Comparative Analysis of Comprehensive Genomic Profile in Thymomas and Recurrent Thymomas Reveals Potentially Actionable Mutations for Target Therapies
Abstract
1. Introduction
- −
- To compare the CGP of recurrent thymoma versus non-recurrent thymoma patients;
- −
- To explore the CGP of both primary and recurrent thymomas and identify associations with clinicopathological variables;
- −
- To evaluate actionable mutations detected in thymomas as targets for new therapeutic approaches.
2. Results
2.1. Clinical and Pathological Characteristics
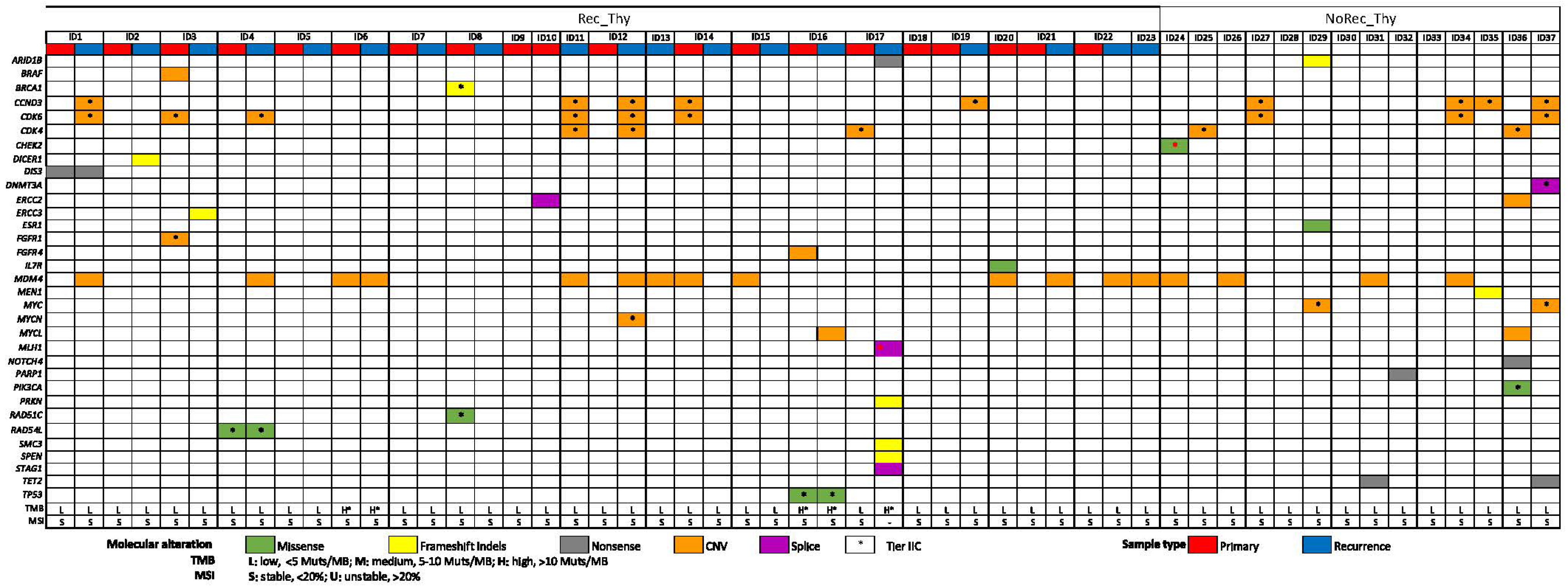
2.2. Overall Genomic Results (Entire Cohort)
2.3. CGP Differences in Recurrent Thymoma vs. Non-Recurrent Thymoma
2.4. CGP Differences in Primary vs. Recurrent Thymoma and Inter-Relationship with Clinic-Pathological Variables
2.5. Actionable Mutations for New Therapeutic Approaches
3. Discussion
Limitations, Points of Strength and Future Clinical Applications
4. Materials and Methods
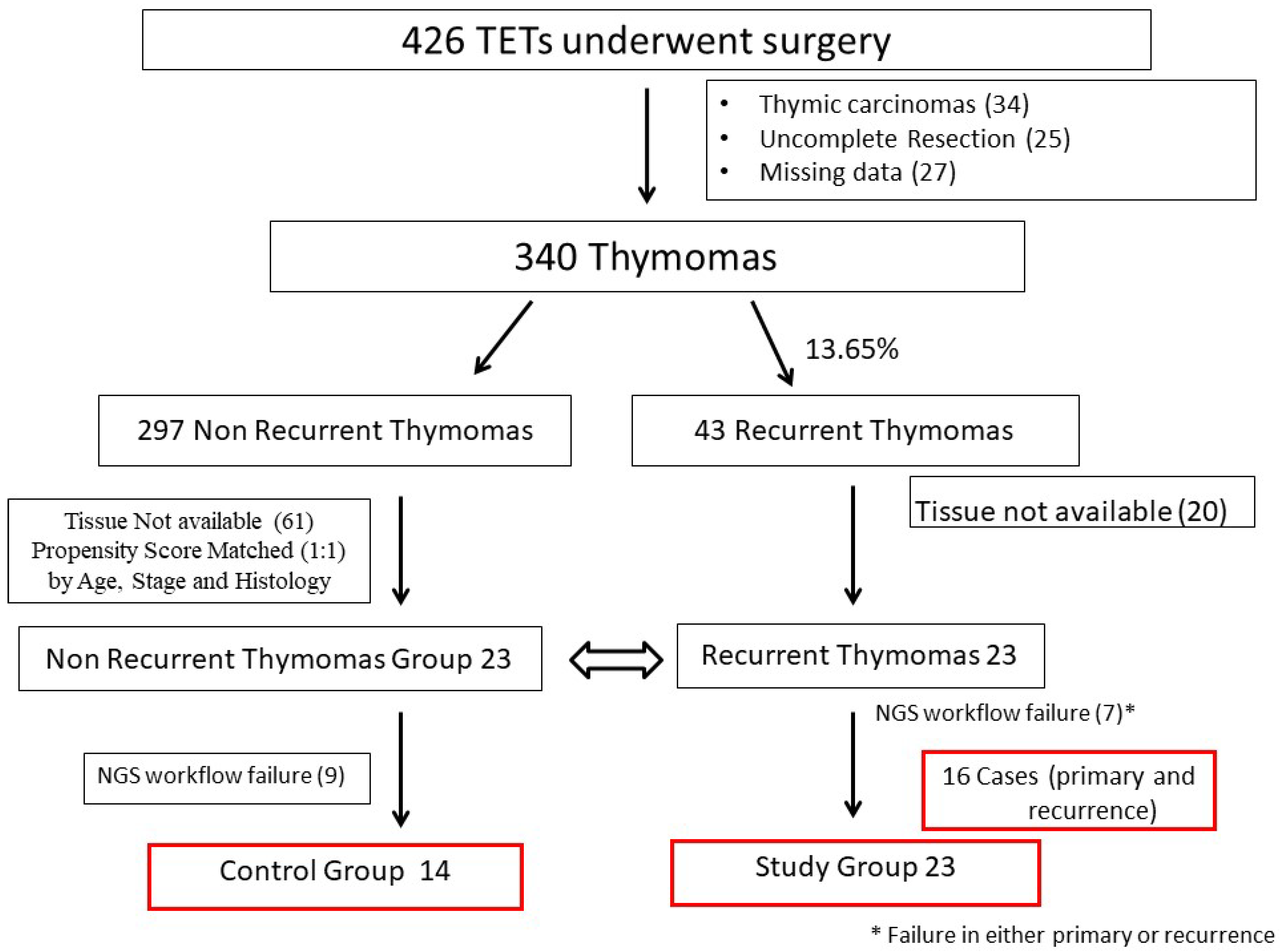
4.1. Study Design and Selection of Cases
4.2. Pathological Review
4.3. Comprehensive Genomic Profiling and Bioinformatics Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Jong, W.K.; Blaauwgeers, J.L.G.; Schaapveld, M.; Timens, W.; Klinkenberg, T.J.; Groen, H.J.M. Thymic Epithelial Tumours: A Population-Based Study of the Incidence, Diagnostic Procedures and Therapy. Eur. J. Cancer 2008, 44, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Marx, A.; Chan, J.K.C.; Coindre, J.M.; Detterbeck, F.; Girard, N.; Harris, N.L.; Jaffe, E.S.; Kurrer, M.O.; Marom, E.M.; Moreira, A.L.; et al. The 2015 World Health Organization Classification of Tumors of the Thymus Continuity and Changes. J. Thorac. Oncol. 2015, 10, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Regnard, J.F.; Magdeleinat, P.; Dromer, C.; Dulmet, E.; De Montpreville, V.; Levi, J.F.; Levasseur, P. Prognostic Factors and Long-Term Results after Thymoma Resection: A Series of 307 Patients. J. Thorac. Cardiovasc. Surg. 1996, 112, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Sandri, A.; Cusumano, G.; Lococo, F.; Alifano, M.; Granone, P.; Margaritora, S.; Cesario, A.; Oliaro, A.; Filosso, P.; Regnard, J.F.; et al. Long-Term Results after Treatment for Recurrent Thymoma: A Multicenter Analysis. J. Thorac. Oncol. 2014, 9, 1796–1804. [Google Scholar] [CrossRef]
