Hyperspectral Imaging Combined with Deep Transfer Learning to Evaluate Flavonoids Content in Ginkgo biloba Leaves
Abstract
:1. Introduction
2. Results
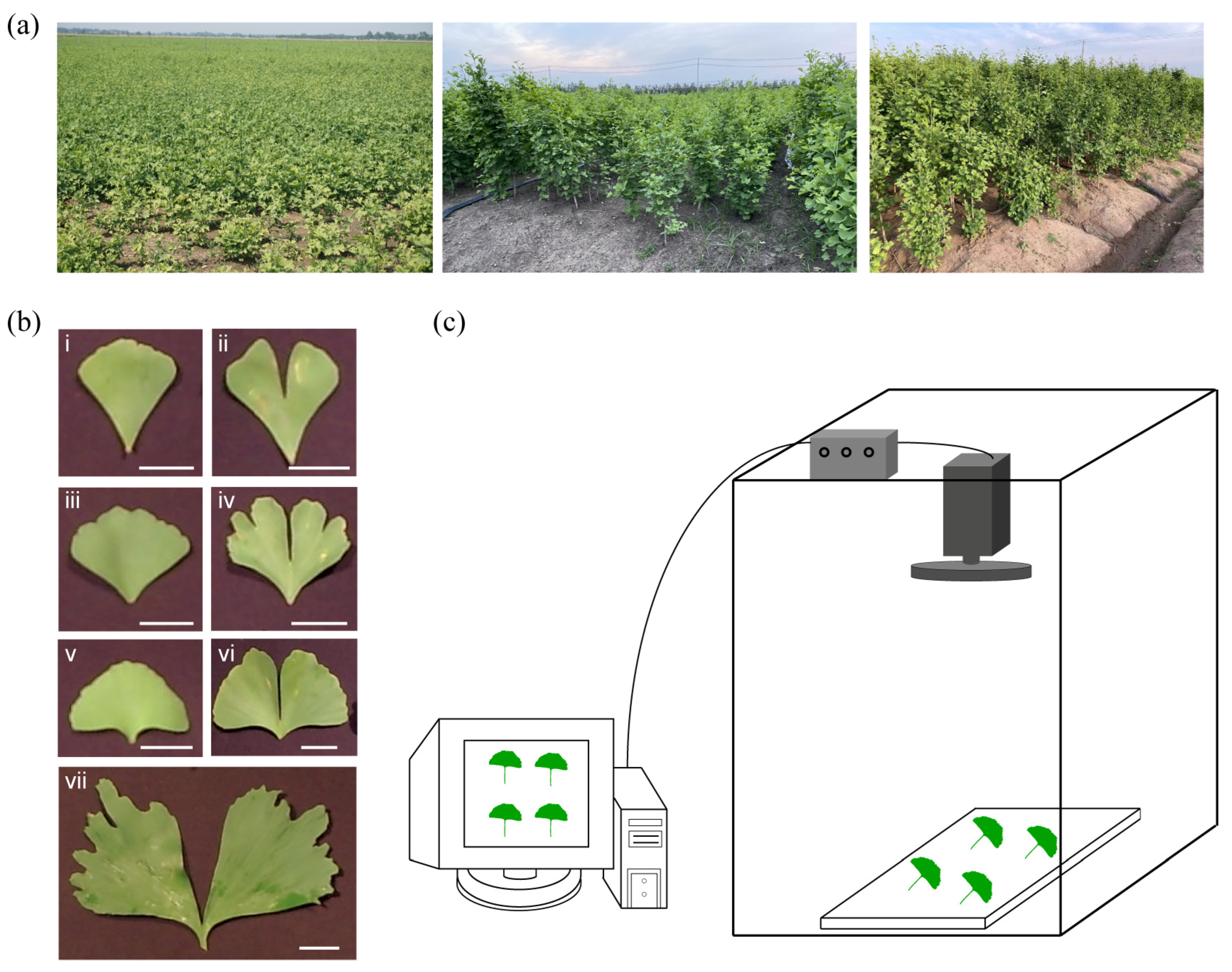
2.1. Differences in Ginkgo Leaf Shapes and Total Flavonoid Content
