Abstract
Lipopolysaccharide (LPS) triggers a severe systemic inflammatory reaction in mammals, with the dimerization of TLR4/MD-2 upon LPS stimulation serving as the pivotal mechanism in the transmission of inflammatory signals. Ginsenoside Rh2 (G-Rh2), one of the active constituents of red ginseng, exerts potent anti-inflammatory activity. However, whether G-Rh2 can block the TLR4 dimerization to exert anti-inflammatory effects remains unclear. Here, we first investigated the non-cytotoxic concentration of G-Rh2 on RAW 264.7 cells, and detected the releases of pro-inflammatory cytokines in LPS-treated RAW 264.7 cells, and then uncovered the mechanisms involved in the anti-inflammatory activity of G-Rh2 through flow cytometry, fluorescent membrane localization, Western blotting, co-immunoprecipitation (Co-IP), molecular docking and surface plasmon resonance (SPR) analysis in LPS-stimulated macrophages. Our results show that G-Rh2 stimulation markedly inhibited the secretion of LPS-induced interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and nitric oxide (NO). Additionally, G-Rh2 blocked the binding of LPS with the membrane of RAW 264.7 cells through direct interaction with TLR4 and MD-2 proteins, leading to the disruption of the dimerization of TLR4 and MD-2, followed by suppression of the TLR4/NF-κB signaling pathway. Our results suggest that G-Rh2 acts as a new inhibitor of TLR4 dimerization and may serve as a promising therapeutic agent against inflammation.
1. Introduction
Toll-like receptor 4 (TLR4), a member of the toll-like receptor (TLR) family, is involved in innate immunity and mediates inflammatory responses by recognizing lipopolysaccharide (LPS) [1,2]. LPS-induced bimolecular TLR4/MD-2 dimerization is the basis of LPS-induced signal transduction. The lipid A component of Escherichia coli LPS features two phosphorylated glucosamines linked by a β (1–6) linkage and acylated by six lipid chains. Among these, five fatty acid chains are deeply embedded within a pocket, while the sixth chain extends to the surface of MD-2, engaging in hydrophobic interactions with the invariant phenylalanine residues of the adjacent TLR4*. Subsequently, the two phosphate groups on lipid A attach to the TLR4/MD-2 complex through electrostatic interactions with positively charged amino acids in TLR4, TLR4* and MD-2 [3]. The formation of the TLR4/MD-2/TLR4*/MD-2* heterodimer ultimately triggers the dimerization of the cytoplasmic domains, culminating in the recruitment of downstream adaptor proteins and the initiation of intracellular signaling cascades, ultimately leading to an immune response [4]. Studies have shown that TLR4 dimerization is crucial for activating downstream inflammatory signaling pathways, including the NF-κB pathway, which in turn promotes the secretion of TNF-α, IL-6 and IL-1β [5,6]. Several studies have demonstrated that a range of substances functioning as TLR4/MD-2 inhibitors can produce anti-inflammatory outcomes by blocking the dimerization of TLR4 with MD-2, such as total tanshinones, isoacteoside and ginsenoside Rb1 [7,8,9]. Therefore, blocking TLR4 dimerization has become a new strategy to inhibit inflammation.
G-Rh2, first isolated from red ginseng, is a steroidal saponin belonging to the protopanaxadiol type, and has various potent biological functions, including antitumor, anti-obesity, anti-inflammatory and antioxidant activities, preventing neurodegenerative diseases and so on [10,11,12,13,14]. In this study, we aimed to explore the anti-inflammatory effect of G-Rh2 by constructing an LPS-induced RAW 264.7 cell inflammatory model in vitro. Furthermore, we determined whether G-Rh2 blocked the TLR4/MD-2 dimerization to exert anti-inflammatory effects.
2. Results
2.1. Effect of G-Rh2 on the Levels of TNF-α, IL-6 and NO
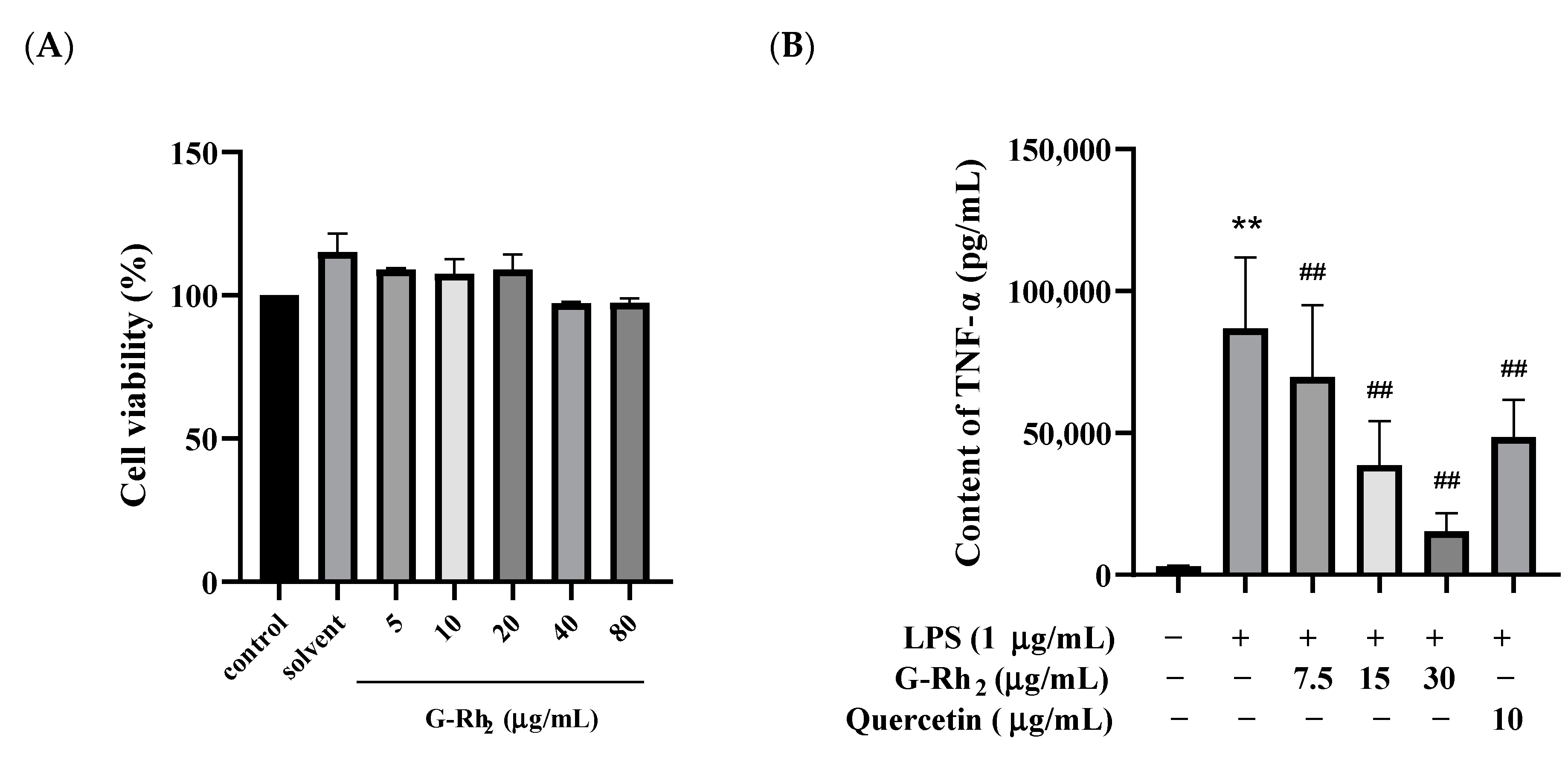
To explore whether G-Rh2 exerted potential cytotoxicity action on RAW 264.7 cells, cell viability was detected by an MTT assay after incubation for 24 h in the absence or presence of different concentrations of G-Rh2. Our findings indicated that G-Rh2, at concentrations ranging from 0 to 80 μg/mL, did not notably impact cell viability in a dose-dependent manner (p > 0.05) (Figure 1A).
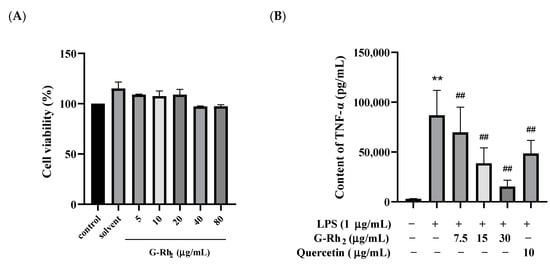
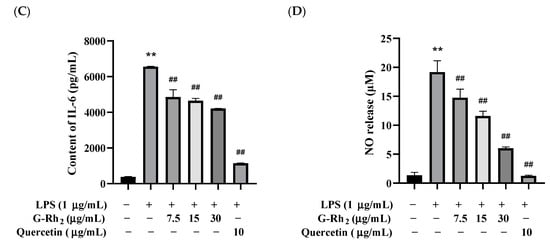
Figure 1.
