Simultaneous Detection of Collagen I Alpha II and Cytokeratin 19 mRNA by Multiplex qPCR in Liquid Biopsy in Diagnosis of Patients with Resectable Solid Tumors
Abstract
:1. Introduction
2. Results
2.1. Preparation of the Standard Curve
2.2. Simplex RT-qPCR Standardization
2.3. Repeatability and Reproducibility of Intra- and Inter-Assay of RT-qPCR
2.4. Absence of Interactions between Real-Time Reactions
2.5. Limit of Detection (LOD) and RT-qPCR Efficiency
2.6. Baseline Characteristics of Patients
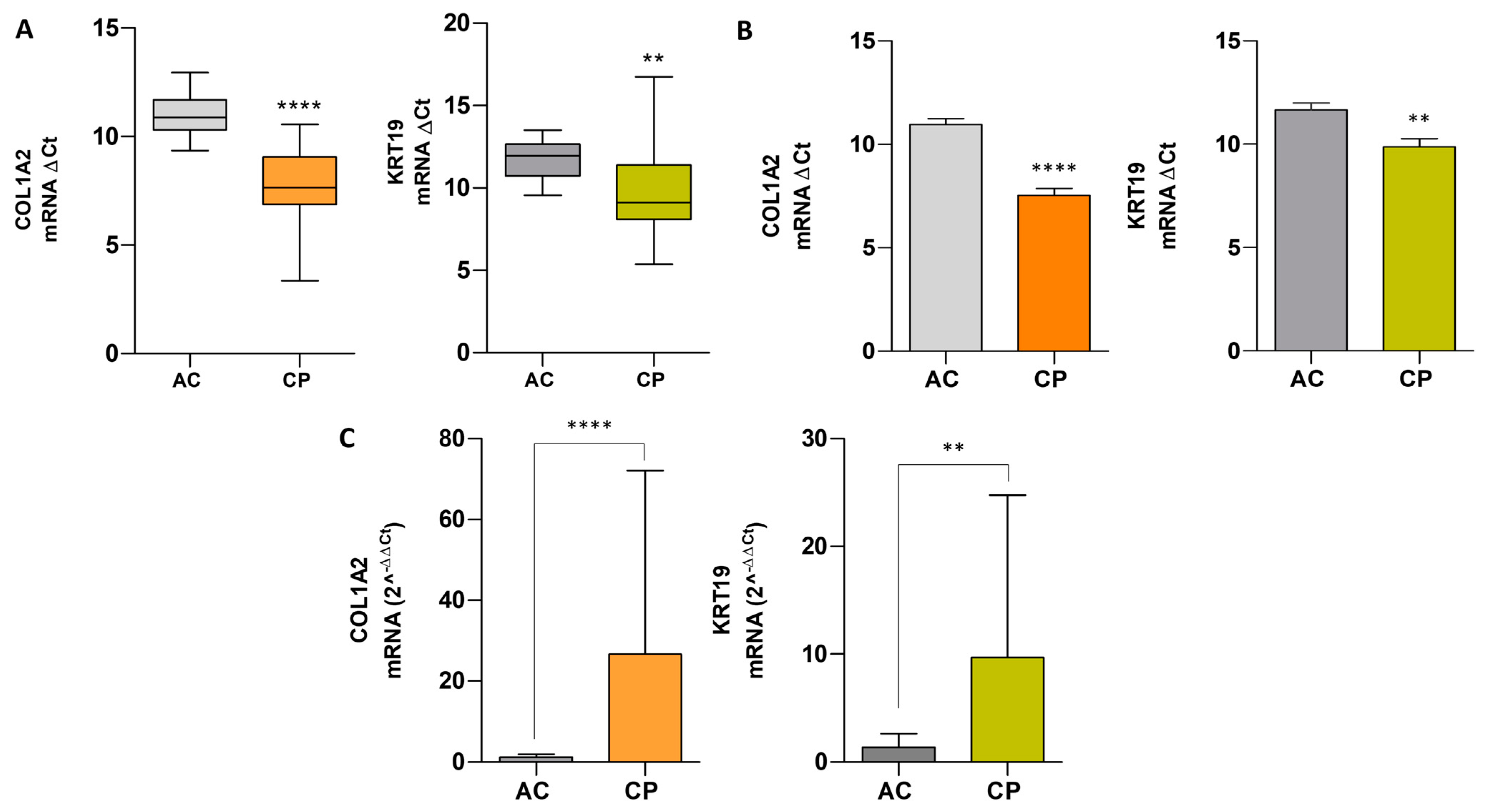
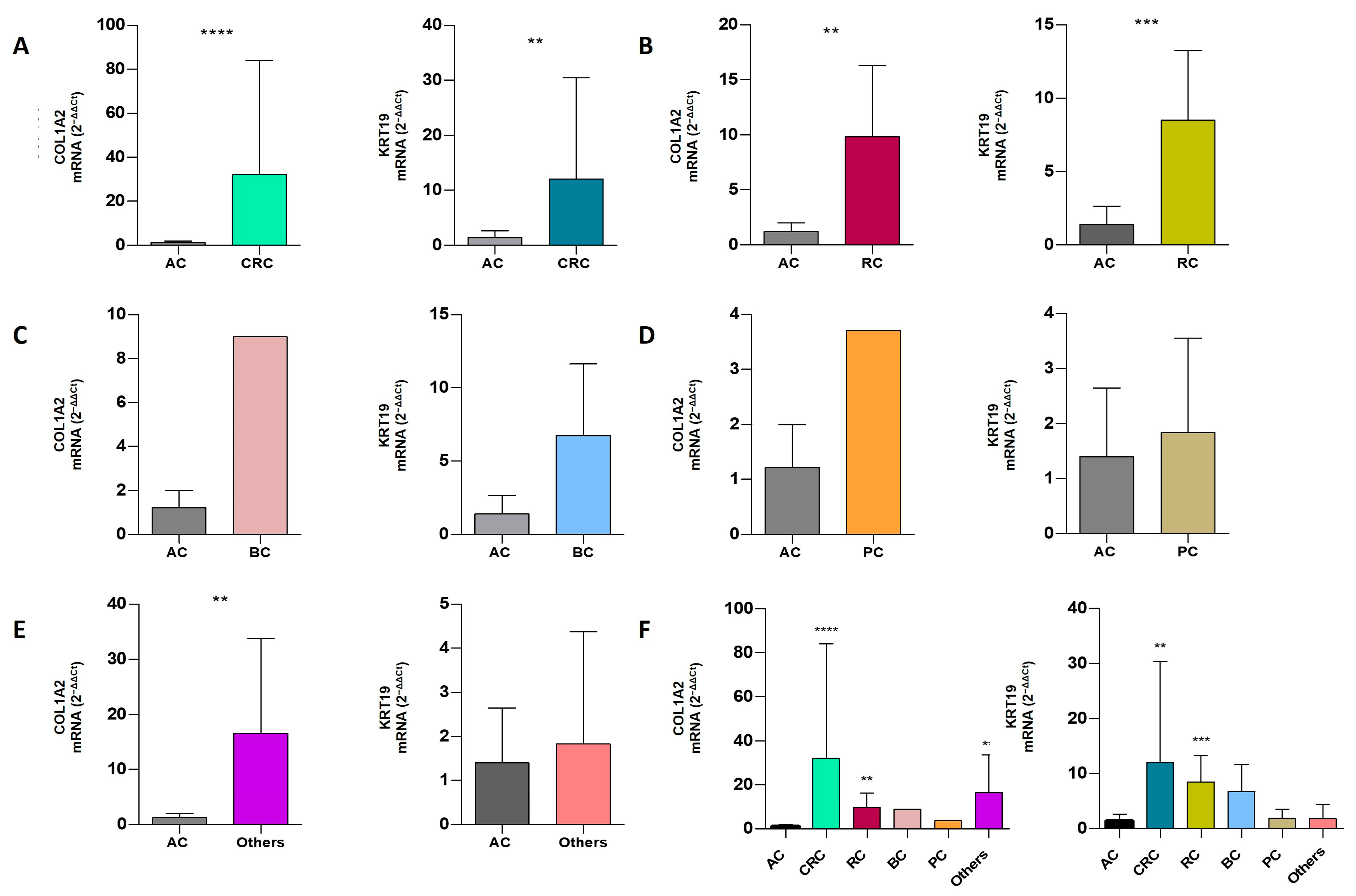
2.7. Comparative Analysis of mRNA Expression Level in Extracellular Vesicles of ACs vs. CPs
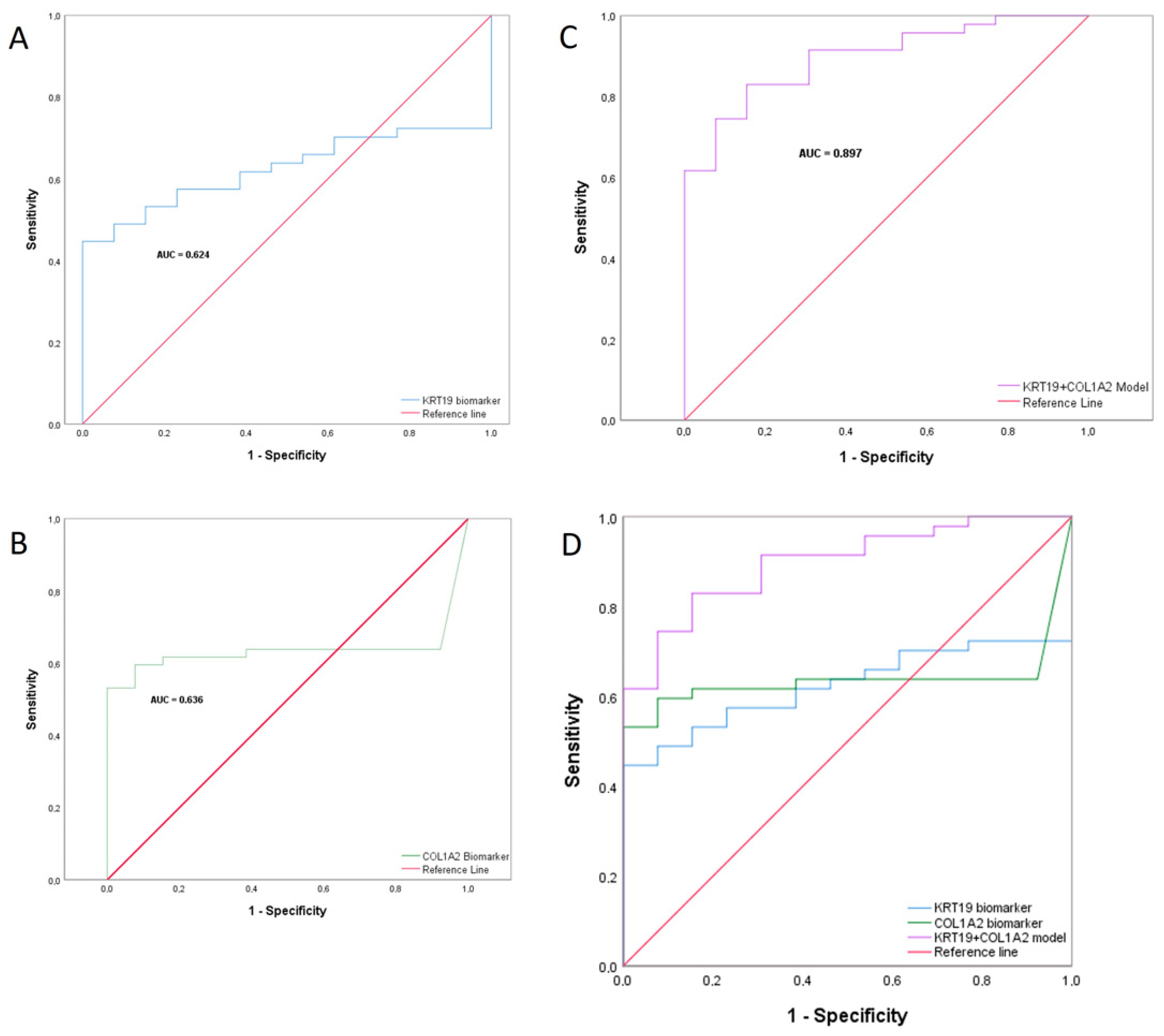
2.8. Combined Biomarker Prediction
3. Discussion
4. Materials and Methods
4.1. Patients and Human Samples
4.2. Blood Sample Collection and RNA Isolation from Extracellular Vesicles
4.3. Multiplex RT-qPCR Assay
4.4. Amplification Efficiency (E)
4.5. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Chen, W.-D.; Parmigiani, G.; Diehl, F.; Beerenwinkel, N.; Antal, T.; Traulsen, A.; Nowak, M.A.; Siegel, C.; Velculescu, V.E.; et al. Comparative lesion sequencing provides insights into tumor evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 4283–4288. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol-Bouts, M.J.; Tammela, T.L.J.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef]
- Denters, M.J.; Deutekom, M.; Essink-Bot, M.L.; Bossuyt, P.M.; Fockens, P.; Dekker, E. FIT false-positives in colorectal cancer screening experience psychological distress up to 6 weeks after colonoscopy. Support. Care Cancer 2013, 21, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Schuler, M.; Popat, S.; Miura, S.; Heeke, S.; Park, K.; Märten, A.; Kim, E.S. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: A database of 693 cases. J. Thorac. Oncol. 