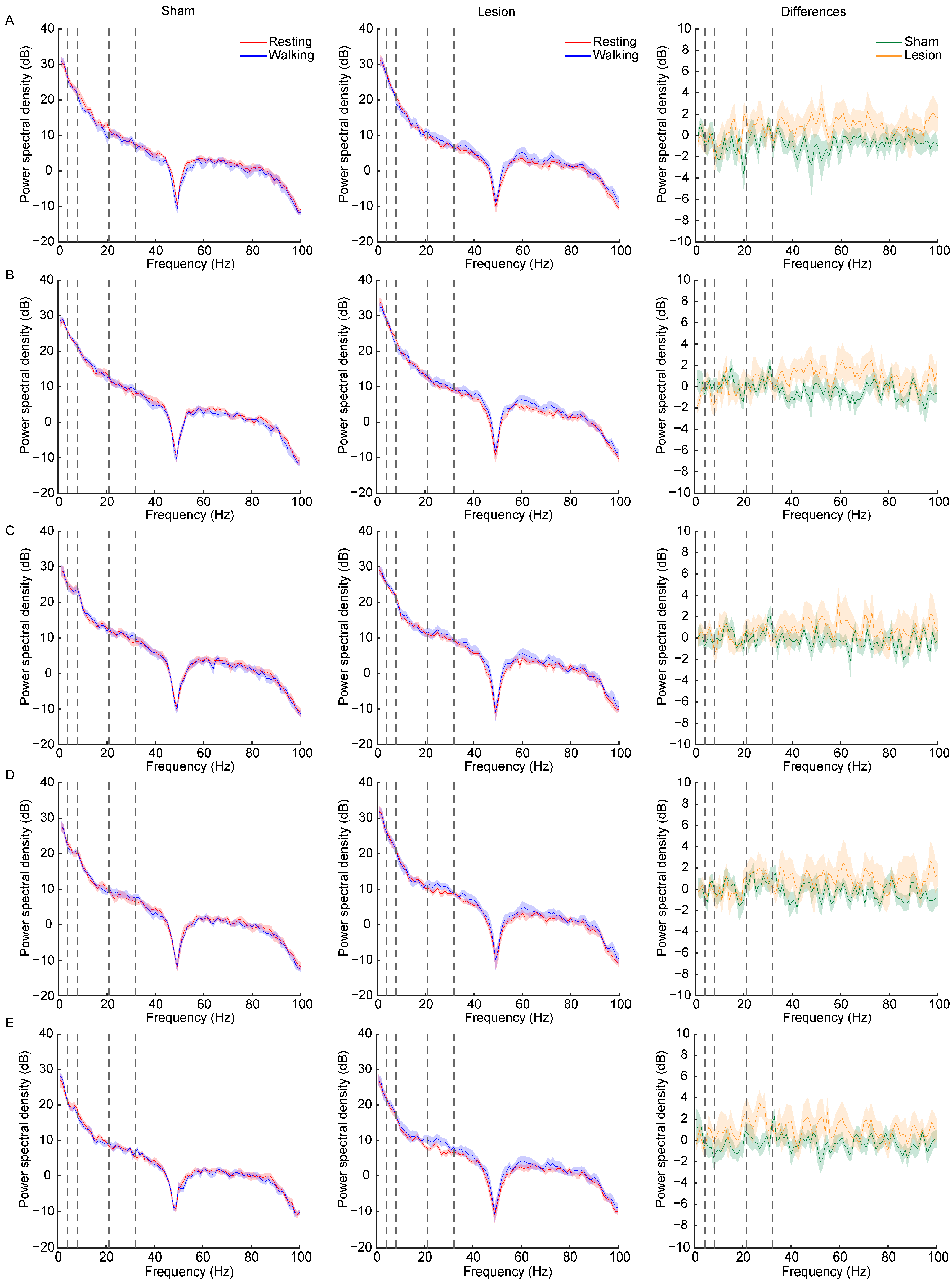
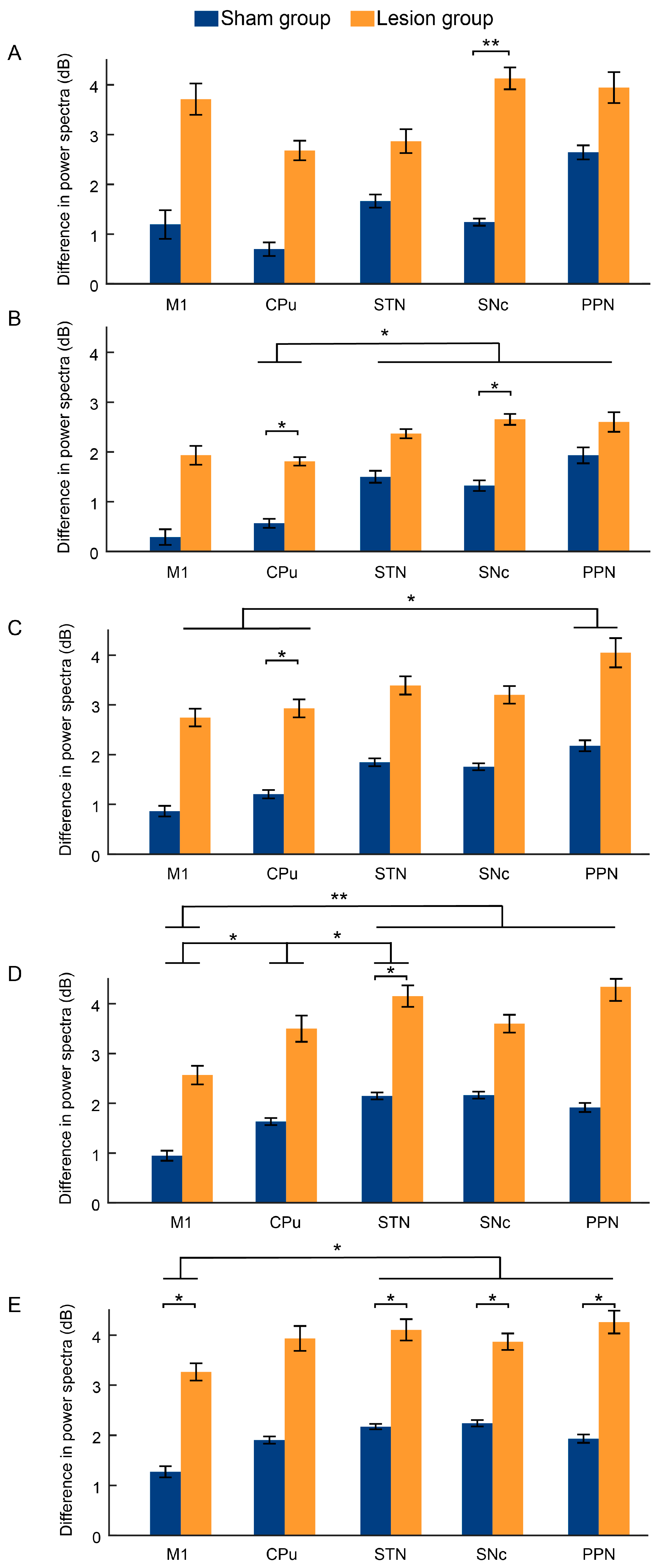
2.3. Dynamic Changes in LFP Power Spectra Following Dopaminergic Lesion across Resting and Walking Phases
3. Discussion
3.1. Loss of Dopaminergic Neurons in the SNc and Motor Function Impairments Resulting from 6-OHDA
3.2. Transient Changes in LFP Bands after 6-OHDA Lesion
4. Materials and Methods
4.1. Animals
4.2. Surgery
4.2.1. Unilateral 6-OHDA Lesions
4.2.2. Electrode Implantation
4.3. Behavioral Tests
4.3.1. Rotarod Test
4.3.2. Pole Test
4.3.3. Beam Traversal Test
4.3.4. Open Field Test
4.3.5. Rearing Cylinder Test
4.3.6. Gait Analysis
4.4. Electrophysiological Recordings and Data Analysis
4.5. Histology and Immunohistochemistry
4.6. Cell Counting
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis; Exon Publications: Brisbane, Australia, 2018; pp. 3–26. [Google Scholar]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Lin, G.; Wang, L.; Marcogliese, P.C.; Bellen, H.J. Sphingolipids in the pathogenesis of parkinson’s disease and parkinsonism. Trends Endocrinol. Metab. 2019, 30, 106–117. [Google Scholar] [CrossRef]
- Lemaire, N.; Hernandez, L.F.; Hu, D.; Kubota, Y.; Howe, M.W.; Graybiel, A.M. Effects of dopamine depletion on lfp oscillations in striatum are task-and learning-dependent and selectively reversed by l-dopa. Proc. Natl. Acad. Sci. USA 2012, 109, 18126–18131. [Google Scholar] [CrossRef] [PubMed]
- Galvan, A.; Devergnas, A.; Wichmann, T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front. Neuroanat. 2015, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhu, G.; Zhao, B.; Bai, Y.; Jiang, Y.; Neumann, W.-J.; Kühn, A.A.; Zhang, J. Local field potentials in parkinson’s disease: A frequency-based review. Neurobiol. Dis. 2021, 155, 105372. [Google Scholar] [CrossRef]
- Moënne-Loccoz, C.; Astudillo-Valenzuela, C.; Skovgård, K.; Salazar-Reyes, C.A.; Barrientos, S.A.; García-Núñez, X.P.; Cenci, M.A.; Petersson, P.; Fuentes-Flores, R.A. Cortico-striatal oscillations are correlated to motor activity levels in both physiological and parkinsonian conditions. Front. Syst. Neurosci. 2020, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Kilavik, B.E.; Ponce-Alvarez, A.; Trachel, R.; Confais, J.; Takerkart, S.; Riehle, A. Context-related frequency modulations of macaque motor cortical lfp beta oscillations. Cereb. Cortex 2012, 22, 2148–2159. [Google Scholar] [CrossRef]
- Kristeva-Feige, R.; Feige, B.; Makeig, S.; Ross, B.; Elbert, T. Oscillatory brain activity during a motor task. Neuroreport 1993, 4, 1291–1294. [Google Scholar] [CrossRef]
