Design, Synthesis, and Characterization of Novel Cannabidiol-Based Derivatives with Potent Antioxidant Activities
Abstract
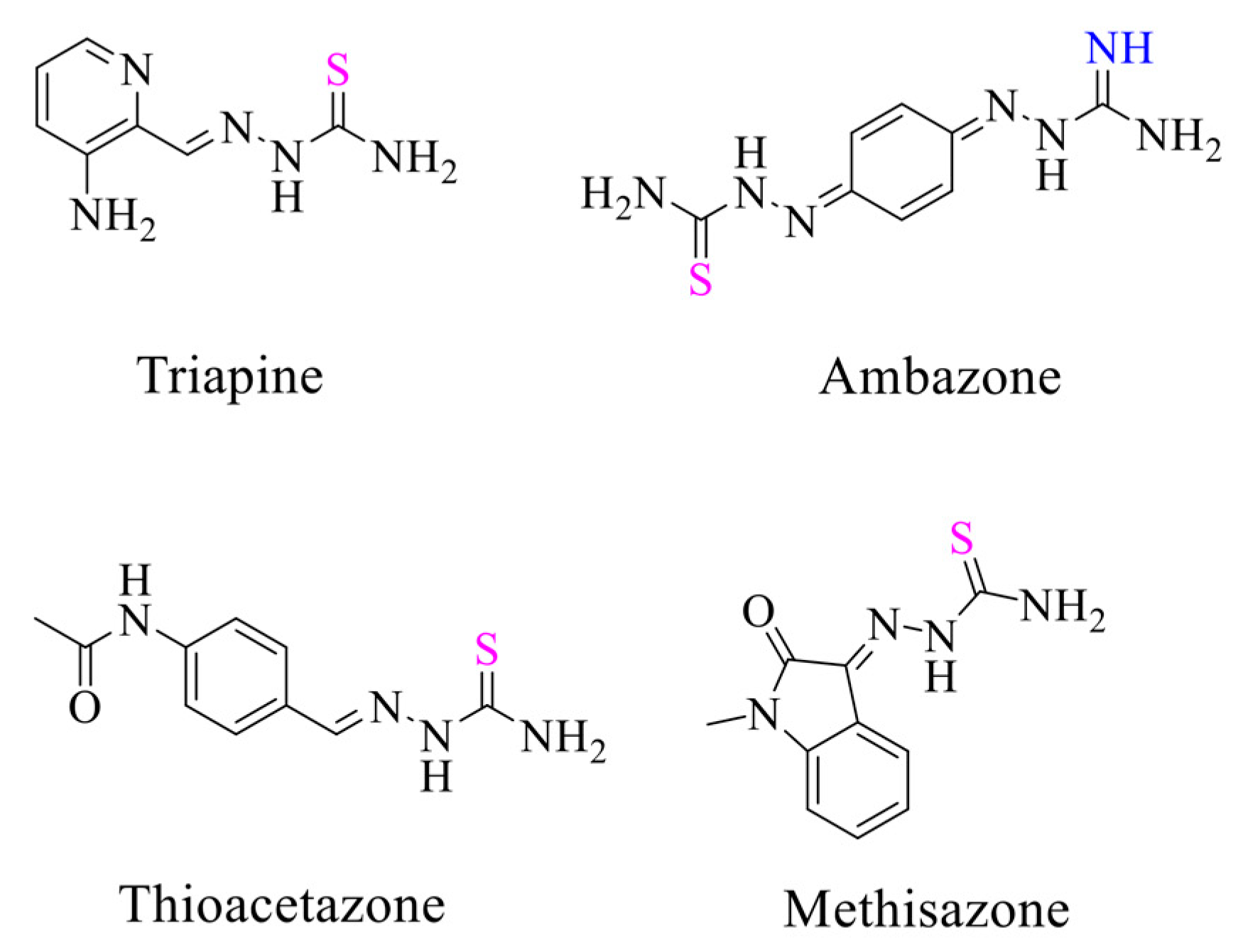
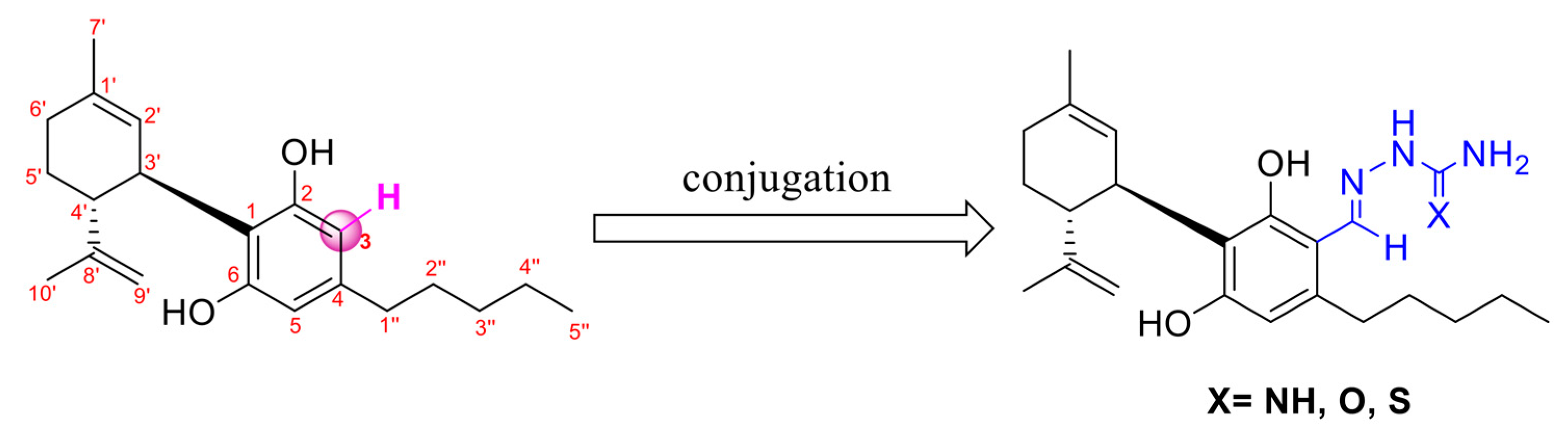
1. Introduction
2. Results
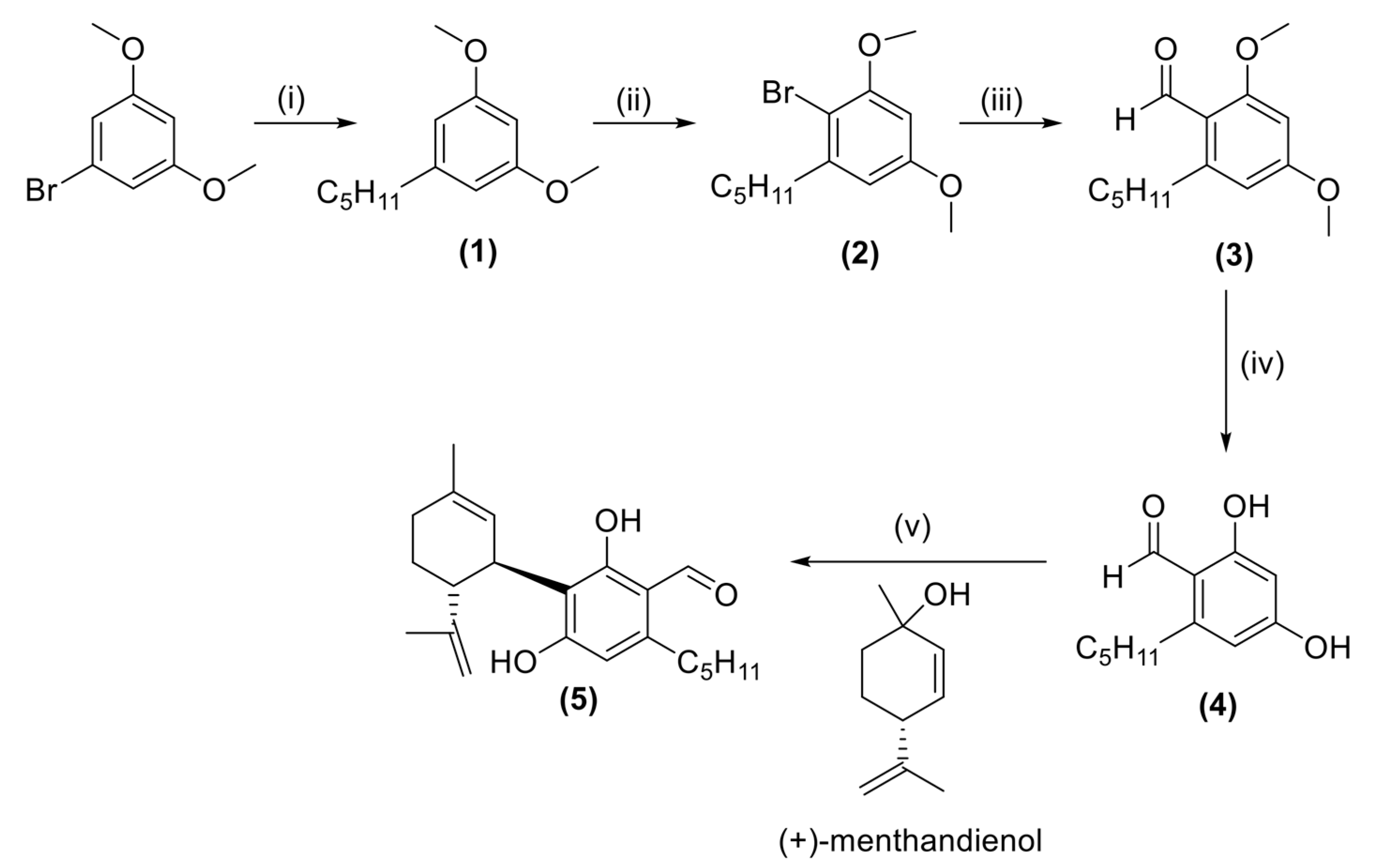
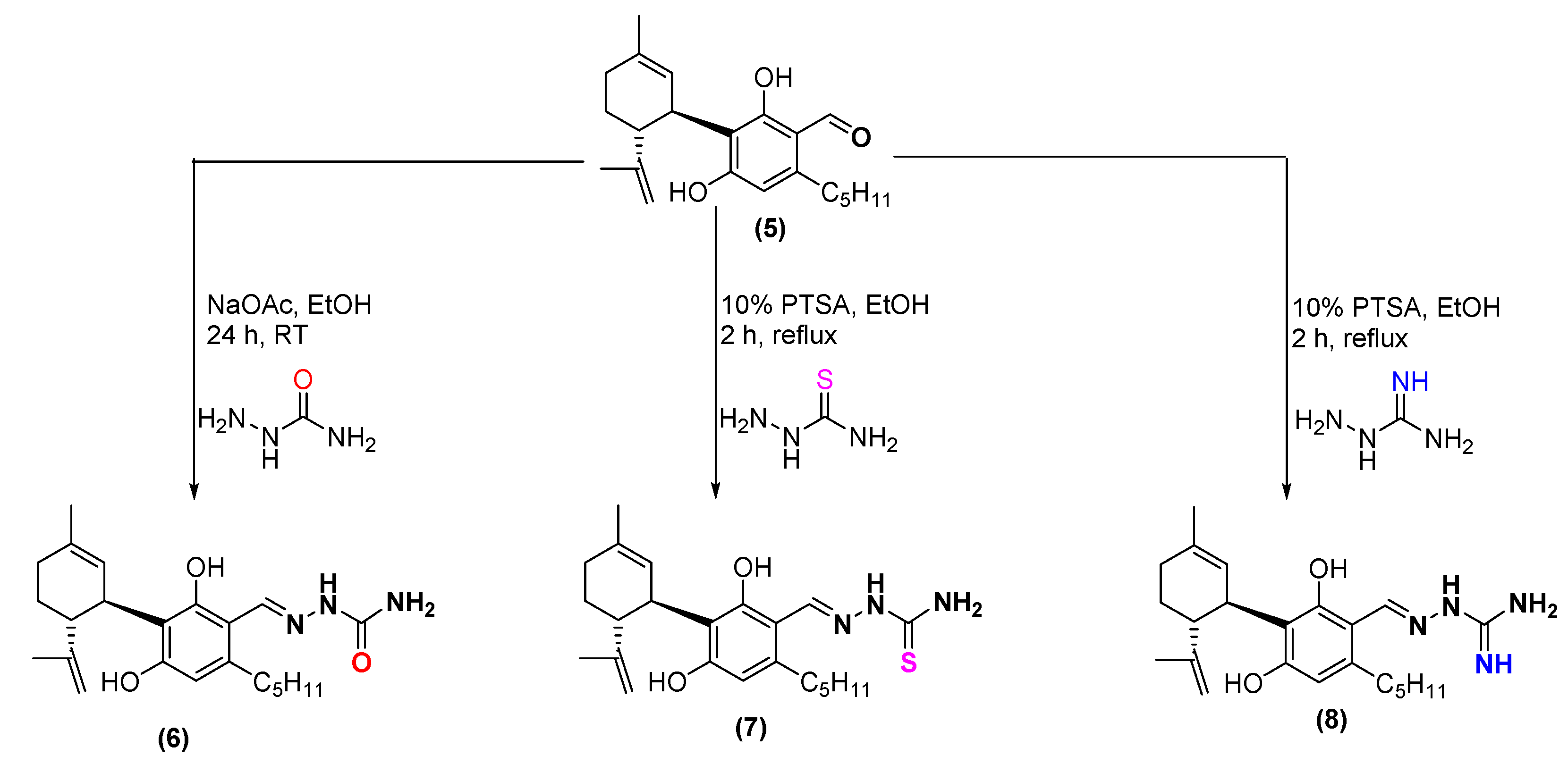
2.1. Synthesis
2.2. Antioxidant Activity by FRAP and DPPH Assays
2.3. Antioxidant Activity by LDL Oxidation
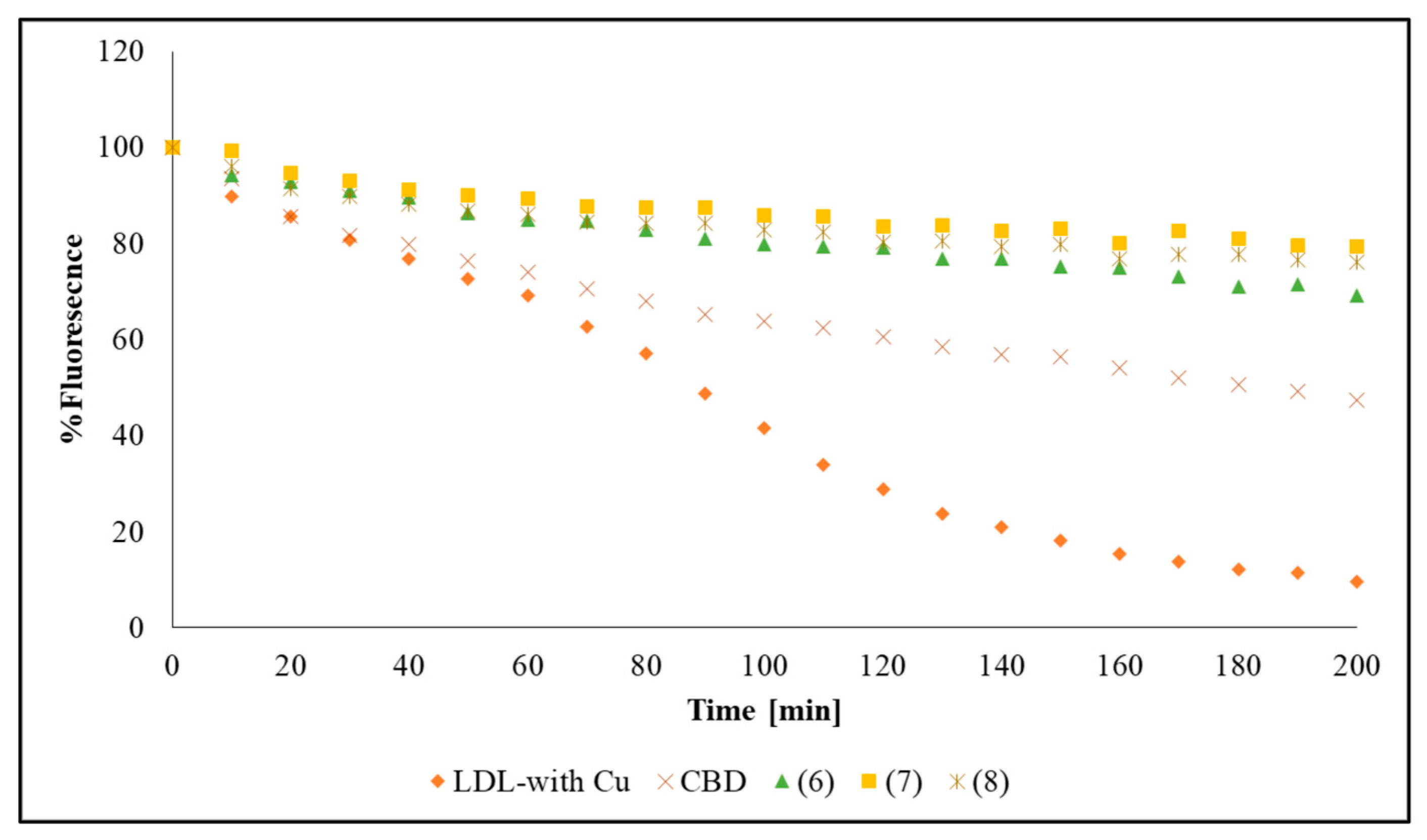
2.4. Effect of Synthesized Compounds on LDL Tryptophan Fluorescence
3. Discussion
4. Materials and Methods
4.1. General
4.2. Preparation of 1,3-Dimethoxy-5-pentylbenzene (1) [58]
4.3. Preparation of 2-Bromo-1,5-dimethoxy-3-pentylbenzene (2)
4.4. Preparation of 2,4-Dimethoxy-6-pentylbenzaldehyde (3)
4.5. Preparation of 2,4-Dihydroxy-6-pentylbenzaldehyde (4)
4.6. Synthesis of 3-Formyl Cannabidiol (CBD-Aldehyde) (5)
4.7. Synthesis of Aminoguanylhydrazone and (Thio)-Semicarbazone Cannabidiol-Aldehyde Derivatives
4.8. Free Radical 1,1-Diphenyl-2-picryl-hydrazyl (DPPH)-Scavenging Assay
4.9. Ferric-Reducing Antioxidant Power (FRAP) Assay
4.10. AAPH and Cu2+-Induced LDL Oxidation
4.11. Measurement of LDL-Tryptophan Fluorescence
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Rong, C.; Lee, Y.; Carmona, N.E.; Cha, D.S.; Ragguett, R.-M.; Rosenblat, J.D.; Mansur, R.B.; Ho, R.C.; McIntyre, R.S. Cannabidiol in medical marijuana: Research vistas and potential opportunities. Pharmacol. Res. 2017, 121, 213–218. [Google Scholar] [CrossRef]
- Shao, K.; Stewart, C.; Grant-Kels, J.M. Cannabis and the skin. Clin. Dermatol. 2021, 39, 784–795. [Google Scholar] [CrossRef]