2020, 15, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Al-Turkmani, M.R.; Godwin, K.N.; Peterson, J.D.; Tsongalis, G.J. Rapid somatic mutation testing in colorectal cancer by use of a fully automated system and single-use cartridge: A comparison with next-generation sequencing. J. Appl. Lab. Med. 2018, 3, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T.; et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun. 2020, 11, 3475. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef]
- Pérez, M.E.; Romero, M.T.H.; Alén, B.O.; Martínez, M.; Prado, R.F.; Bravos, M.D.L.T.; López, C. Detection of lymph node metastasis in lung cancer patients using a one-step nucleic acid amplification assay: A single-centre prospective study. J. Transl. Med. 2019, 17, 233. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Alen, B.O.; Estévez-Pérez, L.S.; Alén, M.O.; Hormaetxe, S.D.; Simón, L.; Concha, Á. Expression of Epithelial and Mesenchymal Markers in Plasmatic Extracellular Vesicles as a Diagnostic Tool for Neoplastic Processes. Int. J. Mol. Sci. 2023, 24, 3578. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hayden, S.R.; Brown, M.D. Likelihood ratio: A powerful tool for incorporating the results of a diagnostic test into clinical decision making. Ann. Emerg. Med. 1999, 33, 575–580. [Google Scholar] [CrossRef]
- Gavert, N.; Ben-Ze’ev, A. Epithelial–mesenchymal transition and the invasive potential of tumors. Trends Mol. Med. 2008, 14, 199–209. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Korkaya, H.; Orsulic, S. Editorial: The tumor microenvironment: Recent advances and novel therapeutic approaches. Front. Cell Dev. Biol. 2020, 8, 586176. [Google Scholar] [CrossRef]