- Margaritora, S.; Cesario, A.; Cusumano, G.; Lococo, F.; Porziella, V.; Meacci, E.; Evoli, A.; Granone, P. Single-Centre 40-Year Results of Redo Operation for Recurrent Thymomas. Eur. J. Cardiothorac. Surg. 2011, 40, 894–900. [Google Scholar] [CrossRef]
- Chiappetta, M.; Lococo, F.; Zanfrini, E.; Moroni, R.; Aprile, V.; Guerrera, F.; Nachira, D.; Congedo, M.T.; Ambrogi, M.C.; Korasidis, S.; et al. The International Thymic Malignancy Interest Group Classification of Thymoma Recurrence: Survival Analysis and Perspectives. J. Thorac. Oncol. 2021, 16, 1936–1945. [Google Scholar] [CrossRef]
- Mizuno, T.; Okumura, M.; Asamura, H.; Yoshida, K.; Niwa, H.; Kondo, K.; Horio, H.; Matsumura, A.; Yokoi, K. Surgical Management of Recurrent Thymic Epithelial Tumors: A Retrospective Analysis Based on the Japanese Nationwide Database. J. Thorac. Oncol. 2015, 10, 199–205. [Google Scholar] [CrossRef]
- Chiappetta, M.; Grossi, U.; Sperduti, I.; Margaritora, S.; Marulli, G.; Fiorelli, A.; Sandri, A.; Mizuno, T.; Cusumano, G.; Hamaji, M.; et al. Which Is the Best Treatment in Recurrent Thymoma? A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1559. [Google Scholar] [CrossRef]
- Giaccone, G.; Wilmink, H.; Paul, M.A.; Van Der Valk, P. Systemic Treatment of Malignant Thymoma: A Decade Experience at a Single Institution. Am. J. Clin. Oncol. 2006, 29, 336–344. [Google Scholar] [CrossRef]
- Bott, M.J.; Wang, H.; Travis, W.; Riely, G.J.; Bains, M.; Downey, R.; Rusch, V.; Huang, J. Management and Outcomes of Relapse after Treatment for Thymoma and Thymic Carcinoma. Ann. Thorac. Surg. 2011, 92, 1984–1992. [Google Scholar] [CrossRef]
- Shimada, M.; Taniguchi, H.; Yamaguchi, H.; Gyotoku, H.; Sasaki, D.; Kaku, N.; Senju, C.; Senju, H.; Imamura, E.; Takemoto, S.; et al. Genetic Profile of Thymic Epithelial Tumors in the Japanese Population: An Exploratory Study Examining Potential Therapeutic Targets. Transl. Lung Cancer Res. 2023, 12, 707–718. [Google Scholar] [CrossRef]
