Identifying microRNAs Possibly Implicated in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: A Review
Abstract
1. Introduction
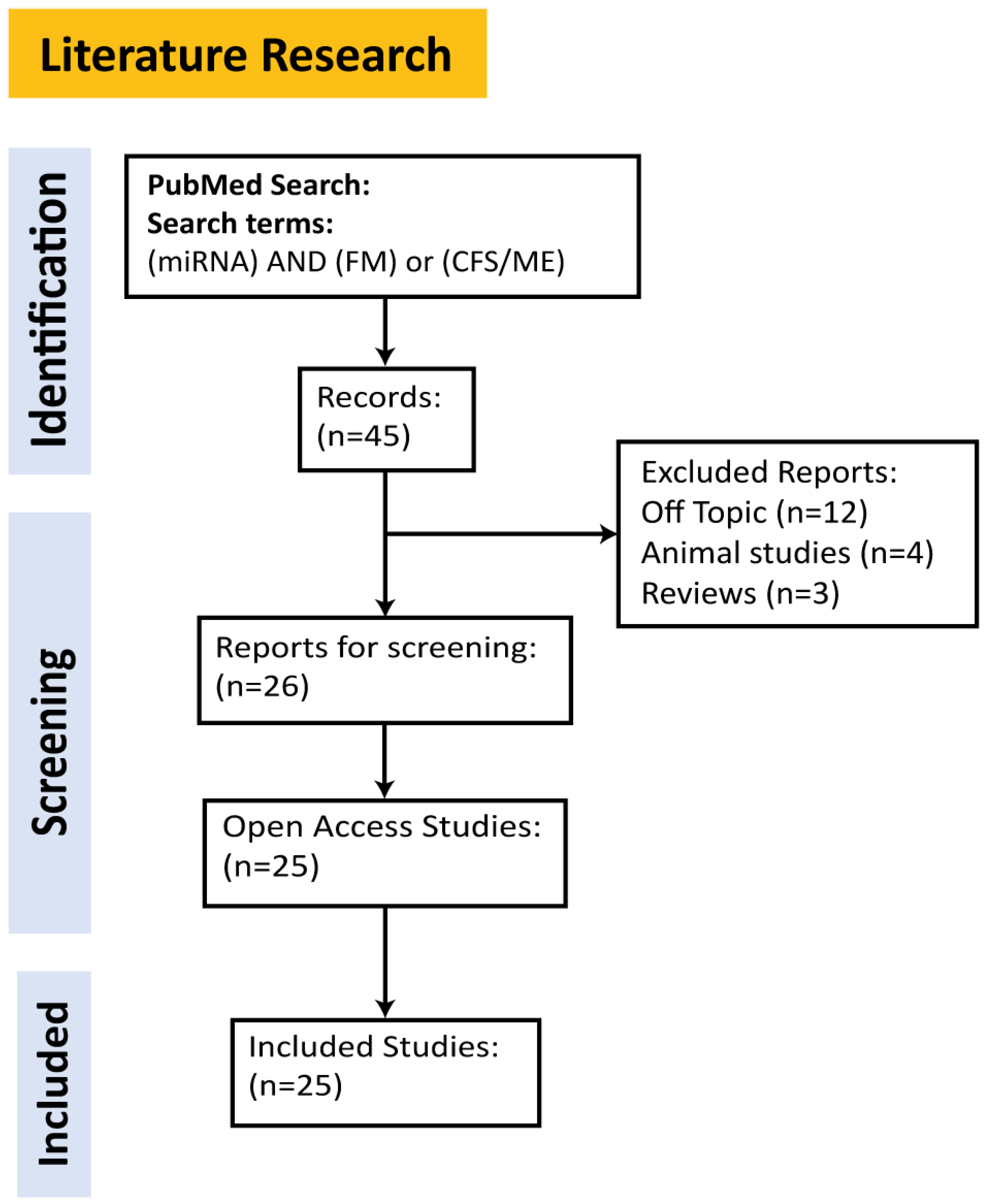
2. Methods
Literature Review for miRNAs in ME/CFS and FM
3. Discussion
3.1. Dysregulated Biological and Cellular Processes Shared by ME/CFS and FM
3.2. miRNAs Potentially Implicated in FM and/or ME/CFS
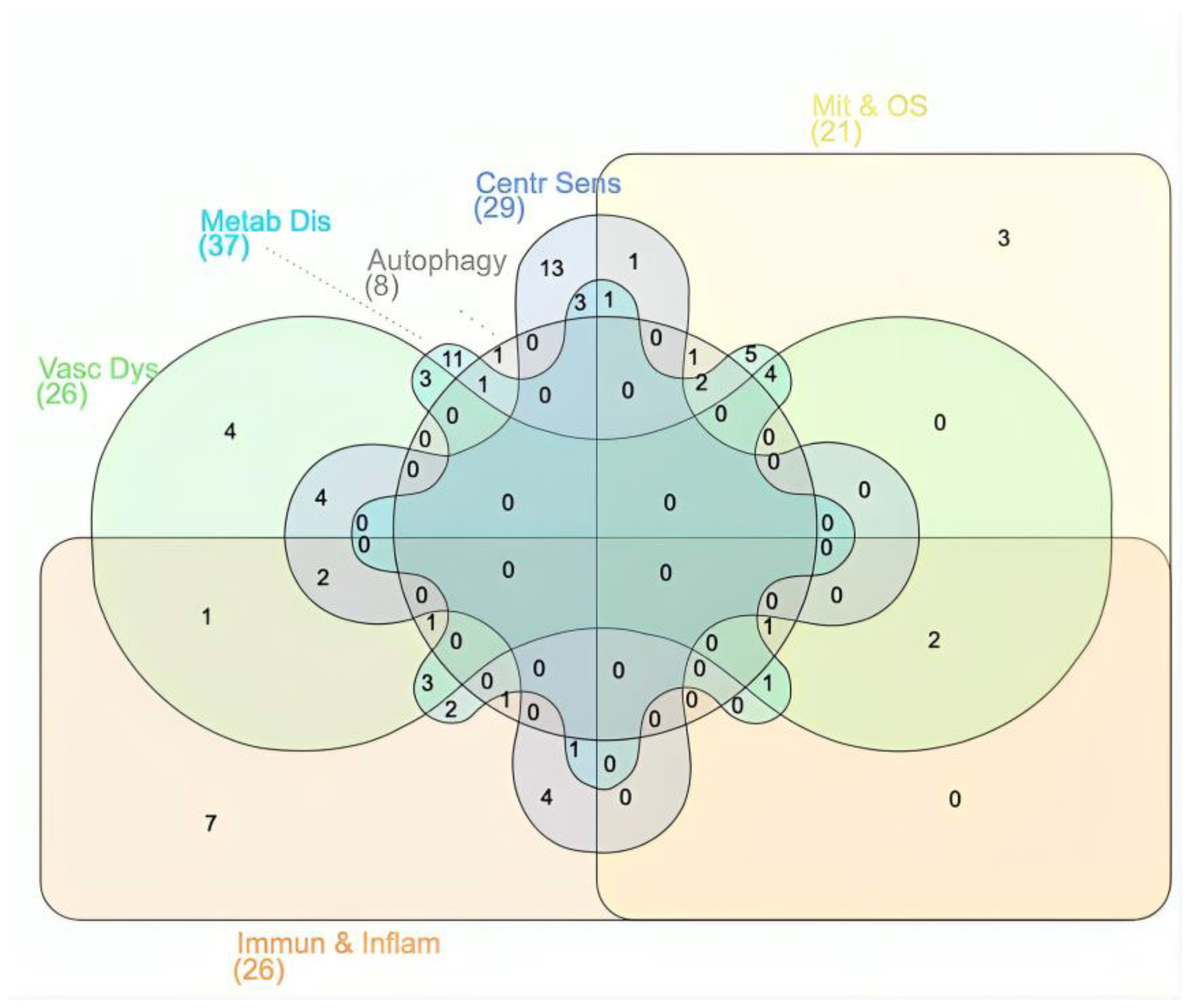
3.3. Sex- and Age-specific Patterns
4. Conclusions
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Avellaneda Fernández, A.; Pérez Martín, Á.; Izquierdo Martínez, M.; Arruti Bustillo, M.; Barbado Hernández, F.J.; de la Cruz Labrado, J.; Díaz-Delgado Peñas, R.; Gutiérrez Rivas, E.; Palacín Delgado, C.; Rivera Redondo, J.; et al. Chronic fatigue syndrome: Aetiology, diagnosis and treatment. BMC Psychiatry 2009, 9, S1. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.P. Worldwide Epidemiology of Fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef]
- Zambolin, F.; Duro-Ocana, P.; Faisal, A.; Bagley, L.; Gregory, W.J.; Jones, A.W.; McPhee, J.S. Fibromyalgia and Chronic Fatigue Syndromes: A systematic review and meta-analysis of cardiorespiratory fitness and neuromuscular function compared with healthy individuals. PLoS ONE 2022, 17, e0276009. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Olivares, P.R.; Adsuar, J.C.; Gusi, N. Impact of fibromyalgia on sexual function in women. J. Back Musculoskelet. Rehabil. 2020, 33, 355–361. [Google Scholar] [CrossRef]
- Saral, I.; Sindel, D.; Esmaeilzadeh, S.; Sertel-Berk, H.O.; Oral, A. The effects of long- and short-term interdisciplinary treatment approaches in women with fibromyalgia: A randomized controlled trial. Rheumatol. Int. 2016, 36, 1379–1389. [Google Scholar] [CrossRef]
- Clayton, E.W. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An IOM Report on Redefining an Illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, F.; Sunnquist, M.; Nacul, L. Rethinking the Standard of Care for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Gen. Intern. Med. 2020, 35, 906–909. [Google Scholar] [CrossRef]
- Brimmer, D.J.; Fridinger, F.; Lin, J.-M.S.; Reeves, W.C. U.S. healthcare providers’ knowledge, attitudes, beliefs, and perceptions concerning Chronic Fatigue Syndrome. BMC Fam. Pract. 2010, 11, 28. [Google Scholar] [CrossRef]
- Van Houdenhove, B.; Luyten, P. Customizing Treatment of Chronic Fatigue Syndrome and Fibromyalgia: The Role of Perpetuating Factors. Psychosomatics 2008, 49, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Arnold, L.M.; Clauw, D.J.; McCarberg, B.H. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin. Proc. 2011, 86, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; McManimen, S.; Sunnquist, M.; Brown, A.; Newton, J.L.; Strand, E.B. Examining the Institute of Medicine’s Recommendations Regarding Chronic Fatigue Syndrome: Clinical Versus Research Criteria. J. Neurol. Psychol. 2015, 2015 (Suppl. S2). Available online: http://www.avensonline.org/wp-content/uploads/JNP-2332-3469-S2-0002.pdf (accessed on 1 August 2024).
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Berwick, R.B.C.; Goebel, A. The diagnosis of fibromyalgia syndrome. Clin. Med. 2022, 22, 570–574. [Google Scholar]
- Bjørklund, G.; Dadar, M.; Pivina, L.; Doşa, M.D.; Semenova, Y.; Maes, M. Environmental, Neuro-immune, and Neuro-oxidative Stress Interactions in Chronic Fatigue Syndrome. Mol. Neurobiol. 2020, 57, 4598–4607. [Google Scholar] [CrossRef]
- Arnold, L.M.; Hudson, J.I.; Hess, E.V.; Ware, A.E.; Fritz, D.A.; Auchenbach, M.B.; Starck, L.O.; Keck, P.E., Jr. Family study of fibromyalgia. Arthritis Rheum. 2004, 50, 944–952. [Google Scholar] [CrossRef]
- Racciatti, D.; Vecchiet, J.; Ceccomancini, A.; Ricci, F.; Pizzigallo, E. Chronic fatigue syndrome following a toxic exposure. Sci. Total Environ. 2001, 270, 27–31. [Google Scholar] [CrossRef]
- Sterzl, I.; Procházková, J.; Hrdá, P.; Bártová, J.; Matucha, P.; Stejskal, V.D. Mercury and nickel allergy: Risk factors in fatigue and autoimmunity. Neuro Endocrinol. Lett. 1999, 20, 221–228. [Google Scholar]
- Tahmaz, N.; Soutar, A.; Cherrie, J.W. Chronic Fatigue and Organophosphate Pesticides in Sheep Farming: A Retrospective Study Amongst People Reporting to a UK Pharmacovigilance Scheme. Ann. Occup. Hyg. 2003, 47, 261–267. [Google Scholar] [CrossRef]
- Dunstan, R.H.; Donohoe, M.; Taylor, W.; Roberts, T.K.; Murdoch, R.N.; Watkins, J.A.; McGregor, N.R. A preliminary investigation of chlorinated hydrocarbons and chronic fatigue syndrome. Med. J. Aust. 1995, 163, 294–297. [Google Scholar] [CrossRef]