- Aravamuthan, B.R.; Bergstrom, D.A.; French, R.A.; Taylor, J.J.; Parr-Brownlie, L.C.; Walters, J.R. Altered neuronal activity relationships between the pedunculopontine nucleus and motor cortex in a rodent model of parkinson’s disease. Exp. Neurol. 2008, 213, 268–280. [Google Scholar] [CrossRef]
- Devergnas, A.; Pittard, D.; Bliwise, D.; Wichmann, T. Relationship between oscillatory activity in the cortico-basal ganglia network and parkinsonism in mptp-treated monkeys. Neurobiol. Dis. 2014, 68, 156–166. [Google Scholar] [CrossRef]
- Parr-Brownlie, L.C.; Itoga, C.A.; Walters, J.R.; Underwood, C.F. Oscillatory waveform sharpness asymmetry changes in motor thalamus and motor cortex in a rat model of parkinson’s disease. Exp. Neurol. 2022, 354, 114089. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.; Mahant, N.; Hutchison, W.D.; Lozano, A.M.; Moro, E.; Hodaie, M.; Lang, A.E.; Dostrovsky, J.O. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in parkinson’s disease. J. Neurophysiol. 2006, 96, 3248–3256. [Google Scholar] [CrossRef] [PubMed]
- Kühn, A.A.; Kempf, F.; Brücke, C.; Doyle, L.G.; Martinez-Torres, I.; Pogosyan, A.; Trottenberg, T.; Kupsch, A.; Schneider, G.-H.; Hariz, M.I. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory β activity in patients with parkinson’s disease in parallel with improvement in motor performance. J. Neurosci. 2008, 28, 6165–6173. [Google Scholar] [CrossRef]
- Mallet, N.; Pogosyan, A.; Sharott, A.; Csicsvari, J.; Bolam, J.P.; Brown, P.; Magill, P.J. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J. Neurosci. 2008, 28, 4795–4806. [Google Scholar] [CrossRef]
- Hammond, C.; Bergman, H.; Brown, P. Pathological synchronization in parkinson’s disease: Networks, models and treatments. Trends Neurosci. 2007, 30, 357–364. [Google Scholar] [CrossRef]
- Swann, N.C.; De Hemptinne, C.; Miocinovic, S.; Qasim, S.; Wang, S.S.; Ziman, N.; Ostrem, J.L.; San Luciano, M.; Galifianakis, N.B.; Starr, P.A. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in parkinson’s disease. J. Neurosci. 2016, 36, 6445–6458. [Google Scholar] [CrossRef]
- Zemel, D.; Gritton, H.; Cheung, C.; Shankar, S.; Kramer, M.; Han, X. Dopamine depletion selectively disrupts interactions between striatal neuron subtypes and lfp oscillations. Cell Rep. 2022, 38, 110265. [Google Scholar] [CrossRef] [PubMed]
- Delaville, C.; McCoy, A.J.; Gerber, C.M.; Cruz, A.V.; Walters, J.R. Subthalamic nucleus activity in the awake hemiparkinsonian rat: Relationships with motor and cognitive networks. J. Neurosci. 2015, 35, 6918–6930. [Google Scholar] [CrossRef]
