Fibrin Scaffolds Perfused with Transforming Growth Factor-β1 as an In Vitro Model to Study Healthy and Tendinopathic Human Tendon Stem/Progenitor Cells
Abstract
:1. Introduction
2. Results
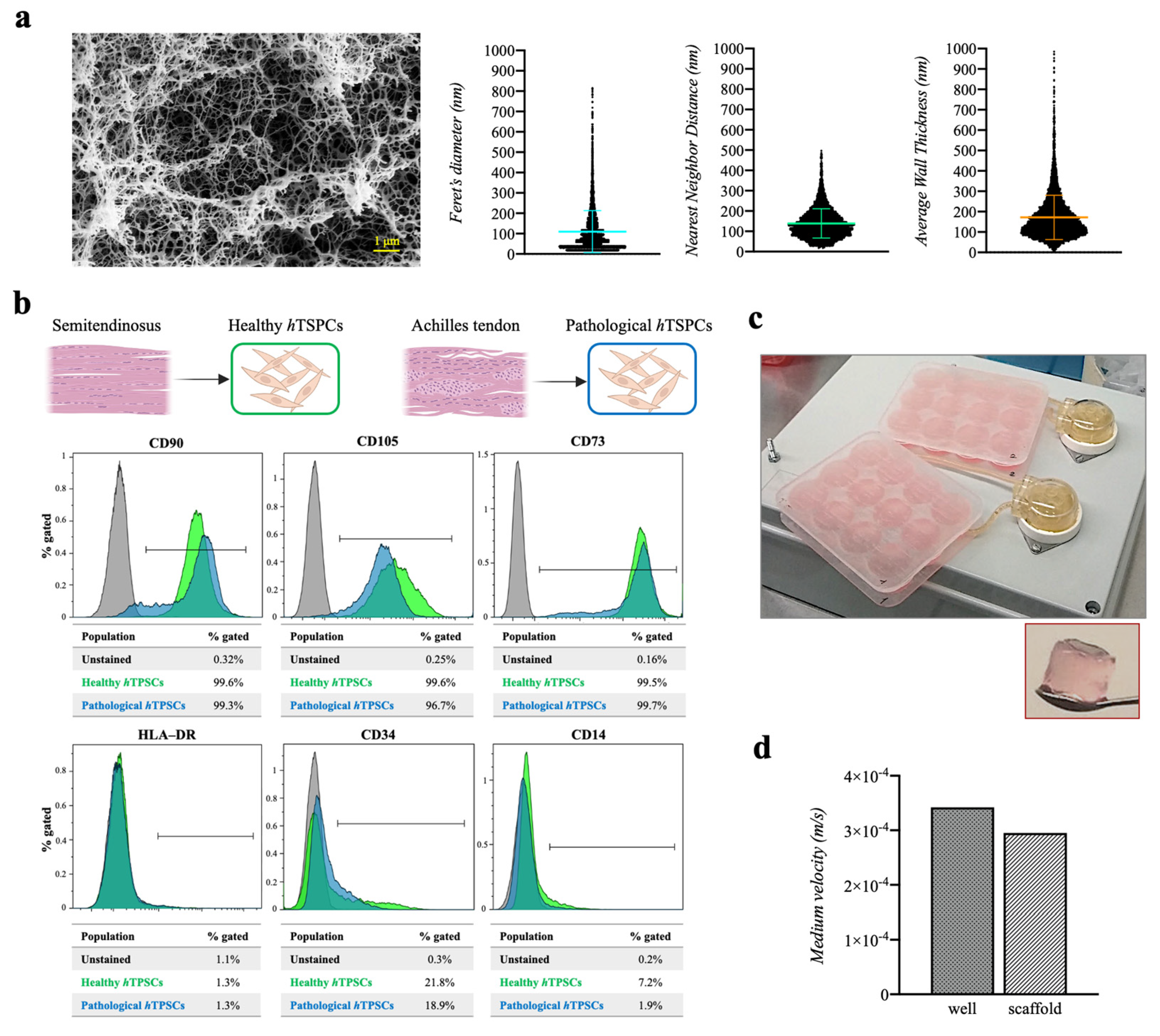
2.1. Three-Dimensional Fibrin Scaffold Characterization
2.2. The hTSPC Immunophenotyping and 3D Culture Establishment
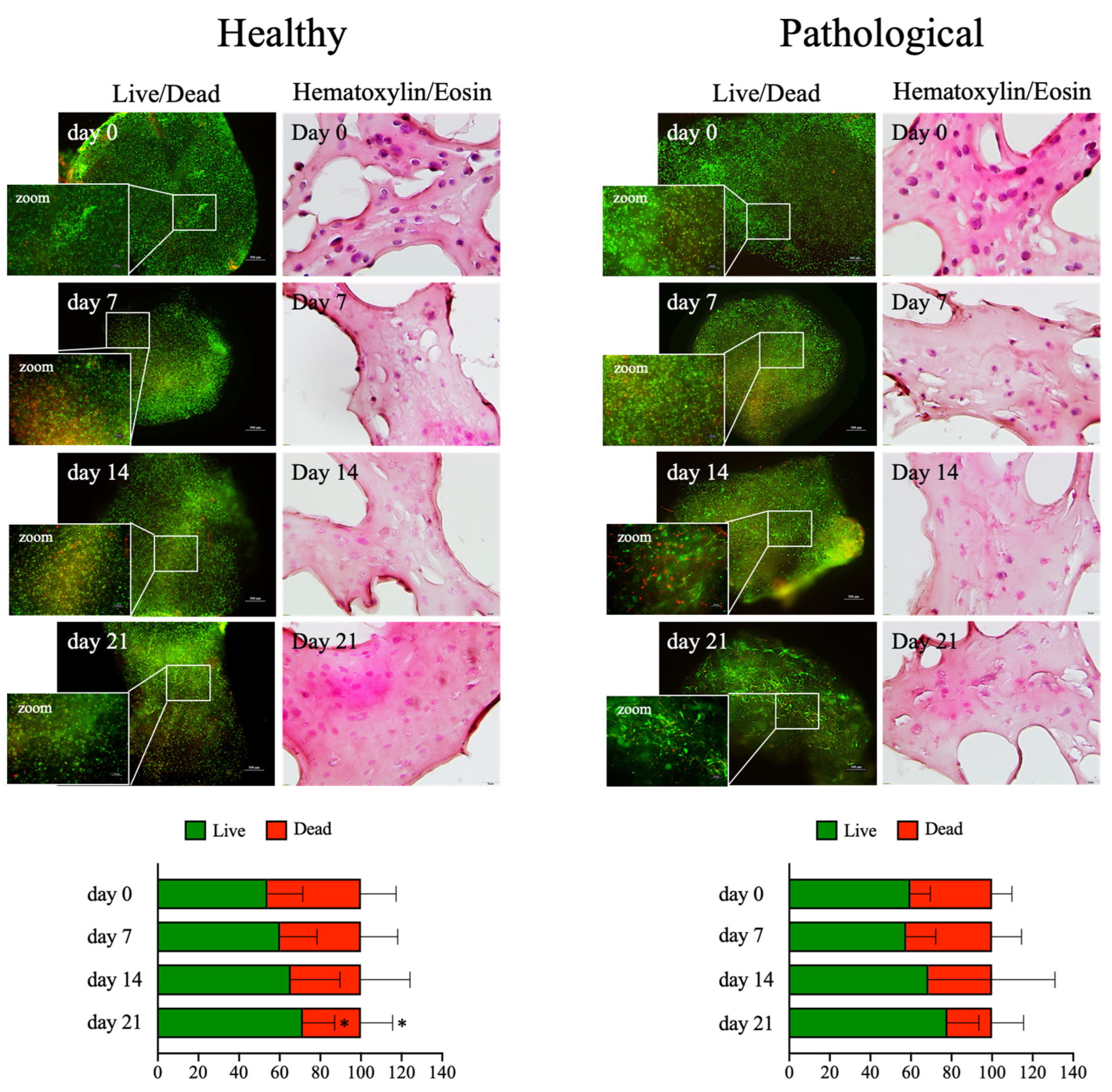
2.3. Perfusion-Based Dynamic Culture Assures Long-Term Viability
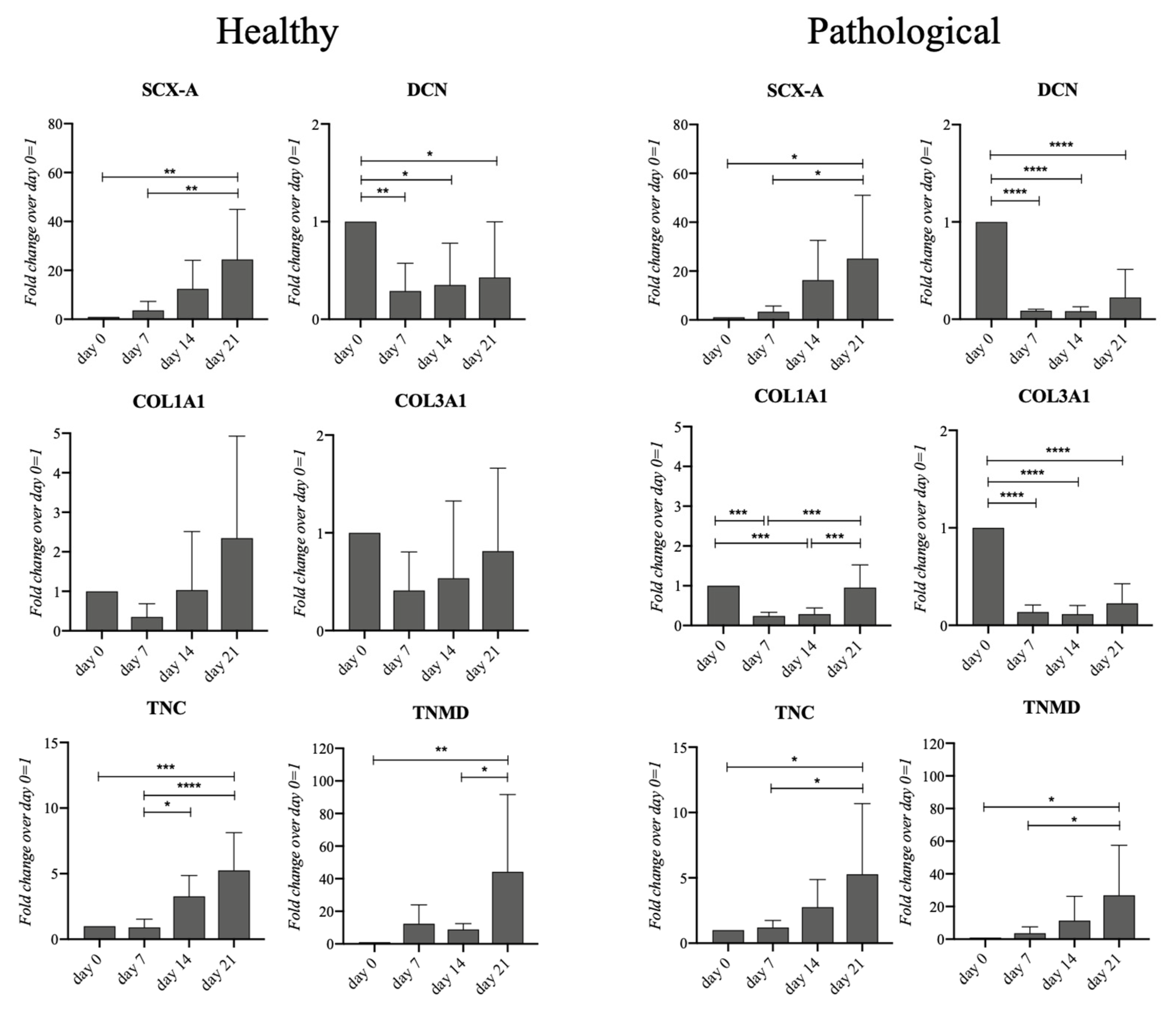
2.4. Healthy and Pathological hTSPCs Display Different Collagen Gene Expression
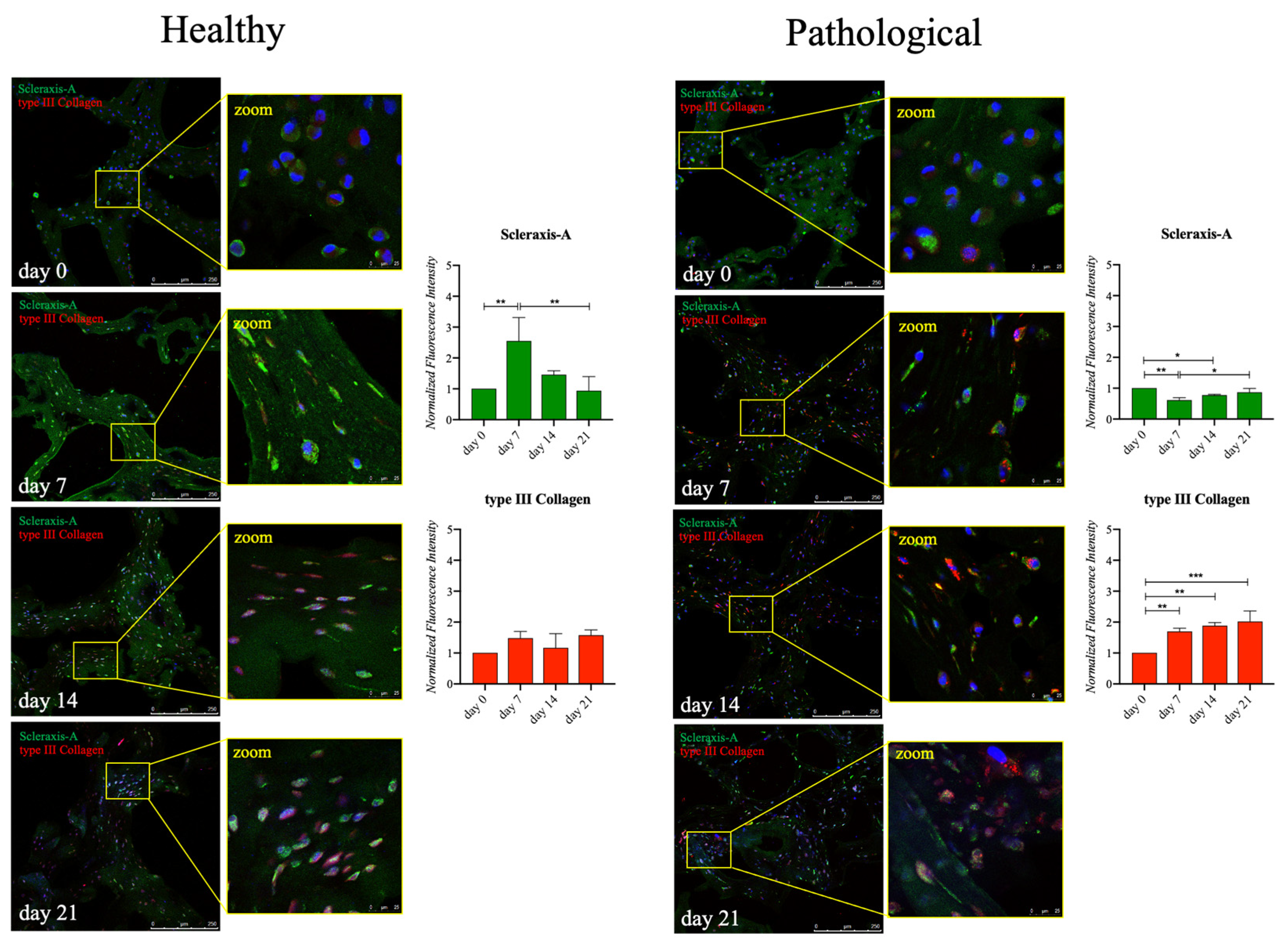
2.5. Healthy and Pathological hTSPCs Show Differences in Tendon-Related Protein Expression
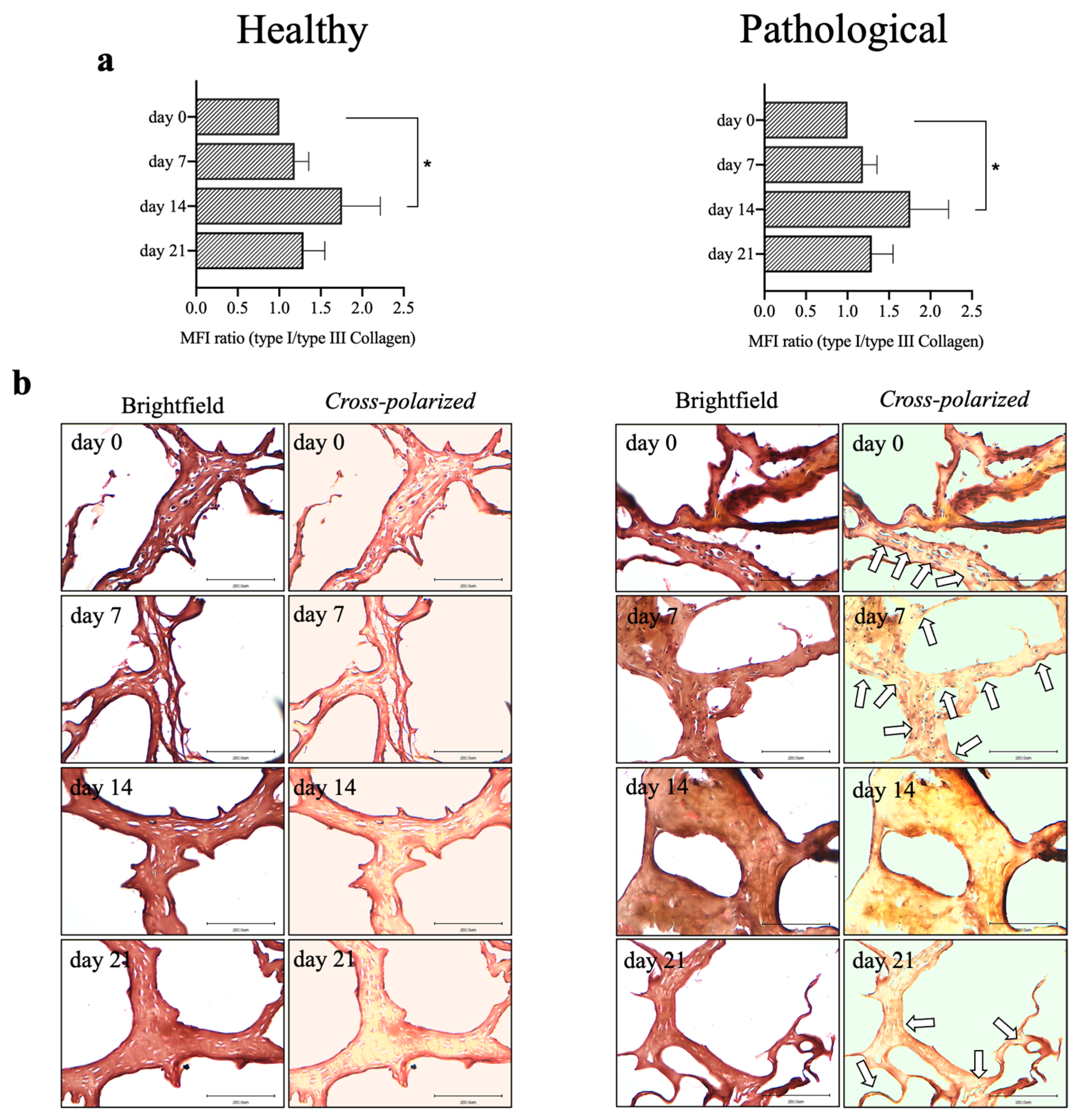
2.6. Pathological hTSPCs Show a Distinct Expression of Collagen Subtypes
2.7. Pathological hTSPCs Exhibit Reduced Overall Cytokine Expression
2.8. Healthy and Pathological hTSPCs Secrete Inflammatory Cytokines Differently
3. Discussion
4. Materials and Methods
4.1. Collection of Human Biopsies
4.2. The hTSPC Harvesting and Characterization
4.3. Flow Cytometry and Gating Strategy of hTSPCs
4.4. Fibrin Scaffold Drying, FE-SEM, and Pores Analysis
4.5. Assembly of 3D Bioengineered Scaffolds and Perfusion Culture System
4.6. Live and Dead Assays
4.7. RNA Isolation and Gene Expression Profiling
4.8. Immunohistochemical Assay
4.9. Hematoxylin and Eosin Staining
4.10. Sirius Red Staining
4.11. Detection of Cytokine Release
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maffulli, N.; Cuozzo, F.; Migliorini, F.; Oliva, F. The Tendon Unit: Biochemical, Biomechanical, Hormonal Influences. J. Orthop. Surg. 2023, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Nourissat, G.; Berenbaum, F.; Duprez, D. Tendon Injury: From Biology to Tendon Repair. Nat. Rev. Rheumatol. 2015, 11, 223–233. [Google Scholar] [CrossRef]
- Oliva, F.; Gatti, S.; Porcellini, G.; Forsyth, N.R.; Maffulli, N. Growth Factors and Tendon Healing. In Medicine and Sport Science; Maffulli, N., Ed.; S. Karger AG: Basilea, Switzerland, 2012; Volume 57, pp. 53–64. ISBN 978-3-8055-9814-9. [Google Scholar]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and Ligament Regeneration and Repair: Clinical Relevance and Developmental Paradigm. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 203–222. [Google Scholar] [CrossRef]
- Citeroni, M.R.; Ciardulli, M.C.; Russo, V.; Della Porta, G.; Mauro, A.; El Khatib, M.; Di Mattia, M.; Galesso, D.; Barbera, C.; Forsyth, N.R.; et al. In Vitro Innovation of Tendon Tissue Engineering Strategies. Int. J. Mol. Sci. 2020, 21, 6726. [Google Scholar] [CrossRef] [PubMed]