- Godfrey, R. Fibromyalgia as a manifestation of petroleum fume toxicity in a family of four. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 1997, 3, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Rasa-Dzelzkaleja, S.; Krumina, A.; Capenko, S.; Nora-Krukle, Z.; Gravelsina, S.; Vilmane, A.; Ievina, L.; Shoenfeld, Y.; Murovska, M. The persistent viral infections in the development and severity of myalgic encephalomyelitis/chronic fatigue syndrome. J. Transl. Med. 2023, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Shikova, E.; Reshkova, V.; Kumanova, A.; Raleva, S.; Alexandrova, D.; Capo, N.; Murovska, M. Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J. Med. Virol. 2020, 92, 3682–3688. [Google Scholar] [CrossRef] [PubMed]
- Hunskar, G.S.; Rortveit, G.; Litleskare, S.; Eide, G.E.; Hanevik, K.; Langeland, N.; Wensaas, K.-A. Prevalence of fibromyalgia 10 years after infection with Giardia lamblia: A controlled prospective cohort study. Scand. J. Pain 2022, 22, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Buskila, D.; Atzeni, F.; Sarzi-Puttini, P. Etiology of fibromyalgia: The possible role of infection and vaccination. Autoimmun. Rev. 2008, 8, 41–43. [Google Scholar] [CrossRef]
- Brenu, E.W.; van Driel, M.L.; Staines, D.R.; Ashton, K.J.; Ramos, S.B.; Keane, J.; Klimas, N.G.; Marshall-Gradisnik, S.M. Immunological abnormalities as potential biomarkers in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. J. Transl. Med. 2011, 9, 81. [Google Scholar] [CrossRef]
- Staud, R. Cytokine and immune system abnormalities in fibromyalgia and other central sensitivity syndromes. Curr. Rheumatol. Rev. 2015, 11, 109–115. [Google Scholar] [CrossRef]
- Van Houdenhove, B.; Luyten, P.; Kempke, S. Chronic fatigue syndrome/fibromyalgia: A “stress-adaptation” model. Fatigue Biomed. Health Behav. 2013, 1, 137–147. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J. Fibromyalgia and nutrition: Therapeutic possibilities? Biomed. Pharmacother. 2018, 103, 531–538. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pen, J.J.; Chirumbolo, S.; Aaseth, J. Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed. Pharmacother. 2019, 109, 1000–1007. [Google Scholar] [CrossRef]
- Zaporozhchenko, I.A.; Rykova, E.Y.; Laktionov, P.P. The Fundamentals of miRNA Biology: Structure, Biogenesis, and Regulatory Functions. Russ. J. Bioorganic Chem. 2020, 46, 1–13. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Kaczmarek, M.P. Heterogenous circulating miRNA changes in ME/CFS converge on a unified cluster of target genes: A computational analysis. PLoS ONE 2023, 18, e0296060. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.; Godderis, L.; Ghosh, M.; Ickmans, K.; Nijs, J. Epigenetic and miRNA Expression Changes in People with Pain: A Systematic Review. J. Pain 2020, 21, 763–780. [Google Scholar] [CrossRef]
- Groven, N.; Fors, E.A.; Reitan, S.K. Patients with Fibromyalgia and Chronic Fatigue Syndrome show increased hsCRP compared to healthy controls. Brain Behav. Immun. 2019, 81, 172–177. [Google Scholar] [CrossRef]
- Atamer, Y.; Sarac, S.; Asık, H.K.; Sahbaz, T. Serum paraoxonase activities, nitric oxide, and malondialdehyde levels are altered in patients with primary fibromyalgia syndrome. Ir. J. Med. Sci. 2023, 192, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Lee, D.S.; Son, C.G. Oxidative Stress is a Convincing Contributor to Idiopathic Chronic Fatigue. Sci. Rep. 2018, 8, 12890. [Google Scholar] [CrossRef]
- Meeus, M.; Nijs, J.; Hermans, L.; Goubert, D.; Calders, P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: Peripheral and central mechanisms as therapeutic targets? Expert Opin. Ther. Targets 2013, 17, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, K.I.; Kim, S.M.; Lee, H.G.; Kim, T.I. Arterial stiffness in female patients with fibromyalgia and its relationship to chronic emotional and physical stress. Korean Circ. J. 2011, 41, 596–602. [Google Scholar] [CrossRef][Green Version]
- Sandvik, M.K.; Sørland, K.; Leirgul, E.; Rekeland, I.G.; Stavland, C.S.; Mella, O.; Fluge, Ø. Endothelial dysfunction in ME/CFS patients. PLoS ONE 2023, 18, e0280942. [Google Scholar] [CrossRef]
- Kavyani, B.; Lidbury, B.A.; Schloeffel, R.; Fisher, P.R.; Missailidis, D.; Annesley, S.J.; Dehhaghi, M.; Heng, B.; Guillemin, G.J. Could the kynurenine pathway be the key missing piece of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) complex puzzle? Cell. Mol. Life Sci. 2022, 79, 412. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Staines, D.; Nilius, B.; Smith, P.; Marshall-Gradisnik, S. Novel identification and characterisation of Transient receptor potential melastatin 3 ion channels on Natural Killer cells and B lymphocytes: Effects on cell signalling in Chronic fatigue syndrome/Myalgic encephalomyelitis patients. Biol. Res. 2016, 49, 27. [Google Scholar] [CrossRef] [PubMed]
- Cagnie, B.; Coppieters, I.; Denecker, S.; Six, J.; Danneels, L.; Meeus, M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin. Arthritis Rheum. 2014, 44, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Holtorf, K. Diagnosis and Treatment of Hypothalamic-Pituitary-Adrenal (HPA) Axis Dysfunction in Patients with Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). J. Chronic Fatigue Syndr. 2007, 14, 59–88. [Google Scholar] [CrossRef]