- Nelson, K.M.; Bisson, J.; Singh, G.; Graham, J.G.; Chen, S.-N.; Friesen, J.B.; Dahlin, J.L.; Niemitz, M.; Walters, M.A.; Pauli, G.F. The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Borges, R.S.; da Silva, A.B.F. Chapter e12—Cannabidiol as an Antioxidant. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2017; p. 122. [Google Scholar]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. Biomed. Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Lolic, M.; Wink, D.; Rosenthal, R.; Axelrod, J. Neuroprotective Antioxidants from Marijuanaa. Ann. N. Y. Acad. Sci. 2000, 899, 274–282. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Lemmer, Y.; Mason, S. A narrative review of the therapeutic and remedial prospects of cannabidiol with emphasis on neurological and neuropsychiatric disorders. J. Cannabis Res. 2024, 6, 14. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Z.; Zuo, J.; Wu, C.; Zha, L.; Xu, Y.; Wang, S.; Shi, J.; Liu, X.-H.; Zhang, J.; et al. Novel cannabidiol−carbamate hybrids as selective BuChE inhibitors: Docking-based fragment reassembly for the development of potential therapeutic agents against Alzheimer’s disease. Eur. J. Med. Chem. 2021, 223, 113735. [Google Scholar] [CrossRef]
- Singla, S.; Sachdeva, R.; Mehta, J.L. Cannabinoids and atherosclerotic coronary heart disease. Clin. Cardiol. 2012, 35, 329–335. [Google Scholar] [CrossRef]
- Musetti, B.; González-Ramos, H.; González, M.; Bahnson, E.M.; Varela, J.; Thomson, L. Cannabis sativa extracts protect LDL from Cu2+-mediated oxidation. J. Cannabis Res. 2020, 2, 37. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Albertini, R.; Moratti, R.; Luca, D.G. Oxidation of Low-Density Lipoprotein in Atherosclerosis from Basic Biochemistry to Clinical Studies. Curr. Mol. Med. 2002, 2, 579–592. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; p. 1. [Google Scholar]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. Haemost. 2018, 16, 418–428. [Google Scholar] [CrossRef]
