The Effect of Statins on Markers of Breast Cancer Proliferation and Apoptosis in Women with In Situ or Early-Stage Invasive Breast Cancer
Abstract
1. Introduction
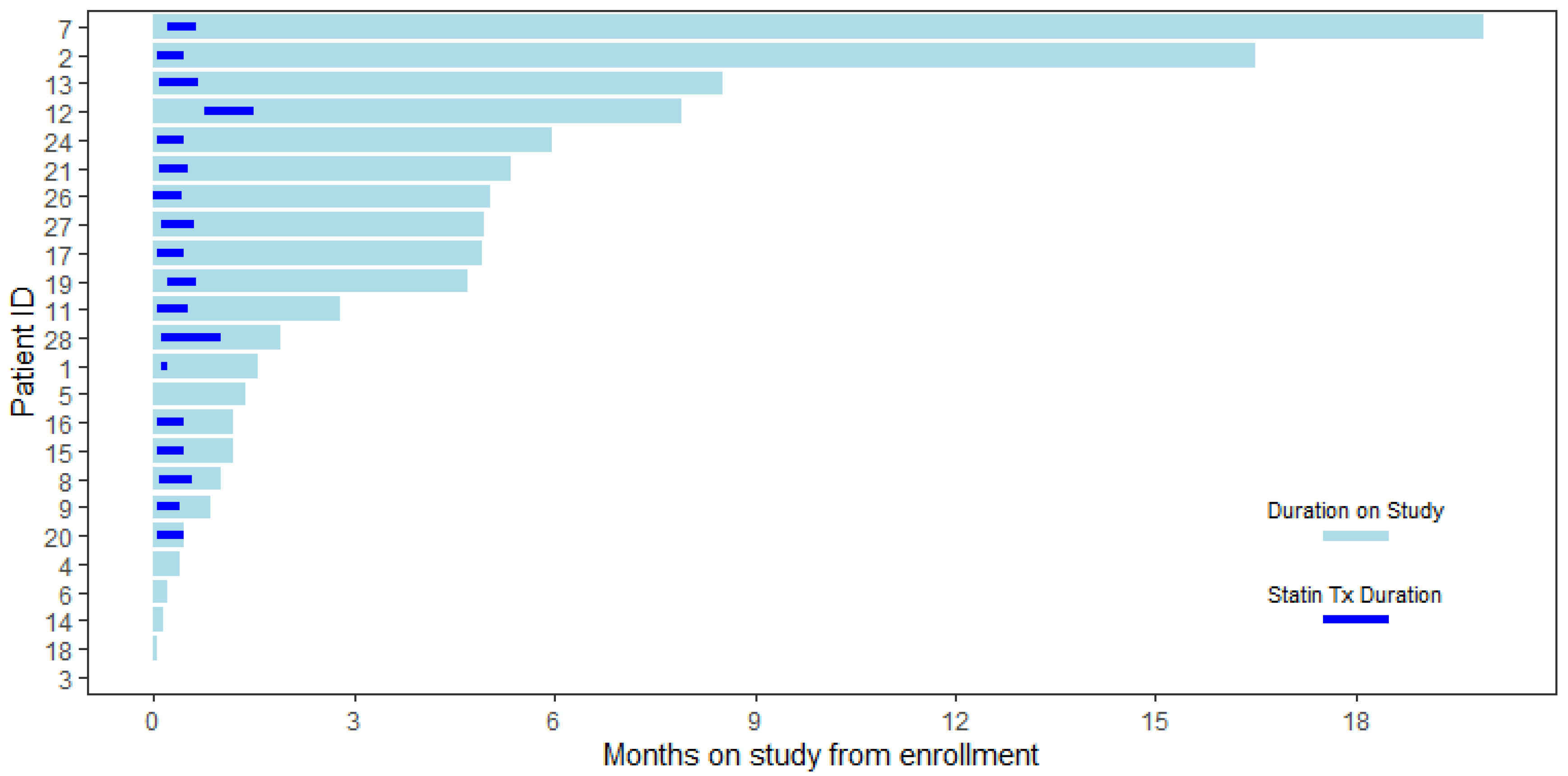
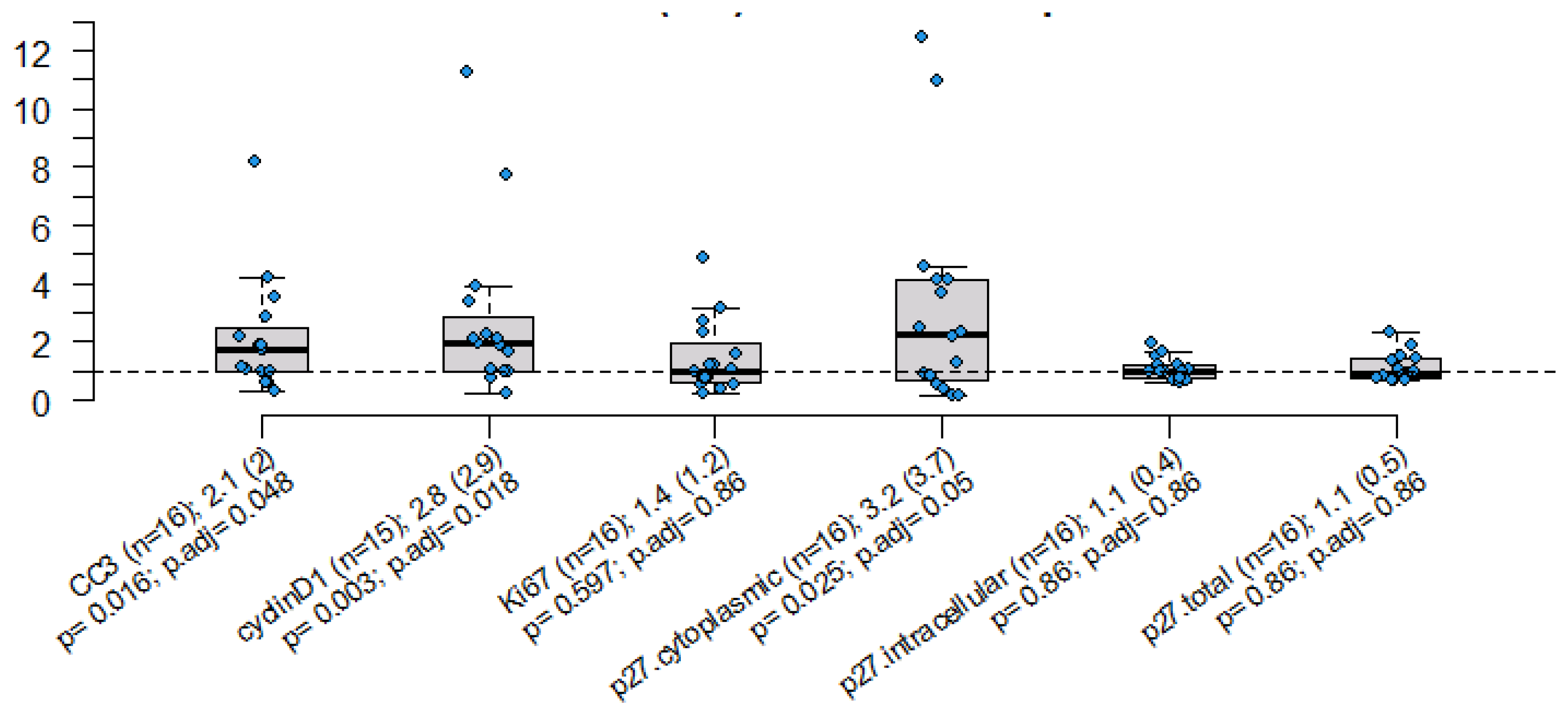
2. Results
3. Discussion
| Study/Reference | Type of Statin and Dose | Duration of Statin Treatment Prior to Surgery | Study Population | Marker of Proliferation | Marker of Apoptosis/Cell Cycle Arrest | Comments |
|---|---|---|---|---|---|---|
| Garwood et al., 2010 [41] | Fluvastatin 20 mg vs. 80 mg | 3–6 weeks | N = 40 DCIS, high and low-grade early-stage IDC (29 women had paired Ki-67 data) | Ki-67 7.2% decrease in high grade tumors | CC3 38% increase (60 vs. 13% in high vs. low-grade tumors) | Significant reduction in Ki-67 expression only among women with “high-grade”, ER-negative tumors No differences in Ki-67 level in the Garwood study based on fluvastatin dose |
| Bjarnadottir et al., 2013 [42] | Atorvastatin 80 mg | 2 weeks | N = 42 Early-stage IDC (26 women had paired Ki-67 data) | Ki-67 Decreased post-treatment (24% vs. 21.9%) | - | Significant reduction in Ki-67 expression only among women with tumors with baseline * HMGCoAR expression |
| Wang et al., 2015 [58] | Simvastatin 20 mg | 5–38 days | N = 15 Early-stage IDC | Ki-67 Decreased post vs. pre-treatment (57.7 ± 35.2 vs. 74.6 ± 59.9) | CC3 Increased post- vs. pre-treatment (23.4 ± 24.3 vs. 8.9 ± 7.4 | Change in Ki-67 not statistically significant |
| Feldt et al., 2015 [43] | Atorvastatin 80 mg | 2 weeks | N = 42 Early-stage IDC (30 and 33 women had paired cyclin D-1 and p27 data respectively) | Cyclin D-1 (cytoplasmic intensity) Decreased in 14/30, unchanged 13/30 and increased 3/30 post treatment | P27 (cytoplasmic intensity) Increased in 12/33, unchanged 18/33, decreased 3/33 | - |
4. Material and Methods
4.1. Trial Design
4.2. Participants
4.3. Study Procedures, Endpoints, and Biomarker Evaluation
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hebert, P.R.; Gaziano, J.M.; Chan, K.S.; Hennekens, C.H. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 1997, 278, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Beyond lipid lowering: The role of statins in vascular protection. Int. J. Cardiol. 