- McKiernan, J.; Donovan, M.J.; O’Neill, V.; Bentink, S.; Noerholm, M.; Belzer, S.; Skog, J.; Kattan, M.W.; Partin, A.; Andriole, G.; et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016, 2, 882–889. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, J.; Zhong, B.; Huang, J.; Jiang, L.; Jiang, Y.; Yuan, J.; Sun, J.; Dai, L.; Yang, C.; et al. Exosomes as potential sources of biomarkers in colorectal cancer. Cancer Lett. 2020, 476, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Saidijam, M.; Kaki, A.; Etemadi, K.; Shabab, N.; Yadegarazari, R. A pilot study of CK19, CK20 and GCC mRNA in the peripheral blood as a colorectal cancer biomarker panel. Int. J. Mol. Cell Med. 2016, 5, 30–36. [Google Scholar] [PubMed]
- Mertz, K.D.; Demichelis, F.; Sboner, A.; Hirsch, M.S.; Cin, P.D.; Struckmann, K.; Storz, M.; Scherrer, S.; Schmid, D.M.; Strebel, R.T.; et al. Association of cytokeratin 7 and 19 expression with genomic stability and favorable prognosis in clear cell renal cell cancer: Cytokeratin Expression in ccRCC. Int. J. Cancer 2008, 123, 569–576. [Google Scholar] [CrossRef]
- El Shobaky, A.; Abbas, M.; Raouf, R.; Zakaria, M.M.; Ali-El-Dein, B. Effect of pathogenic bacteria on reliability of CK-19, CK-20 and UPII as bladder cancer genetic markers: A molecular biology study. Egypt. J. Basic Appl. Sci. 2015, 2, 176–182. [Google Scholar] [CrossRef]
- Kutun, S.; Celik, A.; Kockar, M.C.; Erkorkmaz, U.; Eroğlu, A.; Cetin, A.; Erkosar, B.; Yakicier, C. Expression of CK-19 and CEA mRNA in peripheral blood of gastric cancer patients. Exp. Oncol. 2010, 32, 263–268. [Google Scholar]
- Callau, C.; Lejeune, M.; Korzynska, A.; García, M.; Bueno, G.; Bosch, R.; Jaén, J.; Orero, G.; Salvadó, T.; López, C. Evaluation of cytokeratin-19 in breast cancer tissue samples: A comparison of automatic and manual evaluations of scanned tissue microarray cylinders. Biomed. Eng. Online 2015, 14, S2. [Google Scholar] [CrossRef]
- Stathopoulou, A.; Gizi, A.; Perraki, M.; Apostolaki, S.; Malamos, N.; Mavroudis, D.; Georgoulias, V.; Lianidou, E.S. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin. Cancer Res. 2003, 9, 5145–5151. [Google Scholar] [PubMed]