- Hashem, H.E.; El Bakri, Y. An overview on novel synthetic approaches and medicinal applications of benzimidazole compounds. Arab. J. Chem. 2021, 14, 103418. [Google Scholar] [CrossRef]
- Millimaci, A.M.; Trilles, R.V.; McNeely, J.H.; Brown, L.E.; Beeler, A.B.; Porco, J.A., Jr. Synthesis of Neocannabinoids Using Controlled Friedel–Crafts Reactions. J. Org. Chem. 2023, 88, 13135–13141. [Google Scholar] [CrossRef]
- Usami, N.; Okuda, T.; Yoshida, H.; Kimura, T.; Watanabe, K.; Yoshimura, H.; Yamamoto, I. Synthesis and pharmacological evaluation in mice of halogenated cannabidiol derivatives. Chem. Pharm. Bull. 1999, 47, 1641–1645. [Google Scholar] [CrossRef][Green Version]
- Prandi, C.; Blangetti, M.; Namdar, D.; Koltai, H. Structure-Activity Relationship of Cannabis Derived Compounds for the Treatment of Neuronal Activity-Related Diseases. Molecules 2018, 23, 1526. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Liu, Y.; Xu, Y.; Yang, B.; Li, H.; Chen, L. An overview on synthetic and biological activities of cannabidiol (CBD) and its derivatives. Bioorganic Chem. 2023, 140, 106810. [Google Scholar] [CrossRef]
- Kinney, W.A.; McDonnell, M.E.; Zhong, H.M.; Liu, C.; Yang, L.; Ling, W.; Qian, T.; Chen, Y.; Cai, Z.; Petkanas, D.; et al. Discovery of KLS-13019, a Cannabidiol-Derived Neuroprotective Agent, with Improved Potency, Safety, and Permeability. ACS Med. Chem. Lett. 2016, 7, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Shete, S.S.; Iqbal, F.; Bhardwaj, M.; Nandi, U.; Kumar, A.; Reddy, D.S. Sila-CBD Derivatives as Inhibitors of Heme-Induced NLRP3 Inflammasome: Application in Hemolytic Diseases. ACS Med. Chem. Lett. 2023, 14, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Lavi, Y.; Kogan, N.M.; Topping, L.M.; Liu, C.; McCann, F.E.; Williams, R.O.; Breuer, A.; Yekhtin, Z.; Ezra, A.F.; Gallily, R.; et al. Novel Synthesis of C-Methylated Phytocannabinoids Bearing Anti-inflammatory Properties. J. Med. Chem. 2023, 66, 5536–5549. [Google Scholar] [CrossRef] [PubMed]