- Crofford, L.J. The hypothalamic-pituitary-adrenal stress axis in fibromyalgia and chronic fatigue syndrome. Z. Für Rheumatol. 1998, 57, S67–S71. [Google Scholar] [CrossRef][Green Version]
- Romano, G.F.; Tomassi, S.; Russell, A.; Mondelli, V.; Pariante, C.M. Fibromyalgia and chronic fatigue: The underlying biology and related theoretical issues. Adv. Psychosom. Med. 2015, 34, 61–77. [Google Scholar] [CrossRef]
- Maes, M. Inflammatory and Oxidative and Nitrosative Stress. Cascades as New Drug Targets in Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. Mod. Trends Pharmacopsychiatry 2013, 28, 162–174. [Google Scholar] [CrossRef]
- Banfi, G.; Diani, M.; Pigatto, P.D.; Reali, E. T Cell Subpopulations in the Physiopathology of Fibromyalgia: Evidence and Perspectives. Int. J. Mol. Sci. 2020, 21, 1186. [Google Scholar] [CrossRef]
- Groven, N.; Fors, E.A.; Stunes, A.K.; Reitan, S.K. MCP-1 is increased in patients with CFS and FM, whilst several other immune markers are significantly lower than healthy controls. Brain Behav. Immun.-Health 2020, 4, 100067. [Google Scholar] [CrossRef]
- Brenu, E.W.; Hardcastle, S.L.; Atkinson, G.M.; van Driel, M.L.; Kreijkamp-Kaspers, S.; Ashton, K.J.; Staines, D.R.; Marshall-Gradisnik, S.M. Natural killer cells in patients with severe chronic fatigue syndrome. Autoimmun. Highlights 2013, 4, 69–80. [Google Scholar] [CrossRef]
- Mensah, F.K.F.; Bansal, A.S.; Ford, B.; Cambridge, G. Chronic fatigue syndrome and the immune system: Where are we now? Neurophysiol. Clin. 2017, 47, 131–138. [Google Scholar] [CrossRef]
- Komaroff, A.L. Inflammation correlates with symptoms in chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 8914–8916. [Google Scholar] [CrossRef] [PubMed]
- Tate, W.; Walker, M.; Sweetman, E.; Helliwell, A.; Peppercorn, K.; Edgar, C.; Blair, A.; Chatterjee, A. Molecular Mechanisms of Neuroinflammation in ME/CFS and Long COVID to Sustain Disease and Promote Relapses. Front. Neurol. 2022, 13, 877772. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Russell, I.J.; Vaeroy, H.; Javors, M.; Nyberg, F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992, 35, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Schertzinger, M.; Wesson-Sides, K.; Parkitny, L.; Younger, J. Daily Fluctuations of Progesterone and Testosterone Are Associated with Fibromyalgia Pain Severity. J. Pain 2018, 19, 410–417. [Google Scholar] [CrossRef]
- de Kruijf, M.; Stolk, L.; Zillikens, M.C.; de Rijke, Y.B.; Bierma-Zeinstra, S.M.A.; Hofman, A.; Huygen, F.J.P.M.; Uitterlinden, A.G.; van Meurs, J.B.J. Lower sex hormone levels are associated with more chronic musculoskeletal pain in community-dwelling elderly women. Pain 2016, 157, 1425–1431. [Google Scholar] [CrossRef]
- Boneva, R.S.; Maloney, E.M.; Lin, J.-M.; Jones, J.F.; Wieser, F.; Nater, U.M.; Heim, C.M.; Reeves, W.C. Gynecological History in Chronic Fatigue Syndrome: A Population-Based Case-Control Study. J. Women’s Health 2010, 20, 21–28. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Increased nuclear factor-κB and loss of p53 are key mechanisms in Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med. Hypotheses 2012, 79, 607–613. [Google Scholar] [CrossRef]
- McArdle, A.; McArdle, F.; Jackson, M.J.; Page, S.F.; Fahal, I.; Edwards, R.H.T. Investigation by Polymerase Chain Reaction of Enteroviral Infection in Patients with Chronic Fatigue Syndrome. Clin. Sci. 1996, 90, 295–300. [Google Scholar] [CrossRef]
- Cordero, M.D.; De Miguel, M.; Moreno Fernández, A.M.; Carmona López, I.M.; Garrido Maraver, J.; Cotán, D.; Gómez Izquierdo, L.; Bonal, P.; Campa, F.; Bullon, P.; et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010, 12, R17. [Google Scholar] [CrossRef]
- Oezel, L.; Then, H.; Jung, A.L.; Jabari, S.; Bonaterra, G.A.; Wissniowski, T.T.; Önel, S.F.; Ocker, M.; Thieme, K.; Kinscherf, R.; et al. Fibromyalgia syndrome: Metabolic and autophagic processes in intermittent cold stress mice. Pharmacol. Res. Perspect. 2016, 4, e00248. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, G.; Peterson, D.; Knox, K.; Maynard, M.; Whelan, R.J.; Roy, A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE). Mol. Cell. Neurosci. 2022, 120, 103731. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Szklarski, M.; Hartwig, J.; Sotzny, F.; Lorenz, S.; Meyer, A.; Grabowski, P.; Doehner, W.; Scheibenbogen, C. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020, 7, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Lee, J.H.; Kim, S.M.; Lee, H.G.; Kim, T.I. Assessment of endothelial function in patients with fibromyalgia—Cardiac ultrasound study. Clin. Rheumatol. 2011, 30, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.J.; Kennedy, G.; Chan, K.K.F.; Lang, C.C.; Belch, J.J.F.; Khan, F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int. J. Cardiol. 2012, 154, 335–336. [Google Scholar] [CrossRef]