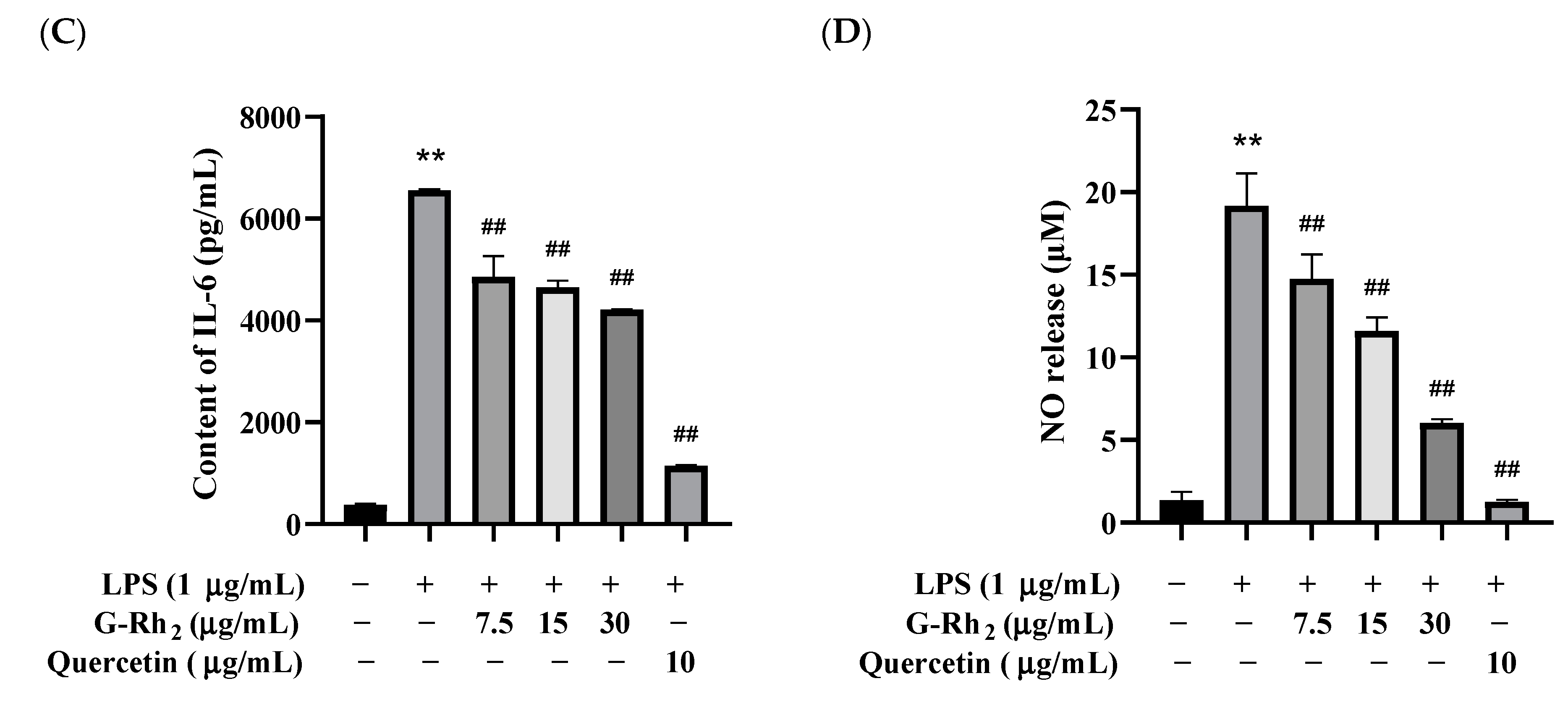
G-Rh2 inhibited LPS-induced inflammatory cytokine expression in RAW 264.7 cells in vitro. After treatment with different concentrations of G-Rh2, (A) the cell viability was measured by MTT. LPS was incubated with RAW 264.7 cells alone or together with G-Rh2 for 12 h, and the secretion levels of (B) TNF-α, (C) IL-6 and (D) NO were detected in the supernatant of the medium. The data are presented as mean values ± standard deviation (n = 3), based on a minimum of three separate experiments (**, p < 0.01, compared with the control group. ##, compared with the LPS group, p < 0.01).
Because TNF-α, IL-6 and NO are the main markers of inflammation, we analyzed the effects of G-Rh2 on TNF-α, IL-6 and NO secretion in LPS-induced RAW 264.7 cells in vitro. As shown in Figure 1B–D, TNF-α, IL-6 and NO secreted by RAW 264.7 cells were significantly increased under LPS stimulation (p < 0.01). Compared with the LPS group, the secretion of TNF-α, IL-6 and NO was significantly decreased by different concentrations of G-Rh2 (p < 0.01). Among these, the inhibitory effects of G-Rh2 (15 and 30 μg/mL) on TNF-α secretion were better than those of the positive drug (quercetin 10 μg/mL).
2.2. Effect of G-Rh2 on the Binding of FITC-LPS to the Cell Membrane
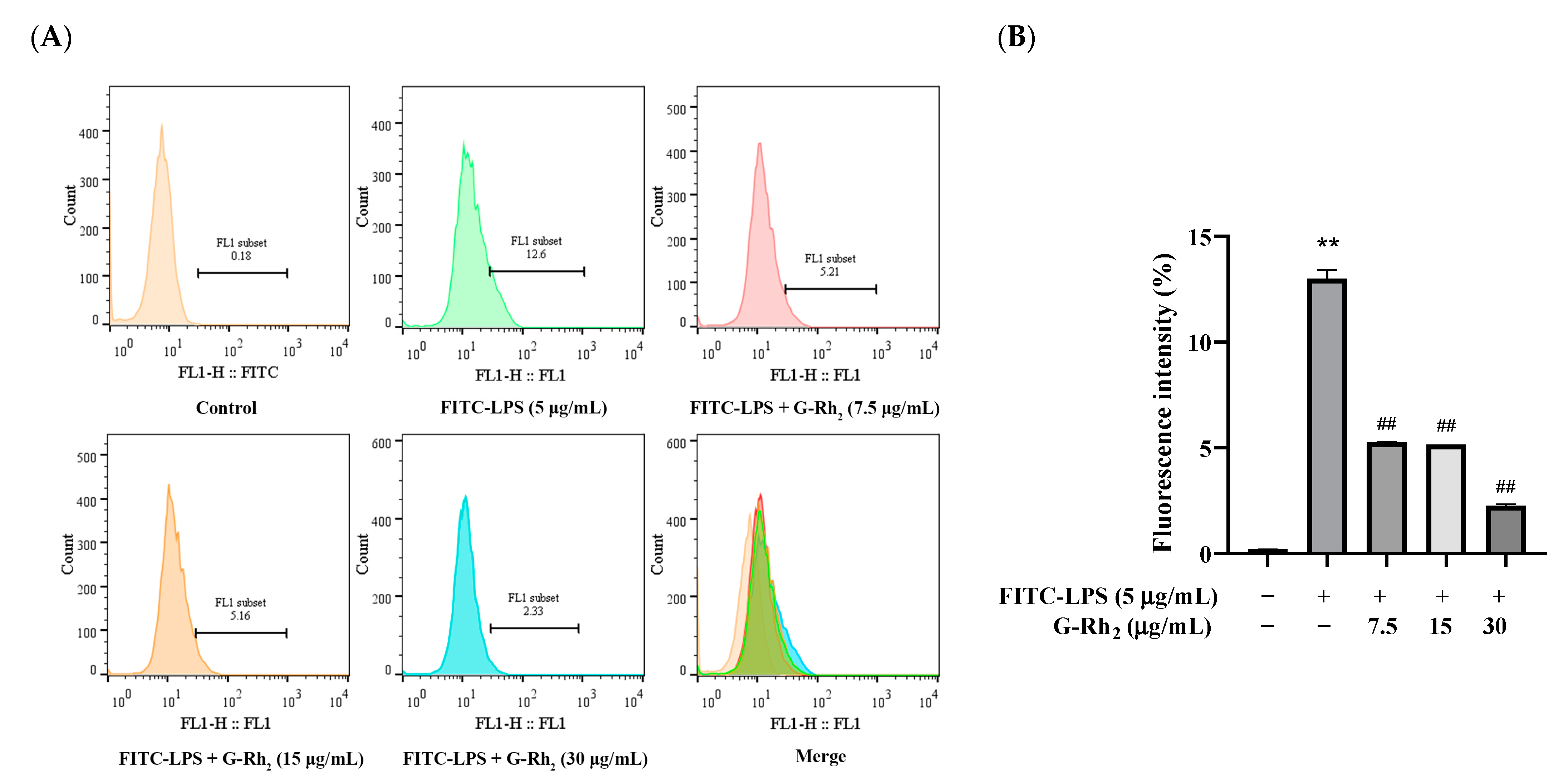
To investigate the effect of G-Rh2 on the binding of FITC-LPS to the cell membrane, firstly, we further detected the effect of G-Rh2 on the binding of FITC-LPS to RAW 264.7 cells by flow cytometry. As shown in Figure 2, the fluorescence intensity in the FITC-LPS stimulation group was significantly increased to 12.6% (p < 0.01). And compared with the FITC-LPS group, the fluorescence intensity of G-Rh2 groups were significantly decreased to 5.21%, 5.16% and 2.33%, respectively (p < 0.01), in a concentration-dependent manner.