2002, 86, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Malfitano, A.M.; Proto, M.C.; Esposito, I.; Gazzerro, P.; Formisano, P.; Pisanti, S.; Santoro, A.; Caruso, M.G.; Bifulco, M. Inhibition of 3-hydroxy-3-methylglutaryl- coenzyme A reductase activity and of Ras farnesylation mediate antitumor effects of anandamide in human breast cancer cells. Endocr. Relat. Cancer 2010, 17, 495–503. [Google Scholar] [CrossRef]
- Agarwal, B.; Halmos, B.; Feoktistov, A.S.; Protiva, P.; Ramey, W.G.; Chen, M.; Pothoulakis, C.; Lamont, J.; Holt, P.R. Mechanism of lovastatin-induced apoptosis in intestinal epithelial cells. Carcinogenesis 2002, 23, 521–528. [Google Scholar] [CrossRef]
- Liao, J.K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Investig. 2002, 110, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Aldieri, E.; Doublier, S.; Bosia, A.; Ghigo, D. Statins-mediated inhibition of rho GTPases as a potential tool in anti-tumor therapy. Mini Rev. Med. Chem. 2008, 8, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Wachtershauser, A.; Akoglu, B.; Stein, J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis 2001, 22, 1061–1067. [Google Scholar] [CrossRef]
- Duncan, R.E.; El-Sohemy, A.; Archer, M.C. Statins and the risk of cancer. JAMA 2006, 295, 2720–2722. [Google Scholar] [CrossRef]
- Kumar, A.S.; Campbell, M.; Benz, C.C.; Esserman, L.J. A call for clinical trials: Lipophilic statins may prove effective in treatment and prevention of particular breast cancer subtypes. J. Clin. Oncol. 2006, 24, 2127–2128. [Google Scholar] [CrossRef]
- Karp, I.; Behlouli, H.; LeLorier, J.; Pilote, L. Statins and cancer risk. Am. J. Med. 2008, 121, 302–309. [Google Scholar] [CrossRef]
- Cauley, J.A.; Zmuda, J.M.; Lui, L.-Y.; Hillier, T.A.; Ness, R.B.; Stone, K.L.; Cummings, S.R.; Bauer, D.C. Lipid-lowering drug use and breast cancer in older women: A prospective study. J. Womens Health 2003, 12, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; McTiernan, A.; Rodabough, R.J.; LaCroix, A.; Bauer, D.C.; Margolis, K.L.; Paskett, E.D.; Vitolins, M.Z.; Furberg, C.D.; Chlebowski, R.T. Statin use and breast cancer: Prospective results from the Women’s Health Initiative. J. Natl. Cancer Inst. 2006, 98, 700–707. [Google Scholar] [CrossRef]
