Isolation and Characterization of Novel Pueroside B Isomers and Other Bioactive Compounds from Pueraria lobata Roots: Structure Elucidation, α-Glucosidase, and α-Amylase Inhibition Studies
Abstract
:1. Introduction
2. Results and Discussion
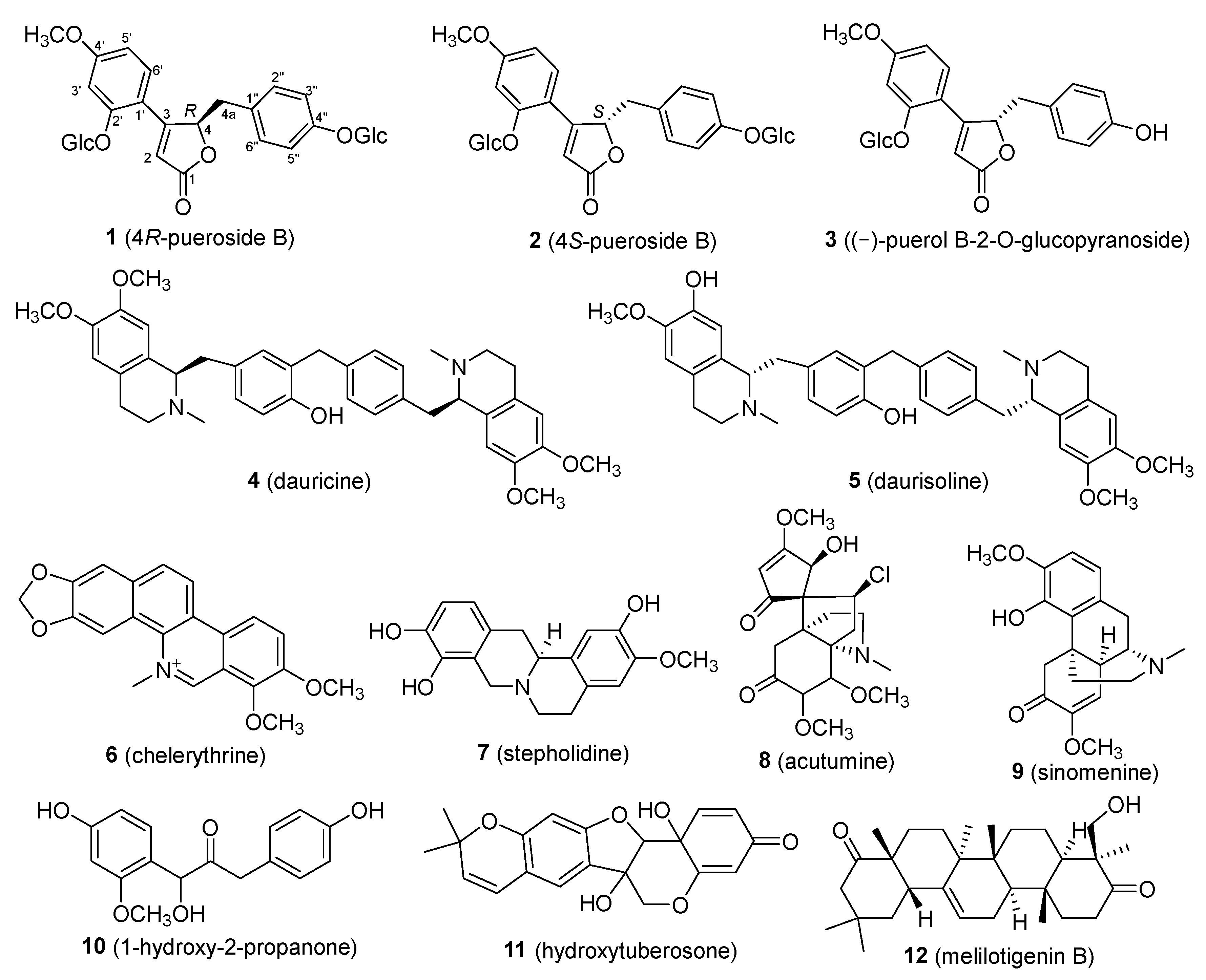
2.1. Isolation and Structural Identification of Chemical Constituents from P. lobata
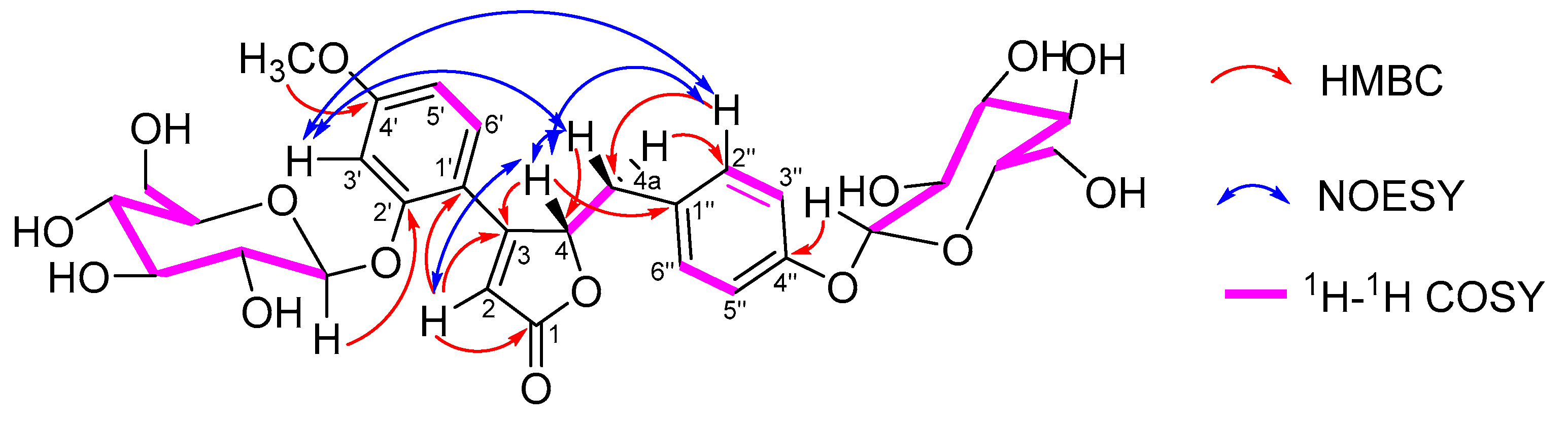
2.2. Isolation and Structural Elucidation of Compound 1
2.3. Mass Spectrometry Fragmentation Patterns of 4R-pueroside B and 4S-pueroside B
2.4. In Vitro Inhibition of α-Glucosidase and α-Amylase
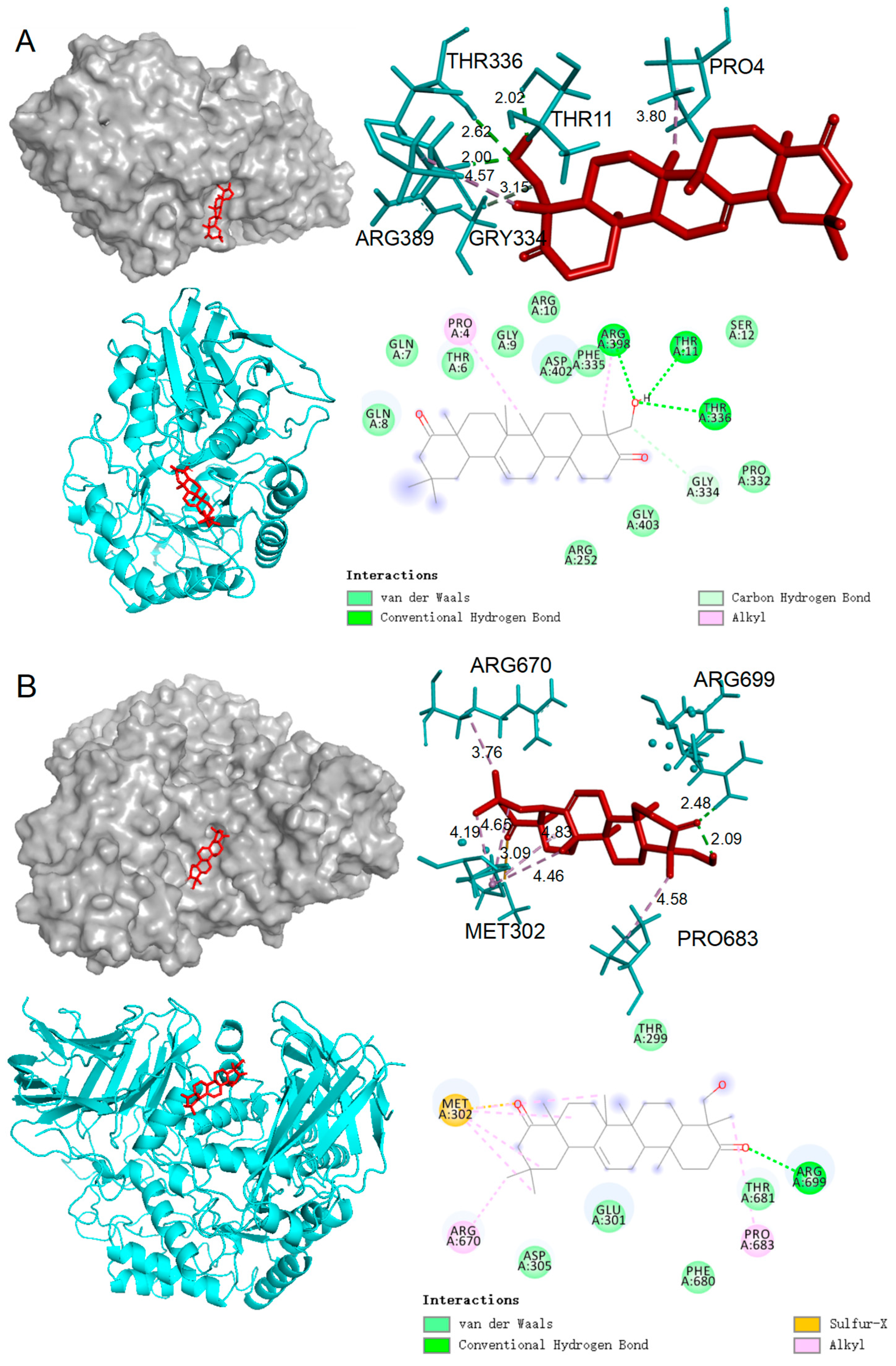
2.5. Molecular Docking Results
2.6. Comparison of Isolated Compounds and Their Activities with Existing Research on P. lobata
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Structure Identification of Compounds
3.5. LC-MS Analysis of Compounds 1 and 2
3.6. Spectral and Physical Data of Compounds
3.7. α-Glucosidase Inhibition Assay
3.8. α-Amylase Inhibition Assay
3.9. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China, 1st ed.; Science Press: Beijing, China, 1995; Volume 41, pp. 224–225. [Google Scholar]
- Wang, J.; Dai, G.; Shang, M.; Wang, Y.; Xia, C.; Duan, B.; Xu, L. Extraction, structural-activity relationships, bioactivities, and application prospects of Pueraria lobata polysaccharides as ingredients for functional products: A review. Int. J. Biol. Macromol. 2023, 243, 125210. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.R.; Pyun, B.J.; Jung, D.H.; Lee, I.S.; Kim, C.S.; Kim, Y.S.; Kim, J.S. Pueraria lobata Extract Protects Hydrogen Peroxide-Induced Human Retinal Pigment Epithelial Cells Death and Membrane Permeability. Evid.-Based Complement. Altern. Med. 2019, 2019, 5710289. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Comparing morphological, chemical and anti-diabetic characteristics of Puerariae lobatae Radix and Puerariae Thomsonii Radix. J. Ethnopharmacol. 2015, 164, 53–63. [Google Scholar] [CrossRef]
