ZmARF16 Regulates ZCN12 to Promote the Accumulation of Florigen and Accelerate Flowering
Abstract
1. Introduction
2. Results
2.1. Analysis of the Gene Structure and Expression of ZmARF16 and ZCN12
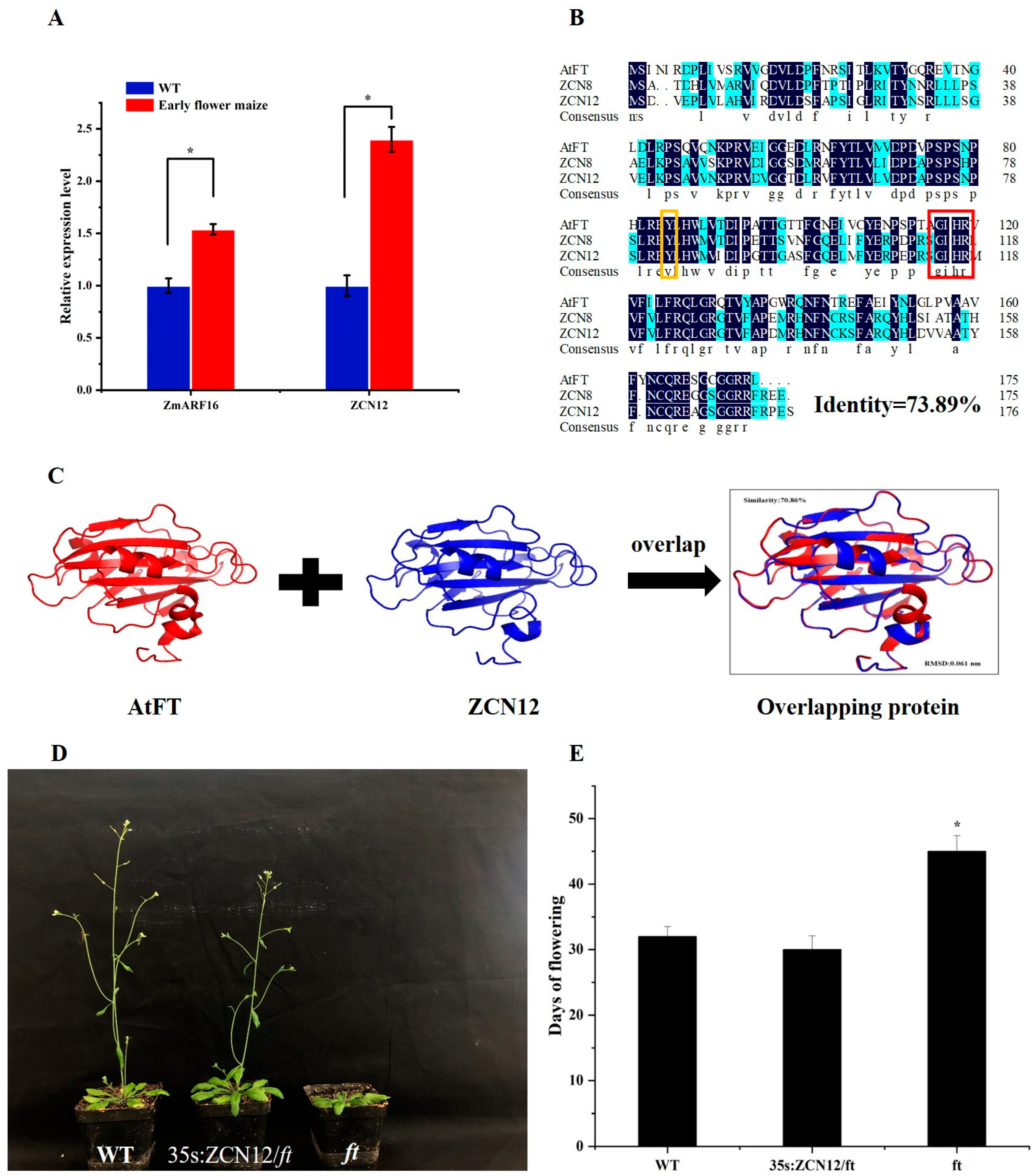
2.2. ZCN12 Can Rescue the Late Flowering Phenotype of the Arabidopsis FT Mutant
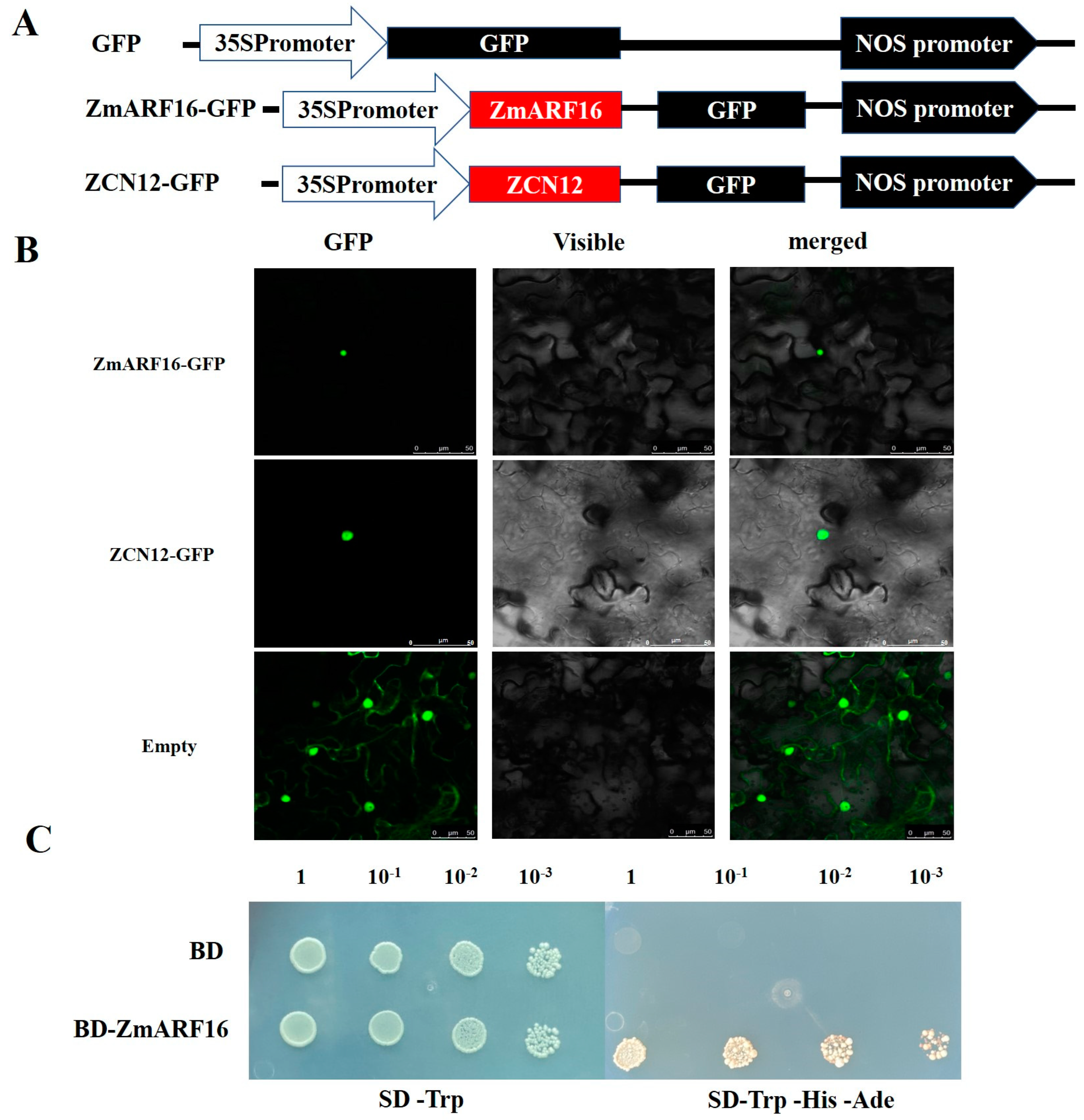
2.3. Identification of the ZmARF16 and ZCN12 Gene
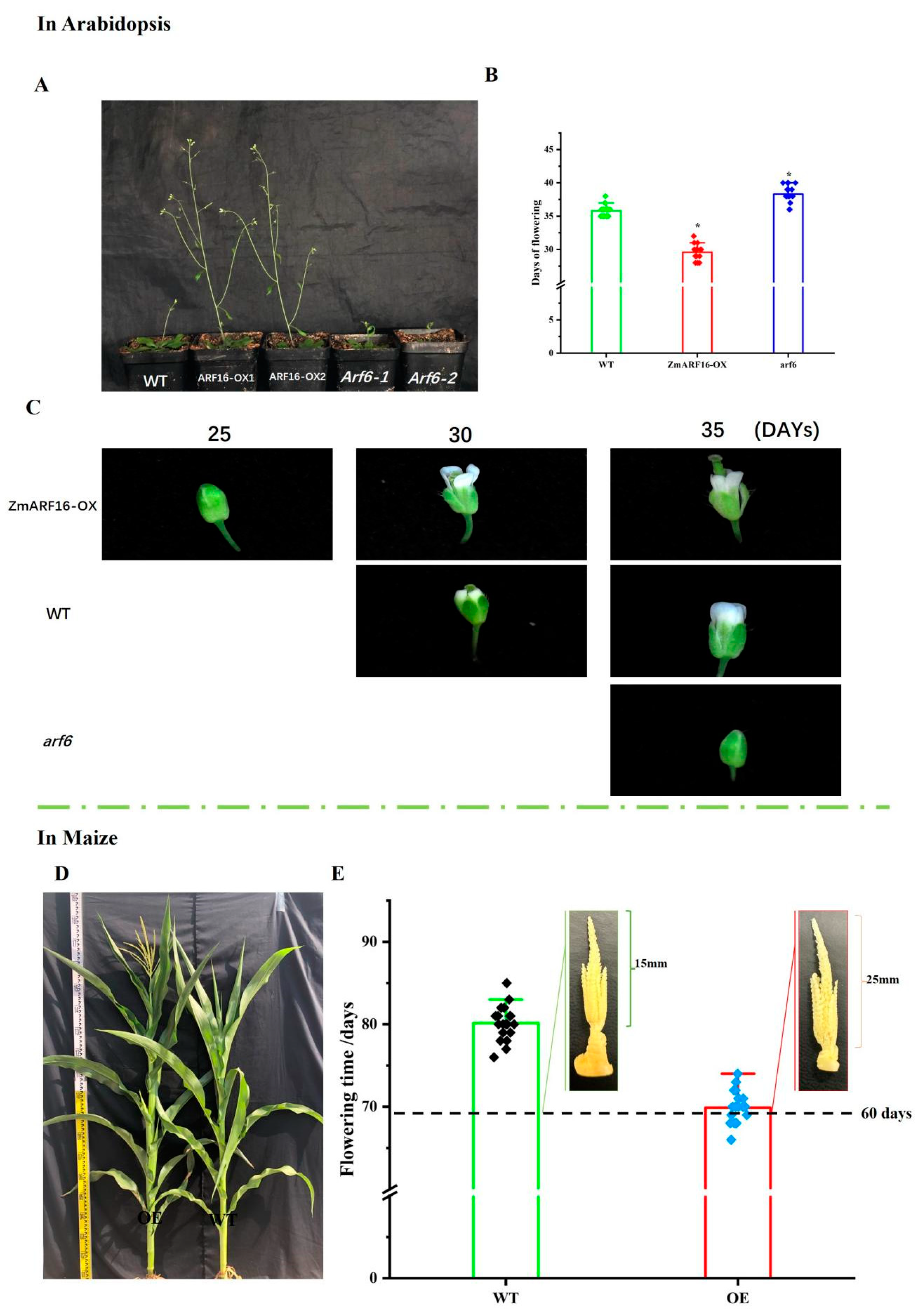
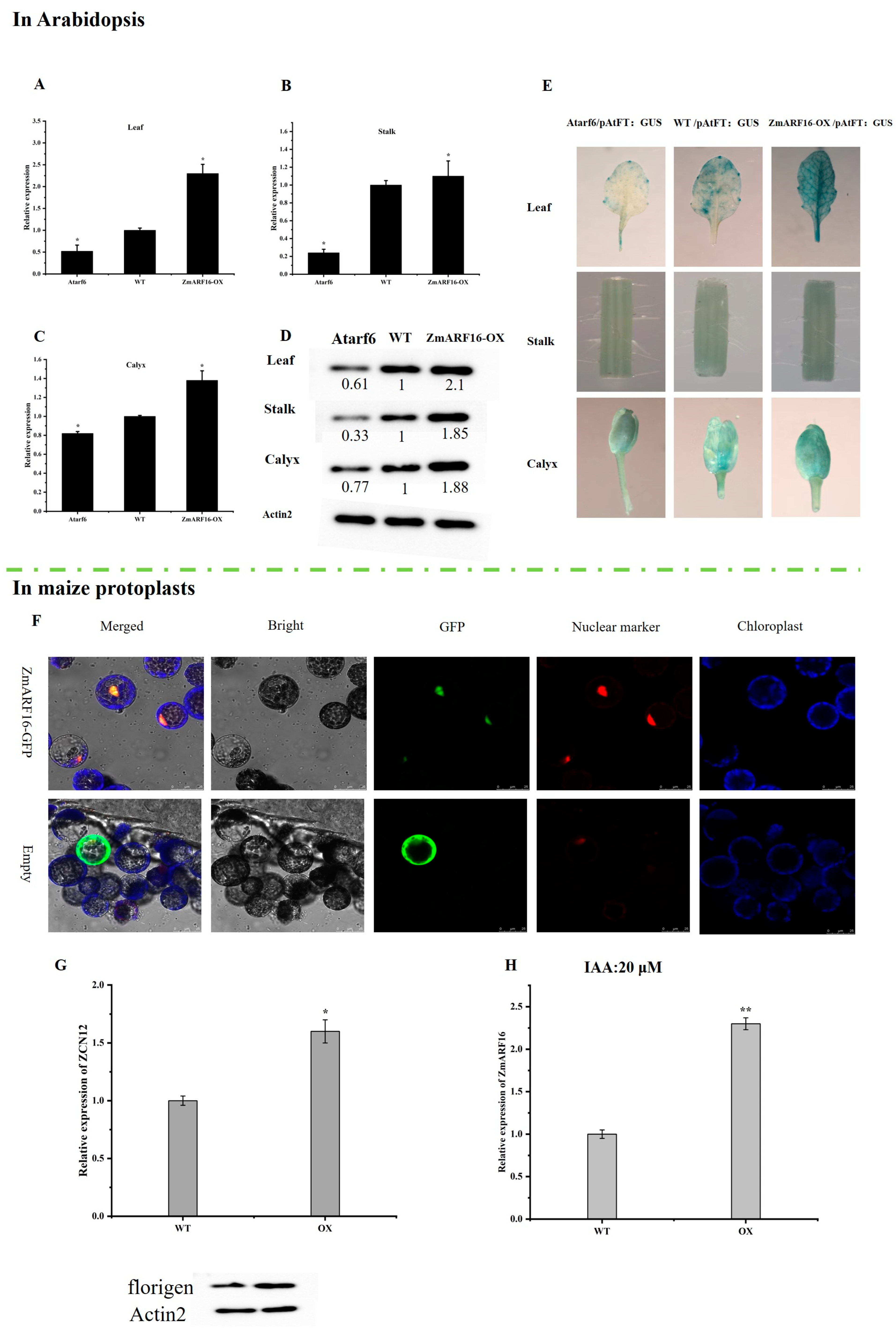
2.4. ZmARF16 Can Promote Flowering Time in Arabidopsis and Maize
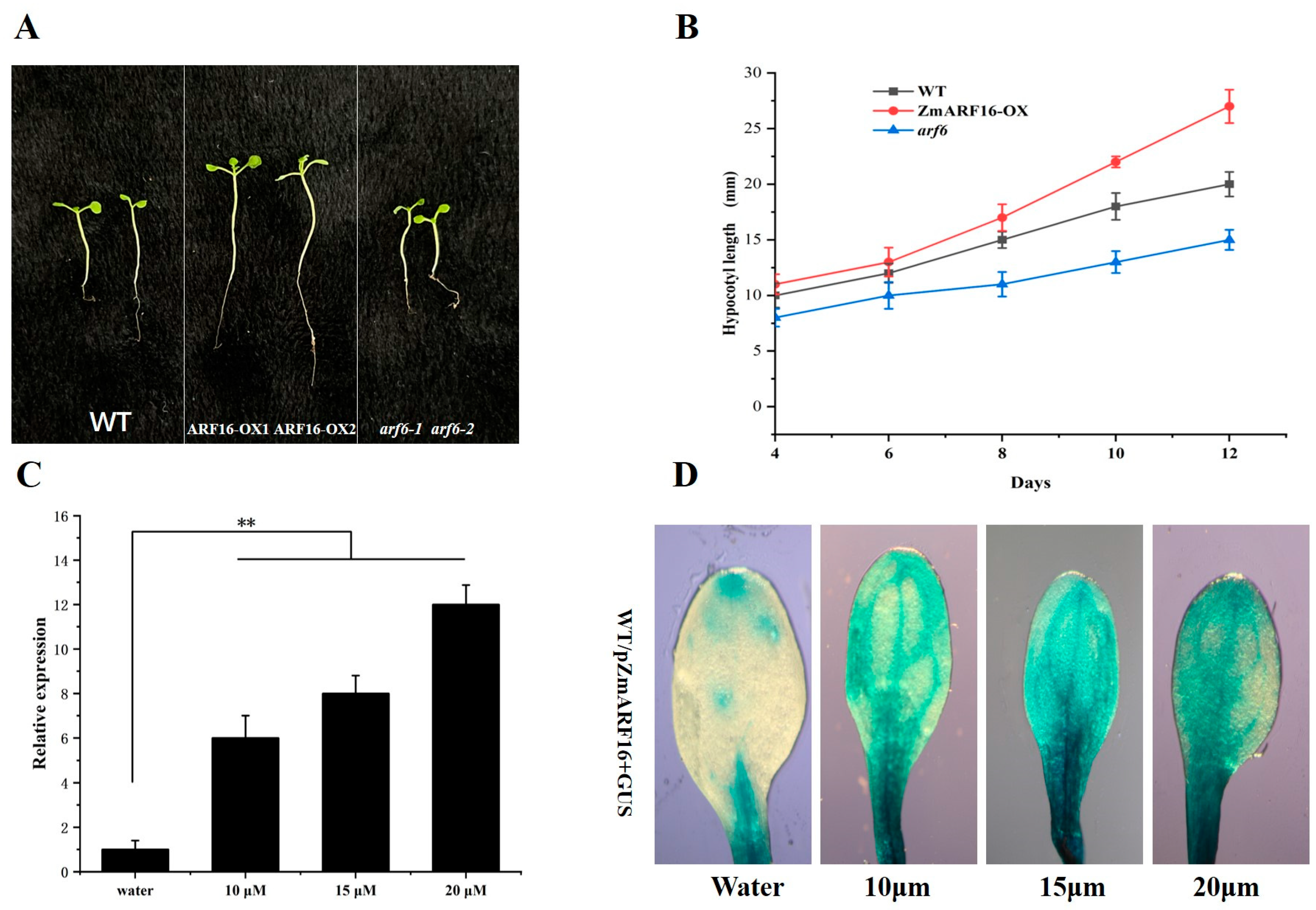
2.5. ZmARF16 Positively Responds to the Auxin Signal in Arabidopsis
2.6. ZmARF16 Promotes the Accumulation of Florigen in Arabidopsis and Maize Protoplasts
2.7. ZmARF16 Positively Regulates ZCN12 and AtFT
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Comparison of Protein Structures
4.3. Flowering Time Determination
4.4. Subcellular Localization
4.5. Auxin Treatment
4.6. Gene Quantitative Analysis
4.7. GUS Staining
4.8. Yeast One Hybridization Test
4.9. Transcriptional Activity Analysis
4.10. The Dual-Luciferase Assay
4.11. Toloniumchloride Staining
4.12. Western Blotting
4.13. Preparation of Maize Protoplasts and Transform
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, H.; Chen, Z.; Dong, Y.; Ku, L.; Abou-Elwafa, S.F.; Ren, Z.; Cao, Y.; Dou, D.; Liu, Z.; Liu, H.; et al. Identification of ZmNF-YC2 and its regulatory network for maize flowering time. J. Exp. Bot. 2021, 72, 7792–7807. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Hempton, A.K.; Imaizumi, T. Photoperiodic flowering in Arabidopsis: Multilayered regulatory mechanisms of CONSTANS and the florigen FLOWERING LOCUS T. Plant Commun. 2023, 4, 100552. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, P.E.; van Es, S.W.; Winkelmolen, T.; Immink, R.G.H.; van Esse, G.W. Flowering time genes branching out. J. Exp. Bot. 2024, 75, 4195–4209. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Nasim, Z.; Susila, H.; Ahn, J.H. Evolution and functional diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in plants. Semin. Cell Dev. Biol. 2021, 109, 20–30. [Google Scholar] [CrossRef]
- Song, C.; Li, G.; Dai, J.; Deng, H. Genome-Wide Analysis of PEBP Genes in Dendrobium huoshanense: Unveiling the Antagonistic Functions of FT/TFL1 in Flowering Time. Front. Genet. 2021, 12, 687689. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Qi, Y. Advances in structure and function of auxin response factor in plants. J. Integr. Plant Biol. 2023, 65, 617–632. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, Y.; Chang, Y.; Zhang, W.; Deng, Y.; Zhang, N.; Zhang, X.; Fan, K.; Hu, X.; Wang, S.; et al. Identification and transcriptome data analysis of ARF family genes in five Orchidaceae species. Plant Mol. Biol. 2023, 112, 85–98. [Google Scholar] [CrossRef]
- Pei, Q.; Li, N.; Yang, Q.; Wu, T.; Feng, S.; Feng, X.; Jing, Z.; Zhou, R.; Gong, K.; Yu, T.; et al. Genome-Wide Identification and Comparative Analysis of ARF Family Genes in Three Apiaceae Species. Front Genet. 2021, 11, 590535. [Google Scholar] [CrossRef]
- Freire-Rios, A.; Tanaka, K.; Crespo, I.; Van der Wijk, E.; Sizentsova, Y.; Levitsky, V.; Lindhoud, S.; Fontana, M.; Hohlbein, J.; Boer, D.R.; et al. Architecture of DNA elements mediating ARF transcription factor binding and auxin-responsive gene expression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 24557–24566. [Google Scholar] [CrossRef]