- Wenger, N.; Vogt, A.; Skrobot, M.; Garulli, E.L.; Kabaoglu, B.; Salchow-Hömmen, C.; Schauer, T.; Kroneberg, D.; Schuhmann, M.K.; Ip, C.W. Rodent models for gait network disorders in parkinson’s disease–a translational perspective. Exp. Neurol. 2022, 352, 114011. [Google Scholar] [CrossRef]
- Weinberger, M.; Hutchison, W.D.; Lozano, A.M.; Hodaie, M.; Dostrovsky, J.O. Increased gamma oscillatory activity in the subthalamic nucleus during tremor in parkinson’s disease patients. J. Neurophysiol. 2009, 101, 789–802. [Google Scholar] [CrossRef]
- Beudel, M.; Little, S.; Pogosyan, A.; Ashkan, K.; Foltynie, T.; Limousin, P.; Zrinzo, L.; Hariz, M.; Bogdanovic, M.; Cheeran, B. Tremor reduction by deep brain stimulation is associated with gamma power suppression in parkinson’s disease. Neuromodulation Technol. Neural Interface 2015, 18, 349–354. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, M.-Y.; Tu, P.-H.; Yeh, C.-H.; Lu, C.-S. F183. Oscillatory β activities in basal ganglia are associated with gait speed in pd. Clin. Neurophysiol. 2018, 129, e137. [Google Scholar] [CrossRef]
- Fraix, V.; Bastin, J.; David, O.; Goetz, L.; Ferraye, M.; Benabid, A.-L.; Chabardes, S.; Pollak, P.; Debû, B. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in parkinson’s disease. PLoS ONE 2013, 8, e83919. [Google Scholar] [CrossRef] [PubMed]
- Whalen, T.C.; Willard, A.M.; Rubin, J.E.; Gittis, A.H. Delta oscillations are a robust biomarker of dopamine depletion severity and motor dysfunction in awake mice. J. Neurophysiol. 2020, 124, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Di Bisceglie Caballero, S.; Ces, A.; Liberge, M.; Ambroggi, F.; Amalric, M.; Ouagazzal, A.-M. Optogenetic globus pallidus stimulation improves motor deficits in 6-hydroxydopamine-lesioned mouse model of parkinson’s disease. Int. J. Mol. Sci. 2023, 24, 7935. [Google Scholar] [CrossRef]
- Broom, L.; Worley, A.; Gao, F.; Hernandez, L.D.; Ashton, C.E.; Shih, L.C.; VanderHorst, V.G. Translational methods to detect asymmetries in temporal and spatial walking metrics in parkinsonian mouse models and human subjects with parkinson’s disease. Sci. Rep. 2019, 9, 2437. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, M.; Di Matteo, V.; Benigno, A.; Crescimanno, G.; Esposito, E.; Di Giovanni, G. The unilateral nigral lesion induces dramatic bilateral modification on rat brain monoamine neurochemistry. Ann. N. Y. Acad. Sci. 2009, 1155, 316–323. [Google Scholar] [CrossRef]