- Kogan, N.M.; Peters, M.; Mechoulam, R. Cannabinoid Quinones—A Review and Novel Observations. Molecules 2021, 26, 1761. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gong, X.; Sun, H.; Wu, C.; Suo, J.; Ji, J.; Jiang, X.; Shen, J.; He, Y.; Aisa, H.A. Discovery of a CB2 and 5-HT1A receptor dual agonist for the treatment of depression and anxiety. Eur. J. Med. Chem. 2024, 265, 116048. [Google Scholar] [CrossRef]
- Lőrincz, E.B.; Tóth, G.; Spolárics, J.; Herczeg, M.; Hodek, J.; Zupkó, I.; Minorics, R.; Ádám, D.; Oláh, A.; Zouboulis, C.C.; et al. Mannich-type modifications of (−)-cannabidiol and (−)-cannabigerol leading to new, bioactive derivatives. Sci. Rep. 2023, 13, 19618. [Google Scholar] [CrossRef]
- Xu, H.; Su, X.; Liu, X.; Zhang, K.; Hou, Z.; Guo, C. Design, synthesis and biological evaluation of novel semicarbazone-selenochroman-4-ones hybrids as potent antifungal agents. Bioorganic Med. Chem. Lett. 2019, 29, 126726. [Google Scholar] [CrossRef]
- Heffeter, P.; Pape, V.F.S.; Enyedy, É.A.; Keppler, B.K.; Szakacs, G.; Kowol, C.R. Anticancer Thiosemicarbazones: Chemical Properties, Interaction with Iron Metabolism, and Resistance Development. Antioxid. Redox Signal. 2019, 30, 1062. [Google Scholar] [CrossRef]
- Alam, M.; Abser, M.N.; Kumer, A.; Bhuiyan, M.M.H.; Akter, P.; Hossain, M.E.; Chakma, U. Synthesis, characterization, antibacterial activity of thiosemicarbazones derivatives and their computational approaches: Quantum calculation, molecular docking, molecular dynamic, ADMET, QSAR. Heliyon 2023, 9, e16222. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A.; Fonseca-Berzal, C.; Martínez-Montiel, M.; Álvarez-Márquez, M.; Gómez-Núñez, M.; Lacueva-Arnedo, M.; Espinosa-Buitrago, T.; Martín-Pérez, T.; Escario, J.A.; Merino-Montiel, P.; et al. Thio- and selenosemicarbazones as antiprotozoal agents against Trypanosoma cruzi and Trichomonas vaginalis. J. Enzym. Inhib. Med. Chem. 2022, 37, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Behnisch, R.; Mietzsch, F.; Schmidt, H. Chemical Studies on Thiosemicarbazones with Particular Reference to Antituberculous Acitivity. Am. Rev. Tuberc. 1950, 61, 1–7. [Google Scholar]
- Grayson, M.L.; Cosgrove, S.; Crowe, S.; Hope, W.; McCarthy, J.; Mills, J.; Mouton, J.W.; Paterson, D. Kucers’ The Use of Antibiotics Sixth Edition: A Clinical Review of Antibacterial, Antifungal and Antiviral Drugs; CRC Press: Boca Raton, FL, USA, 2010; p. 1673. [Google Scholar]
- Sliva, K.; Schnierle, B. From actually toxic to highly specific—Novel drugs against poxviruses. Virol. J. 2007, 4, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kunos, C.A.; Ivy, S.P. Triapine Radiochemotherapy in Advanced Stage Cervical Cancer. Front. Oncol. 2018, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Löber, G.; Hoffmann, H. Ambazone as a membrane active antitumor drug. Biophys. Chem. 1990, 35, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef]
- Kim, T.K.; Kim, J.E.; Youn, U.J.; Han, S.J.; Kim, I.-C.; Cho, C.-G.; Yim, J.H. Total Syntheses of Lobaric Acid and Its Derivatives from the Antarctic Lichen Stereocaulon alpinum. J. Nat. Prod. 2018, 81, 1460–1467. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Yang, C.; Gu, Z.-W.; Yang, M.; Lin, S.-N.; Siuzdak, G.; Smith, C.V. Identification of Modified Tryptophan Residues in Apolipoprotein B-100 Derived from Copper Ion-Oxidized Low-Density Lipoprotein. Biochemistry 1999, 38, 15903–15908. [Google Scholar] [CrossRef]