- Pocobelli, G.; Newcomb, P.A.; Trentham-Dietz, A.; Titus-Ernstoff, L.; Hampton, J.M.; Egan, K.M. Statin use and risk of breast cancer. Cancer 2008, 112, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.S.; Rosenberg, C.A.; Rodabough, R.J.; Greenland, P.; Ockene, I.; Roy, H.K.; Lane, D.S.; Cauley, J.A.; Khandekar, J. Prospective analysis of association between use of statins or other lipid-lowering agents and colorectal cancer risk. Ann. Epidemiol. 2012, 22, 17–27. [Google Scholar] [CrossRef][Green Version]
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [CrossRef]
- Jagtap, D.; Rosenberg, C.A.; Martin, L.W.; Pettinger, M.; Khandekar, J.; Lane, D.; Ockene, I.; Simon, M.S. Prospective analysis of association between use of statins and melanoma risk in the Women’s Health Initiative. Cancer 2012, 118, 5124–5131. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Chlebowski, R.; Cauley, J.A.; Manson, J.E.; Wu, C.; Martin, L.W.; Jay, A.; Bock, C.; Cote, M.; Petrucelli, N.; et al. Prospective analysis of association between statin use and breast cancer risk in the women’s health initiative. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1868–1876. [Google Scholar] [CrossRef][Green Version]
- Bonovas, S.; Filioussi, K.; Tsavaris, N.; Sitaras, N.M. Use of statins and breast cancer: A meta-analysis of seven randomized clinical trials and nine observational studies. J. Clin. Oncol. 2005, 23, 8606–8612. [Google Scholar] [CrossRef]
- Kathiresan, S.; Melander, O.; Anevski, D.; Guiducci, C.; Burtt, N.P.; Roos, C.; Hirschhorn, J.N.; Berglund, G.; Hedblad, B.; Groop, L.; et al. Polymorphisms associated with cholesterol and risk of cardiovascular events. N. Engl. J. Med. 2008, 358, 1240–1249. [Google Scholar] [CrossRef]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar]
- Kumar, A.S.; Benz, C.C.; Shim, V.; Minami, C.A.; Moore, D.H.; Esserman, L.J. Estrogen receptor- negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1028–1033. [Google Scholar] [CrossRef]
- Desai, P.; Lehman, A.; Chlebowski, R.T.; Kwan, M.L.; Arun, M.; Manson, J.E.; Lavasani, S.; Wasswertheil-Smoller, S.; Sarto, G.E.; LeBoff, M.; et al. Statins and breast cancer stage and mortality in the Women’s Health Initiative. Cancer Causes Control. 2015, 26, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Valsecchi, M.E.; Kim, J.; Bianchi, A.L.; Khemasuwan, D.; Desai, A.; Tester, W. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Investig. 2011, 29, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Habel, L.A.; Flick, E.D.; Quesenberry, C.P.; Caan, B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res. Treat. 2008, 109, 573–579. [Google Scholar] [CrossRef]