- Cabanas, H.; Muraki, K.; Eaton, N.; Balinas, C.; Staines, D.; Marshall-Gradisnik, S. Loss of Transient Receptor Potential Melastatin 3 ion channel function in natural killer cells from Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients. Mol. Med. 2018, 24, 44. [Google Scholar] [CrossRef]
- Veldhuis, N.A.; Poole, D.P.; Grace, M.; McIntyre, P.; Bunnett, N.W. The G Protein–Coupled Receptor–Transient Receptor Potential Channel Axis: Molecular Insights for Targeting Disorders of Sensation and Inflammation. Pharmacol. Rev. 2015, 67, 36–73. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Nicotra, L.; Loram, L.C.; Watkins, L.R.; Hutchinson, M.R. Toll-like receptors in chronic pain. Exp. Neurol. 2012, 234, 316–329. [Google Scholar] [CrossRef]
- Lacagnina, M.J.; Watkins, L.R.; Grace, P.M. Toll-like receptors and their role in persistent pain. Pharmacol. Ther. 2018, 184, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eede, F.; Moorkens, G.; Van Houdenhove, B.; Cosyns, P.; Claes, S.J. Hypothalamic-Pituitary-Adrenal Axis Function in Chronic Fatigue Syndrome. Neuropsychobiology 2007, 55, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Riva, R.; Mork, P.J.; Westgaard, R.H.; Rø, M.; Lundberg, U. Fibromyalgia Syndrome is Associated with Hypocortisolism. Int. J. Behav. Med. 2010, 17, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Tomas, C.; Newton, J.; Watson, S. A Review of Hypothalamic-Pituitary-Adrenal Axis Function in Chronic Fatigue Syndrome. ISRN Neurosci. 2013, 2013, 784520. [Google Scholar] [CrossRef] [PubMed]
- Altemus, M.; Dale, J.K.; Michelson, D.; Demitrack, M.A.; Gold, P.W.; Straus, S.E. Abnormalities in response to vasopressin infusion in chronic fatigue syndrome. Psychoneuroendocrinology 2001, 26, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.M.; Boneva, R.S.; Lin, J.-M.S.; Reeves, W.C. Chronic fatigue syndrome is associated with metabolic syndrome: Results from a case-control study in Georgia. Metab. Clin. Exp. 2010, 59, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Loevinger, B.L.; Muller, D.; Alonso, C.; Coe, C.L. Metabolic syndrome in women with chronic pain. Metabolism: Clinical and experimental. Metabolism 2007, 56, 87–93. [Google Scholar] [CrossRef]
- Bjersing, J.L.; Dehlin, M.; Erlandsson, M.; Bokarewa, M.I.; Mannerkorpi, K. Changes in pain and insulin-like growth factor 1 in fibromyalgia during exercise: The involvement of cerebrospinal inflammatory factors and neuropeptides. Arthritis Res. Ther. 2012, 14, R162. [Google Scholar] [CrossRef]
- Cuatrecasas, G.; Gonzalez, M.J.; Alegre, C.; Sesmilo, G.; Fernandez-Solà, J.; Casanueva, F.F.; Garcia-Fructuoso, F.; Poca-Dias, V.; Izquierdo, J.P.; Puig-Domingo, M. High prevalence of growth hormone deficiency in severe fibromyalgia syndromes. J. Clin. Endocrinol. Metab. 2010, 95, 4331–4337. [Google Scholar] [CrossRef]
- Bennett, R.M. Adult growth hormone deficiency in patients with fibromyalgia. Curr. Rheumatol. Rep. 2002, 4, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Germain, A.; Ruppert, D.; Levine, S.M.; Hanson, M.R. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol. Biosyst. 2017, 13, 371–379. [Google Scholar] [CrossRef]
- Nepotchatykh, E.; Caraus, I.; Elremaly, W.; Leveau, C.; Elbakry, M.; Godbout, C.; Rostami-Afshari, B.; Petre, D.; Khatami, N.; Franco, A.; et al. Circulating microRNA expression signatures accurately discriminate myalgic encephalomyelitis from fibromyalgia and comorbid conditions. Sci. Rep. 2023, 13, 1896. [Google Scholar] [CrossRef] [PubMed]
- Almenar-Pérez, E.; Sánchez-Fito, T.; Ovejero, T.; Nathanson, L.; Oltra, E. Impact of Polypharmacy on Candidate Biomarker miRNomes for the Diagnosis of Fibromyalgia and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Striking Back on Treatments. Pharmaceutics 2019, 11, 126. [Google Scholar] [CrossRef]
- Cerdá-Olmedo, G.; Mena-Durán, A.V.; Monsalve, V.; Oltra, E. Identification of a MicroRNA Signature for the Diagnosis of Fibromyalgia. PLoS ONE 2015, 10, e0121903. [Google Scholar] [CrossRef] [PubMed]
- Soffritti, I.; Gravelsina, S.; D’Accolti, M.; Bini, F.; Mazziga, E.; Vilmane, A.; Rasa-Dzelzkaleja, S.; Nora-Krukle, Z.; Krumina, A.; Murovska, M.; et al. Circulating miRNAs Expression in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int. J. Mol. Sci. 2023, 24, 10582. [Google Scholar] [CrossRef]
- Yang, X.; Li, F.; Ma, J.; Liu, Y.; Wang, X.; Wang, R.; Zhang, Y.; Zhang, W.; He, Q.; Song, D.; et al. Study on the Relationship between the miRNA-centered ceRNA Regulatory Network and Fatigue. J. Mol. Neurosci. 2021, 71, 1967–1974. [Google Scholar] [CrossRef]
- Almenar-Pérez, E.; Sarría, L.; Nathanson, L.; Oltra, E. Assessing diagnostic value of microRNAs from peripheral blood mononuclear cells and extracellular vesicles in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Sci. Rep. 2020, 10, 2064. [Google Scholar] [CrossRef]
- Brenu, E.W.; Ashton, K.J.; van Driel, M.; Staines, D.R.; Peterson, D.; Atkinson, G.M.; Marshall-Gradisnik, S.M. Cytotoxic lymphocyte microRNAs as prospective biomarkers for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. J. Affect. Disord. 2012, 141, 261–269. [Google Scholar] [CrossRef]