2.2. Flavonoid Composition Determination of Ginkgo Leaves and Sample Set for HIS
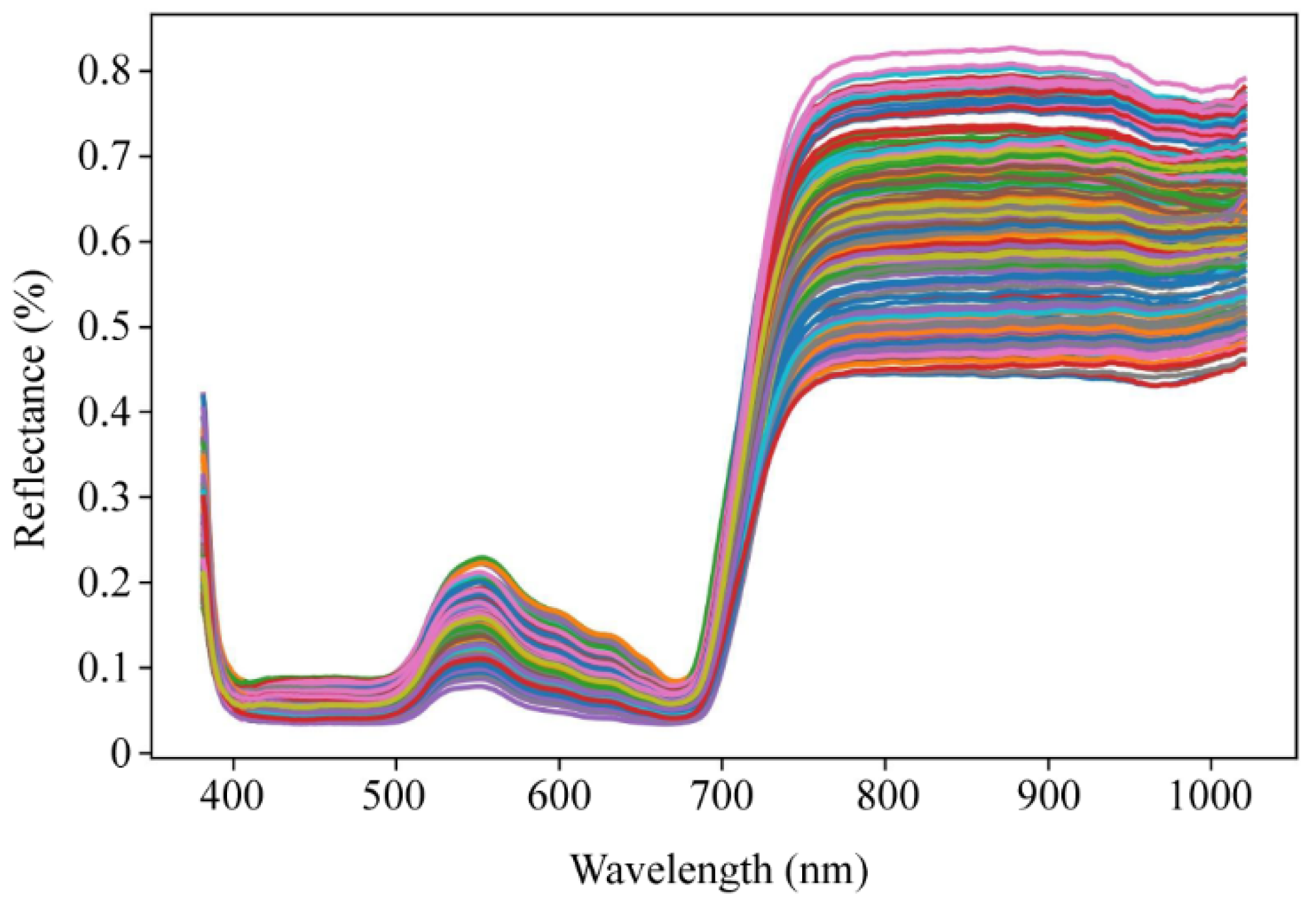
2.3. Spectra Characteristics of Ginkgo Leaves
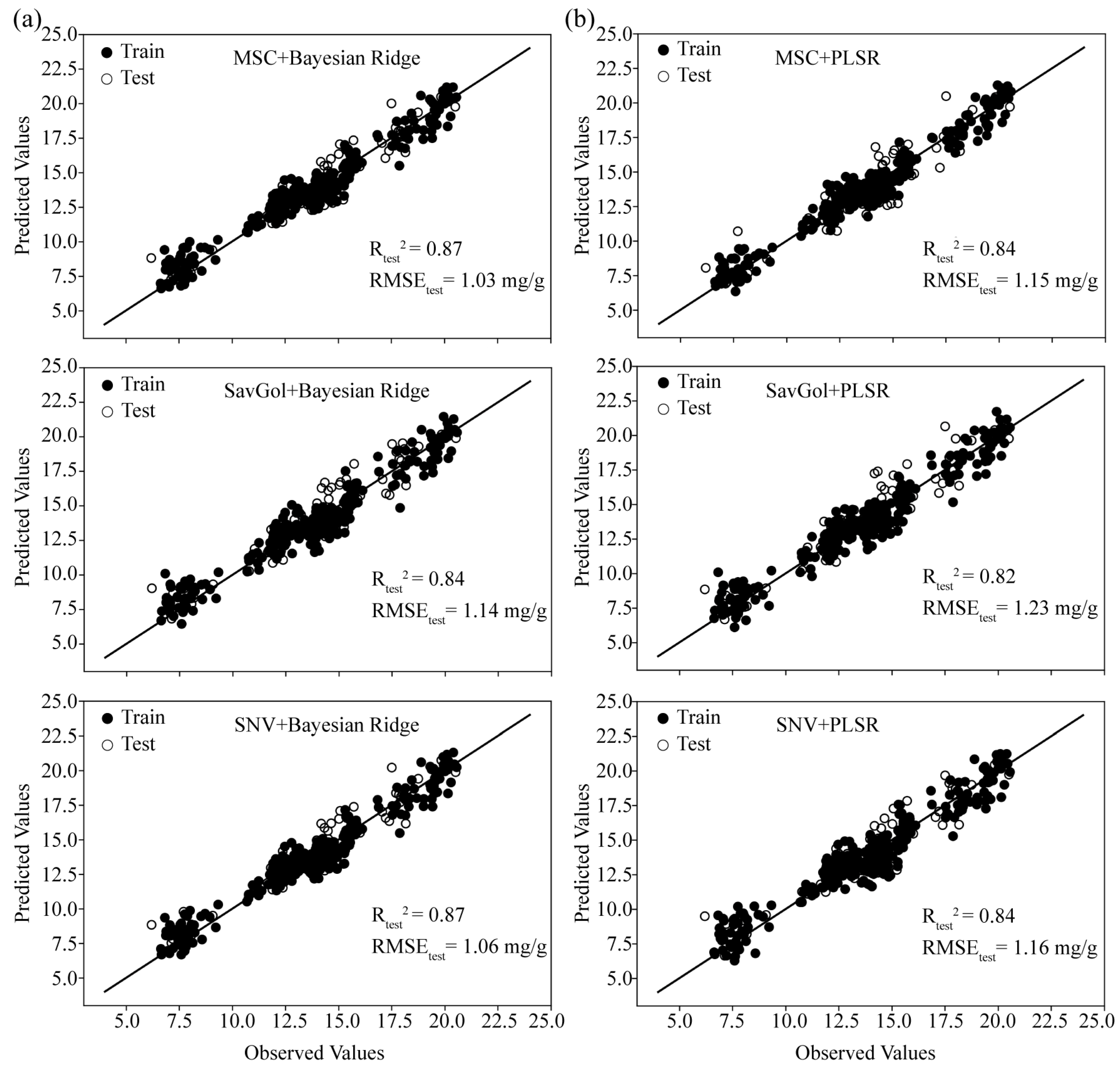
2.4. Analysis of Different Models
2.5. Effects of Different Pretreatments on Model Transfer
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Spectra Acquisition
4.3. Spectral Extraction
- Initially, a visible-light image was extracted from each hyperspectral image.