- Schmitt, A.M.; Anlauf, M.; Rousson, V.; Schmid, S.; Kofler, A.; Riniker, F.; Bauersfeld, J.; Barghorn, A.; Probst-Hensch, N.M.; Moch, H.; et al. WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumors. Am. J. Surg. Pathol. 2007, 31, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Bremmer, F.; Ströbel, P.; Jarry, H.; Strecker, J.; Gaisa, N.; Strauß, A.; Schweyer, S.; Radzun, H.-J.; Behnes, C.-L. CK19 is a sensitive marker for yolk sac tumours of the testis. Diagn. Pathol. 2015, 10, 7. [Google Scholar] [CrossRef]
- Jain, R.; Fischer, S.; Serra, S.; Chetty, R. The use of cytokeratin 19 (CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 9–15. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Pourhashem, Z.; Yardehnavi, N.; Oladnabi, M. Evaluation of cytokeratin 19 as a prognostic tumoral and metastatic marker with focus on improved detection methods. J. Cell Physiol. 2019, 234, 21425–21435. [Google Scholar] [CrossRef]
- Yao, G.; Zhao, K.; Bao, K.; Li, J. Radiation increases COL1A1, COL3A1, and COL1A2 expression in breast cancer. Open Med. 2022, 17, 329–340. [Google Scholar] [CrossRef]
- Yu, G.; Chen, W.; Li, X.; Yu, L.; Xu, Y.; Ruan, Q.; He, Y.; Wang, Y. TWIST1-EP300 expedites gastric cancer cell resistance to apatinib by activating the expression of COL1A2. Anal. Cell Pathol. 2022, 2022, 5374262. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Packham, G.; Murphy, L.B.; Bateman, A.C.; Conti, J.A.; Fine, D.R.; Johnson, C.D.; Benyon, R.C.; Iredale, J.P. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2004, 10, 7427–7437. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, J.; Xiong, L.; Gao, M.; Qin, L.; Dai, C.; Liang, Z.; Wang, Y.; Xue, J.; Wang, Q.; et al. Identification of candidate biomarkers and therapeutic drugs of colorectal cancer by integrated bioinformatics analysis. Med. Oncol. 2020, 37, 104. [Google Scholar] [CrossRef] [PubMed]