- Korasick, D.A.; Westfall, C.S.; Lee, S.G.; Nanao, M.H.; Dumas, R.; Hagen, G.; Guilfoyle, T.J.; Jez, J.M.; Strader, L.C. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc. Natl. Acad. Sci. USA 2014, 111, 5427–5432. [Google Scholar] [CrossRef]
- Leng, Y.; Ye, G.; Zeng, D. Genetic Dissection of Leaf Senescence in Rice. Int. J. Mol. Sci. 2017, 18, 2686. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, M.; Fidanza, M.; Mattsson, J. Identification of Auxin Response Factor-Encoding Genes Expressed in Distinct Phases of Leaf Vein Development and with Overlapping Functions in Leaf Formation. Plants 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Min, Y.; Holappa, L.D.; Walcher-Chevillet, C.L.; Duan, X.; Donaldson, E.; Kong, H.; Kramer, E.M. A role for the Auxin Response Factors ARF6 and ARF8 homologs in petal spur elongation and nectary maturation in Aquilegia. New Phytol. 2020, 227, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Cho, C.; Pandey, S.K.; Park, Y.; Kim, M.J.; Kim, J. LBD16 and LBD18 acting downstream of ARF7 and ARF19 are involved in adventitious root formation in Arabidopsis. BMC Plant Biol. 2019, 19, 46. [Google Scholar] [CrossRef]
- Aksenova, N.P. Problems of Growth and Development in the Studies by M.Kh. Chailakhyan. Russ. J. Plant Physiol. 2002, 49, 434–437. [Google Scholar] [CrossRef]
- Lincoln, R.G.; Mayfield, D.L.; Cunningham, A. Preparation of a Floral Initiating Extract from Xanthium. Science 1961, 133, 756. [Google Scholar] [CrossRef]
- Lee, N.; Imaizumi, T. Uncoupling FT Protein Transport from its Function. Plant Cell Physiol. 2018, 59, 1487–1489. [Google Scholar] [CrossRef]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef]
