Structural and Phylogenetic In Silico Characterization of Vitis vinifera PRR Protein as Potential Target for Plasmopara viticola Infection
Abstract
1. Introduction
2. Results
2.1. Vitis vinifera LecRLK Identification and Classification
2.2. Chromosomal Characterization
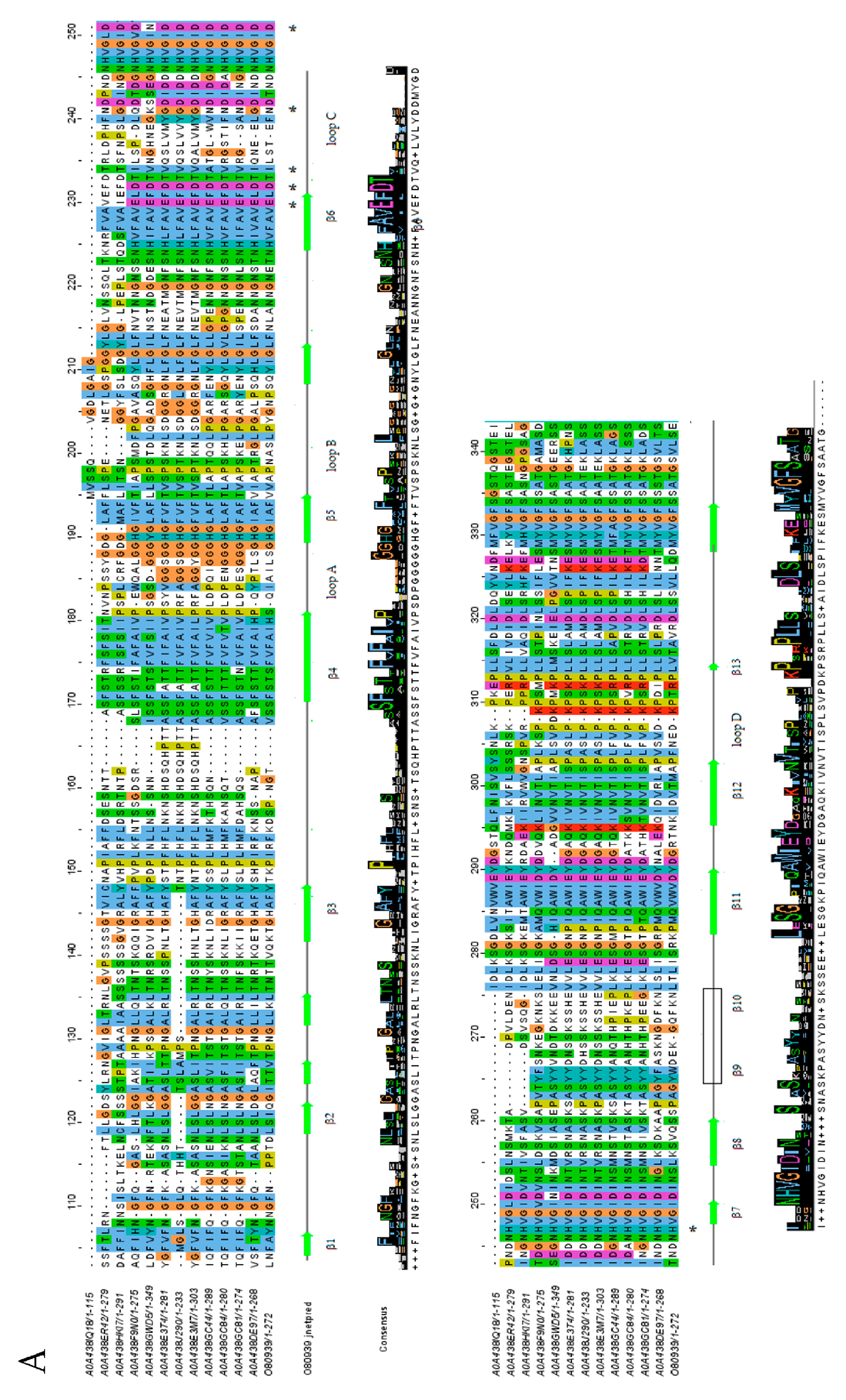
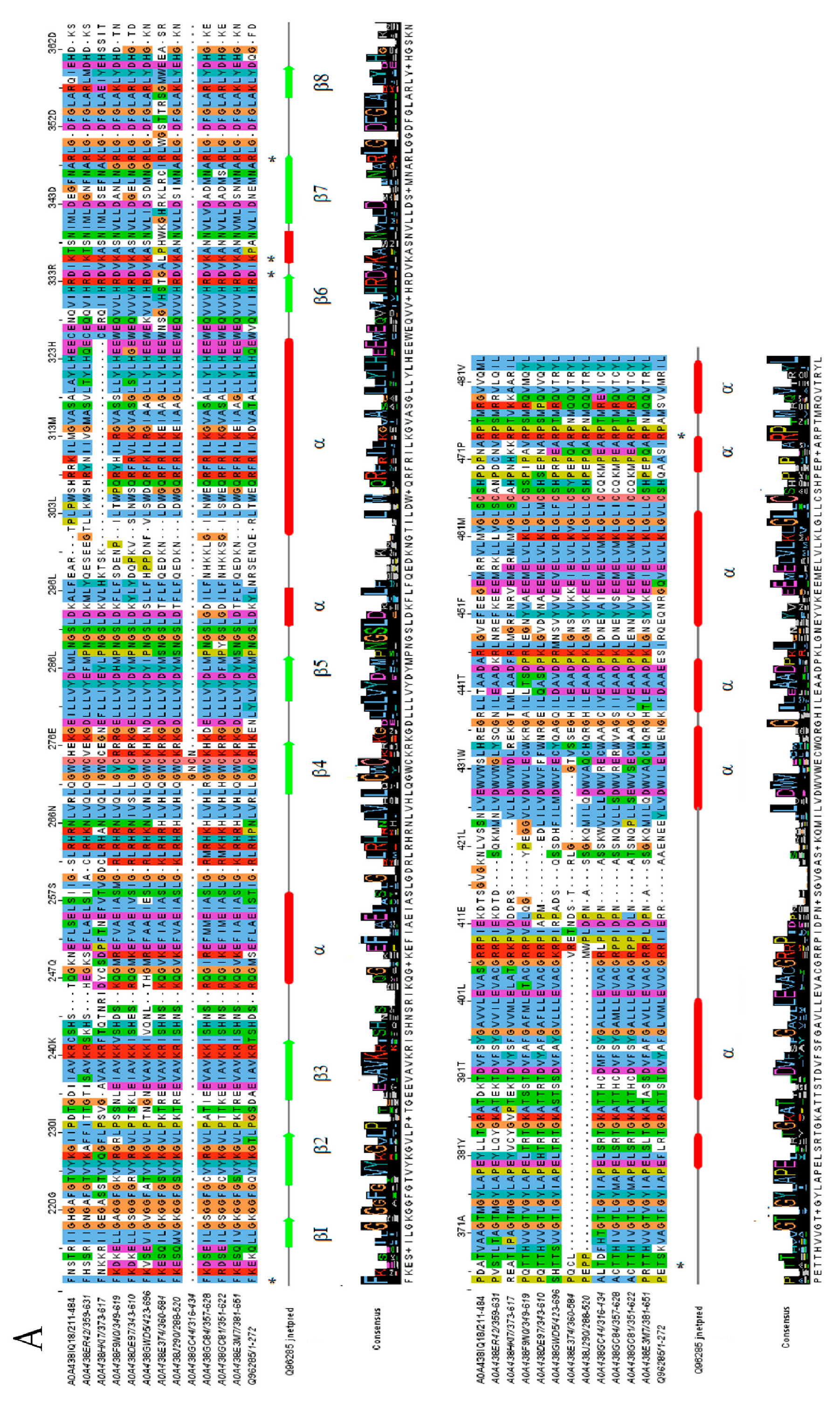
2.3. Protein Domain Architecture
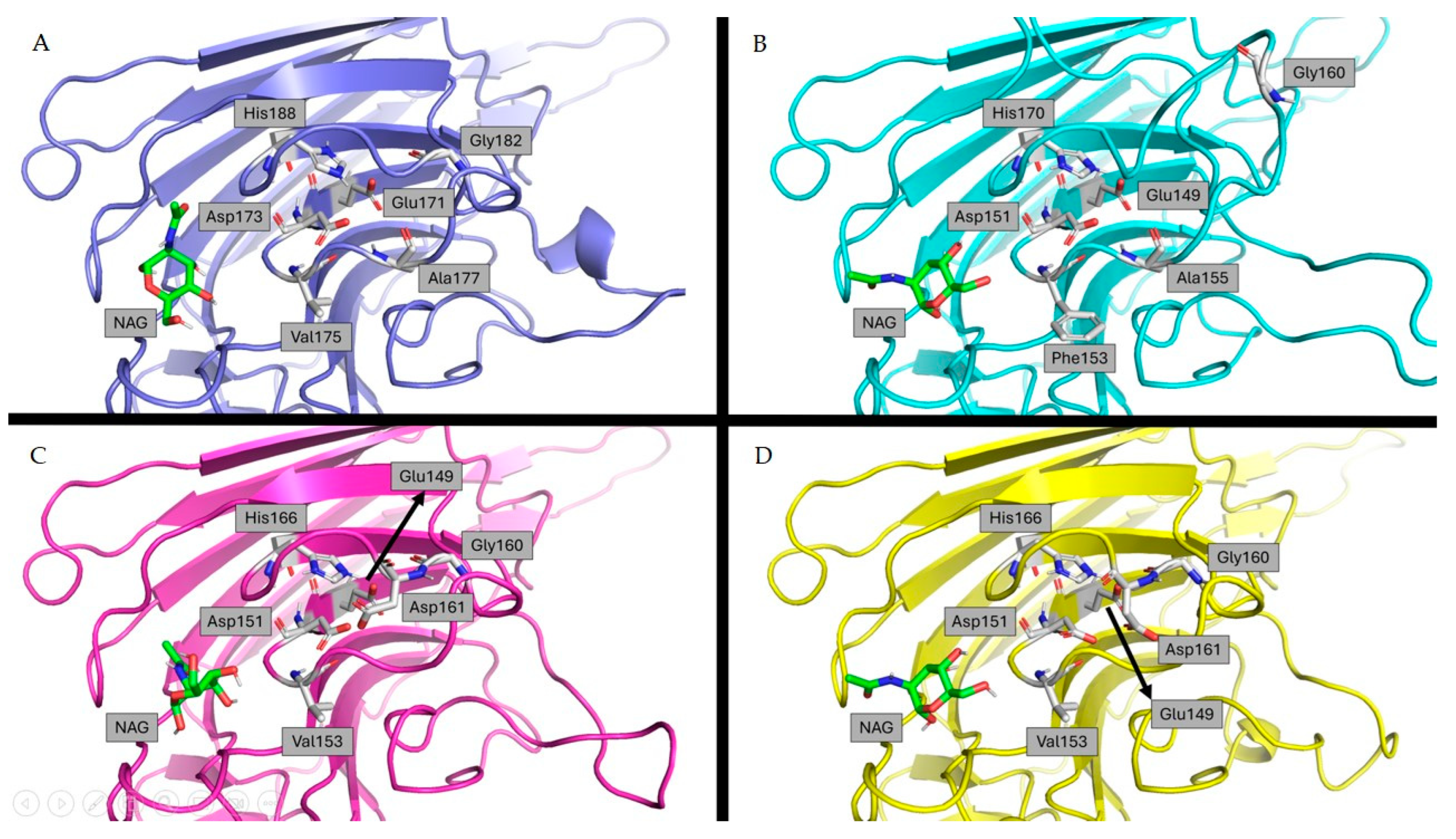
2.4. Carbohydrate Specificity Analysis through Docking
3. Discussion
4. Materials and Methods
4.1. LecRLK Sequence Homolog Search in Vitis vinifera
4.2. Functional Domain Annotation and Functional Motif Prediction of LecRLKs
4.3. Chromosomal Location and Exon–Intron Distribution
4.4. Multiple Sequence Alignment and Phylogenetic Analysis
4.5. Structure Prediction
4.6. Docking Analysis of Carbohydrate Interaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant–Pathogen Interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Alston, J.M.; Sambucci, O. Grapes in the World Economy. In The Grape Genome; Cantu, D., Walker, M.A., Eds.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–24. ISBN 978-3-030-18600-5. [Google Scholar]
- Longo, R.; Carew, A.; Sawyer, S.; Kemp, B.; Kerslake, F. A Review on the Aroma Composition of Vitis vinifera L. Pinot Noir Wines: Origins and Influencing Factors. Crit. Rev. Food Sci. Nutr. 2021, 61, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, H.; Wang, W.; Duan, C.; Wang, J.; He, F. The Influence of Rootstocks on the Scions’ Aromatic Profiles of Vitis vinifera L. Cv. Chardonnay. Sci. Hortic. 2020, 272, 109517. [Google Scholar] [CrossRef]
- Jermini, M.; Blaise, P.; Gessler, C. Quantitative Effect of Leaf Damage Caused by Downy Mildew (Plasmopara viticola) on Growth and Yield Quality of Grapevine ‘Merlot’(Vitis vinifera). Vitis 2010, 49, 77–85. [Google Scholar]
- Koledenkova, K.; Esmaeel, Q.; Jacquard, C.; Nowak, J.; Clément, C.; Ait Barka, E. Plasmopara viticola the Causal Agent of Downy Mildew of Grapevine: From Its Taxonomy to Disease Management. Front. Microbiol. 2022, 13, 889472. [Google Scholar] [CrossRef] [PubMed]
- Moriondo, M.; Orlandini, S.; Giuntoli, A.; Bindi, M. The Effect of Downy and Powdery Mildew on Grapevine (Vitis vinifera L.) Leaf Gas Exchange. J. Phytopathol. 2005, 153, 350–357. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of Pattern Recognition Receptor Signalling in Plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Sun, Y.; Qiao, Z.; Muchero, W.; Chen, J.-G. Lectin Receptor-like Kinases: The Sensor and Mediator at the Plant Cell Surface. Front. Plant Sci. 2020, 11, 596301. [Google Scholar] [CrossRef]