- Ahern, T.P.; Pedersen, L.; Tarp, M.; Cronin-Fenton, D.P.; Garne, J.P.; Silliman, R.A.; Sørensen, H.T.; Lash, T.L. Statin prescriptions and breast cancer recurrence risk: A Danish nationwide prospective cohort study. J. Natl. Cancer Inst. 2011, 103, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Nickels, S.; Vrieling, A.; Seibold, P.; Heinz, J.; Obi, N.; Flesch-Janys, D.; Chang-Claude, J. Mortality and recurrence risk in relation to the use of lipid-lowering drugs in a prospective breast cancer patient cohort. PLoS ONE 2013, 8, e75088. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef]
- Duncan, R.E.; El-Sohemy, A.; Archer, M.C. Statins and cancer development. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1897–1898. [Google Scholar] [CrossRef]
- Crick, D.C.; Andres, D.A.; Danesi, R.; Macchia, M.; Waechter, C.J. Geranylgeraniol overcomes the block of cell proliferation by lovastatin in C6 glioma cells. J. Neurochem. 1998, 70, 2397–2405. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, J.S.; Kim, J.M.; Lee, J.Y.; Jung, H.C.; Song, I.S. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int. J. Cancer 2008, 123, 951–957. [Google Scholar] [CrossRef]
- Keyomarsi, K.; Sandoval, L.; Band, V.; Pardee, A.B. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991, 51, 3602–3609. [Google Scholar] [PubMed]
- Brown, A.J. Cholesterol, statins and cancer. Clin. Exp. Pharmacol. Physiol. 2007, 34, 135–141. [Google Scholar] [CrossRef]
- Hindler, K.; Cleeland, C.S.; Rivera, E.; Collard, C.D. The role of statins in cancer therapy. Oncologist 2006, 11, 306–315. [Google Scholar] [CrossRef]
- Campbell, M.J.; Esserman, L.J.; Zhou, Y.; Shoemaker, M.; Lobo, M.; Borman, E.; Baehner, F.; Kumar, A.S.; Adduci, K.; Marx, C.; et al. Breast cancer growth prevention by statins. Cancer Res. 2006, 66, 8707–8714. [Google Scholar] [CrossRef]
- Corcos, L.; Le Jossic-Corcos, C. Statins: Perspectives in cancer therapeutics. Dig. Liver Dis. 2013, 45, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar] [PubMed]