- Tamaki, S.; Matsuo, S.; Wong, H.L.; Yokoi, S.; Shimamoto, K. Hd3a protein is a mobile flowering signal in rice. Science 2007, 316, 1033–1036. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yasuno, N.; Sato, Y.; Yoda, M.; Yamazaki, R.; Kimizu, M.; Yoshida, H.; Nagamura, Y.; Kyozuka, J. Inflorescence meristem identity in rice is specified by overlapping functions of three AP1/FUL-like MADS box genes and PAP2, a SEPALLATA MADS box gene. Plant Cell 2012, 24, 1848–1859. [Google Scholar] [CrossRef]
- Su, H.; Liang, J.; Abou-Elwafa, S.F.; Cheng, H.; Dou, D.; Ren, Z.; Xie, J.; Chen, Z.; Gao, F.; Ku, L.; et al. ZmCCT regulates photoperiod-dependent flowering and response to stresses in maize. BMC Plant Biol. 2021, 21, 453. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, X.; Zhao, M.; Huang, C.; Li, C.; Li, D.; Yang, C.J.; York, A.M.; Xue, W.; Xu, G.; et al. Stepwise cis-Regulatory Changes in ZCN8 Contribute to Maize Flowering-Time Adaptation. Curr. Biol. 2018, 28, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Ku, L.; Tian, L.; Su, H.; Wang, C.; Wang, X.; Wu, L.; Shi, Y.; Li, G.; Wang, Z.; Wang, H.; et al. Dual functions of the ZmCCT-associated quantitative trait locus in flowering and stress responses under long-day conditions. BMC Plant Biol. 2016, 16, 239. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Zhang, X.; Chen, Q.; Xu, G.; Xu, D.; Wang, C.; Liang, Y.; Wu, L.; Huang, C.; et al. The genetic architecture of leaf number and its genetic relationship to flowering time in maize. New Phytol. 2016, 210, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liu, X.; Jia, W.; Liu, H.; Li, W.; Peng, Y.; Du, Y.; Wang, Y.; Yin, Y.; Zhang, X.; et al. ZmCOL3, a CCT gene represses flowering in maize by interfering with the circadian clock and activating expression of ZmCCT. J. Integr. Plant Biol. 2018, 60, 465–480. [Google Scholar] [CrossRef]