- Wang, Y.; Bouwmeester, K. L-Type Lectin Receptor Kinases: New Forces in Plant Immunity. PLoS Pathog. 2017, 13, e1006433. [Google Scholar] [CrossRef]
- Villano, C.; Aversano, R. Towards Grapevine (Vitis vinifera L.) Mildews Resistance: Molecular Defence Mechanisms and New Breeding Technologies. Italus Hortus 2020, 27, 1–17. [Google Scholar] [CrossRef]
- Goyal, N.; Bhatia, G.; Garewal, N.; Upadhyay, A.; Singh, K. Identification of Defense Related Gene Families and Their Response against Powdery and Downy Mildew Infections in Vitis vinifera. BMC Genom. 2021, 22, 776. [Google Scholar] [CrossRef] [PubMed]
- Thuerig, B.; James, E.E.; Schärer, H.; Langat, M.K.; Mulholland, D.A.; Treutwein, J.; Kleeberg, I.; Ludwig, M.; Jayarajah, P.; Giovannini, O.; et al. Reducing Copper Use in the Environment: The Use of Larixol and Larixyl Acetate to Treat Downy Mildew Caused by Plasmopara viticola in Viticulture. Pest. Manag. Sci. 2018, 74, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- De Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE Signature Matches and ProRule-Associated Functional and Structural Residues in Proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Labbé, J.; Muchero, W.; Yang, X.; Jawdy, S.S.; Kennedy, M.; Johnson, J.; Sreedasyam, A.; Schmutz, J.; Tuskan, G.A.; et al. Genome-Wide Analysis of Lectin Receptor-like Kinases in Populus. BMC Genom. 2016, 17, 699. [Google Scholar] [CrossRef]
- Hofberger, J.A.; Nsibo, D.L.; Govers, F.; Bouwmeester, K.; Schranz, M.E. A Complex Interplay of Tandem- and Whole-Genome Duplication Drives Expansion of the L-Type Lectin Receptor Kinase Gene Family in the Brassicaceae. Genome Biol. Evol. 2015, 7, 720–734. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, R.; Liu, K.; Ahmad, B.; Zhang, X.; Yang, L.; Tian, Y.; Shi, X.; Du, G.; Wang, L. Genomic-Organization and Expression Profiling of Lectin Receptor Kinases Genes Suggest Their Involvement in Multiple Biological Processes. Sci. Hortic. 2024, 329, 113042. [Google Scholar] [CrossRef]