- Evrova, O.; Bürgisser, G.M.; Ebnöther, C.; Adathala, A.; Calcagni, M.; Bachmann, E.; Snedeker, J.G.; Scalera, C.; Giovanoli, P.; Vogel, V.; et al. Elastic and Surgeon Friendly Electrospun Tubes Delivering PDGF-BB Positively Impact Tendon Rupture Healing in a Rabbit Achilles Tendon Model. Biomaterials 2020, 232, 119722. [Google Scholar] [CrossRef] [PubMed]
- Rieber, J.; Meier-Bürgisser, G.; Miescher, I.; Weber, F.E.; Wolint, P.; Yao, Y.; Ongini, E.; Milionis, A.; Snedeker, J.G.; Calcagni, M.; et al. Bioactive and Elastic Emulsion Electrospun DegraPol Tubes Delivering IGF-1 for Tendon Rupture Repair. Int. J. Mol. Sci. 2023, 24, 10272. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Marino, L.; Lamparelli, E.P.; Guida, M.; Forsyth, N.R.; Selleri, C.; Della Porta, G.; Maffulli, N. Dose-Response Tendon-Specific Markers Induction by Growth Differentiation Factor-5 in Human Bone Marrow and Umbilical Cord Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 5905. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Angele, P.; Järvinen, T.A.H.; Docheva, D. Rescue Plan for Achilles: Therapeutics Steering the Fate and Functions of Stem Cells in Tendon Wound Healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef]
- Ruzzini, L.; Abbruzzese, F.; Rainer, A.; Longo, U.G.; Trombetta, M.; Maffulli, N.; Denaro, V. Characterization of Age-Related Changes of Tendon Stem Cells from Adult Human Tendons. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2856–2866. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of Tendon Stem/Progenitor Cells and the Role of the Extracellular Matrix in Their Niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Lu, J.; Chen, H.; Lyu, K.; Jiang, L.; Chen, Y.; Long, L.; Wang, X.; Shi, H.; Li, S. The Functions and Mechanisms of Tendon Stem/Progenitor Cells in Tendon Healing. Stem Cells Int. 2023, 2023, 1258024. [Google Scholar] [CrossRef]
- Wang, B.; Guo, J.; Feng, L.; Suen, C.; Fu, W.; Zhang, J.; Li, G. MiR124 Suppresses Collagen Formation of Human Tendon Derived Stem Cells through Targeting Egr1. Exp. Cell Res. 2016, 347, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Strunz, F.; Yan, Z.; Lu, J.; Brochhausen, C.; Kiderlen, S.; Clausen-Schaumann, H.; Wang, X.; Gomes, M.E.; Alt, V.; et al. Three-Dimensional Self-Assembling Nanofiber Matrix Rejuvenates Aged/Degenerative Human Tendon Stem/Progenitor Cells. Biomaterials 2020, 236, 119802. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yin, H.; Chu, J.; Eglin, D.; Serra, T.; Docheva, D. An Anisotropic Nanocomposite Hydrogel Guides Aligned Orientation and Enhances Tenogenesis of Human Tendon Stem/Progenitor Cells. Biomater. Sci. 2021, 9, 1237–1245. [Google Scholar] [CrossRef]