- Danilevskaya, O.N.; Meng, X.; Hou, Z.; Ananiev, E.V.; Simmons, C.R. A genomic and expression compendium of the expanded PEBP gene family from maize. Plant Physiol. 2008, 146, 250–264. [Google Scholar] [CrossRef]
- Lazakis, C.M.; Coneva, V.; Colasanti, J. ZCN8 encodes a potential orthologue of Arabidopsis FT florigen that integrates both endogenous and photoperiod flowering signals in maize. J. Exp. Bot. 2011, 62, 4833–4842. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Wójcik, A.M.; Gaj, M.D. Epigenetic Regulation of Auxin-Induced Somatic Embryogenesis in Plants. Int. J. Mol. Sci. 2020, 21, 2307. [Google Scholar] [CrossRef]
- Perico, C.; Tan, S.; Langdale, J.A. Developmental regulation of leaf venation patterns: Monocot versus eudicots and the role of auxin. New Phytol. 2022, 234, 783–803. [Google Scholar] [CrossRef]
- Ibañes, M.; Fàbregas, N.; Chory, J.; Caño-Delgado, A.I. Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc. Natl. Acad. Sci. USA 2009, 106, 13630–13635. [Google Scholar] [CrossRef]
- Li, S.B.; Xie, Z.Z.; Hu, C.G.; Zhang, J.Z. A Review of Auxin Response Factors (ARFs) in Plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef]
- Guilfoyle, T.J.; Ulmasov, T.; Hagen, G. The ARF family of transcription factors and their role in plant hormone-responsive transcription. Cell. Mol. Life Sci. 1998, 54, 619–627. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Shi, T.; Chen, M.; Jia, C.; Wang, J.; Hou, Z.; Han, J.; Bian, S. Identification of ARF family in blueberry and its potential involvement of fruit development and pH stress response. BMC Genom. 2022, 23, 329. [Google Scholar] [CrossRef]