- Bjarnadottir, O.; Kimbung, S.; Johansson, I.; Veerla, S.; Jonsson, M.; Bendahl, P.O.; Grabau, D.; Hedenfalk, I.; Borgquist, S. Global Transcriptional Changes Following Statin Treatment in Breast Cancer. Clin. Cancer Res. 2015, 21, 3402–3411. [Google Scholar] [CrossRef]
- Spampanato, C.; DE Maria, S.; Sarnataro, M.; Giordano, E.; Zanfardino, M.; Baiano, S.; Cartenì, M.; Morelli, F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int. J. Oncol. 2012, 40, 935–941. [Google Scholar] [CrossRef]
- Garwood, E.R.; Kumar, A.S.; Baehner, F.L.; Moore, D.H.; Au, A.; Hylton, N.; Flowers, C.I.; Garber, J.; Lesnikoski, B.-A.; Hwang, E.S.; et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res. Treat. 2010, 119, 137–144. [Google Scholar] [CrossRef]
- Bjarnadottir, O.; Romero, Q.; Bendahl, P.O.; Jirström, K.; Rydén, L.; Loman, N.; Uhlén, M.; Johannesson, H.; Rose, C.; Grabau, D.; et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res. Treat. 2013, 138, 499–508. [Google Scholar] [CrossRef]
- Feldt, M.; Bjarnadottir, O.; Kimbung, S.; Jirström, K.; Bendahl, P.O.; Veerla, S.; Grabau, D.; Hedenfalk, I.; Borgquist, S. Statin-induced anti-proliferative effects via cyclin D1 and p27 in a window-of-opportunity breast cancer trial. J. Transl. Med. 2015, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, T.; Brown, Z.; Lovrics, O.; Yang, S.; Kazi, T.; Eskicioglu, C.; Parvez, E. Concurrent use of statins in patients undergoing curative intent treatment for triple negative breast Cancer: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2024, 24, e103–e115. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, S.; Crown, J.; Duffy, M.J. Statins inhibit proliferation and induce apoptosis in triple-negative breast cancer cells. Med. Oncol. 2022, 39, 142. [Google Scholar] [CrossRef] [PubMed]
- Barbalata, C.I.; Tefas, L.R.; Achim, M.; Tomuta, I.; Porfire, A.S. Statins in risk-reduction and treatment of cancer. World J. Clin. Oncol. 2020, 11, 573–588. [Google Scholar] [CrossRef]
- Kato, S.; Smalley, S.; Sadarangani, A.; Chen-Lin, K.; Oliva, B.; Brañes, J.; Carvajal, J.; Gejman, R.; Owen, G.I.; Cuello, M. Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase. J. Cell Mol. Med. 2010, 14, 1180–1193. [Google Scholar]