- Vandepoele, K.; Simillion, C.; Van De Peer, Y. Evidence That Rice and Other Cereals Are Ancient Aneuploids. Plant Cell 2003, 15, 2192–2202. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Ma, Z.; Ramachandran, S. Evolutionary History and Stress Regulation of the Lectin Superfamily in Higher Plants. BMC Evol. Biol. 2010, 10, 79. [Google Scholar] [CrossRef]
- Simillion, C.; Vandepoele, K.; Van Montagu, M.C.E.; Zabeau, M.; Van De Peer, Y. The Hidden Duplication Past of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 13627–13632. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A User-Friendly Online Tool for Drawing Genetic Maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucl. Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. DOG 1.0: Illustrator of Protein Domain Structures. Cell Res. 2009, 19, 271–273. [Google Scholar] [CrossRef]
- Cavada, B.S.; Pinto-Junior, V.R.; Osterne, V.J.S.; Nascimento, K.S. ConA-Like Lectins: High Similarity Proteins as Models to Study Structure/Biological Activities Relationships. IJMS 2018, 20, 30. [Google Scholar] [CrossRef]
- Barre, A.; Hervé, C.; Lescure, B.; Rougé, P. Lectin Receptor Kinases in Plants. Crit. Rev. Plant Sci. 2002, 21, 379–399. [Google Scholar] [CrossRef]
- Drozdetskiy, A.; Cole, C.; Procter, J.; Barton, G.J. JPred4: A Protein Secondary Structure Prediction Server. Nucleic Acids Res. 2015, 43, W389–W394. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Katoch, R.; Tripathi, A. Research Advances and Prospects of Legume Lectins. J. Biosci. 2021, 46, 104. [Google Scholar] [CrossRef]
- Wirthmueller, L.; Maqbool, A.; Banfield, M.J. On the Front Line: Structural Insights into Plant–Pathogen Interactions. Nat. Rev. Microbiol. 2013, 11, 761–776. [Google Scholar] [CrossRef]
- Hervé, C.; Serres, J.; Dabos, P.; Canut, H.; Barre, A.; Rougé, P.; Lescure, B. Characterization of the Arabidopsis lecRK-a Genes: Members of a Superfamily Encoding Putative Receptors with an Extracellular Domain Homologous to Legume Lectins. Plant Mol. Biol. 1999, 39, 671–682. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Geethanandan, K.; Abhilash, J.; Bharath, S.R.; Sadasivan, C.; Haridas, M. X-ray Structure of a Galactose-Specific Lectin from Spatholobous Parviflorous. Int. J. Biol. Macromol. 2011, 49, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Gautier, C.; Lescar, J.; Pérez, S.; Wyns, L.; Loris, R. An Unusual Carbohydrate Binding Site Revealed by the Structures of Two Maackia Amurensis Lectins Complexed with Sialic Acid-Containing Oligosaccharides. J. Biol. Chem. 2000, 275, 17541–17548. [Google Scholar] [CrossRef] [PubMed]