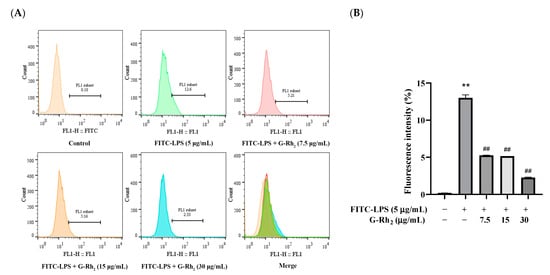
Figure 2.
Effect of G-Rh2 on FITC-LPS binding to RAW 264.7 cells. LPS was incubated with RAW 264.7 cells alone or together with G-Rh2 for 6 h. Cells were collected and the mean fluorescence values of cells in each group were detected by flow cytometry. (A) The bound FITC-LPS with RAW 264.7 was examined by flow cytometry. (B) The fluorescence intensity was analyzed. The results are shown as means ± SD (n = 3) of at least three independent experiments (**, p < 0.01, compared with the mean fluorescence values of cells in the control group. ##, compared with the mean fluorescence values of cells in the LPS group, p < 0.01).
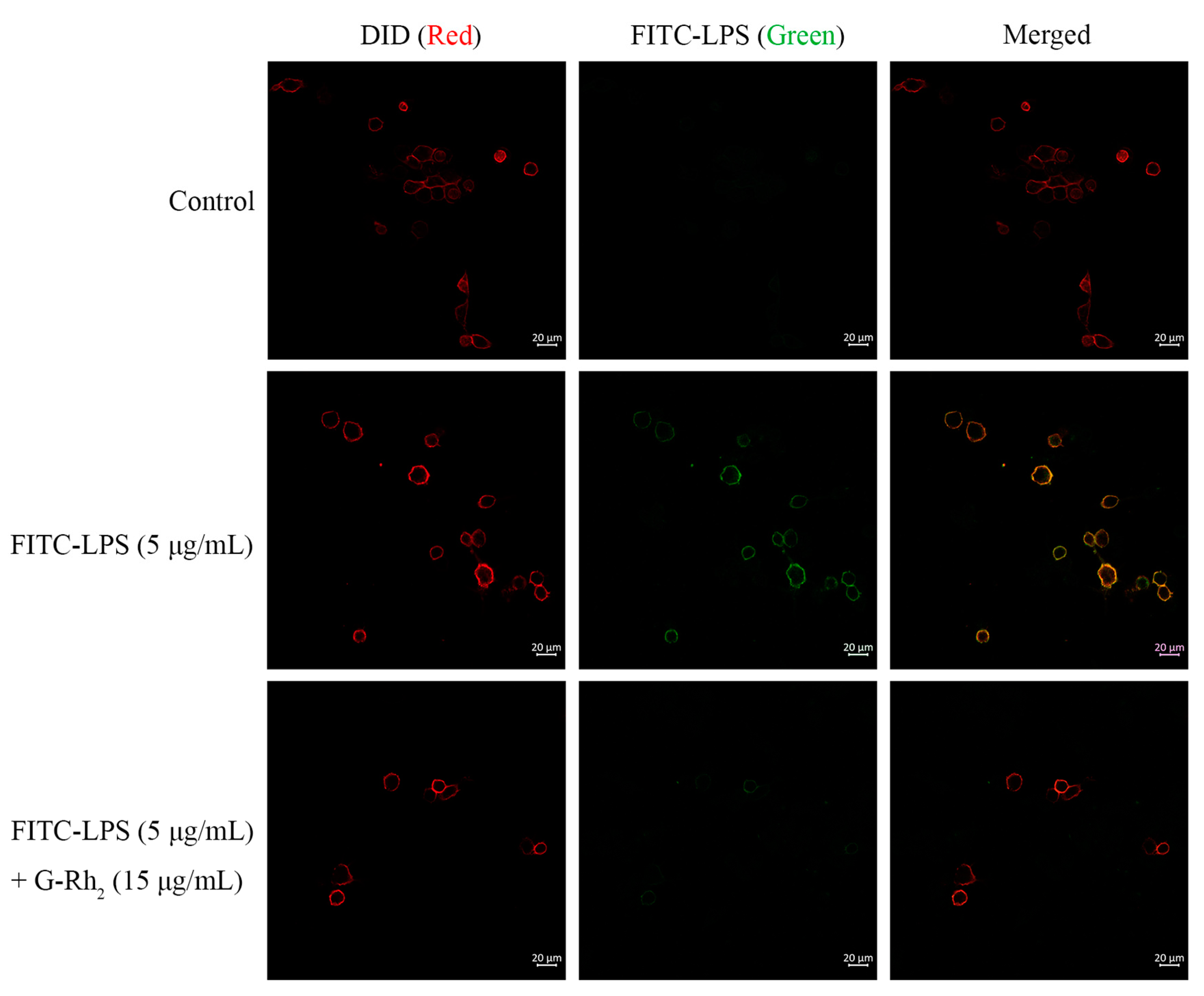
Secondly, we measured the fluorescence intensity of the cell membrane by CLSM, as shown in Figure 3. The cell membranes fluoresced strongly after FITC-LPS stimulation, whereas the G-Rh2 group fluoresced weakly.
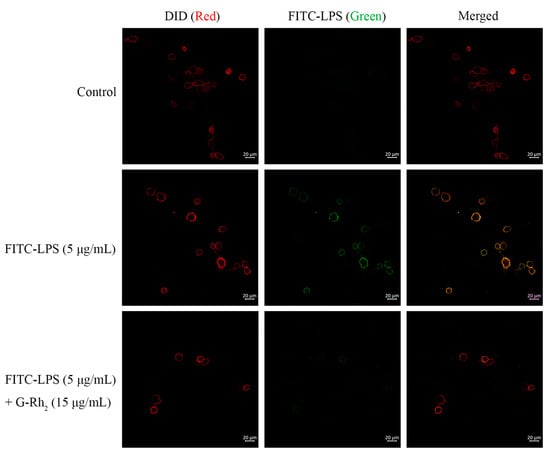
Figure 3.
G-Rh2 blocks the localization of FITC-LPS on the cell membrane. Morphologically, the effect of G-Rh2 on the localization of FITC-LPS on the cell membrane was observed by CLSM. Protein on the cell membrane was observed as red fluorescence circles on the DID line; FITC-LPS with green fluorescence was bound to the membrane, and was observed as green circles on the cell membrane.