- Ahmadi, Y.; Karimian, R.; Panahi, Y. Effects of statins on the chemoresistance-The antagonistic drug-drug interactions versus the anti-cancer effects. Biomed. Pharmacother. 2018, 108, 1856–1865. [Google Scholar] [CrossRef]
- Beckwitt, C.H.; Brufsky, A.; Oltvai, Z.N.; Wells, A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Beckwitt, C.H.; Shiraha, K.; Wells, A. Lipophilic statins limit cancer cell growth and survival, via involvement of Akt signaling. PLoS ONE 2018, 13, e0197422. [Google Scholar] [CrossRef]
- Jiang, P.; Mukthavaram, R.; Chao, Y.; Nomura, N.; Bharati, I.S.; Fogal, V.; Pastorino, S.; Teng, D.; Cong, X.; Pingle, S.C.; et al. In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. Br. J. Cancer 2014, 111, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Luporsi, E.; André, F.; Spyratos, F.; Martin, P.-M.; Jacquemier, J.; Penault-Llorca, F.; Tubiana-Mathieu, N.; Sigal-Zafrani, B.; Arnould, L.; Gompel, A.; et al. Ki-67: Level of evidence and methodological considerations for its role in the clinical management of breast cancer: Analytical and critical review. Breast Cancer Res. Treat. 2012, 132, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.; Belloc, F.; Lacombe, F.; Dumain, P.; Reiffers, J.; Bernard, P.; Boisseau, M.R. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry 1991, 12, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kilickap, S.; Kaya, Y.; Yucel, B.; Tuncer, E.; Babacan, N.A.; Elagoz, S. Higher Ki67 expression is associates with unfavorable prognostic factors and shorter survival in breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. G1 cell-cycle control and cancer. Nature 2004, 432, 298–306. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Lee, C.S.; Buckley, M.F.; Sutherland, R.L. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc. Natl. Acad. Sci. USA 1994, 91, 8022–8026. [Google Scholar] [CrossRef]
- Jelínek, M.; Balušíková, K.; Schmiedlová, M.; Němcová-Fürstová, V.; Šrámek, J.; Stančíková, J.; Zanardi, I.; Ojima, I.; Kovář, J. The role of individual caspases in cell death induction by taxanes in breast cancer cells. Cancer Cell Int. 2015, 15, 8. [Google Scholar] [CrossRef]