- Han, W.; Tao, X.; Weng, T.; Chen, L. Circular RNA PVT1 Inhibits Tendon Stem/Progenitor Cell Senescence by Sponging microRNA-199a-5p. Toxicol. In Vitro 2022, 79, 105297. [Google Scholar] [CrossRef]
- Popov, C.; Burggraf, M.; Kreja, L.; Ignatius, A.; Schieker, M.; Docheva, D. Mechanical Stimulation of Human Tendon Stem/Progenitor Cells Results in Upregulation of Matrix Proteins, Integrins and MMPs, and Activation of P38 and ERK1/2 Kinases. BMC Mol. Biol. 2015, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.W.; Lui, P.P.Y.; Rui, Y.F. Hypoxia-Mediated Efficient Expansion of Human Tendon–Derived Stem Cells In Vitro. Tissue Eng. Part A 2012, 18, 484–498. [Google Scholar] [CrossRef]
- Son, Y.H.; Yang, D.H.; Uricoli, B.; Park, S.-J.; Jeong, G.-J.; Chun, H.J. Three-Dimensional Cell Culture System for Tendon Tissue Engineering. Tissue Eng. Regen. Med. 2023, 20, 553–562. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Lovecchio, J.; Scala, P.; Lamparelli, E.P.; Dale, T.P.; Giudice, V.; Giordano, E.; Selleri, C.; Forsyth, N.R.; Maffulli, N.; et al. 3D Biomimetic Scaffold for Growth Factor Controlled Delivery: An In-Vitro Study of Tenogenic Events on Wharton’s Jelly Mesenchymal Stem Cells. Pharmaceutics 2021, 13, 1448. [Google Scholar] [CrossRef]
- Sun, J.; Mou, C.; Shi, Q.; Chen, B.; Hou, X.; Zhang, W.; Li, X.; Zhuang, Y.; Shi, J.; Chen, Y.; et al. Controlled Release of Collagen-Binding SDF-1α from the Collagen Scaffold Promoted Tendon Regeneration in a Rat Achilles Tendon Defect Model. Biomaterials 2018, 162, 22–33. [Google Scholar] [CrossRef]
- Chen, E.; Yang, L.; Ye, C.; Zhang, W.; Ran, J.; Xue, D.; Wang, Z.; Pan, Z.; Hu, Q. An Asymmetric Chitosan Scaffold for Tendon Tissue Engineering: In Vitro and in Vivo Evaluation with Rat Tendon Stem/Progenitor Cells. Acta Biomater. 2018, 73, 377–387. [Google Scholar] [CrossRef]
- Kim, W.; Kim, G.-E.; Attia Abdou, M.; Kim, S.; Kim, D.; Park, S.; Kim, Y.-K.; Gwon, Y.; Jeong, S.-E.; Kim, M.-S.; et al. Tendon-Inspired Nanotopographic Scaffold for Tissue Regeneration in Rotator Cuff Injuries. ACS Omega 2020, 5, 13913–13925. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; El Khatib, M.; Di Marcantonio, L.; Ancora, M.; Wyrwa, R.; Mauro, A.; Walter, T.; Weisser, J.; Citeroni, M.R.; Lazzaro, F.; et al. Tendon Biomimetic Electrospun PLGA Fleeces Induce an Early Epithelial-Mesenchymal Transition and Tenogenic Differentiation on Amniotic Epithelial Stem Cells. Cells 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Qian, Y.; Jin, Y.; Wang, S.; Li, J.; Yuan, W.-E.; Fan, C. Biomimetic Multilayer Polycaprolactone/Sodium Alginate Hydrogel Scaffolds Loaded with Melatonin Facilitate Tendon Regeneration. Carbohydr. Polym. 2022, 277, 118865. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, S.; Chen, X. Injectable Hydrogels for Tendon and Ligament Tissue Engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1333–1348. [Google Scholar] [CrossRef]