- Bager, C.; Willumsen, N.; Leeming, D.; Smith, V.; Karsdal, M.; Dornan, D.; Bay-Jensen, A. Collagen degradation products measured in serum can separate ovarian and breast cancer patients from healthy controls: A preliminary study. Cancer Biomark. 2015, 15, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Corsa, C.A.; Ponik, S.M.; Prior, J.L.; Piwnica-Worms, D.; Eliceiri, K.W.; Keely, P.J.; Longmore, G.D. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 2013, 15, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Huang, W.; Tian, S.; Chi, X.; Zhao, P.; Liu, F. FCOL1A2 is a novel biomarker to improve clinical prediction in human gastric cancer: Integrating bioinformatics and me-ta-analysis. Pathol. Oncol. Res. 2017, 24, 129–134. [Google Scholar] [CrossRef]
- Willumsen, N.; Bager, C.L.; Leeming, D.J.; Smith, V.; Christiansen, C.; Karsdal, M.A.; Dornan, D.; Bay-Jensen, A. Serum biomarkers reflecting specific tumor tissue remodeling processes are valuable diagnostic tools for lung cancer. Cancer Med. 2014, 3, 1136–1145. [Google Scholar] [CrossRef]
- Liu, J.; Shen, J.-X.; Wu, H.-T.; Li, X.-L.; Wen, X.-F.; Du, C.-W.; Zhang, G.-J. Collagen 1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov. Med. 2018, 25, 211–223. [Google Scholar] [PubMed]
- Ghassemi, H.; Hashemnia, M.; Mousavibahar, S.H.; Hosseini, H.M.; Mirhosseini, S.A. Positive association of matrix proteins alteration with TAZ and the progression of high-grade bladder cancer. Cell J. 2021, 23, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Y.; Li, A. Identification of COL1A1 and COL1A2 as candidate prognostic factors in gastric cancer. World J. Surg. Oncol. 2016, 14, 297. [Google Scholar] [CrossRef]