- Larroque, M.; Barriot, R.; Bottin, A.; Barre, A.; Rougé, P.; Dumas, B.; Gaulin, E. The Unique Architecture and Function of Cellulose-Interacting Proteins in Oomycetes Revealed by Genomic and Structural Analyses. BMC Genom. 2012, 13, 605. [Google Scholar] [CrossRef]
- Mourey, L.; Pédelacq, J.-D.; Birck, C.; Fabre, C.; Rougé, P.; Samama, J.-P. Crystal Structure of the Arcelin-1 Dimer fromPhaseolus Vulgarisat 1.9-Å Resolution. J. Biol. Chem. 1998, 273, 12914–12922. [Google Scholar] [CrossRef][Green Version]
- Manoj, N.; Srinivas, V.R.; Surolia, A.; Vijayan, M.; Suguna, K. Carbohydrate Specificity and Salt-Bridge Mediated Conformational Change in Acidic Winged Bean Agglutinin 1 1Edited by A. Klug. J. Mol. Biol. 2000, 302, 1129–1137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kulkarni, K.A.; Sinha, S.; Katiyar, S.; Surolia, A.; Vijayan, M.; Suguna, K. Structural Basis for the Specificity of Basic Winged Bean Lectin for the Tn-antigen: A Crystallographic, Thermodynamic and Modelling Study. FEBS Lett. 2005, 579, 6775–6780. [Google Scholar] [CrossRef]
- Buts, L.; Dao-Thi, M.-H.; Loris, R.; Wyns, L.; Etzler, M.; Hamelryck, T. Weak Protein-Protein Interactions in Lectins: The Crystal Structure of a Vegetative Lectin from the Legume Dolichos Biflorus. J. Mol. Biol. 2001, 309, 193–201. [Google Scholar] [CrossRef]
- Buts, L.; Garcia-Pino, A.; Wyns, L.; Loris, R. Structural Basis of Carbohydrate Recognition by a Man(A1-2)Man-Specific Lectin from Bowringia Milbraedii. Glycobiology 2006, 16, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Mallis, R.J.; Brazin, K.N.; Fulton, D.B.; Andreotti, A.H. Structural Characterization of a Proline-Driven Conformational Switch within the Itk SH2 Domain. Nat. Struct. Biol. 2002, 9, 900–905. [Google Scholar] [CrossRef]
- Schwab, C.H. Conformations and 3D Pharmacophore Searching. Drug Discov. Today Technol. 2010, 7, e245–e253. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic Atom Type and Bond Type Perception in Molecular Mechanical Calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Schrodinger, L. The PyMOL Molecular Graphics System. Version 2015, 1, 8. [Google Scholar]
- Kang, J.; Gong, P.; Ge, M.; Sadeghnezhad, E.; Liu, Z.; Zhang, M.; Shangguan, L.; Fang, J. The PLCP Gene Family of Grapevine (Vitis vinifera L.): Characterization and Differential Expression in Response to Plasmopara viticola. BMC Plant Biol. 2021, 21, 499. [Google Scholar] [CrossRef]