- Mao, Z.; He, S.; Xu, F.; Wei, X.; Jiang, L.; Liu, Y.; Wang, W.; Li, T.; Xu, P.; Du, S.; et al. Photoexcited CRY1 and phyB interact directly with ARF6 and ARF8 to regulate their DNA-binding activity and auxin-induced hypocotyl elongation in Arabidopsis. New Phytol. 2020, 225, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, S.; Wu, H.; Wang, H. Protein Levels of Several Arabidopsis Auxin Response Factors Are Regulated by Multiple Factors and ABA Promotes ARF6 Protein Ubiquitination. Int. J. Mol. Sci. 2020, 21, 9437. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Tian, Q.; Reed, J.W. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 2006, 133, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, T.; Hagen, G.; Guilfoyle, T.J. Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA 1999, 96, 5844–5849. [Google Scholar] [CrossRef]
- Ben-Targem, M.; Ripper, D.; Bayer, M.; Ragni, L. Auxin and gibberellin signaling cross-talk promotes hypocotyl xylem expansion and cambium homeostasis. J. Exp. Bot. 2021, 72, 3647–3660. [Google Scholar] [CrossRef]
- Xu, F.; He, S.; Zhang, J.; Mao, Z.; Wang, W.; Li, T.; Hua, J.; Du, S.; Xu, P.; Li, L.; et al. Photoactivated CRY1 and phyB Interact Directly with AUX/IAA Proteins to Inhibit Auxin Signaling in Arabidopsis. Mol Plant. 2018, 11, 523–541. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Guan, Y.; Wang, S.; Luo, H.; Li, X.; Li, H.; Zhang, Z. Auxin-induced AUXIN RESPONSE FACTOR4 activates APETALA1 and FRUITFULL to promote flowering in woodland strawberry. Hortic. Res. 2021, 8, 115. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Chen, X.; Li, L.; Liu, K.; Zhao, H.; Wang, Y.; Han, S. ERF72 interacts with ARF6 and BZR1 to regulate hypocotyl elongation in Arabidopsis. J. Exp. Bot. 2018, 69, 3933–3947. [Google Scholar] [CrossRef]