- Breidenbach, A.P.; Dyment, N.A.; Lu, Y.; Rao, M.; Shearn, J.T.; Rowe, D.W.; Kadler, K.E.; Butler, D.L. Fibrin Gels Exhibit Improved Biological, Structural, and Mechanical Properties Compared with Collagen Gels in Cell-Based Tendon Tissue-Engineered Constructs. Tissue Eng. Part A 2015, 21, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gardiner, B.S.; Lin, Z.; Rubenson, J.; Kirk, T.B.; Wang, A.; Xu, J.; Smith, D.W.; Lloyd, D.G.; Zheng, M.H. Bioreactor Design for Tendon/Ligament Engineering. Tissue Eng. Part B Rev. 2013, 19, 133–146. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Wu, Y.-F.; Wang, H.-K.; Yao, C.-C.J.; Chiu, Y.-H.; Sun, J.-S.; Chao, Y.-H. Cyclic Mechanical Stretch Regulates the AMPK/Egr1 Pathway in Tenocytes via Ca2+-Mediated Mechanosensing. Connect. Tissue Res. 2022, 63, 590–602. [Google Scholar] [CrossRef]
- Dyment, N.A.; Barrett, J.G.; Awad, H.A.; Bautista, C.A.; Banes, A.J.; Butler, D.L. A Brief History of Tendon and Ligament Bioreactors: Impact and Future Prospects. J. Orthop. Res. 2020, 38, 2318–2330. [Google Scholar] [CrossRef]
- Wang, T.; Thien, C.; Wang, C.; Ni, M.; Gao, J.; Wang, A.; Jiang, Q.; Tuan, R.S.; Zheng, Q.; Zheng, M.H. 3D Uniaxial Mechanical Stimulation Induces Tenogenic Differentiation of Tendon-derived Stem Cells through a PI3K/AKT Signaling Pathway. FASEB J. 2018, 32, 4804–4814. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Current Progress in Growth Factors and Extracellular Vesicles in Tendon Healing. Int. Wound J. 2023, 20, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Pryce, B.A.; Watson, S.S.; Murchison, N.D.; Staverosky, J.A.; Dünker, N.; Schweitzer, R. Recruitment and Maintenance of Tendon Progenitors by TGFβ Signaling Are Essential for Tendon Formation. Development 2009, 136, 1351–1361. [Google Scholar] [CrossRef]
- Tan, G.-K.; Pryce, B.A.; Stabio, A.; Keene, D.R.; Tufa, S.F.; Schweitzer, R. Cell Autonomous TGFβ Signaling Is Essential for Stem/Progenitor Cell Recruitment into Degenerative Tendons. Stem Cell Rep. 2021, 16, 2942–2957. [Google Scholar] [CrossRef]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming Growth Factor Beta (TGF-β) Isoforms in Wound Healing and Fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Yuan, S.; Bai, L.; Zhang, X.; Chen, P.; Zhang, W. Benzyl Alcohol Accelerates Recovery from Achilles Tendon Injury, Potentially via TGF-Β1/Smad2/3 Pathway. Injury 2020, 51, 1515–1521. [Google Scholar] [CrossRef]