- He, H.; Peng, S.; Song, X.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Protective effect of isoflavones and triterpenoid saponins from Pueraria lobata on liver diseases: A review. Food Sci. Nutr. 2022, 10, 272–285. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Lu, H.; Lai, J.; He, Y.; Liu, S.; Guo, X. Pueraria lobata for diabetes mellitus: Past, present and future. Am. J. Chin. Med. 2019, 47, 1419–1444. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Li, Y.; Qiao, X.; Qian, Y.; Ye, M. Chemistry of the Chinese herbal medicine Puerariae Radix (Ge-Gen): A review. J. Chin. Pharm. Sci. 2014, 23, 347–360. [Google Scholar] [CrossRef]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavón, F.J.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernández-Llebrez, P.; Martínez, A.; Pérez-Valero, V.; et al. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- LiNa, G.; DanDan, W.; Yuan, P.; Rui, W.; XuePing, C.; HuiDong, T.; Rui, Z. Investigating main components and action mechanism of Pueraria lobata based on pharmacology network. Drug Eval. Res. 2019, 42, 1741–1748. [Google Scholar]
- Xuan, T.; Liu, Y.; Liu, R.; Liu, S.; Han, J.; Bai, X.; Wu, J.; Fan, R. Advances in Extraction, Purification, and Analysis Techniques of the Main Components of Kudzu Root: A Comprehensive Review. Molecules 2023, 28, 6577. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y. Glycosylation and methylation in the biosynthesis of isoflavonoids in Pueraria lobata. Front. Plant Sci. 2023, 14, 1330586. [Google Scholar] [CrossRef]
- Wang, Z.; Du, H.; Peng, W.; Yang, S.; Feng, Y.; Ouyang, H.; Zhu, W.; Liu, R. Efficacy and Mechanism of Pueraria lobata and Pueraria thomsonii Polysaccharides in the Treatment of Type 2 Diabetes. Nutrients 2022, 14, 3926. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Zhang, M.; Li, B.; Meng, X.; Xu, S.; Du, H.; Wang, X.; Xu, J.; Ke, H.; Cui, Y.; et al. Metabolomics of the anti-inflammatory effect of Pueraria lobata and Pueraria lobata var. Thomsonii in rats. J. Ethnopharmacol. 