- Nascimento, R.; Maia, M.; Ferreira, A.E.N.; Silva, A.B.; Freire, A.P.; Cordeiro, C.; Silva, M.S.; Figueiredo, A. Early Stage Metabolic Events Associated with the Establishment of Vitis vinifera—Plasmopara viticola Compatible Interaction. Plant Physiol. Biochem. 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Chitarrini, G.; Soini, E.; Riccadonna, S.; Franceschi, P.; Zulini, L.; Masuero, D.; Vecchione, A.; Stefanini, M.; Di Gaspero, G.; Mattivi, F.; et al. Identification of Biomarkers for Defense Response to Plasmopara viticola in a Resistant Grape Variety. Front. Plant Sci. 2017, 8, 1524. [Google Scholar] [CrossRef]
- Shiu, S.-H.; Karlowski, W.M.; Pan, R.; Tzeng, Y.-H.; Mayer, K.F.X.; Li, W.-H. Comparative Analysis of the Receptor-Like Kinase Family in Arabidopsis and Rice. Plant Cell 2004, 16, 1220–1234. [Google Scholar] [CrossRef]
- Vaid, N.; Macovei, A.; Tuteja, N. Knights in Action: Lectin Receptor-Like Kinases in Plant Development and Stress Responses. Mol. Plant 2013, 6, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.-N.; Tseng, K.-C.; Hou, P.-F.; Wu, N.-Y.; Lee, T.-Y.; Chang, W.-C. Mysteries of Gene Regulation: Promoters Are Not the Sole Triggers of Gene Expression. Comput. Struct. Biotechnol. J. 2022, 20, 4910–4920. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, G.; Xiong, L.-R.; Sun, J.-X.; Chen, Y.; Guo, C.-L.; Yu, Y.; He, H.-L.; Cai, R.; Pan, J.-S. Genome-Wide Identification and Characterization of Lectin Receptor-Like Kinase Gene Family in Cucumber and Expression Profiling Analysis under Different Treatments. Genes 2020, 11, 1032. [Google Scholar] [CrossRef]
- Faysal Ahmed, F.; Dola, F.S.; Zohra, F.T.; Rahman, S.M.; Konak, J.N.; Sarkar, M.A.R. Genome-Wide Identification, Classification, and Characterization of Lectin Gene Superfamily in Sweet Orange (Citrus sinensis L.). PLoS ONE 2023, 18, e0294233. [Google Scholar] [CrossRef]
- De Hoff, P.L.; Brill, L.M.; Hirsch, A.M. Plant Lectins: The Ties That Bind in Root Symbiosis and Plant Defense. Mol. Genet. Genom. 2009, 282, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rebaque, D.; Del Hierro, I.; López, G.; Bacete, L.; Vilaplana, F.; Dallabernardina, P.; Pfrengle, F.; Jordá, L.; Sánchez-Vallet, A.; Pérez, R.; et al. Cell Wall-derived Mixed-linked Β-1,3/1,4-glucans Trigger Immune Responses and Disease Resistance in Plants. Plant J. 2021, 106, 601–615. [Google Scholar] [CrossRef]