- Kapacee, Z.; Yeung, C.-Y.C.; Lu, Y.; Crabtree, D.; Holmes, D.F.; Kadler, K.E. Synthesis of Embryonic Tendon-like Tissue by Human Marrow Stromal/Mesenchymal Stem Cells Requires a Three-Dimensional Environment and Transforming Growth Factor Β3. Matrix Biol. 2010, 29, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Durant, T.J.S.; Dyment, N.; McCarthy, M.B.R.; Cote, M.P.; Arciero, R.A.; Mazzocca, A.D.; Rowe, D. Mesenchymal Stem Cell Response to Growth Factor Treatment and Low Oxygen Tension in 3-Dimensional Construct Environment. Muscles Ligaments Tendons J. 2014, 4, 46–51. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Scala, P.; Giudice, V.; Santoro, A.; Selleri, C.; Oliva, F.; Maffulli, N.; Della Porta, G. Stem Cells from Healthy and Tendinopathic Human Tendons: Morphology, Collagen and Cytokines Expression and Their Response to T3 Thyroid Hormone. Cells 2022, 11, 2545. [Google Scholar] [CrossRef]
- Clerici, M.; Ciardulli, M.C.; Lamparelli, E.P.; Lovecchio, J.; Giordano, E.; Dale, T.P.; Forsyth, N.R.; Maffulli, N. Human Tendon Stem/Progenitor Cell-Derived Extracellular Vesicle Production Promoted by Dynamic Culture. Artif. Cells Nanomed. Biotechnol. 2024; in press. [Google Scholar]
- Scala, P.; Manzo, P.; Lamparelli, E.P.; Lovecchio, J.; Ciardulli, M.C.; Giudice, V.; Selleri, C.; Giordano, E.; Rehak, L.; Maffulli, N.; et al. Peripheral Blood Mononuclear Cells Contribute to Myogenesis in a 3D Bioengineered System of Bone Marrow Mesenchymal Stem Cells and Myoblasts. Front. Bioeng. Biotechnol. 2023, 10, 1075715. [Google Scholar] [CrossRef]
- Della Porta, G.; Del Gaudio, P.; De Cicco, F.; Aquino, R.P.; Reverchon, E. Supercritical Drying of Alginate Beads for the Development of Aerogel Biomaterials: Optimization of Process Parameters and Exchange Solvents. Ind. Eng. Chem. Res. 2013, 52, 12003–12009. [Google Scholar] [CrossRef]
- Della Porta, G.; Nguyen, B.B.; Campardelli, R.; Reverchon, E.; Fisher, J.P. Synergistic Effect of Sustained Release of Growth Factors and Dynamic Culture on Osteoblastic Differentiation of Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2015, 103, 2161–2171. [Google Scholar] [CrossRef]
- Lamparelli, E.P.; Lovecchio, J.; Ciardulli, M.C.; Giudice, V.; Dale, T.P.; Selleri, C.; Forsyth, N.; Giordano, E.; Maffulli, N.; Della Porta, G. Chondrogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells in a Perfused Collagen Hydrogel Functionalized with hTGF-Β1-Releasing PLGA Microcarrier. Pharmaceutics 2021, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.; Lovecchio, J.; Ferretti, G.; Giordano, E. Medium Perfusion Flow Improves Osteogenic Commitment of Human Stromal Cells. Stem Cells Int. 2019, 2019, 1304194. [Google Scholar] [CrossRef] [PubMed]