2.3. Effect of G-Rh2 on the Activation of the TLR4/NF-κB Signaling Pathway
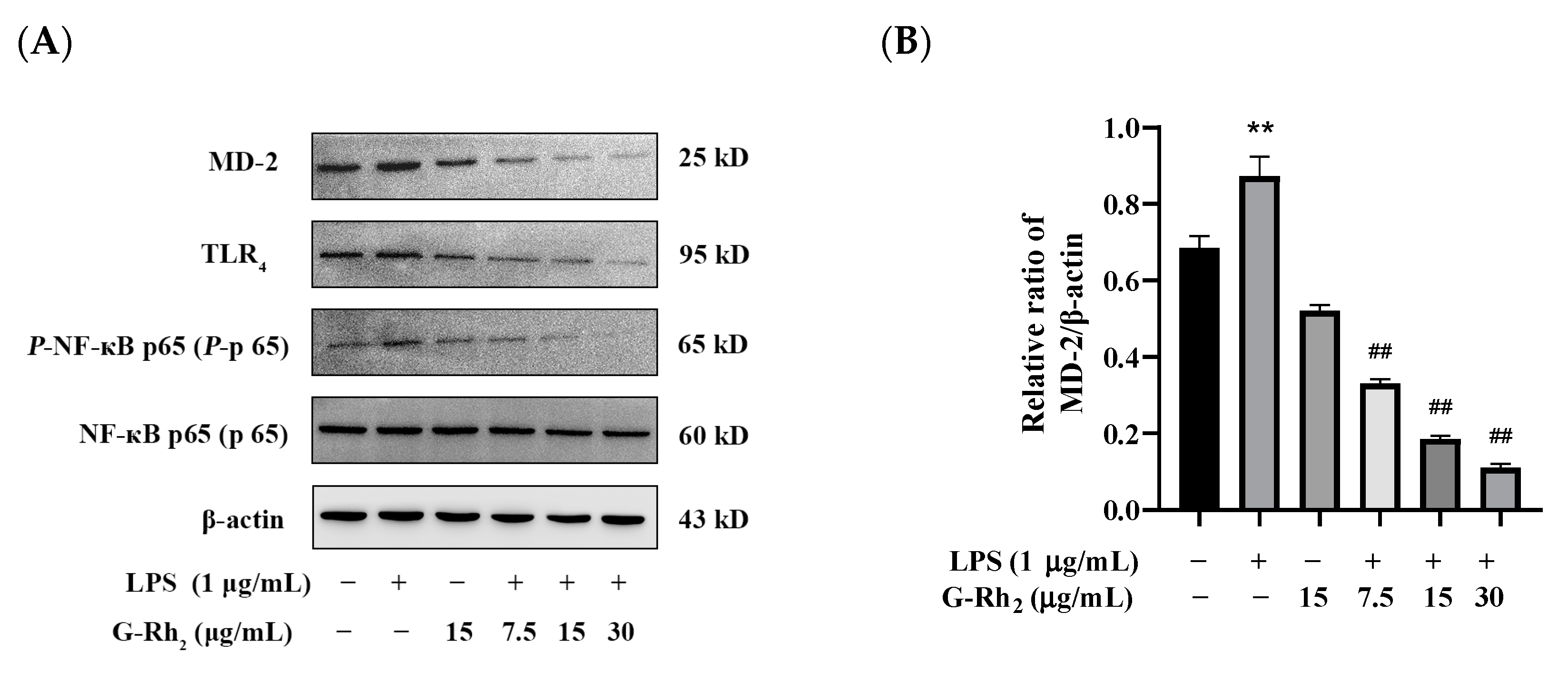
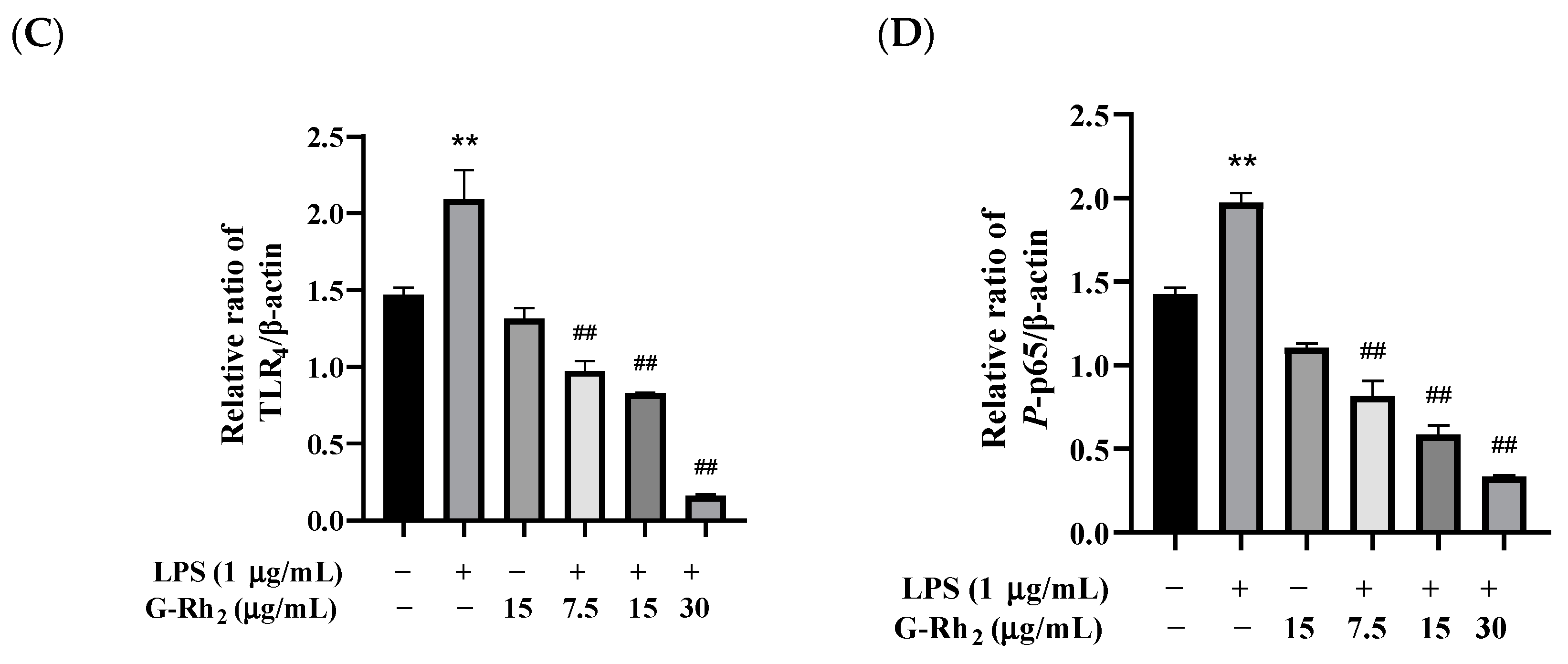
To further confirm that G-Rh2 inhibits the production of pro-inflammatory factors by blocking the TLR4 signaling pathway, we examined the effect of G-Rh2 on the expression of key proteins of the TLR4 signaling pathway. As shown in Figure 4, G-Rh2 dose-dependently inhibited LPS-induced phosphorylation levels of NF-κB p65 and protein expression of TLR4 and MD-2 (p < 0.01).
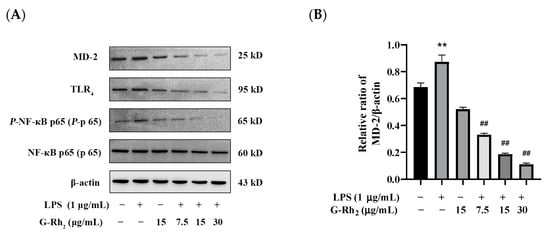
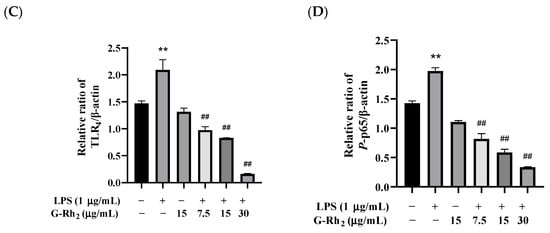
Figure 4.
Effects of G-Rh2 on the expression of TLR4 signaling pathway proteins. (A–D) LPS was incubated with RAW 264.7 cells alone or together with G-Rh2 for 12 h. The effects of G-Rh2 on MD-2, TLR4 and P-NF-κB p65 were analyzed by Western blotting. The results are shown as means ± SD (n = 3) of at least three independent experiments (**, p < 0.01, compared with the control group. ##, compared with the LPS group, p < 0.01).