- Kurokawa, K.; Shukuya, T.; Greenstein, R.A.; Kaplan, B.G.; Wakelee, H.; Ross, J.S.; Miura, K.; Furuta, K.; Kato, S.; Suh, J.; et al. Genomic Characterization of Thymic Epithelial Tumors in a Real-World Dataset. ESMO Open 2023, 8, 101627. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Basse, C.; Schrock, A.; Ramkissoon, S.; Killian, K.; Ross, J.S. Comprehensive Genomic Profiling of 274 Thymic Epithelial Tumors Unveils Oncogenic Pathways and Predictive Biomarkers. Oncologist 2022, 27, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, A.C.; Brandão, M.; Berghmans, T.; Durieux, V.; Jungels, C. Immunotherapy and Targeted Therapies Efficacy in Thymic Epithelial Tumors: A Systematic Review. Biomedicines 2023, 11, 2722. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, Z.T.; Qiu, B.; Detterbeck, F.C.; Roden, A.C.; Ruffini, E.; Okumura, M.; Girard, N.; Xiang, Y.W.; Liu, Y.; et al. A Recurrence Predictive Model for Thymic Tumors and Its Implication for Postoperative Management: A Chinese Alliance for Research in Thymomas Database Study. J. Thorac. Oncol. 2020, 15, 448–456. [Google Scholar] [CrossRef]
- Chiappetta, M.; Sassorossi, C.; Nachira, D.; Lococo, F.; Meacci, E.; Ruffini, E.; Guerrera, F.; Lyberis, P.; Aprile, V.; Lucchi, M.; et al. Survival Outcome After Surgery in Patients with Thymoma Distant Recurrence. J. Thorac. Oncol. 2024, 19, 1086–1094. [Google Scholar] [CrossRef]
- Ak, N.; Aydiner, A. Nivolumab Treatment for Metastatic Thymic Epithelial Tumors. J. Oncol. Pharm. Pract. 2021, 27, 1710–1715. [Google Scholar] [CrossRef]
- Song, X.; Fan, J.; Zhu, L.; Wang, Z.; He, Y.; Zhou, C. The Efficacy and Safety of Immunotherapy in Thymic Epithelial Tumors: More Effective, More Risky: A Systematic Review. J. Thorac. Dis. 2021, 13, 5093–5103. [Google Scholar] [CrossRef]
- Montella, L.; Ottaviano, M.; Morra, R.; Pietroluongo, E.; De Placido, P.; Tortora, M.; Sorrentino, C.; Facchini, G.; De Placido, S.; Giuliano, M.; et al. The Never-Ending History of Octreotide in Thymic Tumors: A Vintage or A Contemporary Drug? Cancers 2022, 14, 774. [Google Scholar] [CrossRef]
- Morfouace, M.; Stevovic, A.; Vinches, M.; Golfinopoulos, V.; Jin, D.X.; Holmes, O.; Erlich, R.; Fayette, J.; Croce, S.; Ray-Coquard, I.; et al. First Results of the EORTC-SPECTA/Arcagen Study Exploring the Genomics of Rare Cancers in Collaboration with the European Reference Network EURACAN. ESMO Open 2020, 5, e001075. [Google Scholar] [CrossRef]