- La Spada, F.; Aloi, F.; Coniglione, M.; Pane, A.; Cacciola, S.O. Natural Biostimulants Elicit Plant Immune System in an Integrated Management Strategy of the Postharvest Green Mold of Orange Fruits Incited by Penicillium Digitatum. Front. Plant Sci. 2021, 12, 684722. [Google Scholar] [CrossRef]
- Bodin, E.; Bellée, A.; Dufour, M.-C.; André, O.; Corio-Costet, M.-F. Grapevine Stimulation: A Multidisciplinary Approach to Investigate the Effects of Biostimulants and a Plant Defense Stimulator. J. Agric. Food Chem. 2020, 68, 15085–15096. [Google Scholar] [CrossRef]
- Jacquens, L.; Trouvelot, S.; Lemaitre-Guillier, C.; Krzyzaniak, Y.; Clément, G.; Citerne, S.; Mouille, G.; Moreau, E.; Héloir, M.-C.; Adrian, M. Biostimulation Can Prime Elicitor Induced Resistance of Grapevine Leaves to Downy Mildew. Front. Plant Sci. 2022, 13, 998273. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen Derived Elicitors: Searching for Receptors in Plants. Mol. Plant Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology Knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher Sequence Analysis Tools Framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; Von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- The UniProt Consortium; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
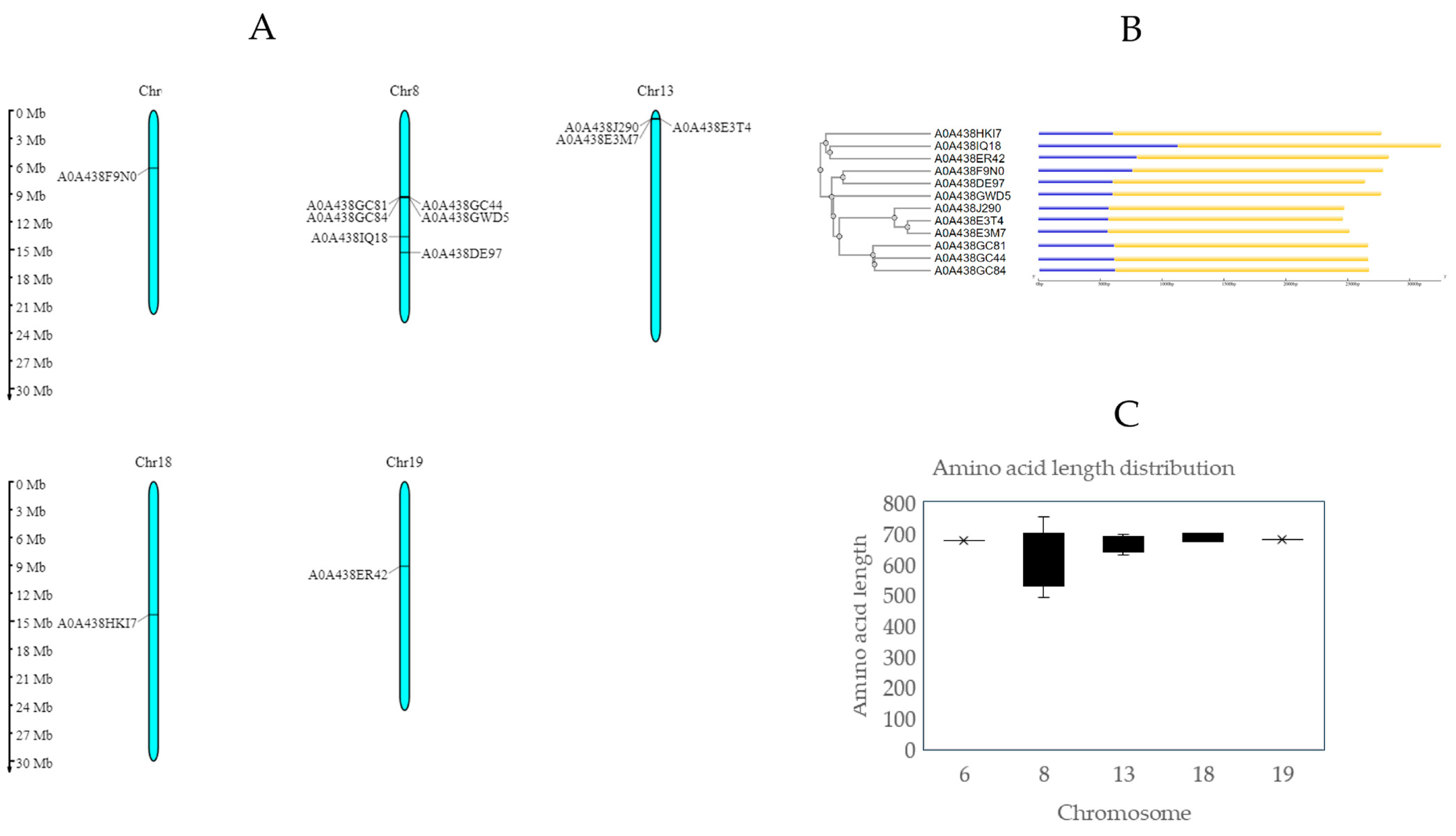
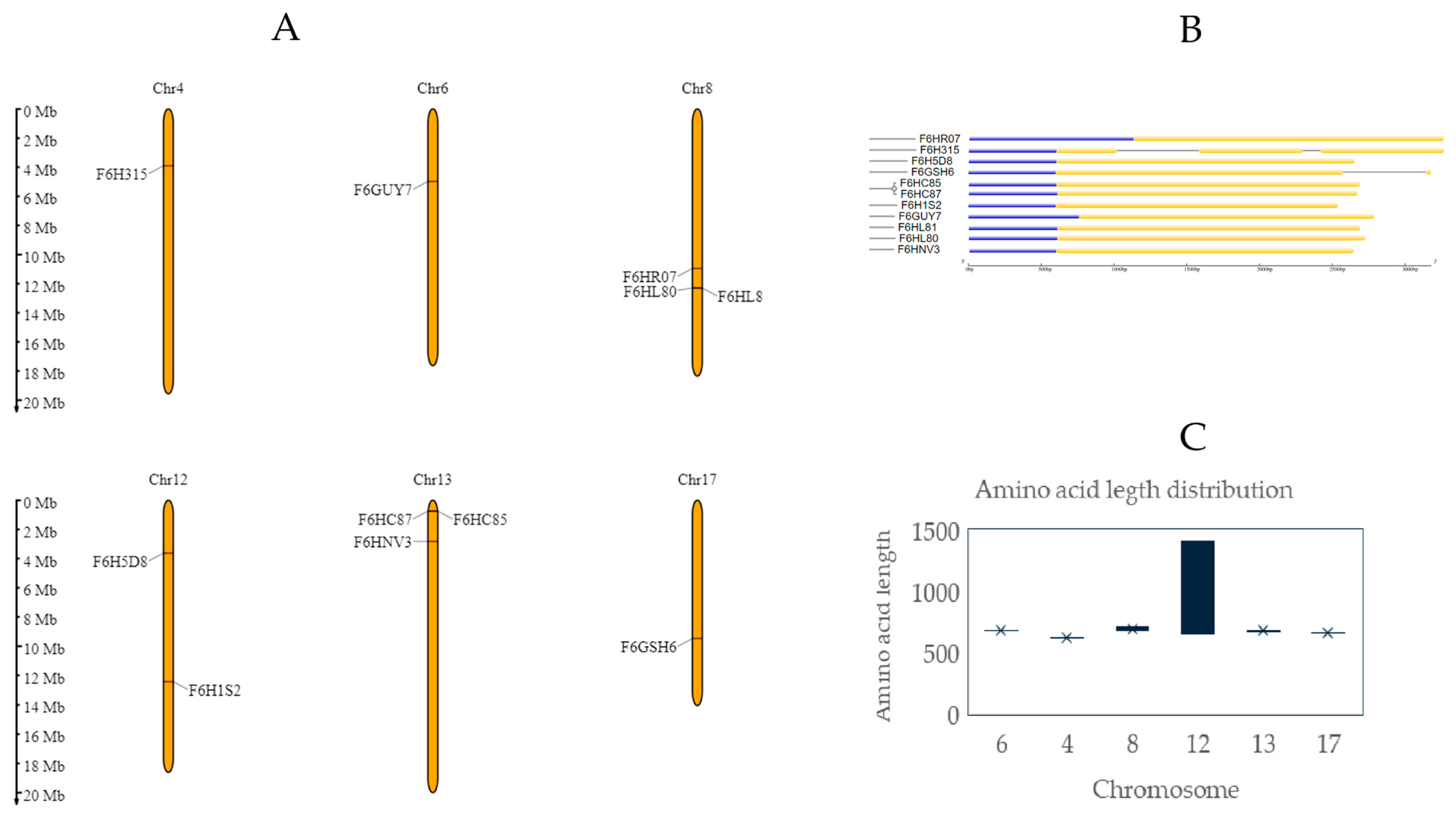




| UniProt ID | Strand | Chromosome | Start | End | (bp) | ORF (aa) | Transcript ID Ensembl Plants Vitis vinifera (PN40024.v4) | Gene Identifier (Phytozome Genome ID Vitis vinifera v2.1) |
|---|---|---|---|---|---|---|---|---|
| A0A438F9N0 | Forward | 6 | 6,049,633 | 6,052,122 | 2490 | 675 | Vitvi06g00512_P001 | VIT_206s0004g05170 (PAC: 38062906) |
| A0A438GC44 | Reverse | 8 | 9,132,636 | 9,134,678 | 2043 | 490 | Vitvi08g04118_t001 | VIT_208s0058g00280 (PAC: 38023734) |
| A0A438GC81 | Reverse | 8 | 9,126,997 | 9,129,042 | 2046 | 682 | Vitvi08g00740_t001 | - |
| A0A438GC84 | Reverse | 8 | 9,161,497 | 9,163,515 | 2019 | 678 | Vitvi08g00743_t001 | - |
| A0A438GWD5 | Reverse | 8 | 9,165,420 | 9,167,591 | 2172 | 753 | Vitvi08g00744_t001 | VIT_208s0058g00290 (PAC: 38023825) |
| A0A438IQ18 | Forward | 8 | 13,330,945 | 13,334,344 | 3400 | 540 | Vitvi08g01059_t001 | VIT_208s0040g02210 (PAC: 38022968) |