- Citro, V.; Clerici, M.; Boccaccini, A.R.; Della Porta, G.; Maffulli, N.; Forsyth, N.R. Tendon Tissue Engineering: An Overview of Biologics to Promote Tendon Healing and Repair. J. Tissue Eng. 2023, 14, 20417314231196275. [Google Scholar] [CrossRef]
- Lui, P.P. Stem Cell Technology for Tendon Regeneration: Current Status, Challenges, and Future Research Directions. Stem Cells Cloning Adv. Appl. 2015, 8, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for Tendon Repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Peneda Pacheco, D.; Suárez Vargas, N.; Visentin, S.; Petrini, P. From Tissue Engineering to Engineering Tissues: The Role and Application of In Vitro Models. Biomater. Sci. 2021, 9, 70–83. [Google Scholar] [CrossRef]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. Immunological Characterization of Multipotent Mesenchymal Stromal Cells—The International Society for Cellular Therapy (ISCT) Working Proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Tendon Injury and Tendinopathy: Healing and Repair. J. Bone Jt. Surg. 2005, 87, 187–202. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Chen, X.; Li, G.; Chan, K.-M.; Heng, B.C.; Yin, Z.; Ouyang, H.-W. Concise Review: Stem Cell Fate Guided By Bioactive Molecules for Tendon Regeneration. Stem Cells Transl. Med. 2018, 7, 404–414. [Google Scholar] [CrossRef]
- Li, H.; Luo, S.; Wang, H.; Chen, Y.; Ding, M.; Lu, J.; Jiang, L.; Lyu, K.; Huang, S.; Shi, H.; et al. The Mechanisms and Functions of TGF-Β1 in Tendon Healing. Injury 2023, 54, 111052. [Google Scholar] [CrossRef]
- Hsia, H.C.; Schwarzbauer, J.E. Meet the Tenascins: Multifunctional and Mysterious. J. Biol. Chem. 2005, 280, 26641–26644. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, S.; Zhang, C.; Lu, P.; Hu, J.; Yin, Z.; Ma, Y.; Chen, X.; OuYang, H. Crucial Transcription Factors in Tendon Development and Differentiation: Their Potential for Tendon Regeneration. Cell Tissue Res. 2014, 356, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Shukunami, C.; Takimoto, A.; Oro, M.; Hiraki, Y. Scleraxis Positively Regulates the Expression of Tenomodulin, a Differentiation Marker of Tenocytes. Dev. Biol. 2006, 298, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Delgado Caceres, M.; Angerpointner, K.; Galler, M.; Lin, D.; Michel, P.A.; Brochhausen, C.; Lu, X.; Varadarajan, A.R.; Warfsmann, J.; Stange, R.; et al. Tenomodulin Knockout Mice Exhibit Worse Late Healing Outcomes with Augmented Trauma-Induced Heterotopic Ossification of Achilles Tendon. Cell Death Dis. 2021, 12, 1049. [Google Scholar] [CrossRef]