- Morfouace, M.; Novello, S.; Stevovic, A.; Dooms, C.; Janžič, U.; Berghmans, T.; Dziadziuszko, R.; Gorlia, T.; Felip, E.; Paz-Ares, L.; et al. Results of Screening in Early and Advanced Thoracic Malignancies in the EORTC Pan-European SPECTAlung Platform. Sci. Rep. 2022, 12, 8342. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, D.; Markovic, J.; Ceriman, V.; Peric, J.; Pavlovic, S.; Soldatovic, I. Correlation of Genomic Alterations and PD-L1 Expression in Thymoma. J. Thorac. Dis. 2020, 12, 7561–7570. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Giaccone, G.; De Pas, T. Thymic Epithelial Tumors: From Biology to Treatment. Cancer Treat. Rev. 2020, 86, 102014. [Google Scholar] [CrossRef]
- Jardim, D.L.; Millis, S.Z.; Ross, J.S.; Woo, M.S.-A.; Ali, S.M.; Kurzrock, R. Cyclin Pathway Genomic Alterations Across 190,247 Solid Tumors: Leveraging Large-Scale Data to Inform Therapeutic Directions. Oncologist 2021, 26, e78–e89. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Ambrogi, V.; Mineo, D.; Mellone, P.; Campioni, M.; Citro, G.; Mineo, T.C. Analysis of Cell Cycle Regulator Proteins in Encapsulated Thymomas. Clin. Cancer Res. 2005, 11, 5078–5083. [Google Scholar] [CrossRef][Green Version]
- Besse, B.; Garassino, M.C.; Rajan, A.; Novello, S.; Mazieres, J.; Weiss, G.J.; Kocs, D.M.; Barnett, J.M.; Davite, C.; Crivori, P.; et al. Efficacy of Milciclib (PHA-848125AC), a Pan-Cyclin d-Dependent Kinase Inhibitor, in Two Phase II Studies with Thymic Carcinoma (TC) and B3 Thymoma (B3T) Patients. J. Clin. Oncol. 2018, 36, 8519. [Google Scholar] [CrossRef]
- Aesif, S.W.; Aubry, M.C.; Yi, E.S.; Kloft-Nelson, S.M.; Jenkins, S.M.; Spears, G.M.; Greipp, P.T.; Sukov, W.R.; Roden, A.C. Loss of P16INK4A Expression and Homozygous CDKN2A Deletion Are Associated with Worse Outcome and Younger Age in Thymic Carcinomas. J. Thorac. Oncol. 2017, 12, 860–871. [Google Scholar] [CrossRef]
- CBioPortal for Cancer Genomics. Available online: https://www.cbioportal.org/ (accessed on 6 August 2024).
- Markey, M.P. Regulation of MDM4. Front. Biosci. (Landmark Ed) 2011, 16, 1144–1156. [Google Scholar] [CrossRef]
- Yi, E.J.; Park, J.H.; Lee, H.W.; Cho, S.Y.; Na, I.I.; Kang, M.C. BRCA1 Gene Mutation in Thymic Malignant Melanoma. Ann. Thorac. Surg. 2013, 96, 677–680. [Google Scholar] [CrossRef]