- Nazempour, N.; Taleqani, M.H.; Taheri, N.; Najafabadi, A.H.H.A.A.; Shokrollahi, A.; Zamani, A.; Dolatabadi, N.F.; Peymani, M.; Mahdevar, M. The role of cell surface proteins gene expression in diagnosis, prognosis, and drug resistance of colorectal cancer: In silico analysis and validation. Exp. Mol. Pathol. 2021, 123, 104688. [Google Scholar] [CrossRef] [PubMed]
- Asociación Española de Normalización y Certificación; AENOR. Quality Management Systems—Requirements (ISO 9001:2015), 1st ed.; AENOR: Madrid, Spain, 2015. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]


| Dilutions | ARN (ng) | Ct Values for the Different Dilutions of Human XpressRef Universal Total ARN | |||||
|---|---|---|---|---|---|---|---|
| β-Actin Mean Ct ± SD | CV (%) | KRT19 Mean Ct ± SD | CV (%) | COL1A2 Mean Ct ± SD | CV (%) | ||
| Intra-assay | 200 | 17.02 ± 0.44 | 2.58 | 19.83 ± 0.23 | 1.18 | 17.91 ± 0.11 | 0.63 |
| 20 | 19.82 ± 0.44 | 2.21 | 22.50 ± 0.22 | 0.96 | 21.32 ± 0.37 | 1.75 | |
| 2 | 23.45 ± 0.16 | 0.68 | 26.65 ± 0.26 | 0.99 | 24.80 ± 0.38 | 1.55 | |
| 0.2 | 27.11 ± 0.29 | 1.07 | 29.61 ± 0.33 | 1.12 | 28.44 ± 0.24 | 0.85 | |
| 0.02 | 30.19 ± 0.23 | 0.77 | 33.32 ± 0.17 | 0.51 | 31.78 ± 0.27 | 0.85 | |
| 0.002 | 33.13 ± 0.23 | 0.69 | 36.50 ± 1.15 | 3.16 | 35.31 ± 1.05 | 3.00 | |
| 0.0002 | 36.61 ± 0.31 | 0.84 | - | - | - | - | |
| Inter-assay | 200 | 16.89 ± 0.39 | 2.33 | 19.97 ± 0.29 | 1.45 | 19.22 ± 0.68 | 3.52 |
| 20 | 20.04 ± 0.44 | 2.17 | 23.30 ± 0.39 | 1.66 | 22.65 ± 0.68 | 3.01 | |
| 2 | 23.29 ± 0.26 | 1.10 | 26.63 ± 0.31 | 1.15 | 25.70 ± 0.88 | 3.42 | |
| 0.2 | 27.15 ± 0.35 | 1.30 | 30.16 ± 0.28 | 0.93 | 29.68 ± 0.67 | 2.24 | |
| 0.02 | 30.89 ± 0.23 | 0.74 | 33.96 ± 0.61 | 1.81 | 33.38 ± 0.81 | 2.44 | |
| 0.002 | 33.45 ± 0.43 | 1.27 | 36.68 ± 0.92 | 2.50 | 35.77 ± 0.95 | 2.66 | |
| 0.0002 | 36.63 ± 0.57 | 1.54 | - | - | - | - | |
| Target | Multiplex | Slope | R2 | Efficiency |
|---|---|---|---|---|
| COL1A2 | Singleplex | −3.325 | 0.987 | 99.9% |
| KRT19 | Singleplex | −3.240 | 0.996 | 103.5% |
| ACTB | Singleplex | −3.453 | 0.999 | 94.8% |