2023, 306, 116144. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Khan, S.; Liu, Y.; Xue, M.; Nabi, G.; Kumar, S.; Alshwmi, M.; Qarluq, A.W. Molecular mechanisms of anticancer activities of puerarin. Cancer Manag. Res. 2020, 12, 79–90. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Y.; Song, M.; Sun, Y.; Li, C.; Kang, W. Screening the Marker Components in Psoralea corylifolia L. with the Aids of Spectrum-Effect Relationship and Component Knock-Out by UPLC-MS/MS. Int. J. Mol. Sci. 2018, 19, 3439. [Google Scholar] [CrossRef] [PubMed]
- Serea, D.; Condurache, N.N.; Aprodu, I.; Constantin, O.E.; Bahrim, G.-E.; Stănciuc, N.; Stanciu, S.; Rapeanu, G. Thermal Stability and Inhibitory Action of Red Grape Skin Phytochemicals against Enzymes Associated with Metabolic Syndrome. Antioxidants 2022, 11, 118. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Kong, K.W.; Xiang, P.; He, X.; Zhang, X. Probiotic fermentation improves the bioactivities and bioaccessibility of polyphenols in Dendrobium officinale under in vitro simulated gastrointestinal digestion and fecal fermentation. Front. Nutr. 2022, 9, 1005912. [Google Scholar] [CrossRef]
- Seong, S.H.; Roy, A.; Jung, H.A.; Jung, H.J.; Choi, J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory activities of Pueraria lobata root and its constituents. J. Ethnopharmacol. 2016, 194, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Liu, D.; Zhou, H.; Li, Y.; Yuan, W.; Xu, S.; Wang, J.; Liang, X.; Weng, J. Profiling of Antidiabetic Bioactive Flavonoid Compounds from an Edible Plant Kudzu (Pueraria lobata). J. Agric. Food Chem. 2024, 72, 15704–15714. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, P.; Wen, W.; Qiu, H.; Chu, C.; Shao, Q.; Tong, S. Screening, characterization of trace α-glucosidase inhibitors from the root of Pueraria lobata and evaluation of their hypoglycemic activity. Food Biosci. 2023, 53, 102641. [Google Scholar] [CrossRef]