2.4. Effect of G-Rh2 on the Dimerization of TLR4 and MD-2
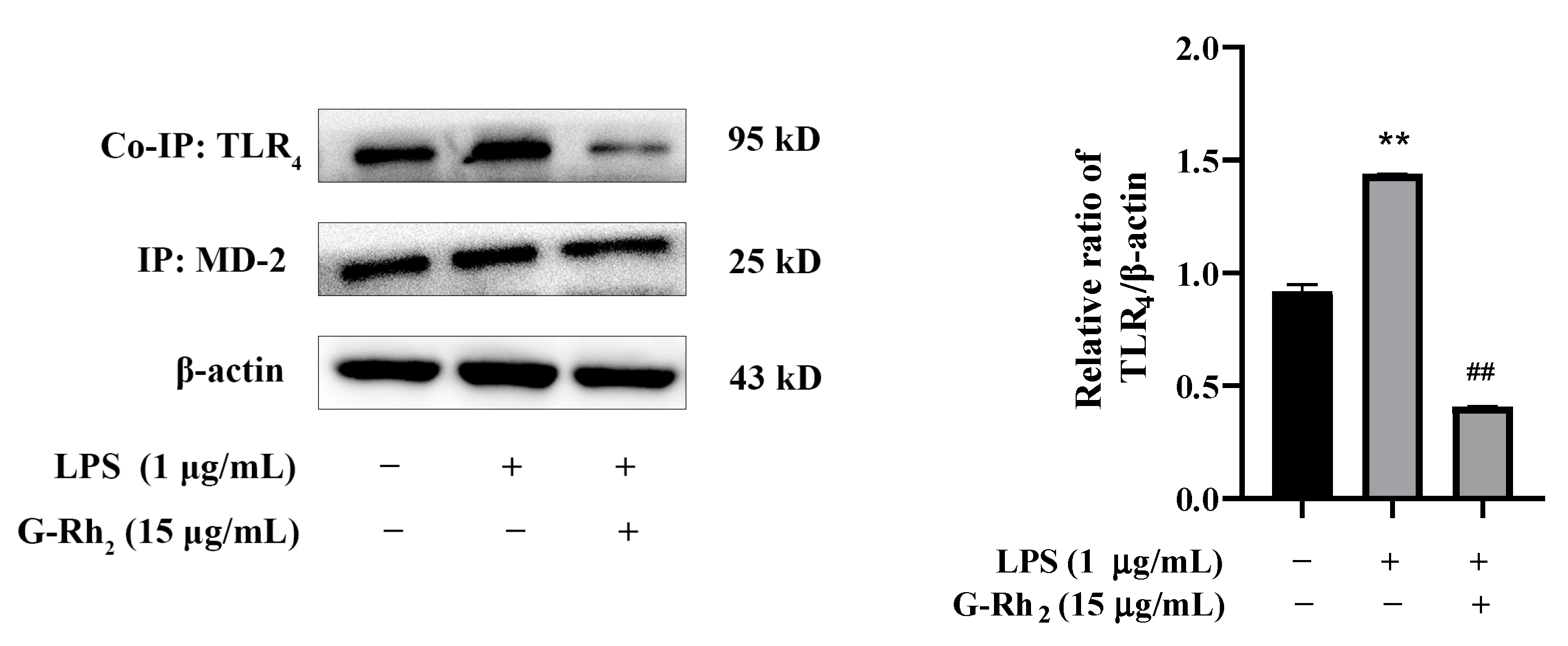
Only the dimerization of TLR4 and MD-2 can trigger TLR4 intracellular signaling pathways and finally induce NF-κB into the nucleus [15]. Therefore, we further investigated the effect of G-Rh2 on LPS-induced dimerization of TLR4 and MD-2 by means of Co-IP. As shown in Figure 5, the co-precipitation of TLR4/MD-2 was significantly increased in the LPS group (p < 0.01), while G-Rh2 (15 μg/mL) treatment significantly inhibited LPS-induced TLR4/MD-2 complex formation (p < 0.01). The results indicated that G-Rh2 could prevent the transmission of LPS signals to cell membranes and inhibit the dimerization of TLR4 and MD-2.
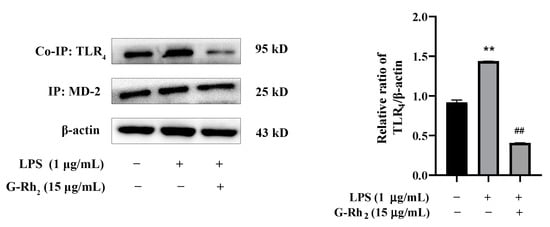
Figure 5.
G-Rh2 inhibits the formation of the TLR4/MD-2 complex. A Co-IP assay detected the effect of G-Rh2 on the complex formation of TLR4 and MD-2. The results are shown as means ± SD (n = 3) of at least three independent experiments (**, p < 0.01, compared with the control group. ##, compared with the LPS group, p < 0.01).
2.5. G-Rh2 Binds to TLR4/MD-2, Blocking the Formation of LPS-TLR4/MD-2 Complexes
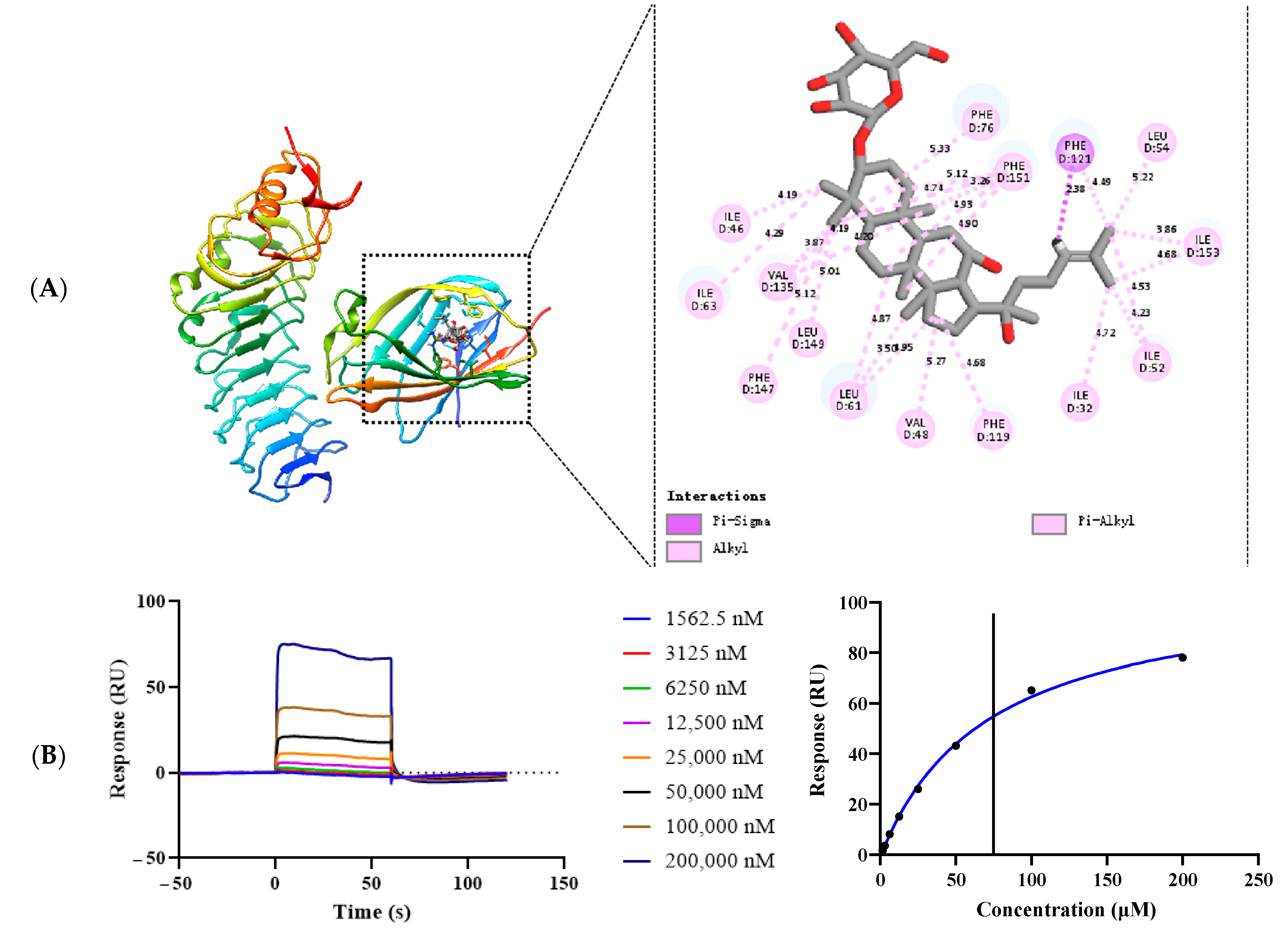
To investigate the mechanism of G-Rh2 inhibiting the dimerization of TLR4 and MD-2, we performed molecular simulations of complexes formed by G-Rh2 and TLR4/MD-2 using molecular docking software (version 1.1.2). As shown in Figure 6A, G-Rh2 is mated into the hydrophobic pocket of TLR4/MD-2, which binds to TLR4/MD-2 and may present an amino acid binding site (ILE32, ILE46, VAL48, ILE52, LEU54, LEU61, ILE63, PHE76, PHE119, PHE121, VAL135, PHE147, LEU149, PHE151, ILE153).
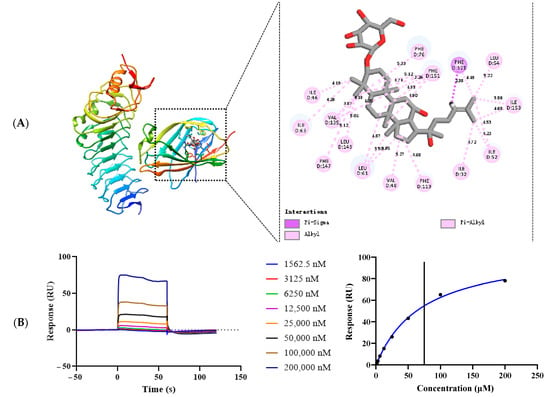
Figure 6.
G-Rh2 targets TLR4/MD-2. (A) The amino acid interaction site between G-Rh2 and TLR4/MD-2 was investigated by molecular docking. (B) The interaction affinity between G-Rh2 and TLR4/MD-2 was studied with an SPR experiment.
Next, we evaluated the interaction between G-Rh2 and TLR4/MD-2 through SPR experiments; as shown in Figure 6B, G-Rh2 binds to TLR4/MD-2 protein in a dose-dependent manner and presents a “fast up, fast down” binding pattern. G-Rh2 and TLR4/MD-2 have a specific binding force and an affinity of KD = 71.20 µM.