- Gieβauf, A.; Steiner, E.; Esterbauer, H. Early destruction of tryptophan residues of apolipoprotein B is a vitamin E-independent process during copper-mediated oxidation of LDL. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1995, 1256, 221–232. [Google Scholar] [CrossRef]
- Fang, S.; Kang, W.-T.; Li, H.; Cai, Q.; Liang, W.; Zeng, M.; Yu, Q.; Zhong, R.; Tao, Y.; Liu, S.; et al. Development of cannabidiol derivatives as potent broad-spectrum antibacterial agents with membrane-disruptive mechanism. Eur. J. Med. Chem. 2024, 266, 116149. [Google Scholar] [CrossRef] [PubMed]
- Pirrung, M.C. Synthetic Access to Cannabidiol and Analogs as Active Pharmaceutical Ingredients. J. Med. Chem. 2020, 63, 12131–12136. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.; Pertwee, R.; Weller, A.; Tam, J.; Haj, C.; Smoum, R. Cannabidiolic Acid Esters Compositions and Uses Thereof. WIPO (PCT) Application No WO2018235079A1, 27 December 2018. p. 1. [Google Scholar]
- Lin, H.; Annamalai, T.; Bansod, P.; Tse-Dinh, Y.-C.; Sun, D. Synthesis and antibacterial evaluation of anziaic acid and its analogues as topoisomerase I inhibitors. MedChemComm 2013, 4, 1613–1618. [Google Scholar] [CrossRef]
- Le, N.A. Lipoprotein-associated oxidative stress: A new twist to the postprandial hypothesis. Int. J. Mol. Sci. 2014, 16, 401–419. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Printz, D.J.; Boyd, D.; Joy, L.; Steinberg, D. Macrophage oxidation of low density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis 1986, 6, 505–510. [Google Scholar] [CrossRef]
- Hong, C.G.; Florida, E.; Li, H.; Parel, P.M.; Mehta, N.N.; Sorokin, A.V. Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 1023651. [Google Scholar] [CrossRef]
- Reyftmann, J.-P.; Santus, R.; Mazière, J.-C.; Morlière, P.; Salmon, S.; Candide, C.; Mazière, C.; Haigle, J. Sensitivity of tryptophan and related compounds to oxidation induced by lipid autoperoxidation. Application to human serum low- and high-density lipoproteins. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1990, 1042, 159–167. [Google Scholar] [CrossRef]