- From the visible light image, a gray-scale image was extracted, and an EGI (Enhanced Greenness Index) image was obtained through the 2G-R-B operation. Otsu’s algorithm was employed to automatically determine the threshold for segmenting the gray-scale image, yielding the gray mask (i.e., the locational region of the black cloth within the image). Similarly, Otsu’s algorithm was used to ascertain the threshold for segmenting the EGI image, resulting in the EGI mask (the locational region within the yellow-green color spectrum of the image). The intersection of these two locational regions was taken to obtain the mask for the leaf’s position.
- Based on the mask position information of the foreground, the hyperspectral image calculated the hyperspectral mean for each pixel point of the leaf, thus obtaining the spectral information for each leaf. This spectral information was then supplemented with corresponding flavonoid data to produce the final dataset used for modeling.
4.4. Measurements of Flavonoids Content
4.5. Spectral Preprocessing
- Average spectral data:
- Univariate linear operation:
- Corrected spectrum:
- Determine a window of fixed size (2m + 1), considering all data within this window as a collective set.
- For each measurement point x = [−m, 1 − m, …, −1, 0, 1, …, m], the following formula is employed for fitting:
- By calculating the least squares residuals between the fitted curve and the spectrum and setting them to the minimum as boundary conditions, the optimal coefficient matrix B can be obtained through the computation B = X(XTX)−1XT. The convolution of B with the sample spectrum is then performed to achieve SavGol filtering.
4.6. Model Establishment
4.7. Model Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Wang, X.; Wang, G.; Cui, P.; Wu, S.; Ai, C.; Hu, N.; Li, A.; He, B.; Shao, X.; et al. The nearly complete genome of Ginkgo biloba illuminates gymnosperm evolution. Nat. Plants 2021, 7, 748–756. [Google Scholar]
- Wu, D.; Feng, J.; Lai, M.; Ouyang, J.; Liao, D.; Yu, W.; Wang, G.; Cao, F.; Jacobs, D.; Zeng, S. Combined application of bud and leaf growth fertilizer improves leaf flavonoids yield of Ginkgo biloba. Ind. Crops Prod. 2020, 150, 112379. [Google Scholar]
- Lu, J.; Xu, Y.; Meng, Z.; Cao, M.; Liu, S.; Kato-Noguchi, H.; Yu, W.; Jin, B.; Wang, L. Integration of morphological, physiological and multi-omics analysis reveals the optimal planting density improving leaf yield and active compound accumulation in Ginkgo biloba. Ind. Crops Prod. 2021, 172, 114055. [Google Scholar]
- Xu, N.; Liu, S.; Lu, Z.; Pang, S.; Wang, L.; Wang, L.; Li, W. Gene expression profiles and flavonoid accumulation during salt stress in Ginkgo biloba seedlings. Plants 2020, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, L.; Pang, S.; Jia, Z.; Wang, L.; Li, W.; Jin, B. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2020, 151, 112483. [Google Scholar]
- Lu, J.; Tong, P.; Xu, Y.; Liu, S.; Jin, B.; Cao, F.; Wang, L. SA-responsive transcription factor GbMYB36 promotes flavonol accumulation in Ginkgo biloba. For. Res. 2023, 3, 19. [Google Scholar]
- van Beek, T.A.; Montoro, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [PubMed]