- Hu, J.; Israeli, A.; Ori, N.; Sun, T.P. The Interaction between DELLA and ARF/IAA Mediates Crosstalk between Gibberellin and Auxin Signaling to Control Fruit Initiation in Tomato. Plant cell 2018, 30, 1710–1728. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, Y.; Han, S.; Chang, S.; Gao, Z.; Qi, Y.; Qian, Q. OsARF4 regulates leaf inclination via auxin and brassinosteroid pathways in rice. Front. Plant Sci. 2022, 13, 979033. [Google Scholar] [CrossRef]
- Bieleszová, K.; Pařízková, B.; Kubeš, M.; Husičková, A.; Kubala, M.; Ma, Q.; Sedlářová, M.; Robert, S.; Doležal, K.; Strnad, M.; et al. New fluorescently labeled auxins exhibit promising anti-auxin activity. New Biotechnol. 2019, 48, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Banasiak, A.; Biedroń, M.; Dolzblasz, A.; Berezowski, M.A. Ontogenetic Changes in Auxin Biosynthesis and Distribution Determine the Organogenic Activity of the Shoot Apical Meristem in pin1 Mutants. Int. J. Mol. Sci. 2019, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Ckurshumova, W.; Smirnova, T.; Marcos, D.; Zayed, Y.; Berleth, T. Irrepressible MONOPTEROS/ARF5 promotes de novo shoot formation. New Phytol. 2014, 204, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Carey, N.S.; Krogan, N.T. The role of AUXIN RESPONSE FACTORs in the development and differential growth of inflorescence stems. Plant Signal. Behav. 2017, 12, e1307492. [Google Scholar] [CrossRef]
- Han, S.; Cho, H.; Noh, J.; Qi, J.; Jung, H.J.; Nam, H.; Lee, S.; Hwang, D.; Greb, T.; Hwang, I. BIL1-mediated MP phosphorylation integrates PXY and cytokinin signalling in secondary growth. Nat. Plants 2018, 4, 605–614. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, R.; Zi, H.; Li, Y.; Cao, X.; Li, D.; Guo, L.; Tong, J.; Pan, Y.; Jiao, Y.; et al. AUXIN RESPONSE FACTOR3 Regulates Floral Meristem Determinacy by Repressing Cytokinin Biosynthesis and Signaling. Plant Cell 2018, 30, 324–346. [Google Scholar] [CrossRef]