- Nohara, T.; Kinjo, J.; Frusawa, J.; Sakai, Y.; Inoue, M.; Shirataki, Y.; Shibashi, Y.; Yokoe, I.; Komatsu, M. But-2-enolides from Pueraria lobata and revised structures of puerosides A, B and sophoroside A. Phytochemistry 1993, 33, 1207–1210. [Google Scholar] [CrossRef]
- Sun, Y.J.; Cao, S.J.; Liang, F.N.; Li, J.Y.; Zhang, X.Y.; Li, W.; Ding, L.Q.; Qiu, F. Puerol and pueroside derivatives from Pueraria lobata and their anti-inflammatory activity. Phytochemistry 2023, 205, 113507. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.P.; Chen, Y.W.; Li, X.J.; Long, J.G. A new alkaloid of Menispermum dauricum DC-dauriciline. Acta Pharm. Sin. 1991, 26, 387–390. [Google Scholar]
- Gong, T.; Wu, Z.Y. Pharmacological effects of daurisoline methyl bromide, a preliminary report. Acta Pharm. Sin. 1979, 14, 439–442. [Google Scholar]
- Liu, Y.; Li, J.; Wang, D.; Xie, F.; Guo, Z. Alkaloids from the stem of Macleaya cordata against Exserohilum turcicum. Nat. Prod. Res. Dev. 2021, 33, 977. [Google Scholar]
- El-Kawi, M.A.; Slatkin, D.J.; Schiff, P.L., Jr.; Dasgupta, S.; Chattopadhyay, S.K.; Ray, A.B. Additional alkaloids of Pachygone ovata. J. Nat. Prod. 1984, 47, 459–464. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Inanaga, S.; Kato, M.; Shimizu, T.; Hakoshima, T.; Isogai, A. Dechloroacutumine from cultured roots of Menispermum dauricum. Phytochemistry 1998, 49, 1293–1297. [Google Scholar] [CrossRef]
- Jin, J.; Xu, R.; Wu, X.; Fang, X.; Kong, W.; Zhang, K.; Cheng, J. Design and synthesis of sinomenine D-ring tetrazole-isoxazole and tetrazole-triazole derivatives via 1,3-dipolar cycloaddition reaction. Tetrahedron 2023, 132, 133261. [Google Scholar] [CrossRef]
- Bezuidenhout, S.C.; Bezuidenhoudt, B.C.; Ferreira, D. α-Hydroxydihydrochalcones and related 1,3-diarylpropan-2-ones from Xanthocercis zambesiaca. Phytochemistry 1988, 27, 2329–2334. [Google Scholar] [CrossRef]