- Yao, X.; Shang, E.; Zhou, G.; Tang, Y.; Guo, S.; Su, S.; Jin, C.; Qiao, D.; Qin, Y.; Duan, J.A. Comparative characterization of total flavonol glycosides and terpene lactones at different ages, from different cultivation sources and genders of Ginkgo biloba leaves. Int. J. Mol. Sci. 2012, 13, 10305–10315. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, X.; Li, Y.; Gao, X.; Wang, J.; Qian, M.; Tong, X.; Wang, S.; Wang, Y.; Feng, J.; et al. Quality evaluation of ginkgo biloba leaves based on non-targeted metabolomics and representative ingredient quantification. J. Chromatogr. B 2023, 1214, 123549. [Google Scholar]
- Yu, W.; Liu, H.; Luo, J.; Zhang, S.; Xiang, P.; Wang, W.; Cai, J.; Lu, Z.; Zhou, Z.; Hu, J.; et al. Partial root-zone simulated drought induces greater flavonoid accumulation than full root-zone simulated water deficiency in the leaves of Ginkgo biloba. Environ. Exp. Bot. 2022, 201, 104998. [Google Scholar]
- Miao, S.F.; Yu, J.P.; Du, Z.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Supercritical fluid extraction and micronization of ginkgo flavonoids from ginkgo biloba leaves. Ind. Eng. Chem. Res. 2010, 49, 5461–5466. [Google Scholar]
- Wang, J.; Cao, F.; Su, E.; Wu, C.; Zhao, L.; Ying, R. Improving flavonoid extraction from Ginkgo biloba leaves by prefermentation processing. J. Agr. Food. Chem. 2013, 61, 5783–5791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, Y.; Mao, X.; Yu, W.; Lu, J.; Wang, L. Integration of morphological, physiological, cytological, metabolome and transcriptome analyses reveal age inhibited accumulation of flavonoid biosynthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2022, 187, 115405. [Google Scholar] [CrossRef]
- Tong, J.H.; Li, J.B.; Jiang, H.Y. Machine vision techniques for the evaluation of seedling quality based on leaf area. Biosyst. Eng. 2013, 115, 369–379. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Chen, Y.; Dai, S.; Li, X.; Kenji, I.; Lu, Z.; Li, M. Real-time monitoring of optimum timing for harvesting fresh tea leaves based on machine vision. Int. J. Agr. Biol. Eng. 2019, 12, 6–9. [Google Scholar] [CrossRef]
- Taghavi Namin, S.; Esmaeilzadeh, M.; Najafi, M.; Brown, T.B.; Borevitz, J.O. Deep phenotyping: Deep learning for temporal phenotype/genotype classification. Plant Methods 2018, 14, 66. [Google Scholar]
- Banerjee, B.P.; Joshi, S.; Thoday-Kennedy, E.; Pasam, R.K.; Tibbits, J.; Hayden, M.; Spangenberg, G.; Kant, S. High-throughput phenotyping using digital and hyperspectral imaging-derived biomarkers for genotypic nitrogen response. J. Exp. Bot. 2020, 71, 4604–4615. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Vergara-Díaz, O.; Gracia-Romero, A.; Rezzouk, F.Z.; Segarra, J.; Buchaillot, M.L.; Chang-Espino, M.; Vatter, T.; Sanchez-Bragado, R.; et al. Crop phenotyping in a context of global change: What to measure and how to do it. J. Integr. Plant Biol. 2022, 64, 592–618. [Google Scholar]
- Liu, Y.; Sun, Y.; Xie, A.; Yu, H.; Yin, Y.; Li, X.; Duan, X. Potential of hyperspectral imaging for rapid prediction of anthocyanin content of purple-fleshed sweet potato slices during drying process. Food Anal. Method. 2017, 10, 3836–3846. [Google Scholar]
- Bai, Y.; Xiong, Y.; Huang, J.; Zhou, J.; Zhang, B. Accurate prediction of soluble solid content of apples from multiple geographical regions by combining deep learning with spectral fingerprint features. Postharvest Biol. Technol. 2019, 156, 110943. [Google Scholar]
- Cui, Y.; Wu, J.; Chen, Y.; Ji, F.; Li, X.; Yang, J.; Hong, S.B.; Zhu, Z.; Zang, Y. Optimization of near-infrared reflectance models in determining flavonoid composition of okra (Abelmoschus esculentus L.) pods. Food Chem. 2023, 418, 135953. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, L.; Chu, B.; Zhang, C. Determination of total polysaccharides and total flavonoids in Chrysanthemum morifolium using near-infrared hyperspectral imaging and multivariate analysis. Molecules 2018, 23, 2395. [Google Scholar] [CrossRef]