- Faria, M.H.; Simieli, L.; Rietdyk, S.; Penedo, T.; Santinelli, F.B.; Barbieri, F.A. (A) symmetry during gait initiation in people with parkinson’s disease: A motor and cortical activity exploratory study. Front. Aging Neurosci. 2023, 15, 1142540. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; McGinley, J.; Huxham, F.; Collier, J.; Iansek, R. Constraints on the kinetic, kinematic and spatiotemporal parameters of gait in parkinson’s disease. Hum. Mov. Sci. 1999, 18, 461–483. [Google Scholar] [CrossRef]
- Sitek, O.; Kalichová, M.; Hedbávný, P.; Boušek, T.; Baláž, M. Kinematic relations during double support phase in parkinsonian gait. Appl. Sci. 2022, 12, 949. [Google Scholar] [CrossRef]
- Keloth, S.M.; Arjunan, S.P.; Kumar, D.K. Variance of the gait parameters and fraction of double-support interval for determining the severity of parkinson’s disease. Appl. Sci. 2020, 10, 577. [Google Scholar] [CrossRef]
- d’Août, K.; Vereecke, E.; Schoonaert, K.; De Clercq, D.; Van Elsacker, L.; Aerts, P. Locomotion in bonobos (pan paniscus): Differences and similarities between bipedal and quadrupedal terrestrial walking, and a comparison with other locomotor modes. J. Anat. 2004, 204, 353–361. [Google Scholar] [CrossRef]
- Ruder, L.; Takeoka, A.; Arber, S. Long-distance descending spinal neurons ensure quadrupedal locomotor stability. Neuron 2016, 92, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Lu, G.; Hu, X.; Tsang, K.S.; Kwong, W.H.; Wu, F.X.; Meng, H.W.; Jiang, S.; Liu, S.W.; Ng, H.K. Quantitative assessment of gait and neurochemical correlation in a classical murine model of parkinson’s disease. BMC Neurosci. 2012, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Maricelli, J.W.; Lu, Q.L.; Lin, D.C.; Rodgers, B.D. Trendelenburg-like gait, instability and altered step patterns in a mouse model for limb girdle muscular dystrophy 2i. PLoS ONE 2016, 11, e0161984. [Google Scholar] [CrossRef] [PubMed]
- Isaias, I.U.; Volkmann, J.; Marzegan, A.; Marotta, G.; Cavallari, P.; Pezzoli, G. The influence of dopaminergic striatal innervation on upper limb locomotor synergies. PLoS ONE 2012, 7, e51464. [Google Scholar] [CrossRef]
- Costa, R.M.; Lin, S.-C.; Sotnikova, T.D.; Cyr, M.; Gainetdinov, R.R.; Caron, M.G.; Nicolelis, M.A. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 2006, 52, 359–369. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Wang, X.; Yao, X.; Han, H.; Gao, Y.; Chang, H.; Xiang, T.; Sun, S.; Wang, Y. Altered local field potential relationship between the parafascicular thalamic nucleus and dorsal striatum in hemiparkinsonian rats. Neurosci. Bull. 2019, 35, 315–324. [Google Scholar] [CrossRef]