- Prasad, A.V.K.; Singh, A.; Kapil, R.S.; Popli, S.P. Studies in medicinal plants. Part XIII. Hydroxytuberosone a novel pterocarpanone from Pueraria tuberosa DC. Indian J. Chem. 1984, 23B, 1165–1167. [Google Scholar]
- Macías, F.A.; Simonet, A.M.; Galindo, J.C.G.; Pacheco, P.C.; Sánchez, J.A. Bioactive polar triterpenoids from Melilotus messanensis. Phytochemistry 1998, 49, 709–717. [Google Scholar] [CrossRef]
- Fan, M.; Quan, H.; Shao, F.; Meng, X.; Zhu, W.; Zhang, P.; Liu, R. A New Pueroside from Pueraria lobata. Chem. Nat. Compd. 2022, 58, 604–606. [Google Scholar] [CrossRef]
- Sohretoglu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Visvanathan, R.; Houghton, M.J.; Barber, E.; Williamson, G. Structure-function relationships in (poly)phenol-enzyme binding: Direct inhibition of human salivary and pancreatic alpha-amylases. Food Res. Int. 2024, 188, 114504. [Google Scholar] [CrossRef]
- He, W.; Xu, Y.; Wu, D.; Wang, D.; Gao, H.; Wang, L.; Zhu, W. New alkaloids from the diversity-enhanced extracts of an endophytic fungus Aspergillus flavus GZWMJZ-288. Bioorg. Chem. 2021, 107, 104623. [Google Scholar] [CrossRef]
- García-Chacón, J.M.; Tello, E.; Coy-Barrera, E.; Peterson, D.G.; Osorio, C. Mono-n-butyl malate-derived compounds from camu-camu (Myrciaria dubia) malic acid: The alkyl-dependent antihyperglycemic-related activity. ACS Omega 2022, 7, 39335–39346. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Pham, Q.T.; Luong, T.N.H.; Le, H.K.; Vo, V.G. Potential Antidiabetic Activity of Extracts and Isolated Compound from Adenosma bracteosum (Bonati). Biomolecules 2020, 10, 201. [Google Scholar] [CrossRef]
- Li, G.; Fang, Y.; Ma, Y.; Dawa, Y.; Wang, Q.; Gan, J.; Dang, J. Screening and Isolation of Potential Anti-Inflammatory Compounds from Saxifraga atrata via Affinity Ultrafiltration-HPLC and Multi-Target Molecular Docking Analyses. Nutrients 2022, 14, 2405. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.; Zhou, H.; Shao, J.; Ji, W.; Wang, Z.; Liang, D.; Zhao, C. Lignan constituents with α-amylase and α-glucosidase inhibitory activities from the fruits of Viburnum urceolatum. Phytochemistry 2023, 216, 113895. [Google Scholar] [CrossRef]