- Abeysekera, S.K.; Robinson, A.; Ooi, M.P.L.; Kuang, Y.C.; Manley-Harris, M.; Holmes, W.; Hirst, E.; Nowak, J.; Caddie, M.; Steinhorn, G.; et al. Sparse reproducible machine learning for near infrared hyperspectral imaging: Estimating the tetrahydrocannabinolic acid concentration in Cannabis sativa L. Ind. Crops Prod. 2023, 192, 116137. [Google Scholar] [CrossRef]
- Ooi, M.P.L.; Robinson, A.; Manley-Harris, M.; Hill, S.; Raymond, L.; Kuang, Y.C.; Steinhorn, G.; Caddie, M.; Nowak, J.; Holmes, W.; et al. Robust statistical analysis to predict and estimate the concentration of the cannabidiolic acid in Cannabis sativa L.: A comparative study. Ind. Crops Prod. 2022, 189, 115744. [Google Scholar] [CrossRef]
- Xiao, Q.; Tang, W.; Zhang, C.; Zhou, L.; Feng, L.; Shen, J.; Yan, T.; Gao, P.; He, Y.; Wu, N. Spectral preprocessing combined with deep transfer learning to evaluate chlorophyll content in cotton leaves. Plant Phenomics 2022, 2022, 9813841. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, J.; Wang, Y. Recent trends of multi-source and non-destructive information for quality authentication of herbs and spices. Food Chem. 2023, 398, 133939. [Google Scholar]
- Li, L.; Jang, X.; Li, B.; Liu, Y. Wavelength selection method for near-infrared spectroscopy based on standard-sample calibration transfer of mango and apple. Comput. Electron. Agric. 2021, 190, 106448. [Google Scholar] [CrossRef]
- Phiri, D.; Morgenroth, J.; Xu, C.; Hermosilla, T. Effects of pre-processing methods on Landsat OLI-8 land cover classification using OBIA and random forests classifier. Int. J. Appl. Earth. Obs. 2018, 73, 170–178. [Google Scholar]
- Lu, Z.; Zhu, L.; Lu, J.; Shen, N.; Wang, L.; Liu, S.; Wang, Q.; Yu, W.; Kato-Noguchi, H.; Li, W. Rejuvenation increases leaf biomass and flavonoid accumulation in Ginkgo biloba. Hortic. Res. 2022, 9, uhab018. [Google Scholar]
- Corti, M.; Gallina, P.M.; Cavalli, D.; Cabassi, G. Hyperspectral imaging of spinach canopy under combined water and nitrogen stress to estimate biomass, water, and nitrogen content. Biosyst. Eng. 2017, 158, 38–50. [Google Scholar]
- Zhou, X.; Sun, J.; Tian, Y.; Lu, B.; Hang, Y.; Chen, Q. Hyperspectral technique combined with deep learning algorithm for detection of compound heavy metals in lettuce. Food Chem. 2020, 321, 126503. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, T.; Fu, F.F.; El-Kassaby, Y.A.; Wang, G. Temporospatial flavonoids metabolism variation in Ginkgo biloba leaves. Front. Genet. 2020, 11, 589326. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, A.; Yang, C.; de la Guardia, M.; Zhang, W.; Li, X.; He, Y. Proximal hyperspectral sensing of abiotic stresses in plants. Sci. Total Environ. 2023, 861, 160652. [Google Scholar] [PubMed]
- Li, X.; Jin, J.; Sun, C.; Ye, D.; Liu, Y. Simultaneous determination of six main types of lipid-soluble pigments in green tea by visible and near-infrared spectroscopy. Food Chem. 2018, 270, 236–242. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Zhou, L.; Cheng, H.; Ye, X.; He, Y. Developing deep learning based regression approaches for determination of chemical compositions in dry black goji berries (Lycium ruthenicum Murr.) using near-infrared hyperspectral imaging. Food Chem. 2020, 319, 126536. [Google Scholar] [CrossRef]