- Bore, J.C.; Campbell, B.A.; Cho, H.; Gopalakrishnan, R.; Machado, A.G.; Baker, K.B. Prediction of mild parkinsonism revealed by neural oscillatory changes and machine learning. J. Neurophysiol. 2020, 124, 1698–1705. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Li, M.; Lv, S.; Xie, Z.; Li, N.; Wang, N.; Wang, J.; Luo, F.; Zhang, W. Eltoprazine prevents levodopa-induced dyskinesias by reducing causal interactions for theta oscillations in the dorsolateral striatum and substantia nigra pars reticulate. Neuropharmacology 2019, 148, 1–10. [Google Scholar] [CrossRef]
- Alonso-Frech, F.; Zamarbide, I.; Alegre, M.; Rodriguez-Oroz, M.C.; Guridi, J.; Manrique, M.; Valencia, M.; Artieda, J.; Obeso, J.A. Slow oscillatory activity and levodopa-induced dyskinesias in parkinson’s disease. Brain 2006, 129, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Foffani, G.; Pesenti, A.; Tamma, F.; Bianchi, A.; Pellegrini, M.; Locatelli, M.; Moxon, K.; Villani, R. Rhythm-specific pharmacological modulation of subthalamic activity in parkinson’s disease. Exp. Neurol. 2004, 189, 369–379. [Google Scholar] [CrossRef]
- Raz, A.; Vaadia, E.; Bergman, H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine vervet model of parkinsonism. J. Neurosci. 2000, 20, 8559–8571. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Levin, J.; Mehrkens, J.H.; Bötzel, K. Alpha frequency modulation in the human basal ganglia is dependent on motor task. Eur. J. Neurosci. 2011, 33, 960–967. [Google Scholar] [CrossRef]
- Thevathasan, W.; Pogosyan, A.; Hyam, J.A.; Jenkinson, N.; Foltynie, T.; Limousin, P.; Bogdanovic, M.; Zrinzo, L.; Green, A.L.; Aziz, T.Z. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain 2012, 135, 148–160. [Google Scholar] [CrossRef]
- Tattersall, T.L.; Stratton, P.G.; Coyne, T.J.; Cook, R.; Silberstein, P.; Silburn, P.A.; Windels, F.; Sah, P. Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat. Neurosci. 2014, 17, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kammermeier, S.; Plate, A.; Mehrkens, J.H.; Ilmberger, J.; Bötzel, K. Pattern of local field potential activity in the globus pallidus internum of dystonic patients during walking on a treadmill. Exp. Neurol. 2011, 232, 162–167. [Google Scholar] [CrossRef]