- Blauensteiner, J.; Bertinat, R.; León, L.E.; Riederer, M.; Sepúlveda, N.; Westermeier, F. Altered endothelial dysfunction-related miRs in plasma from ME/CFS patients. Sci. Rep. 2021, 11, 10604. [Google Scholar] [CrossRef]
- Erbacher, C.; Vaknine, S.; Moshitzky, G.; Lobentanzer, S.; Eisenberg, L.; Evdokimov, D.; Sommer, C.; Greenberg, D.S.; Soreq, H.; Üçeyler, N. Distinct CholinomiR Blood Cell Signature as a Potential Modulator of the Cholinergic System in Women with Fibromyalgia Syndrome. Cells 2022, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Brenu, E.W.; Ashton, K.J.; Batovska, J.; Staines, D.R.; Marshall-Gradisnik, S.M. High-Throughput Sequencing of Plasma MicroRNA in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. PLoS ONE 2014, 9, e102783. [Google Scholar] [CrossRef] [PubMed]
- Nepotchatykh, E.; Elremaly, W.; Caraus, I.; Godbout, C.; Leveau, C.; Chalder, L.; Beaudin, C.; Kanamaru, E.; Kosovskaia, R.; Lauzon, S.; et al. Profile of circulating microRNAs in myalgic encephalomyelitis and their relation to symptom severity, and disease pathophysiology. Sci. Rep. 2020, 10, 19620. [Google Scholar] [CrossRef]
- Al-Rawaf, H.A.; Alghadir, A.H.; Gabr, S.A. MicroRNAs as Biomarkers of Pain Intensity in Patients with Chronic Fatigue Syndrome. Pain Pract. 2019, 19, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Fathy, W.; Abdelaleem, E.A.; Nasser, M.; Yehia, A.; Elanwar, R. The Impact of Micro RNA-320a Serum Level on Severity of Symptoms and Cerebral Processing of Pain in Patients with Fibromyalgia. Pain Med. 2022, 23, 2061–2072. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrerag, D.; Royoa, F.; Valeroh, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. eBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef]
- Berg, F.; Moser, D.A.; Hagena, V.; Streit, F.; Mosch, B.; Kumsta, R.; Herpertz, S.; Diers, M. MicroRNA-Related Polymorphism and Their Association with Fibromyalgia. Genes 2023, 14, 1312. [Google Scholar] [CrossRef]
- Masotti, A.; Baldassarre, A.; Guzzo, M.P.; Iannuccelli, C.; Barbato, C.; Di Franco, M. Circulating microRNA Profiles as Liquid Biopsies for the Characterization and Diagnosis of Fibromyalgia Syndrome. Mol. Neurobiol. 2017, 54, 7129–7136. [Google Scholar] [CrossRef]
- Andersen, H.H.; Duroux, M.; Gazerani, P. Serum MicroRNA Signatures in Migraineurs During Attacks and in Pain-Free Periods. Mol. Neurobiol. 2016, 53, 1494–1500. [Google Scholar] [CrossRef]
- Petty, R.D.; McCarthy, N.E.; Le Dieu, R.; Kerr, J.R. MicroRNAs hsa-miR-99b, hsa-miR-330, hsa-miR-126 and hsa-miR-30c: Potential Diagnostic Biomarkers in Natural Killer (NK) Cells of Patients with Chronic Fatigue Syndrome (CFS)/Myalgic Encephalomyelitis (ME). PLoS ONE 2016, 11, e0150904. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; Shivapurkar, N. Exercise–induced changes in cerebrospinal fluid miRNAs in Gulf War Illness, Chronic Fatigue Syndrome and sedentary control subjects. Sci. Rep. 2017, 7, 15338. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Evdokimov, D.; Frank, J.; Sommer, C.; Üçeyler, N. MiR103a-3p and miR107 are related to adaptive coping in a cluster of fibromyalgia patients. PLoS ONE 2020, 15, e0239286. [Google Scholar] [CrossRef]
- Ding, X.; Lin, Y.; Yan, B.; Jiao, X.; Liu, Q.; Miao, H.; Wu, Y.; Zhou, C. LncRNA XR_351665 Contributes to Chronic Pain-Induced Depression by Upregulating DNMT1 via Sponging miR-152-3p. J. Pain 2023, 24, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Bjersing, J.L.; Lundborg, C.; Bokarewa, M.I.; Mannerkorpi, K. Profile of Cerebrospinal microRNAs in Fibromyalgia. PLoS ONE 2013, 8, e78762. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Dubey, R.; Saini, N. Cooperative and individualistic functions of the microRNAs in the miR-23a~27a~24-2 cluster and its implication in human diseases. Mol. Cancer 2010, 9, 232. [Google Scholar] [CrossRef]
- Rodriguez, R.E. Morphine and microRNA Activity: Is There a Relation with Addiction? Front. Genet. 2012, 3, 223. [Google Scholar] [CrossRef]
- Zhu, S.; Pan, W.; Song, X.; Liu, Y.; Shao, X.; Tang, Y.; Liang, D.; He, D.; Wang, H.; Liu, W.; et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat. Med. 2012, 18, 1077–1086. [Google Scholar] [CrossRef]
- Bjersing, J.L.; Bokarewa, M.I.; Mannerkorpi, K. Profile of circulating microRNAs in fibromyalgia and their relation to symptom severity: An exploratory study. Rheumatol. Int. 2015, 35, 635–642. [Google Scholar] [CrossRef]
- Linnstaedt, S.D.; Riker, K.D.; Walker, M.G.; Nyland, J.E.; Zimny, E.; Lewandowski, C.; Hendry, P.L.; Damiron, K.; Pearson, C.; Velilla, M.-A.; et al. MicroRNA 320a Predicts Chronic Axial and Widespread Pain Development Following Motor Vehicle Collision in a Stress-Dependent Manner. J. Orthop. Sports Phys. Ther. 2016, 46, 911–919. [Google Scholar] [CrossRef]