- Hendra, R.; Army, M.K.; Frimayanti, N.; Teruna, H.Y.; Abdulah, R.; Nugraha, A.S. α-glucosidase and α-amylase inhibitory activity of flavonols from Stenochlaena palustris (Burm. f.) Bedd. Saudi Pharm. J. 2024, 32, 101940. [Google Scholar] [CrossRef]
- Bautista-Aguilera, Ó.M.; Ismaili, L.; Chioua, M.; Andrys, R.; Schmidt, M.; Bzonek, P.; Martínez-Grau, M.Á.; Beadle, C.D.; Vetman, T.; López-Muñoz, F.; et al. Acetylcholinesterase Inhibition of Diversely Functionalized Quinolinones for Alzheimer’s Disease Therapy. Int. J. Mol. Sci. 2020, 21, 3913. [Google Scholar] [CrossRef]


| No. | δH (ppm, J in Hz) | δC (ppm), Type |
|---|---|---|
| 1 | - | 172.9, C |
| 2 | 6.47 (br s) | 114.8, CH |
| 3 | - | 163.7, C |
| 4 | 6.02 (m) | 82.1, CH |
| 4a | 2.71 (dd, 14.7, 6.5) | 38.1, CH2 |
| 3.15 (m) | ||
| 1′ | - | 112.0, C |
| 2′ | - | 157.1, C |
| 3′ | 6.90 (d, 2.4) | 101.4, CH |
| 4′ | - | 162.6, C |
| 5′ | 6.74 (dd, 8.7, 2.4) | 108.0, CH |
| 6′ | 7.47 (d, 8.7) | 130.8, CH |
| 1″ | - | 128.7, C |
| 2″, 6″ | 6.94 (d, 8.6) | 130.6, CH |
| 3″, 5″ | 6.87 (d, 8.6) | 115.5, CH |
| 4″ | - | 156.2, C |
| Glc-1‴ | 4.81 (d, 7.6) | 100.3, CH |
| 2‴ | 3.32 (m) | 73.2, CH |
| 3‴ | 3.44 (m) | 77.4, CH |
| 4‴ | 3.15 (m) | 69.9, CH |
| 5‴ | 3.24 (m) | 76.9, CH |
| 6‴ | 3.67 (m) | 60.8, CH2 |
| 3.45 (m) | ||
| Glc-1⁗ | 5.12 (d, 7.7) | 99.9, CH |
| 2⁗ | 3.20 (m) | 73.2, CH |
| 3⁗ | 3.14 (m) | 76.9, CH |
| 4⁗ | 3.32 (m) | 69.6, CH |
| 5⁗ | 3.33 (m) | 76.6, CH |
| 6⁗ | 3.73 (m) | 60.6, CH2 |
| 3.45 (m) | ||
| 4′-OCH3 | 3.84 (s) | 55.5, CH3 |
| Compounds | IC50 (μM) 1 | |
|---|---|---|
| α-Glucosidase | α-Amylase | |
| 1 | 61.33 ± 1.15 | 81.65 ± 1.72 |
| 2 | >100 | >100 |
| 3 | 72.87 ± 0.79 | 83.62 ± 1.15 |
| 4 | 44.15 ± 1.03 | 60.88 ± 1.68 |