- Xue, H.; Meng, J.; Lei, P.; Cao, Y.; An, X.; Jia, M.; Li, Y.; Liu, H.; Sheen, J.; Liu, X.; et al. ARF2-PIF5 interaction controls transcriptional reprogramming in the ABS3-mediated plant senescence pathway. EMBO J. 2022, 41, e110988. [Google Scholar] [CrossRef]
- Cancé, C.; Martin-Arevalillo, R.; Boubekeur, K.; Dumas, R. Auxin response factors are keys to the many auxin doors. New Phytol. 2022, 235, 402–419. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Zhao, Y.; Gao, B.; Wei, X.; Jiao, P.; Zhang, H.; Liu, S.; Guan, S.; Ma, Y. ZmARF16 Regulates ZCN12 to Promote the Accumulation of Florigen and Accelerate Flowering. Int. J. Mol. Sci. 2024, 25, 9607. https://doi.org/10.3390/ijms25179607
Jiang Z, Zhao Y, Gao B, Wei X, Jiao P, Zhang H, Liu S, Guan S, Ma Y. ZmARF16 Regulates ZCN12 to Promote the Accumulation of Florigen and Accelerate Flowering. International Journal of Molecular Sciences. 2024; 25(17):9607. https://doi.org/10.3390/ijms25179607
Chicago/Turabian StyleJiang, Zhenzhong, Yang Zhao, Bai Gao, Xiaotong Wei, Peng Jiao, Honglin Zhang, Siyan Liu, Shuyan Guan, and Yiyong Ma. 2024. "ZmARF16 Regulates ZCN12 to Promote the Accumulation of Florigen and Accelerate Flowering" International Journal of Molecular Sciences 25, no. 17: 9607. https://doi.org/10.3390/ijms25179607
APA StyleJiang, Z., Zhao, Y., Gao, B., Wei, X., Jiao, P., Zhang, H., Liu, S., Guan, S., & Ma, Y. (2024). ZmARF16 Regulates ZCN12 to Promote the Accumulation of Florigen and Accelerate Flowering. International Journal of Molecular Sciences, 25(17), 9607. https://doi.org/10.3390/ijms25179607