- Talebi, F.; Ghourbani, S.; Vojgani, M.; Noorbakhsh, F. miR-320 and Inflammation Regulation in Experimental Autoimmune Encephalomyelitis Through Interference with Tumor Growth Factor-β Signaling Pathway. Immunoregulation 2020, 2, 111–120. [Google Scholar] [CrossRef]
- Wang, X.; Qian, R.; Zhang, W.; Chen, S.; Jin, H.; Hu, R. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Hu, C.; Zhou, J.; Xu, D.; Hou, Y.; Wu, C.; Zhao, J.; Li, M.; Zeng, X.; et al. MicroRNA-320a: An important regulator in the fibrotic process in interstitial lung disease of systemic sclerosis. Arthritis Res. Ther. 2021, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Favereaux, A.; Thoumine, O.; Bouali-Benazzouz, R.; Roques, V.; Papon, M.A.; Salam, S.A.; Drutel, G.; Léger, C.; Calas, A.; Nagy, F.; et al. Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: Role in pain. EMBO J. 2011, 30, 3830–3841. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, L.T.; Sørensen, A.E.; Hardikar, A.A.; Joglekar, M.V. The microRNA-29 family: Role in metabolism and metabolic disease. Am. J. Physiol.-Cell Physiol. 2022, 323, C367–C377. [Google Scholar] [CrossRef]
- Horita, M.; Farquharson, C.; Stephen, L.A. The role of miR-29 family in disease. J. Cell Biochem. 2021, 122, 696–715. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.J.; Graça, F.; Cruz, A.; Silvestre, J.G.; Labeit, S.; Miyabara, E.H.; Yan, C.Y.I.; Wang, D.Z.; Moriscot, A.S. miR-29c improves skeletal muscle mass and function throughout myocyte proliferation and differentiation and by repressing atrophy-related genes. Acta Physiol. 2019, 226, e13278. [Google Scholar] [CrossRef]
- Jin, Y.; Tymen, S.D.; Chen, D.; Fang, Z.J.; Zhao, Y.; Dragas, D.; Dai, Y.; Marucha, P.T.; Zhou, X. MicroRNA-99 Family Targets AKT/mTOR Signaling Pathway in Dermal Wound Healing. PLoS ONE 2013, 8, e64434. [Google Scholar] [CrossRef]
- Nandagopal, N.; Ali, A.K.; Komal, A.K.; Lee, S.-H. The Critical Role of IL-15–PI3K–mTOR Pathway in Natural Killer Cell Effector Functions. Front. Immunol. 2014, 5, 187. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, S.; Zhang, W.; Ni, L.; Hu, Z.; Sheng, Z.; Yin, B. MiR-128 inhibits the osteogenic differentiation in osteoporosis by down-regulating SIRT6 expression. Biosci. Rep. 2019, 39, BSR20191405. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.G.; Karam, R.; Huang, L.; Bhardwaj, A.; Lou, C.H.; Shum, E.Y.; Song, H.-W.; Corbett, M.A.; Gifford, W.D.; Gecz, J.; et al. Identification of a MicroRNA that Activates Gene Expression by Repressing Nonsense-Mediated RNA Decay. Mol. Cell 2011, 42, 500–510. [Google Scholar] [CrossRef]
- Liao, Y.; Tsai, H.; Chou, P.; Wang, S.; Chen, H.; Lin, Y.; Chiang, I.; Chang, T.; Hsu, S.; Chou, M.; et al. CCL3 promotes angiogenesis by dysregulation of miR-374b/ VEGF-A axis in human osteosarcoma cells. Oncotarget 2016, 7, 4310–4325. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhou, Z.; Liu, X.; Richards, R.G.; Alini, M.; Peng, S.; Liu, S.; Zou, X.; Li, Z.; Grad, S. Identification and Characterization of Serum microRNAs as Biomarkers for Human Disc Degeneration: An RNA Sequencing Analysis. Diagnostics 2020, 10, 1063. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Nisenbaum, R.; Hoaglin, D.C.; Unger, E.R.; Emmons, C.; Randall, B.; Stewart, J.A.; Abbey, S.; Jones, J.F.; Gantz, N.; et al. Prevalence and Incidence of Chronic Fatigue Syndrome in Wichita, Kansas. Arch. Intern. Med. 2003, 163, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, E.M.; Geraghty, K.; Kingdon, C.C.; Palla, L.; Nacul, L. A logistic regression analysis of risk factors in ME/CFS pathogenesis. BMC Neurol. 2019, 19, 275. [Google Scholar] [CrossRef]
- Wolfe, F.; Ross, K.; Anderson, J.; Russell, I.J.; Hebert, L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995, 38, 19–28. [Google Scholar] [CrossRef]
- Haddad, H.W.; Mallepalli, N.R.; Scheinuk, J.E.; Bhargava, P.; Cornett, E.M.; Urits, I.; Kaye, A.D. The Role of Nutrient Supplementation in the Management of Chronic Pain in Fibromyalgia: A Narrative Review. Pain Ther. 2021, 10, 827–848. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Craft, R.M.; Mogil, J.S.; Aloisi, A.M. Sex differences in pain and analgesia: The role of gonadal hormones. Eur. J. Pain 2004, 8, 397–411. [Google Scholar] [CrossRef]
- Osborne, N.R.; Davis, K.D. Chapter Eight–Sex and gender differences in pain. In International Review of Neurobiology; Moro, E., Arabia, G., Tartaglia, M.C., Ferretti, M.T., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 164, pp. 277–307. [Google Scholar] [CrossRef]
- Martin, V.T. Ovarian hormones and pain response: A review of clinical and basic science studies. Gend. Med. 2009, 6, 168–192. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Kousteni, S. Perspective: Nonreproductive Sites of Action of Reproductive Hormones*. Endocrinology 2001, 142, 2200–2204. [Google Scholar] [CrossRef]