- Lofredi, R.; Neumann, W.J.; Brücke, C.; Huebl, J.; Krauss, J.K.; Schneider, G.H.; Kühn, A.A. Pallidal beta bursts in parkinson’s disease and dystonia. Mov. Disord. 2019, 34, 420–424. [Google Scholar] [CrossRef]
- Darmani, G.; Drummond, N.M.; Ramezanpour, H.; Saha, U.; Hoque, T.; Udupa, K.; Sarica, C.; Zeng, K.; Cortez Grippe, T.; Nankoo, J.F. Long--term recording of subthalamic aperiodic activities and beta bursts in parkinson’s disease. Mov. Disord. 2023, 38, 232–243. [Google Scholar] [CrossRef]
- Stein, E.; Bar-Gad, I. Beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp. Neurol. 2013, 245, 52–59. [Google Scholar] [CrossRef]
- Brown, P. Bad oscillations in parkinson’s disease. J. Neural Transm. Suppl. 2006, 70, 27–30. [Google Scholar]
- Ray, N.; Jenkinson, N.; Wang, S.; Holland, P.; Brittain, J.; Joint, C.; Stein, J.; Aziz, T. Local field potential beta activity in the subthalamic nucleus of patients with parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp. Neurol. 2008, 213, 108–113. [Google Scholar] [CrossRef]
- Doyle, L.; Kühn, A.; Hariz, M.; Kupsch, A.; Schneider, G.H.; Brown, P. Levodopa--induced modulation of subthalamic beta oscillations during self--paced movements in patients with parkinson’s disease. Eur. J. Neurosci. 2005, 21, 1403–1412. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Wen, P.; Wang, Q.; Yang, Y.; Xiao, H.; Xie, Z.; Li, X.; Wang, N.; Wang, J. The network of causal interactions for beta oscillations in the pedunculopontine nucleus, primary motor cortex, and subthalamic nucleus of walking parkinsonian rats. Exp. Neurol. 2016, 282, 27–36. [Google Scholar] [CrossRef]
- Avila, I.; Parr-Brownlie, L.C.; Brazhnik, E.; Castañeda, E.; Bergstrom, D.A.; Walters, J.R. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Exp. Neurol. 2010, 221, 307–319. [Google Scholar] [CrossRef]
- Salvadè, A.; D’Angelo, V.; Di Giovanni, G.; Tinkhauser, G.; Sancesario, G.; Städler, C.; Möller, J.C.; Stefani, A.; Kaelin-Lang, A.; Galati, S. Distinct roles of cortical and pallidal β and γ frequencies in hemiparkinsonian and dyskinetic rats. Exp. Neurol. 2016, 275, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Florin, E.; Erasmi, R.; Reck, C.; Maarouf, M.; Schnitzler, A.; Fink, G.; Timmermann, L. Does increased gamma activity in patients suffering from parkinson’s disease counteract the movement inhibiting beta activity? Neuroscience 2013, 237, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Baudrexel, S.; Witte, T.; Seifried, C.; von Wegner, F.; Beissner, F.; Klein, J.C.; Steinmetz, H.; Deichmann, R.; Roeper, J.; Hilker, R. Resting state fmri reveals increased subthalamic nucleus–motor cortex connectivity in parkinson’s disease. Neuroimage 2011, 55, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Deli, A.; Fischer, P.; Wiest, C.; Huang, Y.; Martin, S.; Khawaldeh, S.; Aziz, T.Z.; Green, A.L.; Brown, P. Gait-phase modulates alpha and beta oscillations in the pedunculopontine nucleus. J. Neurosci. 