| COL1A2 | Triplex | −3.043 | 0.957 | 113.1% |
| KRT19 | Triplex | −3.594 | 0.979 | 89.8% |
| ACTB | Triplex | −3.330 | 0.995 | 99.7% |
| Clinical and Pathological Features of Individuals Included in This Study (n = 60) | ||||
|---|---|---|---|---|
| Clinic Pathological Features | AC (n = 13) | CPs (n = 47) | ||
| Age Median (Range) | 41.46 (26–58) | 70.36 (46–90) | ||
| Gender | ||||
| Female | 9 (69.23%) | 24 (51.06%) | ||
| Male | 4 (30.77%) | 23 (48.94%) | ||
| Curative Resections | - | 47 (100%) | ||
| Clinical and Pathological Features of Patients with Tumor Process (n = 47) | ||||
| Colorectal Cancer (CRC) (n = 31) | Prostate Cancer (PC) (n = 3) | |||
| Age mean (range) | 71.45 (40–90) | Age mean (range) | 71.66 (64–86) | |
| Histological Type | Histological Type | |||
| Adenocarcinoma | 31 | Adenocarcinoma | 3 | |
| Clinical stage | Clinical stage | |||
| Tis */I/II | 20 | II | 2 | |
| III | 11 | III ** | 1 | |
| Renal Cancer (RC) (n = 6) | Bladder Cancer (BC) (n = 4) | |||
| Age mean (range) | 61.66 (44–79) | Age mean (range) | 72.00 (58–86) | |
| Histological Type | Histological Type | |||
| CCRCC | 6 | Urothelial | 4 | |
| Clinical stage | Clinical stage | |||
| I/II | 3 | 0a | 1 | |
| III | 3 | II | 3 | |
| Others (n = 3) | ||||
| Age | Sex | Histological Type | Clinical Stage | |
| Leiomyosarcoma | 74 | female | Renal leiomyosarcoma | III |
| Breast cancer | 74 | female | Ductal invasive | I |
| Penile cancer | 66 | male | Squamous | II |
| Groups | N | Amplification | Mean ± SD | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| COL1A2 | KRT19 | COL1A2 and KRT19 | COL1A2 | KRT19 | COL1A2 | KRT19 | ||
| AC | 13 | 12 (92.30%) | 13 (100%) | 12 (92.30%) | 1.21 ± 0.78 | 1.40 ± 1.25 | - | - |
| CP | 47 | 30 (63.83%) | 38 (80.85%) | 21 (44.68%) | 26.61 ± 45.46 | 9.69 ± 15.07 | 0.0001 | 0.0031 |
| CRC | 31 | 22 (70.96%) | 24 (77.42%) | 15 (48.39%) | 32.11 ± 51.92 | 12.06 ± 18.35 | 0.0001 | 0.0054 |