- Craft, R.M. Modulation of pain by estrogens. Pain 2007, 132, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.R.; Stohler, C.S.; Nichols, T.E.; Bueller, J.A.; Koeppe, R.A.; Zubieta, J.-K. Pronociceptive and Antinociceptive Effects of Estradiol through Endogenous Opioid Neurotransmission in Women. J. Neurosci. 2006, 26, 5777–5785. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S.; Wilson, S.G.; Chesler, E.J.; Rankin, A.L.; Nemmani, K.V.S.; Lariviere, W.R.; Groce, M.K.; Wallace, M.R.; Kaplan, L.; Staud, R.; et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc. Natl. Acad. Sci. USA 2003, 100, 4867–4872. [Google Scholar] [CrossRef]
- Paredes, S.; Cantillo, S.; Candido, K.D.; Knezevic, N.N. An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. Int. J. Mol. Sci. 2019, 20, 5729. [Google Scholar] [CrossRef] [PubMed]
- Athnaiel, O.; Cantillo, S.; Paredes, S.; Knezevic, N.N. The Role of Sex Hormones in Pain-Related Conditions. Int. J. Mol. Sci. 2023, 24, 1866. [Google Scholar] [CrossRef]
- Dessein, P.H.; Shipton, E.A. Hydrocortisone and chronic fatigue syndrome. Lancet 1999, 353, 1618. [Google Scholar] [CrossRef]
- Bakken, I.J.; Tveito, K.; Gunnes, N.; Ghaderi, S.; Stoltenberg, C.; Trogstad, L.; Håberg, S.E.; Magnus, P. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: A population-based registry study from Norway 2008–2012. BMC Med. 2014, 12, 167. [Google Scholar] [CrossRef]
- Cronan, T.A.; Serber, E.R.; Walen, H.R.; Jaffe, M. The Influence of Age on Fibromyalgia Symptoms. J. Aging Health 2002, 14, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Palacios, N.; Molsberry, S.; Fitzgerald, K.C.; Komaroff, A.L. Different risk factors distinguish myalgic encephalomyelitis/chronic fatigue syndrome from severe fatigue. Sci. Rep. 2023, 13, 2469. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.; Brown, A.; McManimen, S.; Jason, L.A.; Newton, J.L.; Strand, E.B. The Relationship between Age and Illness Duration in Chronic Fatigue Syndrome. Diagnostics 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Ceko, M.; Bushnell, M.C.; Fitzcharles, M.-A.; Schweinhardt, P. Fibromyalgia interacts with age to change the brain. NeuroImage Clin. 2013, 3, 249–260. [Google Scholar] [CrossRef]
- Kuchinad, A.; Schweinhardt, P.; Seminowicz, D.A.; Wood, P.B.; Chizh, B.A.; Bushnell, M.C. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 4004–4007. [Google Scholar] [CrossRef] [PubMed]
- Corran, T.M.; Farrell, M.J.; Helme, R.D.; Gibson, S.J. The Classification of Patients with Chronic Pain: Age as a Contributing Factor. Clin. J. Pain. 1997, 13, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Moatar, A.I.; Chis, A.R.; Romanescu, M.; Ciordas, P.-D.; Nitusca, D.; Marian, C.; Oancea, C.; Sirbu, I.-O. Plasma miR-195-5p predicts the severity of Covid-19 in hospitalized patients. Sci. Rep. 2023, 13, 13806. [Google Scholar] [CrossRef]
- Kasimir, F.; Toomey, D.; Liu, Z.; Kaiping, A.C.; Ariza, M.E.; Prusty, B.K. Tissue specific signature of HHV-6 infection in ME/CFS. Front. Mol. Biosci. 2022, 9, 1044964. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, T.; Cai, J.; Huang, C.; Zhan, S.; Liu, J. Bioinformatics and systems biology approach to identify the pathogenetic link of Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Immunol. 2022, 13, 952987. [Google Scholar] [CrossRef]
- Cheema, A.K.; Sarria, L.; Bekheit, M.; Collado, F.; Almenar-Pérez, E.; Martín-Martínez, E.; Alegre, J.; Castro-Marrero, J.; Fletcher, M.A.; Klimas, N.G.; et al. Unravelling myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Gender-specific changes in the microRNA expression profiling in ME/CFS. J. Cell. Mol. Med. 2020, 24, 5865–5877. [Google Scholar] [CrossRef]
- Takakura, S.; Oka, T.; Sudo, N. Changes in circulating microRNA after recumbent isometric yoga practice by patients with myalgic encephalomyelitis/chronic fatigue syndrome: An explorative pilot study. Biopsychosoc. Med. 2019, 13, 29. [Google Scholar] [CrossRef]
- Leinders, M.; Doppler, K.; Klein, T.; Deckart, M.; Rittner, H.; Sommer, C.; Üçeyler, N. Increased cutaneous miR-let-7d expression correlates with small nerve fiber pathology in patients with fibromyalgia syndrome. Pain 2016, 157, 2493–2503. [Google Scholar] [CrossRef]
- Frietze, S.; O'Geen, H.; Blahnik, K.R.; Jin, V.X.; Farnham, P.J. ZNF274 Recruits the Histone Methyltransferase SETDB1 to the 3′ Ends of ZNF Genes. PLoS ONE 2010, 5, e15082. [Google Scholar] [CrossRef] [PubMed]
- Shlopov, B.V.; Gumanovskaya, M.L.; Hasty, K.A. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis Rheum. 2000, 43, 195–205. [Google Scholar] [CrossRef]
- Fallowfield, J.A.; Mizuno, M.; Kendall, T.J.; Constandinou, C.M.; Benyon, R.C.; Duffield, J.S.; Iredale, J.P. Scar-Associated Macrophages Are a Major Source of Hepatic Matrix Metalloproteinase-13 and Facilitate the Resolution of Murine Hepatic Fibrosis. J. Immunol. 2007, 178, 5288–5295. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334, 297–314. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Thomson, R.; Finkelstein, A. Human trypanolytic factor APOL1 forms pH-gated cation-selective channels in planar lipid bilayers: Relevance to trypanosome lysis. Proc. Natl. Acad. Sci. USA 2015, 112, 2894–2899. [Google Scholar] [CrossRef] [PubMed]