- Nicodème, F.; Geffroy, S.; Conti, M.; Delobel, B.; Soenen, V.; Grardel, N.; Porte, H.; Copin, M.C.; Laï, J.L.; Andrieux, J. Familial Occurrence of Thymoma and Autoimmune Diseases with the Constitutional Translocation t(14;20)(Q24.1;P12.3). Genes Chromosomes Cancer 2005, 44, 154–160. [Google Scholar] [CrossRef]
- Enkner, F.; Pichlhöfer, B.; Zaharie, A.T.; Krunic, M.; Holper, T.M.; Janik, S.; Moser, B.; Schlangen, K.; Neudert, B.; Walter, K.; et al. Molecular Profiling of Thymoma and Thymic Carcinoma: Genetic Differences and Potential Novel Therapeutic Targets. Pathol. Oncol. Res. 2017, 23, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Kamath, S.D.; Munshi, H.G.; Mohindra, N.A. Metastatic Thymoma Harboring a Deleterious BRCA2 Mutation Derives Durable Clinical Benefit from Olaparib. Oncologist 2020, 25, 301–305. [Google Scholar] [CrossRef]
- Cimpean, A.M.; Raica, M.; Encica, S.; Cornea, R.; Bocan, V. Immunohistochemical Expression of Vascular Endothelial Growth Factor A (VEGF), and Its Receptors (VEGFR1, 2) in Normal and Pathologic Conditions of the Human Thymus. Ann. Anat. 2008, 190, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Rajan, A.; Berman, A.; Tomita, Y.; Brzezniak, C.; Lee, M.J.; Lee, S.; Ling, A.; Spittler, A.J.; Carter, C.A.; et al. Sunitinib in Patients with Chemotherapy-Refractory Thymoma and Thymic Carcinoma: An Open-Label Phase 2 Trial. Lancet Oncol. 2015, 16, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Radovich, M.; Solzak, J.P.; Hancock, B.A.; Conces, M.L.; Atale, R.; Porter, R.F.; Zhu, J.; Glasscock, J.; Kesler, K.A.; Badve, S.S.; et al. A Large MicroRNA Cluster on Chromosome 19 Is a Transcriptional Hallmark of WHO Type A and AB Thymomas. Br. J. Cancer 2016, 114, 477–484. [Google Scholar] [CrossRef]
- Rajan, A.; Carter, C.A.; Berman, A.; Cao, L.; Kelly, R.J.; Thomas, A.; Khozin, S.; Chavez, A.L.; Bergagnini, I.; Scepura, B.; et al. Cixutumumab for Patients with Recurrent or Refractory Advanced Thymic Epithelial Tumours: A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2014, 15, 191–200. [Google Scholar] [CrossRef]
- Zucali, P.A.; De Pas, T.; Palmieri, G.; Favaretto, A.; Chella, A.; Tiseo, M.; Caruso, M.; Simonelli, M.; Perrino, M.; De Vincenzo, F.; et al. Phase II Study of Everolimus in Patients with Thymoma and Thymic Carcinoma Previously Treated with Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2018, 36, 342–349. [Google Scholar] [CrossRef]