- Korcari, A.; Muscat, S.; McGinn, E.; Buckley, M.R.; Loiselle, A.E. Depletion of Scleraxis-Lineage Cells during Tendon Healing Transiently Impairs Multi-Scale Restoration of Tendon Structure during Early Healing. PLoS ONE 2022, 17, e0274227. [Google Scholar] [CrossRef]
- Docheva, D.; Hunziker, E.B.; Fässler, R.; Brandau, O. Tenomodulin Is Necessary for Tenocyte Proliferation and Tendon Maturation. Mol. Cell. Biol. 2005, 25, 699–705. [Google Scholar] [CrossRef]
- Buckley, M.R.; Evans, E.B.; Matuszewski, P.E.; Chen, Y.-L.; Satchel, L.N.; Elliott, D.M.; Soslowsky, L.J.; Dodge, G.R. Distributions of Types I, II and III Collagen by Region in the Human Supraspinatus Tendon. Connect. Tissue Res. 2013, 54, 374–379. [Google Scholar] [CrossRef]
- John, T.; Lodka, D.; Kohl, B.; Ertel, W.; Jammrath, J.; Conrad, C.; Stoll, C.; Busch, C.; Schulze-Tanzil, G. Effect of Pro-inflammatory and Immunoregulatory Cytokines on Human Tenocytes. J. Orthop. Res. 2010, 28, 1071–1077. [Google Scholar] [CrossRef]
- Al-Sadi, O.; Schulze-Tanzil, G.; Kohl, B.; Lohan, A.; Lemke, M.; Ertel, W.; John, T. Tenocytes, pro-Inflammatory Cytokines and Leukocytes: A Relationship? Muscles Ligaments Tendons J. 2011, 1, 68–76. [Google Scholar]
- Tarafder, S.; Chen, E.; Jun, Y.; Kao, K.; Sim, K.H.; Back, J.; Lee, F.Y.; Lee, C.H. Tendon Stem/Progenitor Cells Regulate Inflammation in Tendon Healing via JNK and STAT3 Signaling. FASEB J. 2017, 31, 3991–3998. [Google Scholar] [CrossRef] [PubMed]
- Perucca Orfei, C.; Bowles, A.C.; Kouroupis, D.; Willman, M.A.; Ragni, E.; Kaplan, L.D.; Best, T.M.; Correa, D.; De Girolamo, L. Human Tendon Stem/Progenitor Cell Features and Functionality Are Highly Influenced by In Vitro Culture Conditions. Front. Bioeng. Biotechnol. 2021, 9, 711964. [Google Scholar] [CrossRef]
- Vinhas, A.; Rodrigues, M.T.; Gomes, M.E. Exploring Stem Cells and Inflammation in Tendon Repair and Regeneration. In Cell Biology and Translational Medicine, Volume 2; Turksen, K., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1089, pp. 37–46. ISBN 978-3-030-04169-4. [Google Scholar]
- Ellis, I.M.; Schnabel, L.V.; Berglund, A.K. Defining the Profile: Characterizing Cytokines in Tendon Injury to Improve Clinical Therapy. J. Immunol. Regen. Med. 2022, 16, 100059. [Google Scholar] [CrossRef]
- Kharaz, Y.A.; Canty-Laird, E.G.; Tew, S.R.; Comerford, E.J. Variations in Internal Structure, Composition and Protein Distribution between Intra- and Extra-articular Knee Ligaments and Tendons. J. Anat. 2018, 232, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Haeri, M.; Haeri, M. ImageJ Plugin for Analysis of Porous Scaffolds Used in Tissue Engineering. J. Open Res. Softw. 2015, 3, e1. [Google Scholar] [CrossRef]