- Feng, L.; Wu, B.; He, Y.; Zhang, C. Hyperspectral imaging combined with deep transfer learning for rice disease detection. Front. Plant Sci. 2021, 12, 693521. [Google Scholar] [CrossRef]
- Conrad, A.O.; Li, W.; Lee, D.Y.; Wang, G.L.; Rodriguez-Saona, L.; Bonello, P. Machine learning-based presymptomatic detection of rice sheath blight using spectral profiles. Plant Phenomics 2020, 2020, 8954085. [Google Scholar]
- Vašát, R.; Kodešová, R.; Klement, A.; Borůvka, L. Simple but efficient signal pre-processing in soil organic carbon spectroscopic estimation. Geoderma 2017, 298, 46–53. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Li, P.; Zeng, F.; Wang, H. Hyperspectral detection of salted sea cucumber adulteration using different spectral preprocessing techniques and SVM method. LWT 2021, 152, 112295. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhou, G.; Liu, C.; Ji, Z.; Li, Z.; Li, W. Strategies for the content determination of capsaicin and the identification of adulterated pepper powder using a hand-held near-infrared spectrometer. Food Res. Int. 2023, 163, 112192. [Google Scholar]
- Khodabakhshian, R.; Lavasani, H.S.; Weller, P. A methodological approach to preprocessing FTIR spectra of adulterated sesame oil. Food Chem. 2023, 419, 136055. [Google Scholar] [CrossRef] [PubMed]
- Valkama, E.; Salminen, J.P.; Koricheva, J.; Pihlaja, K. Changes in leaf trichomes and epicuticular flavonoids during leaf development in three birch taxa. Ann. Bot. 2004, 94, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Valares Masa, C.; Sosa Díaz, T.; Alías Gallego, J.C.; Chaves Lobón, N. Quantitative variation of flavonoids and diterpenes in leaves and stems of Cistus ladanifer L. at different ages. Molecules 2016, 21, 275. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Park, M.Y.; Wang, L.J.; Koo, Y.; Chen, X.Y.; Weigel, D.; Poethig, R.S. miRNA control of vegetative phase change in trees. PLoS Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.P.; Tang, L.J.; Wu, H.L.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Ensemble preprocessing of near-infrared (NIR) spectra for multivariate calibration. Anal. Chim. Acta 2008, 616, 138–143. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Lister, S.J.; Barnes, R.J. On the scales associated with near-infrared reflectance difference spectra. Appl. Spectrosc. 1995, 49, 765–772. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Near infrared spectroscopy for determination of various physical, chemical and biochemical properties in Mediterranean soils. Soil Biol. Biochem. 2008, 40, 1923–1930. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Jiang, Y.; Jin, B.; Sun, C.; Wang, L. Hyperspectral Imaging Combined with Deep Transfer Learning to Evaluate Flavonoids Content in Ginkgo biloba Leaves. Int. J. Mol. Sci. 2024, 25, 9584. https://doi.org/10.3390/ijms25179584
Lu J, Jiang Y, Jin B, Sun C, Wang L. Hyperspectral Imaging Combined with Deep Transfer Learning to Evaluate Flavonoids Content in Ginkgo biloba Leaves. International Journal of Molecular Sciences. 2024; 25(17):9584. https://doi.org/10.3390/ijms25179584
Chicago/Turabian StyleLu, Jinkai, Yanbing Jiang, Biao Jin, Chengming Sun, and Li Wang. 2024. "Hyperspectral Imaging Combined with Deep Transfer Learning to Evaluate Flavonoids Content in Ginkgo biloba Leaves" International Journal of Molecular Sciences 25, no. 17: 9584. https://doi.org/10.3390/ijms25179584