- Abu Zaid, M.I.; Radovich, M.; Althouse, S.; Liu, H.; Spittler, A.J.; Solzak, J.; Badve, S.; Loehrer, P.J. A Phase II Study of Buparlisib in Relapsed or Refractory Thymomas. Front. Oncol. 2022, 12, 891383. [Google Scholar] [CrossRef]
- Piana, D.; Iavarone, F.; De Paolis, E.; Daniele, G.; Parisella, F.; Minucci, A.; Greco, V.; Urbani, A. Phenotyping Tumor Heterogeneity through Proteogenomics: Study Models and Challenges. Int. J. Mol. Sci. 2024, 25, 8830. [Google Scholar] [CrossRef]
- Koga, K.; Matsuno, Y.; Noguchi, M.; Mukai, K.; Asamura, H.; Goya, T.; Shimosato, Y. A Review of 79 Thymomas: Modification of Staging System and Reappraisal of Conventional Division into Invasive and Non-Invasive Thymoma. Pathol. Int. 1994, 44, 359–367. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Union for International Cancer Control: Geneva, Switzerland, 2017; pp. 1–272. [Google Scholar]
- Marx, A.; Ströbel, P.; Badve, S.S.; Chalabreysse, L.; Chan, J.K.C.; Chen, G.; De Leval, L.; Detterbeck, F.; Girard, N.; Huang, J.; et al. ITMIG Consensus Statement on the Use of the WHO Histological Classification of Thymoma and Thymic Carcinoma: Refined Definitions, Histological Criteria, and Reporting. J. Thorac. Oncol. 2014, 9, 596–611. [Google Scholar] [CrossRef]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alföldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A Genomic Mutational Constraint Map Using Variation in 76,156 Human Genomes. Nature 2023, 625, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Sondka, Z.; Dhir, N.B.; Carvalho-Silva, D.; Jupe, S.; Madhumita; McLaren, K.; Starkey, M.; Ward, S.; Wilding, J.; Ahmed, M.; et al. COSMIC: A Curated Database of Somatic Variants and Clinical Data for Cancer. Nucleic Acids Res. 2024, 52, D1210–D1217. [Google Scholar] [CrossRef]
- Suehnholz, S.P.; Nissan, M.H.; Zhang, H.; Kundra, R.; Nandakumar, S.; Lu, C.; Carrero, S.; Dhaneshwar, A.; Fernandez, N.; Xu, B.W.; et al. Quantifying the Expanding Landscape of Clinical Actionability for Patients with Cancer. Cancer Discov. 2024, 14, 49–65. [Google Scholar] [CrossRef]