| A0A438DE97 | Reverse | 8 | 15,007,752 | 15,009,794 | 2043 | 670 | Vitvi08g01241_P001 | VIT_208s0007g00810 (PAC: 38022999) |
| A0A438E3M7 | Reverse | 13 | 921,179 | 923,266 | 2088 | 695 | Vitvi13g00096_P001 | VIT_213s0067g01640 (PAC:38053159) |
| A0A438E3T4 | Reverse | 13 | 896,560 | 898,584 | 2025 | 628 | Vitvi13g00095_P001 | VIT_213s0067g01590 (PAC: 38053471) |
| A0A438J290 | Reverse | 13 | 885,704 | 887,737 | 2034 | 677 | Vitvi13g04016_t001 | VIT_213s0067g01640 (PAC: 38053159) |
| A0A438HKI7 | Reverse | 18 | 14,033,250 | 14,035,346 | 2097 | 671 | Vitvi18g01267_t001 | VIT_218s0166g00120 (PAC:38038848) |
| A0A438ER42 | Forward | 19 | 8,937,885 | 8,940,483 | 2599 | 679 | Vitvi19g00690_P001 | VIT_219s0015g00620 (PAC: 38059114) |
| UniProt ID | Strand | Chromosome | Start | End | (bp) | ORF (aa) | Transcript ID Ensembl Plants Vitis vinifera (PN40024.v4) | Gene Identifier (Phytozome Genome ID Vitis vinifera v2.1) |
|---|---|---|---|---|---|---|---|---|
| F6H315 | Reverse | 4 | 4,770,650 | 4,773,320 | 1986 | 619 | Vitvi04g01901_t001 | VIT_204s0008g05320 (PAC: 38068899) |
| F6GUY7 | Reverse | 6 | 6,049,633 | 6,052,122 | 2490 | 675 | Vitvi06g00512_t001 | VIT_206s0004g05170 (PAC: 38062906) |
| F6HL80 | Reverse | 8 | 15,017,384 | 15,019,462 | 2079 | 692 | Vitvi08g01243_t001 | VIT_208s0007g00830 (PAC: 38025253) |
| F6HL81 | Reverse | 8 | 15,007,752 | 15,009,794 | 2043 | 680 | Vitvi08g01241_t001 | VIT_208s0007g00810 (PAC: 38022999) |
| F6HR07 | Forward | 8 | 13,330,945 | 13,334,344 | 3400 | 709 | Vitvi08g01059_t001 | VIT_208s0040g02210 (PAC: 38022968) |
| F6H1S2 | Forward | 12 | 15,204,429 | 15,206,366 | 1938 | 645 | Vitvi12g01656_t001 | VIT_212s0055g00500 (PAC: 38044712) |
| F6H5D8 | Reverse | 12 | 4,409,439 | 4,411,493 | 2055 | 1406 | Vitvi12g00300_t001 | VIT_212s0028g03580 (PAC: 38043165) |
| F6HNV3 | Reverse | 13 | 3,441,440 | 3,443,479 | 2040 | 679 | Vitvi13g00332_t001 | VIT_213s0019g02040 (PAC: 38053268) |
| F6HC85 | Reverse | 13 | 921,179 | 923,266 | 2088 | 673 | Vitvi13g00096_t001 | VIT_213s0067g01640 (PAC: 38053159) |
| F6HC87 | Reverse | 13 | 896,560 | 898,584 | 2025 | 674 | Vitvi13g00095_t001 | - |
| F6GSH6 | Reverse | 17 | 11,526,350 | 11,528,329 | 1980 | 659 | Vitvi17g00905_t001 | VIT_217s0000g09170 (PAC: 38030737) |
| UniProt ID | PDB Homology Model Code | Carbohydrate Specificity | Reference |
|---|---|---|---|
| A0A438F9N0 | 3IPV | Gal | [35] |
| A0A438GC44 | 1DBN | Gal-α-1,3-Gal | [37] |