- Atmaramani, R.R.; Black, B.J.; de la Peña, J.B.; Campbell, Z.T.; Pancrazio, J.J. Conserved Expression of Nav1.7 and Nav1.8 Contribute to the Spontaneous and Thermally Evoked Excitability in IL-6 and NGF-Sensitized Adult Dorsal Root Ganglion Neurons In Vitro. Bioengineering 2020, 7, 44. [Google Scholar] [CrossRef]
- Kochumon, S.; Al Madhoun, A.; Al-Rashed, F.; Azim, R.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Adipose tissue gene expression of CXCL10 and CXCL11 modulates inflammatory markers in obesity: Implications for metabolic inflammation and insulin resistance. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820930902. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Garbi, N.; Reinheckel, T.; Moldenhauer, G.; Hämmerling, G.J.; Momburg, F. A Transporter Associated with Antigen-Processing Independent Vacuolar Pathway for the MHC Class I-Mediated Presentation of Endogenous Transmembrane Proteins. J. Immunol. 2007, 178, 7932–7942. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Cronk, J.C.; Derecki, N.C.; Ji, E.; Xu, Y.; Lampano, A.E.; Smirnov, I.; Baker, W.; Norris, G.T.; Marin, I.; Coddington, N.; et al. Methyl-CpG Binding Protein 2 Regulates Microglia and Macrophage Gene Expression in Response to Inflammatory Stimuli. Immunity 2015, 42, 679–691. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, X.; Yang, B.; Li, Z.; Xue, Y.; Zhou, Y.; Huang, J.; Zhao, X.; Zhou, J.; Yan, Y.; et al. MicroRNA Directly Enhances Mitochondrial Translation during Muscle Differentiation. Cell 2014, 158, 607–619. [Google Scholar] [CrossRef]
- Ying, S.-W.; Futter, M.; Rosenblum, K.; Webber, M.J.; Hunt, S.P.; Bliss, T.V.P.; Bramham, C.R. Brain-Derived Neurotrophic Factor Induces Long-Term Potentiation in Intact Adult Hippocampus: Requirement for ERK Activation Coupled to CREB and Upregulation of Arc Synthesis. J. Neurosci. 2002, 22, 1532–1540. [Google Scholar] [CrossRef]
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. 2007, 214, 149–160. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Goumans, M.-J.; Liu, Z.; Dijke, P.T. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2008, 19, 116–127. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Alcaide, P.; Liu, L.; Sun, J.; He, A.; Luscinskas, F.W.; Shi, G.-P. Regulation of Endothelial Cell Adhesion Molecule Expression by Mast Cells, Macrophages, and Neutrophils. PLoS ONE 2011, 6, e14525. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Parola, M.; Bardini, P.; Piccini, A.; Borghi, R.; Guglielmotto, M.; Santoro, G.; Davit, A.; Danni, O.; Smith, M.A.; et al. β-Site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 2005, 92, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-Y.; Kim, J.-Y.; Kim, K.-W.; Park, M.-K.; Moon, Y.; Kim, W.-U.; Kim, H.-Y. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-κB- and PI3-kinase/Akt-dependent pathways. Arthritis Res. Ther. 2004, 6, R120–R128. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Harty, J.T.; Badovinac, V.P. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 2008, 8, 107–119. [Google Scholar] [CrossRef]
- Salvadores, N.; Gerónimo-Olvera, C.; Court, F.A. Axonal Degeneration in AD: The Contribution of Aβ and Tau. Front. Aging Neurosci. 2020, 12, 581767. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, Z.; Barrantes, I.d.B.; de la Pompa, J.L.; Anderson, D.J. neurogenin1 Is Essential for the Determination of Neuronal Precursors for Proximal Cranial Sensory Ganglia. Neuron 1998, 20, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Brindley, D.N.; Pilquil, C. Lipid phosphate phosphatases and signaling. J. Lipid Res. 2009, 50, S225–S230. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP Channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsamou, M.; Kremers, F.A.C.; Samaritakis, K.A.; Roggen, E.L. Identifying microRNAs Possibly Implicated in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: A Review. Int. J. Mol. Sci. 2024, 25, 9551. https://doi.org/10.3390/ijms25179551
Tsamou M, Kremers FAC, Samaritakis KA, Roggen EL. Identifying microRNAs Possibly Implicated in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: A Review. International Journal of Molecular Sciences. 2024; 25(17):9551. https://doi.org/10.3390/ijms25179551
Chicago/Turabian StyleTsamou, Maria, Fabiënne A. C. Kremers, Keano A. Samaritakis, and Erwin L. Roggen. 2024. "Identifying microRNAs Possibly Implicated in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: A Review" International Journal of Molecular Sciences 25, no. 17: 9551. https://doi.org/10.3390/ijms25179551
APA StyleTsamou, M., Kremers, F. A. C., Samaritakis, K. A., & Roggen, E. L. (2024). Identifying microRNAs Possibly Implicated in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: A Review. International Journal of Molecular Sciences, 25(17), 9551. https://doi.org/10.3390/ijms25179551