- Chakravarty, D.; Gao, J.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving Access to Variant Interpretations and Supporting Evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Kuzbari, Z.; Bandlamudi, C.; Loveday, C.; Garrett, A.; Mehine, M.; George, A.; Hanson, H.; Snape, K.; Kulkarni, A.; Allen, S.; et al. Germline-Focused Analysis of Tumour-Detected Variants in 49,264 Cancer Patients: ESMO Precision Medicine Working Group Recommendations. Ann. Oncol. 2023, 34, 215–227. [Google Scholar] [CrossRef]
| Rec_Thy (n = 23 pts) | No Rec_Thy (n = 14 pts) | |
|---|---|---|
| GENDER M F | 13 (56.5%) 10 (43.5%) | 10 (71.4%) 4 (28.6%) |
| AGE (median, range) | 51 y (27 y–83 y) | 59 y (16 y–82 y) |
| Myasthenia Gravis (MG) | 8 (34.8%) | 7 (50.0%) |
| MASAOKA * II III IV | 5 (21.8%) 12 (52.2%) 3 (13.0%) | 5 (35.7%) 7 (52.2%) 2 (14.3%) |
| NEOADJUVANT TREATMENT | 10/20 (50.0%) | 6/14 (42.9%) |
| HISTOLOGY WHO AB B1 B2 B3 | 0 (0%) 9 (39.2%) 7 (30.4%) 7 (30.4%) | 1 (7.1%) 2 (14.3%) 9 (64.3%) 2 (14.3%) |
| ^ RISK CLASS * Low-Risk High-Risk | 5 (25.0%) 15 (75.0%) | 5 (35.7%) 9 (64.3%) |
| STAGE * II III IV | 4 (20.0%) 11 (55.0%) 5 (25.0%) | 6 (42.8%) 5 (35.7%) 3 (21.5%) |
| DFI (median, range) ** | 32 m (6 m–132 m) | / |
| ADJUVANT TREATMENT | 7 (30.0%) | 7 (50.0%) |
| GROUP | All Patients (#37) | Rec_Thy(#23) | NoRec_Thy (#14) | p-Value |
|---|---|---|---|---|
| Pathway cell cycle | 26 (70%) | 17 (73.9%) | 9 (64.3%) | p = 0.53 |
| Pathway DNA repair | 8 (22%) | 5 (21.7%) | 3 (21.4%) | p = 0.98 |
| At least 1 alteration | 30 (81%) | 19 (82.6%) | 11 (78.6%) | p = 0.76 |
| Clinically relevant alteration | 18 (49%) | 10 (43%) | 8 (57%) | p = 0.83 |
| GROUP | Primary_Thy | Recurrent_Thy | p-Value |
|---|---|---|---|
| Pathway cell cycle | 6 (37.5%) | 9 (56.2%) | p = 0.30 |
| Pathway DNA repair | 2 (12.5%) | 3 (18.7%) | p = 0.23 |
| At least 1 alteration | 9 (56.2%) | 11 (68.7%) | p = 0.84 |
| Pathway Cell Cycle | Pathway DNA Repair | At Least 1 Alteration | |
|---|---|---|---|
| Rec_Thy (n = 23) | 11/23 (47.8%) | 3/23 (13.0%) | 14/23 (60.9%) |
| Masaoka Stage II (n = 5) III-IV (n = 18) | p = 0.121 5/5 (100.0%) 12/18 (66.6%) | p = 0.019 3/5 (60.0%) 2/18 (11.1%) | p = 0.351 5/5 (100.0%) 14/18 (77.8%) |
| Age <51 (n = 11) >51 (n = 12) | p = 0. 896 5/11 (45.6%) 6/12 (50.0%) | p = 0.635 1/11 (9.1%) 2/12 (16.7%) | p = 0. 582 8/11 (72.7%) 6/12 (50.0%) |