3. Discussion
G-Rh2, a protopanaxadiol saponin from ginseng, has been reported to exhibit anti-inflammatory and anticancer effects [16,17,18]. This provides strong support for us to further explore its anti-inflammatory mechanism. Therefore, the aim of this study was to verify the anti-inflammatory activity of G-Rh2, and to explore the underlying mechanisms in a cell inflammation model. Our results revealed that G-Rh2 could significantly decrease the secretion of TNF-α, IL-6 and NO in LPS-induced RAW 264.7 cells and indicated G-Rh2 exerted quite evident anti-inflammatory effects. Both TNF-α and IL-1β are reported to be NF-κB target genes, and the expression of these two target genes is significantly increased in LPS-stimulated macrophages [19]. Quercetin inhibits the NF-κB, Akt and JNK signaling pathway, thereby reducing the expression of TNF-α and IL-1β in LPS-stimulated RAW 264.7 cells [20]. We speculate that G-Rh2 could block the binding of LPS with the membrane of RAW 264.7 cells through direct interaction with TLR4 and MD-2 proteins, followed by suppression of the TLR4/MD-2 mediated downstream NF-κB signaling pathway, so as to better reduce the expression of TNF-α.
LPS, a macromolecular glycolipid, is unable to cross cell membranes by itself but must bind to membrane receptors to exert its biological effects [21,22]. To further explore the anti-inflammatory mechanism of G-Rh2 in LPS-induced RAW 264.7 cells, we next detected the effect of G-Rh2 on the binding of LPS to RAW 264.7 cells by flow cytometry, and further determined the effect of G-Rh2 on the binding of LPS to cell membranes with laser confocal technology. Flow cytometry showed that G-Rh2 could significantly inhibit the fluorescence intensity of FITC-LPS binding to the cells, indicating that G-Rh2 could significantly inhibit the binding of LPS to RAW 264.7 cells. Laser confocal analysis showed that G-Rh2 could significantly inhibit the fluorescence intensity of FITC-LPS binding to cell membranes. These results suggest that G-Rh2 may inhibit the binding of LPS to cell membranes to alleviate LPS-induced cell inflammation.
TLR4 is responsible for the recognition of LPS and then induces the activation of the NF-κB signaling pathway [23]. Therefore, we then explored the effects of G-Rh2 on the TLR4/NF-κB signaling pathway. Regarding the results, G-Rh2 significantly inhibited the protein expression of TLR4 and MD-2 and phosphorylation of NF-κB p65 in LPS-induced RAW 264.7 cells. These results indicated that G-Rh2 may block the combination of TLR4 and MD-2, and then inhibit TLR4/NF-κB signaling pathway activation. To further explore whether G-Rh2 interfered with the combination of TLR4 and MD-2, we explored whether G-Rh2 influences the LPS-induced dimerization of TLR4 and MD-2 by Co-IP. The findings demonstrated that the co-precipitation of TLR4/MD-2 exhibited a marked increase in the LPS group. Conversely, the administration of G-Rh2 markedly impeded the formation of the LPS-induced TLR4/MD-2 complex.
According to the previous report, LPS-induced TLR4/MD-2 dimerization is the key to inflammation signaling transduction [24]. A previous study has reported that anthocyanins mostly fit into the hydrophobic pocket of MD-2 and bind to TLR4, which results in the inhibition of NF-κB activity to attenuate lipopolysaccharide-induced inflammation [25]. TTAK-242 inhibits the activation of the TLR4 signaling pathway by binding to the Cys747 amino acid site of TLR4’s intracellular TIR domain [26,27]. However, how G-Rh2 affects the dimerization of TLR4/MD-2 is not clear. The molecular docking study predicted that G-Rh2 would bind to the hydrophobic pocket of MD-2, which led to G-Rh2 occupying the position of LPS in the hydrophobic pocket of MD-2, resulting in the inability of TLR4 to dimerize. It has been demonstrated that amino acid residues 119–132 of MD-2 play a key role in the recognition and binding of LPS, while amino acids 46~50, 79~83 and 90~105 are key binding sites of MD-2 and TLR4 [3]. G-Rh2 interacts with MD-2 amino acids PHE119 and PHE121 that bind to important sites of LPS, blocking the binding of LPS to MD-2. In addition, G-Rh2 can block the binding of TLR4 to MD-2 ILE46 and VAL48 amino acids, blocking the formation of a complex between TLR4 and MD-2.
It was found that G-Rh2 bound to TLR4/MD-2 dose-dependently in SPR studies, and the binding interaction occurred in a “fast up and fast down” manner, indicating that G-Rh2 specifically bound to TLR4/MD-2 with a strong affinity, KD = 71.20 µM. Shin et al. showed that the KD value for the binding of LPS to MD-2 was 2.33 μM. Although G-Rh2 has a lower affinity than LPS, the results of Western blot and Co-IP showed that G-Rh2 significantly inhibited the formation of the TLR4/MD-2 complex and suppressed the downstream signal transduction. Combined with the results of molecular docking, G-Rh2 is mated into the hydrophobic pocket of TLR4/MD-2. We conjectured that the binding affinity between G-Rh2 and MD-2 hydrophobic pockets may be higher than that of LPS, which leads to fewer LPS lipid chains falling into hydrophobic pockets; TLR4 then cannot form a dimerization structure, and then its downstream signal transduction is inhibited. Regarding the crystal structure of LPS/MD-2/TLR4, the affinity between LPS and TLR4/MD-2 mainly includes the interaction between five lipid chains of LPS and residues of the hydrophobic pocket of MD-2, and the hydrophobic interaction between another lipid chain and the conserved phenylalanine of TLR4* [3]. More important is that the two phosphate groups on lipid A bind to the TLR4/MD-2 complex through interactions with positively charged amino acids in TLR4, TLR4* and MD-2. A previous study has demonstrated that these two phosphate groups within the lipid A moiety exert a profound influence on the endotoxic properties of LPS. The removal of either phosphate group results in a 100-fold reduction in endotoxic activity, with the remaining monophosphoryl LPS exhibiting relatively weak stimulatory properties with regard to the innate immune response [28]. This suggests that the affinity of LPS may depend mainly on two phosphate groups, while the affinity of LPS binding to MD-2 may be low. Therefore, LPS may have a lower affinity than G-Rh2 when only the capacity for binding to the hydrophobic pockets of MD-2 is compared.
Recently, lipid A derivatives like lipid IVa and eritoran, which possess four fatty acid chains, have been demonstrated to selectively interact with the hydrophobic cavity of MD-2. This interaction serves to disrupt TLR4 dimerization, with them acting as antagonists of the TLR4/MD-2 complex [29]. This also supports our hypothesis that when the number of lipid chains of LPS falling into MD-2 pockets is reduced, this becomes an inhibitor of TLR4 signaling transduction.
These results suggest that G-Rh2 is targeted by preempting LPS and TLR4/MD-2, and that G-Rh2 targeting TLR4/MD-2 blocks the binding of LPS to TLR4/MD-2 and the dimerization of TLR4/MD-2, which will inevitably attenuate the LPS-induced TIR wobble and the recruitment of downstream adapters. This will ultimately inhibit the activation of the NF-κB signaling pathway.
4. Materials and Methods
4.1. Main Chemicals and Reagents
The 10 × RIPA buffer and Griess reagent nitrite measurement kit were acquired from Cell Signaling Technology Co., Ltd. (Danvers, MA, USA). Mouse TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were acquired from R&D systems Co., Ltd. (Minneapolis, MN, USA). Lipopolysaccharide (LPS) and FITC-LPS (O11:B4) were purchased from Sigma Chemical Co., Ltd. (St. Louis, MO, USA). Fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin–streptomycin solution, Dulbecco’s Phosphate Buffered Saline (DPBS), GlutaMAX™-1 and 0.25% Trypsin-EDTA, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Gibco Co., Ltd. (Waltham, MA, USA). The BCA protein assay kit was purchased from CWBIO (Beijing, China). Primary TLR4, MD-2, NF-κB p65, phosphorylation-NF-κB p65 (p-NF-κB p65) antibody, goat anti-rabbit and anti-mouse antibody were purchased from Immunoway Biotechnology. The Co-Immunoprecipitation Kit and DID cell membrane dye were purchased from Shanghai Unionway technology Co., Ltd. (Shanghai, China). His-Flag TLR4/MD-2 protein was acquired from BD Co., Ltd. (Franklin Lakes, NJ, USA). G-Rh2 (purity of 95%) was donated by professor YongRi Jin of Jilin university.