2021, 41, 8390–8402. [Google Scholar] [CrossRef]
- Jing, W.; Wang, Y.; Fang, G.; Chen, M.; Xue, M.; Guo, D.; Yao, D.; Xia, Y. Eeg bands of wakeful rest, slow-wave and rapid-eye-movement sleep at different brain areas in rats. Front. Comput. Neurosci. 2016, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Utts, J.; Heckard, R.F. Statistical Ideas and Methods; Thomson-Brooks/Cole: Belmont, CA, USA, 2005. [Google Scholar]


| Band | Factor | F | p-Value | Partial η2 | LSD |
|---|---|---|---|---|---|
| Delta | group(1,18) 1 | 4.516 | 0.048 | 0.201 | Lesion > Sham 2 |
| channel(4,72) | 1.608 | 0.204 | 0.082 | NA 3 | |
| group*channel(4,72) | 0.635 | 0.639 | 0.034 | NA | |
| Theta | group(1,18) | 4.191 | 0.056 | 0.189 | Lesion > Sham |
| channel(4,72) | 3.016 | 0.048 | 0.144 | PPN/SNc/STN > CPu | |
| group*channel(4,72) | 0.420 | 0.794 | 0.023 | NA | |
| Alpha | group(1,18) | 3.999 | 0.061 | 0.182 | Lesion > Sham |
| channel(4,72) | 3.457 | 0.050 | 0.161 | PPN > CPu/M1 | |
| group*channel(4,72) | 0.095 | 0.984 | 0.005 | NA | |
| Beta | group(1,18) | 4.425 | 0.050 | 0.197 | Lesion > Sham |
| channel(4,72) | 6.491 | 0.001 | 0.265 | PPN/SNc/STN > M1; CPu > M1; STN > CPu | |
| group*channel(4,72) | 0.708 | 0.589 | 0.038 | NA | |
| Gamma | group(1,18) | 5.804 | 0.027 | 0.244 | Lesion > Sham |
| channel(4,72) | 3.616 | 0.023 | 0.167 | PPN/SNc/STN > M1 | |
| group*channel(4,72) | 0.432 | 0.785 | 0.023 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Lu, L.; Qing, T.; Shi, S.; Fang, G. Transient Increases in Neural Oscillations and Motor Deficits in a Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 9545. https://doi.org/10.3390/ijms25179545
Wu Y, Lu L, Qing T, Shi S, Fang G. Transient Increases in Neural Oscillations and Motor Deficits in a Mouse Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2024; 25(17):9545. https://doi.org/10.3390/ijms25179545
Chicago/Turabian StyleWu, Yue, Lidi Lu, Tao Qing, Suxin Shi, and Guangzhan Fang. 2024. "Transient Increases in Neural Oscillations and Motor Deficits in a Mouse Model of Parkinson’s Disease" International Journal of Molecular Sciences 25, no. 17: 9545. https://doi.org/10.3390/ijms25179545