- Smith, C.; Mitchinson, M.J.; Aruoma, O.I.; Halliwell, B. Stimulation of lipid peroxidation and hydroxyl-radical generation by the contents of human atherosclerotic lesions. Biochem. J. 1992, 286, 901–905. [Google Scholar] [CrossRef]
- Atkin, M.A.; Gasper, A.; Ullegaddi, R.; Powers, H.J. Oxidative Susceptibility of Unfractionated Serum or Plasma: Response to Antioxidants In Vitro and to Antioxidant Supplementation. Clin. Chem. 2005, 51, 2138–2144. [Google Scholar] [CrossRef][Green Version]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Siskos, M.; Gerothanassis, I.P. 1H-NMR as a Structural and Analytical Tool of Intra- and Intermolecular Hydrogen Bonds of Phenol-Containing Natural Products and Model Compounds. Molecules 2014, 19, 13643–13682. [Google Scholar] [CrossRef] [PubMed]
- Porte, A.L.; Gutowsky, H.S.; Hunsberger, I.M. The Determination of Double-bond Character in Cyclic Systems. V. Proton Chemical Shifts in Chelated Derivatives of Benzene. Naphthalene and Phenanthrene1,2. J. Am. Chem. Soc. 1960, 82, 5057–5063. [Google Scholar] [CrossRef]
- LDL/VLDL Purification Kit (Ultracentrifugation Free) Product Manual. Available online: https://www.cellbiolabs.com/sites/default/files/STA-606-ldl-vldl-purification-kit.pdf (accessed on 1 November 2022).
- Pauvert, Y.; Charette, A.B. Asymmetric Synthesis of (−)-Cannabidiol (CBD), (−)-Δ9-Tetrahydrocannabinol (Δ9-THC) and Their cis Analogs Using an Enantioselective Organocatalyzed Diels–Alder Reaction. Org. Lett. 2024, 26, 6081–6085. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-T.; Wu, J.-H.; Wang, S.-Y.; Kang, P.-L.; Yang, N.-S.; Shyur, L.-F. Antioxidant Activity of Extracts from Acacia confusa Bark and Heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Esterbauer, H.; Striegl, G.; Puhl, H.; Rotheneder, M. Continuous Monitoring of in Vztro Oxidation of Human Low Density Lipoprotein. Free Radic. Res. Commun. 1989, 6, 67–75. [Google Scholar] [CrossRef]