| 5 | 45.23 ± 1.27 | 66.39 ± 2.31 |
| 6 | 52.30 ± 1.59 | 71.41 ± 1.24 |
| 7 | 58.77 ± 1.55 | 78.22 ± 1.98 |
| 8 | 66.54 ± 2.03 | 80.69 ± 1.85 |
| 9 | 57.62 ± 0.88 | 66.75 ± 1.45 |
| 10 | 43.58 ± 0.76 | 63.57 ± 1.37 |
| 11 | 32.18 ± 0.71 | 38.87 ± 1.08 |
| 12 | 23.25 ± 0.38 | 30.89 ± 0.57 |
| Acarbose 2 | 27.05 ± 0.56 | 36.68 ± 0.77 |
| Compounds | PDB ID 1 | Binding Energy (kcal/mol) | Compounds | PDB ID 1 | Binding Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | 3W37 | −5.71 | 7 | 3W37 | −6.95 |
| 2QV4 | −5.43 | 2QV4 | −6.55 | ||
| 2 | 3W37 | −2.00 | 8 | 3W37 | −5.50 |
| 2QV4 | −3.80 | 2QV4 | −5.36 | ||
| 3 | 3W37 | −5.95 | 9 | 3W37 | −6.20 |
| 2QV4 | −5.62 | 2QV4 | −5.87 | ||
| 4 | 3W37 | −7.26 | 10 | 3W37 | −6.89 |
| 2QV4 | −7.35 | 2QV4 | −5.28 | ||
| 5 | 3W37 | −6.59 | 11 | 3W37 | −7.04 |
| 2QV4 | −6.16 | 2QV4 | −6.88 | ||
| 6 | 3W37 | −7.65 | 12 | 3W37 | −8.15 |
| 2QV4 | −6.03 | 2QV4 | −8.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Lei, M.; Yin, Q.; Nan, H.; Qian, G. Isolation and Characterization of Novel Pueroside B Isomers and Other Bioactive Compounds from Pueraria lobata Roots: Structure Elucidation, α-Glucosidase, and α-Amylase Inhibition Studies. Int. J. Mol. Sci. 2024, 25, 9602. https://doi.org/10.3390/ijms25179602
Dai W, Lei M, Yin Q, Nan H, Qian G. Isolation and Characterization of Novel Pueroside B Isomers and Other Bioactive Compounds from Pueraria lobata Roots: Structure Elucidation, α-Glucosidase, and α-Amylase Inhibition Studies. International Journal of Molecular Sciences. 2024; 25(17):9602. https://doi.org/10.3390/ijms25179602
Chicago/Turabian StyleDai, Wei, Manqiu Lei, Qiuxiong Yin, Haijun Nan, and Guoqiang Qian. 2024. "Isolation and Characterization of Novel Pueroside B Isomers and Other Bioactive Compounds from Pueraria lobata Roots: Structure Elucidation, α-Glucosidase, and α-Amylase Inhibition Studies" International Journal of Molecular Sciences 25, no. 17: 9602. https://doi.org/10.3390/ijms25179602