- Samavedi, S.; Poindexter, L.K.; Van Dyke, M.; Goldstein, A.S. Synthetic Biomaterials for Regenerative Medicine Applications. In Regenerative Medicine Applications in Organ Transplantation; Elsevier: Amsterdam, The Netherlands, 2014; pp. 81–99. ISBN 978-0-12-398523-1. [Google Scholar]
- Spaepen, P.; De Boodt, S.; Aerts, J.-M.; Sloten, J.V. Digital Image Processing of Live/Dead Staining. In Mammalian Cell Viability; Stoddart, M.J., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 740, pp. 209–230. ISBN 978-1-61779-107-9. [Google Scholar]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [PubMed]
- Sundjaja, J.H.; Shrestha, R.; Krishan, K. McNemar And Mann-Whitney U Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- de Winter, J.C.F. Using the Student’s t-Test with Extremely Small Sample Sizes. Pract. Assess. Res. Eval. 2019, 18, 10. [Google Scholar] [CrossRef]



| Target | Gene Bank Accession Number | Sequences | Product Size | Primer Efficiency |
|---|---|---|---|---|
| IL-6 | NM-000600.5 | Fwd: ACTTGCCTGGTGAAAATCAT Rev: CAGGAACTGGATCAGGACTT | 135 | 106 |
| TNF | NM-000594.4 | Fwd: GCCCATGTTGTAGCAAACCC Rev: TATCTCTCAGCTCCACGCCA | 97 | 105 |
| IL-12A | NM-000882.4 | Fwd: TCAGAATTCGGGCAGTGACT Rev: AGTCCCAQTCCTTCTTTCCCC | 163 | 110 |
| IL-1β | NM-000576.3 | Fwd: GGAGAATGACCTGAGCACCT Rev: GGAGGTGGAGAGCTTTCAGT | 185 | 110 |
| IL-10 | NM-000572.3 | Fwd: AAGACCCAGACATCAAGGCG Rev: AATCGATGACAGCGCCGTAG | 85 | 110 |
| TGF-β1 | NM-000660.7 | Fwd: GCACTCGCCAGAGTGGTTAT Rev: AAGCCCTCAATTTCCCCTCC | 81 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciardulli, M.C.; Lovecchio, J.; Parolini, O.; Giordano, E.; Maffulli, N.; Della Porta, G. Fibrin Scaffolds Perfused with Transforming Growth Factor-β1 as an In Vitro Model to Study Healthy and Tendinopathic Human Tendon Stem/Progenitor Cells. Int. J. Mol. Sci. 2024, 25, 9563. https://doi.org/10.3390/ijms25179563
Ciardulli MC, Lovecchio J, Parolini O, Giordano E, Maffulli N, Della Porta G. Fibrin Scaffolds Perfused with Transforming Growth Factor-β1 as an In Vitro Model to Study Healthy and Tendinopathic Human Tendon Stem/Progenitor Cells. International Journal of Molecular Sciences. 2024; 25(17):9563. https://doi.org/10.3390/ijms25179563
Chicago/Turabian StyleCiardulli, Maria Camilla, Joseph Lovecchio, Ornella Parolini, Emanuele Giordano, Nicola Maffulli, and Giovanna Della Porta. 2024. "Fibrin Scaffolds Perfused with Transforming Growth Factor-β1 as an In Vitro Model to Study Healthy and Tendinopathic Human Tendon Stem/Progenitor Cells" International Journal of Molecular Sciences 25, no. 17: 9563. https://doi.org/10.3390/ijms25179563