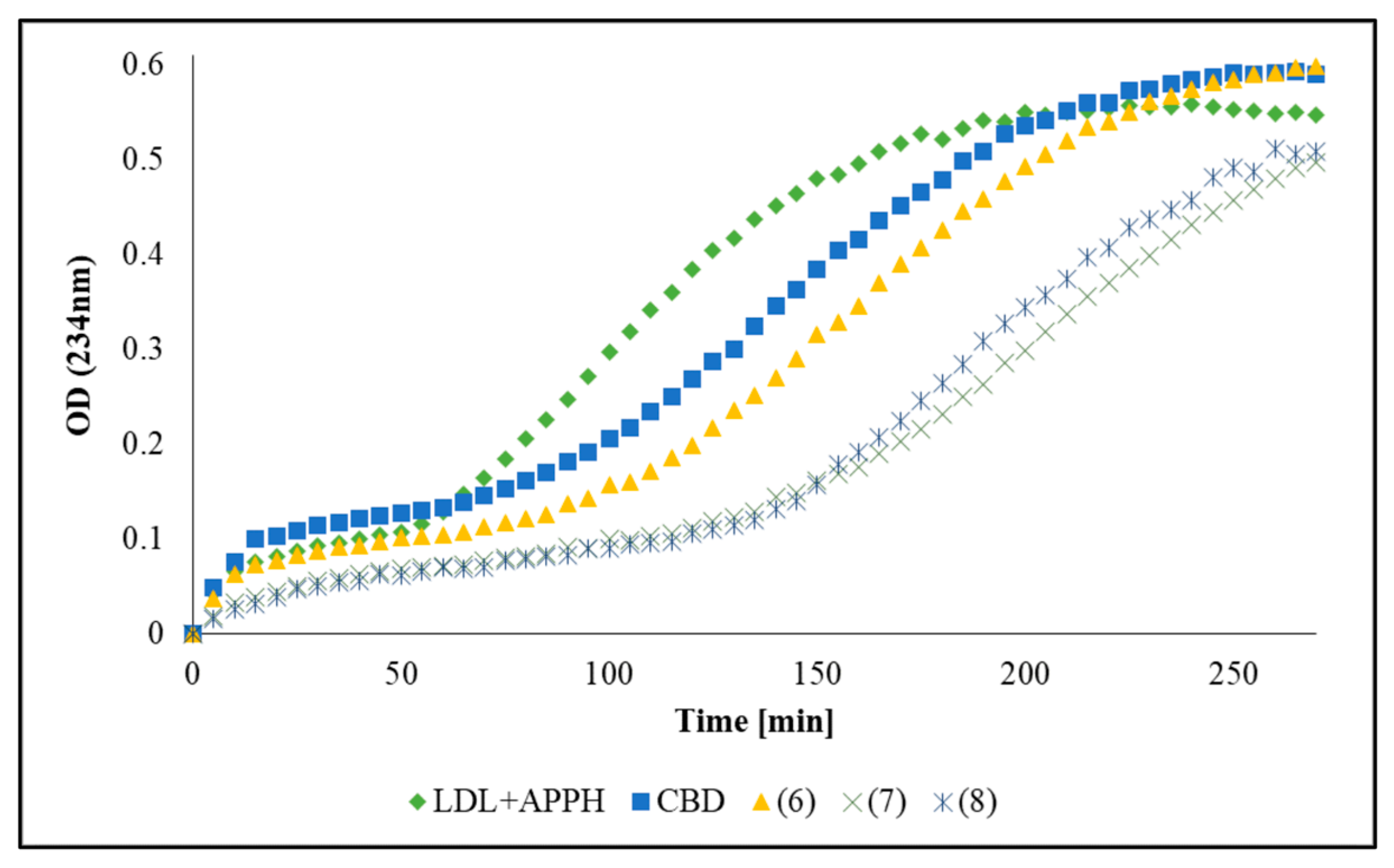
| Compound | Lag Time [min] ± SD | Oxidation Rate | %Inhibition |
|---|---|---|---|
| LDL | 22.76 ± 1.7 | 0.0081 | 0.0 |
| CBD | 55.81 ± 1.3 | 0.0052 | 36.07 |
| (5) | 24.52 ± 0.8 | 0.0080 | 1.64 |
| (6) | 80.17 ± 0.6 | 0.0053 | 35.25 |
| (7) | 107.84 ± 10.1 | 0.0058 | 29.30 |
| Compound | Lag Time [min] ± SD | Oxidation Rate | %Inhibition |
|---|---|---|---|
| LDL | 29.79 ± 2.2 | 0.0042 | 0.0 |
| CBD | 37.24 ± 3.7 | 0.0034 | 19.05 |
| (5) | 60.09 ± 1.8 | 0.0035 | 16.67 |
| (6) | 98.23 ± 6.4 | 0.0034 | 19.05 |
| (7) | 87.25 ± 5.3 | 0.0034 | 19.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peretz, E.; Musa, S. Design, Synthesis, and Characterization of Novel Cannabidiol-Based Derivatives with Potent Antioxidant Activities. Int. J. Mol. Sci. 2024, 25, 9579. https://doi.org/10.3390/ijms25179579
Peretz E, Musa S. Design, Synthesis, and Characterization of Novel Cannabidiol-Based Derivatives with Potent Antioxidant Activities. International Journal of Molecular Sciences. 2024; 25(17):9579. https://doi.org/10.3390/ijms25179579
Chicago/Turabian StylePeretz, Eliav, and Sanaa Musa. 2024. "Design, Synthesis, and Characterization of Novel Cannabidiol-Based Derivatives with Potent Antioxidant Activities" International Journal of Molecular Sciences 25, no. 17: 9579. https://doi.org/10.3390/ijms25179579
APA StylePeretz, E., & Musa, S. (2024). Design, Synthesis, and Characterization of Novel Cannabidiol-Based Derivatives with Potent Antioxidant Activities. International Journal of Molecular Sciences, 25(17), 9579. https://doi.org/10.3390/ijms25179579