4.2. Cell Culture and Viability Assay
RAW 264.7 cells were obtained from the Kunming Cell Bank of Chinese Academy of Sciences and cultured in DMEM, which was supplemented with 10% FBS, 100 U/mL penicillin and 100 U/mL streptomycin at 37 °C in a humidified incubator with 5% CO2 [30]. The MTT assay was used for measurement of cell viability, as described by Yan et al. [31]. Cells were distributed in 96-well plates at a density of 5 × 105 cells/mL and incubated for 12 h. G-Rh2 was prepared at 6 concentrations of G-Rh2 (0, 5, 10, 20, 40, 80 μg/mL) to be added to the well (nine wells were repeated for each dose group) for 24 h. Next, an MTT assay was performed in accordance with the original method. The absorbance of each well was measured at 490 nm using a microplate reader (Multiskan GO Thermo Scientiific, Waltham, MA, USA).
4.3. Detection of Pro-Inflammatory Factors
The dosage of G-Rh2 was determined by the MTT assay and a previous report [32]. In order to assay the production of TNF-α, IL-6 and NO, the supernatant of RAW 264.7 cells was collected after they were co-treated with G-Rh2 (7.5, 15 and 30 μg/mL) or quercetin (10 μg/mL) in conjunction with LPS (1 μg/mL) for a period of 12 h [33]. TNF-α and IL-6 levels were quantified using ELISA kits, following the manufacturer’s instructions. NO production was determined using a Griess reagent nitrite measurement kit.
4.4. Flow Cytometric Analysis of FITC-LPS Binding
The RAW 264.7 cells (1 × 106 cells/mL) were seeded into a 6-well plate for 6 h, then incubated with FITC-LPS (5 μg/mL) with or without G-Rh2 (7.5, 15, 30 μg/mL) for the same time. Finally, cells in each well were collected and centrifuged at 1000 rpm for 3 min. The final cells were suspended with PBS, and the bound FITC-LPS was examined by flow cytometry (Beckman, Brea, CA, USA) according to reference [34].
4.5. Determination of Membrane Localization of FITC-LPS
The localization of FITC-LPS on the cell membrane was determined with reference to Hua et al. [35]. In short, RAW 264.7 cells (1 × 104 cells/mL) were seeded in 6-well plates with cover slides placed in advance for 6 h, then incubated with FITC-LPS (5 μg/mL) with or without G-Rh2 (15 μg/mL) for the same time. Subsequently, the supernatant was removed and the cells were rinsed twice with PBS and stained with 0.2 μM DID. In the end, the cells were fixed in 4% PFA, and the localization of FITC-LPS on the cell membrane was observed with a confocal laser scanning microscope (CLSM) (ZEISS LSM900, excitation 488 nm and 507 nm, Oberkochen, Germany).
4.6. Western Blot Assay
A Western blot assay was carried out as previously described [31]. Briefly speaking, RAW 264.7 cells (6 × 105 cells/mL) were seeded in 6-well plates for 12 h. Then, the cells were incubated with G-Rh2 and LPS for the same time as in the previous experiment. The total proteins were lysed, collected, determined, separated and transferred to PVDF membranes as previously described [31]. Next, the membranes were blocked with 5% nonfat milk and incubated with primary antibodies (TLR4, MD-2, NF-κB p65, p-NF-κB p65) and secondary antibodies. Finally, the membranes were detected by enhanced chemiluminescence. The protein levels were normalized against the included β-actin standards and subsequently analyzed using ImageJ software (Version 1.54j) (available at https://imagej.net/, accessed on 24 February 2023).
4.7. Co-Immunoprecipitation (Co-IP)
The investigation of TLR4/MD-2 complex formation was carried out as previously described [36]. Briefly, RAW 264.7 cells (6 × 105 cells/mL) were seeded into a 6-well plate for 6 h, then incubated with G-Rh2 (15 μg/mL) in the presence or absence of LPS (1 μg/mL) for 12 h. Subsequently, the cells were lysed with buffer containing protease and phosohatase inhibitors. Cell extract was incubated with adequate amounts of anti-MD-2 antibody at 4 °C overnight, and then precipitated with protein A/G magnetic beads under the same conditions. After boiling, the released protein was detected by immunoblotting using anti-TLR4 antibody. The experimental procedure was consistent with the Western blot assay as before.
4.8. Molecular Docking Study
Molecular docking of G-Rh2 with TLR4/MD-2 was conducted with reference to Yao et al. [37] and performed in Autodock Vina software (version 1.1.2). Firstly, the three-dimensional structures of the compound G-Rh2 were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov, accessed on 9 November 2022). Next, the crystal structures of TLR4/MD-2 (PDB ID: 2Z65) were downloaded from the PDB database (https://www.rcsb.org/, accessed on 9 November 2022). Then, we imported them into AutoDockTools software (version 1.5.6) to remove water molecules, add nonpolar hydrogens and calculate the Gasteiger charges of the structures, and saved them as PDBQT files. Lastly, the docking procedure was undertaken for the purpose of analyzing the results using both PyMOL (version 2.5) and AutoDock Vina software.
4.9. SPR Analysis
A Biacore T200 Biomolecular Interaction Analysis system (Company, Shanghai, China) and Series Sensor Chip CM5 (10246576) were used to determine the binding affinity of G-Rh2 to recombinant human TLR4/MD-2. SPR analysis was carried out as previously described [38]. Briefly, the TLR4/MD-2 protein (in acetate acid buffer pH 5.0) was loaded onto the sensors, and then an EDC and NHS mixture was added for activation of the chip. Different concentrations of G-Rh2 (200, 100, 50, 25, 12.5, 6.25, 3.125, 1.562 and 0 μM) were prepared with a running buffer (PBS, 0.2% Tween-20, 5% DMSO, pH 7.4). The sensor and sample plate were positioned within the instrument. Following the manufacturer’s guidelines, interactions were assessed at a flow rate of 50 μL/min, comprising an association phase of 60 s and a dissociation phase of 60 s. The collected data were analyzed using BIAcore T200 Evaluation software (version 3.2.1). Binding kinetic parameters, including KD values, were determined through global fitting of the kinetic data obtained from different concentrations of Blumeatin, employing a 1:1 Langmuir binding model.
4.10. Statistical Analysis
Statistical analysis was conducted using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The results are reported as mean ± standard deviation (SD) and were evaluated through one-way analysis of variance (ANOVA). p < 0.05 was deemed indicative of statistical significance.
5. Conclusions
In summary, our results showed G-Rh2 possessed a good anti-inflammatory effect and G-Rh2 could block the binding of LPS with the membrane of RAW 264.7 cells through direct interaction with TLR4 and MD-2 proteins, leading to the disruption of the dimerization of TLR4 and MD-2, followed by suppression of the TLR4/MD-2 mediated downstream NF-κB signaling pathway to exert anti-inflammatory effects. Our findings indicate that G-Rh2 is a novel TLR4/MD2 inhibitor and could be a potential therapeutic candidate for inflammation.
Author Contributions
This study was designed and carried out by S.P. and L.W. The experiment and data analysis were carried out by S.P., W.Q. and L.W. Drafting of the manuscript was performed by S.P., L.W., L.P. and Q.Y. Manuscript reviews and editing were carried out by H.Y., H.W., L.P. and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32160849, No. 32273051 and No. 31760747).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
No individual data are presented in this manuscript.
Data Availability Statement
All material and data are stored at Guizhou University, College of Pharmacy, Guiyang, People’s Republic of China, and may be shared upon request directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
G-Rh2: ginsenoside Rh2; DEX: dexamethasone; LPS: lipopolysaccharide; FBS: fetal bovine serum; DMEM: Dulbecco’s Modified Eagle’s Medium; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide; TLRs: toll-like receptors; MD-2: myeloid differentiation factor 2; NO: nitric oxide; DPBS: Dulbecco’s Phosphate Buffered Saline; ELISA: enzyme-linked immunosorbent assay; Co-IP: co-immunoprecipitation; SPR: surface plasmon resonance; SD: standard deviation; CLSM: confocal laser scanning microscope.