| Myasthenia Gravis Yes (n = 8) No (n = 15) | p = 0.661 3/8 (37.5%) 8/15 (53.3%) | p = 0.960 1/8 (12.5%) 2/15 (13.3%) | p = 0.695 4/8 (50.0%) 10/15 (66.7%) |
| RISK Class * Low (n = 5) High (n = 18) | p = 0.121 4/5 (80.0%) 9/18 (50.0%) | p = 0.770 1/5 (20.0%) 4/18 (22.2%) | p = 0.201 5/5 (100.0%) 11/18 (61.1%) |
| DFI <32 months (n = 9) >32 months (n = 14) | p = 0.022 9/9 (100.0%) 8/14 (57.1%) | p = 0.960 2/9 (22.2%) 3/14 (21.4%) | p = 0.082 9/9 (100.0%) 10/14 (71.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lococo, F.; De Paolis, E.; Evangelista, J.; Dell’Amore, A.; Giannarelli, D.; Chiappetta, M.; Campanella, A.; Sassorossi, C.; Cancellieri, A.; Calabrese, F.; et al. Comparative Analysis of Comprehensive Genomic Profile in Thymomas and Recurrent Thymomas Reveals Potentially Actionable Mutations for Target Therapies. Int. J. Mol. Sci. 2024, 25, 9560. https://doi.org/10.3390/ijms25179560
Lococo F, De Paolis E, Evangelista J, Dell’Amore A, Giannarelli D, Chiappetta M, Campanella A, Sassorossi C, Cancellieri A, Calabrese F, et al. Comparative Analysis of Comprehensive Genomic Profile in Thymomas and Recurrent Thymomas Reveals Potentially Actionable Mutations for Target Therapies. International Journal of Molecular Sciences. 2024; 25(17):9560. https://doi.org/10.3390/ijms25179560
Chicago/Turabian StyleLococo, Filippo, Elisa De Paolis, Jessica Evangelista, Andrea Dell’Amore, Diana Giannarelli, Marco Chiappetta, Annalisa Campanella, Carolina Sassorossi, Alessandra Cancellieri, Fiorella Calabrese, and et al. 2024. "Comparative Analysis of Comprehensive Genomic Profile in Thymomas and Recurrent Thymomas Reveals Potentially Actionable Mutations for Target Therapies" International Journal of Molecular Sciences 25, no. 17: 9560. https://doi.org/10.3390/ijms25179560
APA StyleLococo, F., De Paolis, E., Evangelista, J., Dell’Amore, A., Giannarelli, D., Chiappetta, M., Campanella, A., Sassorossi, C., Cancellieri, A., Calabrese, F., Conca, A., Vita, E., Minucci, A., Bria, E., Castello, A., Urbani, A., Rea, F., Margaritora, S., & Scambia, G. (2024). Comparative Analysis of Comprehensive Genomic Profile in Thymomas and Recurrent Thymomas Reveals Potentially Actionable Mutations for Target Therapies. International Journal of Molecular Sciences, 25(17), 9560. https://doi.org/10.3390/ijms25179560