| A0A438GC81 | 1DBN | Gal-α-1,3-Gal | [37] |
| A0A438GC84 | 3IPV | Gal | [35] |
| A0A438GWD5 | 1AVB | GluNAc | [38] |
| A0A438IQ18 | 1F9K * | Man | [39] |
| A0A438DE97 | 3IPV | Gal | [35] |
| A0A438E3M7 | 2D3S | GalNAc | [40] |
| A0A438E3T4 | 3IPV | Gal | [35] |
| A0A438J290 | 2D3S | GalNAc | [40] |
| A0A438HKI7 | 2D3S | GalNAc | [40] |
| A0A438ER42 | 3IPV | Gal | [35] |
| F6H315 | 1DBN | Gal-α-1,3-Gal | [37] |
| F6GUY7 | 3IPV | Gal | [35] |
| F6HL80 | 1G8W | GluNAc | [41] |
| F6HL81 | 3IPV | Gal | [35] |
| F6HR07 | 2FMD | Man | [42] |
| F6H1S2 | 1DBN | Gal-α-1,3-Gal | [37] |
| F6H5D8 | −2D3S/3IPV | GalNAc/Gal | [35,40] |
| F6HNV3 | 1LUI | GalNAc | [43] |
| F6HC85 | 2D3S | GalNAc | [40] |
| F6HC87 | 2D3S | GalNAc | [40] |
| F6GSH6 | 1G8W | GluNAc | [41] |
| UniProt ID | Affinity (kcal/mol) |
|---|---|
| A0A438E3M7 | −4.8 |
| A0A438HKI7 | n.d. |
| A0A438J290 | n.d. |
| F6HC85 | −5.1 |
| F6HC87 | −5.6 |
| F6HVN3 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Navarro, S.M.; de Iceta Soler, X.; Martínez-Martínez, M.; Olazábal-Morán, M.; Santos-Moriano, P.; Gómez, S. Structural and Phylogenetic In Silico Characterization of Vitis vinifera PRR Protein as Potential Target for Plasmopara viticola Infection. Int. J. Mol. Sci. 2024, 25, 9553. https://doi.org/10.3390/ijms25179553
Martínez-Navarro SM, de Iceta Soler X, Martínez-Martínez M, Olazábal-Morán M, Santos-Moriano P, Gómez S. Structural and Phylogenetic In Silico Characterization of Vitis vinifera PRR Protein as Potential Target for Plasmopara viticola Infection. International Journal of Molecular Sciences. 2024; 25(17):9553. https://doi.org/10.3390/ijms25179553
Chicago/Turabian StyleMartínez-Navarro, Sofía M., Xavier de Iceta Soler, Mónica Martínez-Martínez, Manuel Olazábal-Morán, Paloma Santos-Moriano, and Sara Gómez. 2024. "Structural and Phylogenetic In Silico Characterization of Vitis vinifera PRR Protein as Potential Target for Plasmopara viticola Infection" International Journal of Molecular Sciences 25, no. 17: 9553. https://doi.org/10.3390/ijms25179553
APA StyleMartínez-Navarro, S. M., de Iceta Soler, X., Martínez-Martínez, M., Olazábal-Morán, M., Santos-Moriano, P., & Gómez, S. (2024). Structural and Phylogenetic In Silico Characterization of Vitis vinifera PRR Protein as Potential Target for Plasmopara viticola Infection. International Journal of Molecular Sciences, 25(17), 9553. https://doi.org/10.3390/ijms25179553