References
- Zhang, Y.S.; Liang, X.J.; Bao, X.F.; Xiao, W.; Chen, G.L. Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, Y.; Zeng, Y.; Yang, X.; Yu, F.M.; Wang, B. Immunomodulatory peptides from thick-shelled mussel (Mytilus coruscus): Isolation, identification, molecular docking and immunomodulatory effects on RAW 264.7 cells. Food Biosci. 2024, 172, 103874. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Billod, J.M.; Lacetera, A.; Guzmán-Caldentey, J.; Martín-Santamaría, S. Computational Approaches to Toll-like Receptor 4 Modulation. Molecules 2016, 21, 994. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, X.; Sun, W.; Kang, N.; Liu, Y.; Yang, S.; Xu, Q.M.; Wang, C.; Chen, X. Total tanshinones exhibits anti-inflammatory effects through blocking TLR4 dimerization via the MyD88 pathway. Cell Death Dis. 2017, 8, e3004. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cui, Y.; Kang, N.; Liu, X.; Liu, Y.; Zou, Y.; Zhang, Z.; Li, X.; Yang, S.; Li, J.; et al. Isoacteoside, a dihydroxyphenylethyl glycoside, exhibits anti-inflammatory effects through blocking toll-like receptor 4 dimerization. Br. J. Pharmacol. 2017, 174, 2880–2896. [Google Scholar] [CrossRef]
- Gao, H.; Kang, N.; Hu, C.; Zhang, Z.; Xu, Q.; Liu, Y.; Yang, S. Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine 2020, 69, 153197. [Google Scholar] [CrossRef]
- Choi, S.; Kim, T.W.; Singh, S.V. Ginsenoside Rh2-mediated G1 phase cell cycle arrest in human breast cancer cells is caused by p15 Ink4B and p27 Kip1-dependent inhibition of cyclin-dependent kinases. Pharm. Res. 2009, 26, 2280–2288. [Google Scholar] [CrossRef]
- Li, L.C.; Piao, H.M.; Zheng, M.Y.; Lin, Z.H.; Choi, Y.H.; Yan, G.H. Ginsenoside Rh2 attenuates allergic airway inflammation by modulating nuclear factor-κB activation in a murine model of asthma. Mol. Med. Rep. 2015, 12, 6946–6954. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Kim, S.J.; Mun, A.R.; Go, H.K.; Kim, G.B.; Kim, S.Z.; Jang, S.I.; Lee, S.J.; Kim, J.S.; Kang, H.S. Korean red ginseng and its primary ginsenosides inhibit ethanol-induced oxidative injury by suppression of the MAPK pathway in TIB-73 cells. J. Ethnopharmacol. 2012, 141, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, D.; Lee, H.L.; Kim, C.E.; Jung, K.; Kang, K.S. Beneficial effects of for the treatment and prevention of neurodegenerative diseases: Past findings and future directions. J. Ginseng Res. 2018, 42, 239–247. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kim, S.H.; Lee, M.S.; Kim, S.H.; Yang, H.J.; Kim, M.J.; Kim, H.S.; Ha, J.; Kim, M.S.; Kwon, D.Y. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem. Biophys. Res. Commun. 2007, 364, 1002–1008. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takeda, K. Current Views of Toll-like Receptor Signaling Pathways. Gastroenterol. Res. Pract. 2010, 2010, 240365. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.M.; Dai, C.X.; Shang, Y.S.; Xie, J. Ginsenoside Rh2 alleviates tumor-associated depression in a mouse model of colorectal carcinoma. Am. J. Transl. Res. 2016, 8, 2189–2195. [Google Scholar] [PubMed]
- Hsieh, Y.H.; Deng, J.S.; Chang, Y.S.; Huang, G.J. Ginsenoside Rh2 Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 Signaling Pathways in Mice. Nutrients 2018, 10, 1208. [Google Scholar] [CrossRef]
- Chen, X.Q.; Xu, T.T.; Lv, X.Y.; Zhang, J.W.; Liu, S.J. Ginsenoside Rh2 alleviates ulcerative colitis by regulating the STAT3/miR-214 signaling pathway. J. Ethnopharmacol. 2021, 274, 113997. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tsai, M.H.; Sheu, W.H.; Hsieh, S.C.; Chiang, A.N. The therapeutic potential and mechanisms of action of quercetin in relation to lipopolysaccharide-induced sepsis in vitro and in vivo. PLoS ONE 2013, 8, e80744. [Google Scholar] [CrossRef]
- Zhao, Q.L.; Yang, F.; Pu, Q.Y.; Zhao, R.; Jiang, S.; Tang, Y.P. Integrative metabolomics and gut microbiota analyses reveal the protective effects of DHA-enriched phosphatidylserine on bisphenol A-induced intestinal damage. J. Funct. Foods 2024, 117, 106229. [Google Scholar] [CrossRef]
- Triantafilou, M.; Triantafilou, K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002, 23, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Yi, Z.J.; Zhang, W.F.; Yang, L.; Qi, F.; Yu, T.; Zhu, Z.; Li, M.J.; Cheng, Y.; Zhao, L.; et al. Single-Cell Sequencing Reveals MYOF-Enriched Monocyte/Macrophage Subcluster as a Favorable Prognostic Factor in Sepsis. Adv. Biol. 2024, 8, 2300673. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xing, S.; Du, J.; Wang, M.; Han, J.; Li, Z. Synthesis and evaluation of the anti-inflammatory activity of novel 8-quinolinesulfonamide derivatives as TLR4/MD-2 inhibitors with efficacy in adjuvant-induced arthritis. Bioorg. Chem. 2021, 114, 105037. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, W.A.H.M.; Lee, K.T.; Choi, Y.H.; Jin, C.Y.; Kim, G.Y. Anthocyanins isolated from Hibiscus syriacus L. attenuate lipopolysaccharide-induced inflammation and endotoxic shock by inhibiting the TLR4/MD2-mediated NF-κB signaling pathway. Phytomedicine 2020, 76, 153237. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Ii, M.; Kitazaki, T.; Iizawa, Y.; Kimura, H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 2008, 584, 40–48. [Google Scholar] [CrossRef]
- Hua, F.; Tang, H.; Wang, J.; Prunty, M.C.; Hua, X.D.; Sayeed, I.; Stein, D.G. TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J. Cereb. Blood Flow. Metab. 2015, 35, 536–542. [Google Scholar] [CrossRef]
- Jin, M.S.; Lee, J.O. Structures of the toll-like receptor family and its ligand complexes. Immunity 2008, 29, 182–191. [Google Scholar] [CrossRef]
- Matsushima, N.; Miyashita, H.; Enkhbayar, P.; Kretsinger, R.H. Comparative Geometrical Analysis of Leucine-Rich Repeat Structures in the Nod-like and Toll-like Receptors in Vertebrate Innate Immunity. Biomolecules 2015, 5, 1955–1978. [Google Scholar] [CrossRef]
- Wu, M.R.; Jiang, Y.H.; Wang, J.N.; Luo, T.; Yi, Y.; Wang, H.X.; Wang, L.M. The Effect and Mechanism of Corilagin from Salisb Shell on LPS-Induced Inflammation in Raw 264.7 Cells. Foods 2023, 12, 979. [Google Scholar]
- Yan, N.; Wen, D.S.; Zhao, Y.R.; Xu, S.J. Epimedium sagittatum inhibits TLR4/MD-2 mediated NF-kappaB signaling pathway with anti-inflammatory activity. BMC Complement. Altern. Med. 2018, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, M.; Ryu, J.; Choi, C. Ginsenosides compound K and Rh2 inhibit tumor necrosis factor-alpha-induced activation of the NF-κB and JNK pathways in human astroglial cells. Neurosci. Lett. 2007, 421, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.Y.K.; Huang, L.T.; Du, L.J.; Feng, J.F.; Li, J.; Luo, Y.Y.; Xu, Q.M.; Yang, S.L.; Gao, H.W.; Feng, Y.L. Dihydrotanshinone exhibits an anti-inflammatory effect and through blocking TLR4 dimerization. Pharmacol. Res. 2019, 142, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dou, H.; Gong, W.; Liu, X.; Yu, Z.; Li, E.; Tan, R.; Hou, Y. Bis-N-norgliovictin, a small-molecule compound from marine fungus, inhibits LPS-induced inflammation in macrophages and improves survival in sepsis. Eur. J. Pharmacol. 2013, 705, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Wu, J.; Wu, H.; Su, C.; Li, X.; Ao, Q.; Zeng, Q.; Zhu, X.; Zhang, X. Exposure to hydroxyapatite nanoparticles enhances Toll-like receptor 4 signal transduction and overcomes endotoxin tolerance in vitro and in vivo. Acta Biomater. 2021, 135, 650–662. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chen, L.F.; Yu, P.T.; Zhang, Y.L.; Fang, B.; Wu, C.; Luo, W.; Chen, X.X.; Li, C.L.; Liang, G. Discovery of 3-(Indol-5-yl)-indazole Derivatives as Novel Myeloid Differentiation Protein 2/Toll-like Receptor 4 Antagonists for Treatment of Acute Lung Injury. J. Med. Chem. 2019, 62, 5453–5469. [Google Scholar] [CrossRef]
- Yao, T.; Wang, Q.; Han, S.; Lu, Y.; Xu, Y.; Wang, Y. Potential Molecular Mechanisms of Ephedra Herb in the Treatment of Nephrotic Syndrome Based on Network Pharmacology and Molecular Docking. Biomed. Res. Int. 2022, 2022, 9214589. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, X.O.; Chen, G.Z.; Jiang, L.L.; Wang, Z.; Fang, Q.L.; Liu, X.; Wang, J.Y.; Zhang, Y.L.; Wu, W.C.; et al. MD-2 as the target of a novel small molecule, L6H21, in the attenuation of LPS-induced inflammatory response and sepsis. Br. J. Pharmacol. 2015, 172, 4391–4405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).