- Wang, T.; Seah, S.; Loh, X.; Chan, C.; Hartman, M.; Goh, B.; Lee, S. Simvastatin-induced breast cancer cell death and deactivation of PI3K/Akt and MAPK/ERK signalling are reversed by metabolic products of the mevalonate pathway. Oncotarget 2016, 7, 2532–2544. [Google Scholar] [CrossRef]

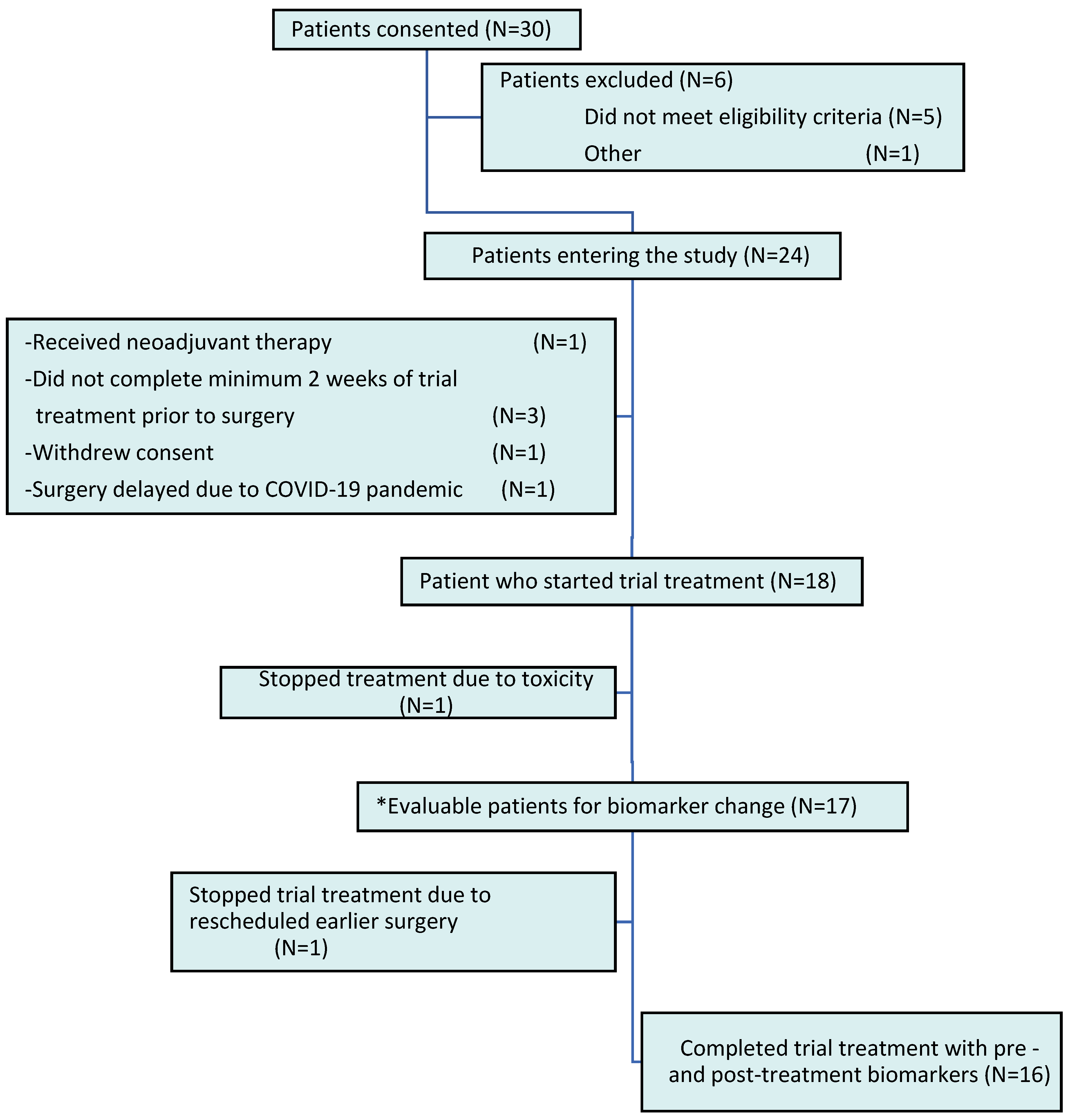
| Variable | All (N = 24) | Non-Evaluable (N = 7) | Evaluable (N = 17) | p-Value |
|---|---|---|---|---|
| AGE | 0.075 | |||
| Median (range) | 61 (42, 73) | 55 (42, 71) | 63 (48, 73) | |
| RACE | 0.126 | |||
| Black or African American | 5 (21%) | 3 (43%) | 2 (12%) | |
| White | 19 (79%) | 4 (57%) | 15 (88%) | |
| STAGE | 0.03 | |||
| DCIS (0) | 7 (29%) | 1 (14%) | 6 (35%) | |
| I | 14 (58%) | 3 (43%) | 11 (65%) | |
| II | 3 (12%) | 3 (43%) | 0 (0%) | |
| ER | 1 | |||
| Negative | 3 (12%) | 1 (14%) | 2 (12%) | |
| Positive | 21 (88%) | 6 (86%) | 15 (88%) | |
| PR | 0.625 | |||
| Negative | 7 (29%) | 1 (14%) | 6 (35%) | |
| Positive | 17 (71%) | 6 (86%) | 11 (65%) | |
| ⊥ HER2 | 0.38 | |||
| ** Negative | 17 (71%) | 5 (71%) | 12 (71%) | |
| Positive | 1 (4%) | 1 (14%) | 0 (0%) | |
| Unknown | 6 (25%) | 1 (14%) | 5 (29%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, A.; Boerner, J.; Assad, H.; Chen, W.; Simon, M.S. The Effect of Statins on Markers of Breast Cancer Proliferation and Apoptosis in Women with In Situ or Early-Stage Invasive Breast Cancer. Int. J. Mol. Sci. 2024, 25, 9587. https://doi.org/10.3390/ijms25179587
Kamal A, Boerner J, Assad H, Chen W, Simon MS. The Effect of Statins on Markers of Breast Cancer Proliferation and Apoptosis in Women with In Situ or Early-Stage Invasive Breast Cancer. International Journal of Molecular Sciences. 2024; 25(17):9587. https://doi.org/10.3390/ijms25179587
Chicago/Turabian StyleKamal, Anam, Julie Boerner, Hadeel Assad, Wei Chen, and Michael S. Simon. 2024. "The Effect of Statins on Markers of Breast Cancer Proliferation and Apoptosis in Women with In Situ or Early-Stage Invasive Breast Cancer" International Journal of Molecular Sciences 25, no. 17: 9587. https://doi.org/10.3390/ijms25179587
APA StyleKamal, A., Boerner, J., Assad, H., Chen, W., & Simon, M. S. (2024). The Effect of Statins on Markers of Breast Cancer Proliferation and Apoptosis in Women with In Situ or Early-Stage Invasive Breast Cancer. International Journal of Molecular Sciences, 25(17), 9587. https://doi.org/10.3390/ijms25179587