| RC | 6 | 3 (50%) | 5 (83.33%) | 1 (16.67%) | 9.82 ± 6.50 | 8.50 ± 4.75 | 0.0088 | 0.0008 |
| BC | 4 | 1 (25%) | 4 (100%) | 1 (25%) | nd | 6.74 ± 4.89 | nd | 0.1567 |
| PC | 3 | 1 (33.33%) | 3 (100%) | 1 (33.33%) | nd | 1.84 ± 1.71 | nd | 0.5054 |
| Others | 3 | 3 (100%) | 2 (66.67%) | 2 (66.67%) | 16.58 ± 17.14 | nd | 0.0044 | nd |
| Total | 60 | 42 (70%) | 51 (85%) | 33 (55%) | nd | nd | nd | nd |
| Sensitivity | 0.830 | (0.699–0.911) |
| Specificity | 0.846 | (0.578–0.957) |
| Positive Predictive Value | 0.951 | (0.839–0.986) |
| Negative Predictive Value | 0.579 | (0.363–0.769) |
| Diagnostic Accuracy | 0.833 | (0.720–0.907) |
| Positive Likelihood Ratio | 5.394 | (2.004–14.520) |
| Negative Likelihood Ratio | 0.201 | (0.152–0.265) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estévez Pérez, L.S.; Alén, B.O.; Otero Alén, M.; Hormaetxe, S.D.; Simón, L.; Concha, Á. Simultaneous Detection of Collagen I Alpha II and Cytokeratin 19 mRNA by Multiplex qPCR in Liquid Biopsy in Diagnosis of Patients with Resectable Solid Tumors. Int. J. Mol. Sci. 2024, 25, 9567. https://doi.org/10.3390/ijms25179567
Estévez Pérez LS, Alén BO, Otero Alén M, Hormaetxe SD, Simón L, Concha Á. Simultaneous Detection of Collagen I Alpha II and Cytokeratin 19 mRNA by Multiplex qPCR in Liquid Biopsy in Diagnosis of Patients with Resectable Solid Tumors. International Journal of Molecular Sciences. 2024; 25(17):9567. https://doi.org/10.3390/ijms25179567
Chicago/Turabian StyleEstévez Pérez, Lara Sofía, Begoña O. Alén, María Otero Alén, Saioa Domínguez Hormaetxe, Laureano Simón, and Ángel Concha. 2024. "Simultaneous Detection of Collagen I Alpha II and Cytokeratin 19 mRNA by Multiplex qPCR in Liquid Biopsy in Diagnosis of Patients with Resectable Solid Tumors" International Journal of Molecular Sciences 25, no. 17: 9567. https://doi.org/10.3390/ijms25179567






