A Spironolactone-Based Prototype of an Innovative Biomedical Patch for Wound Dressing Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization and Fabrication of Electrospun Membranes
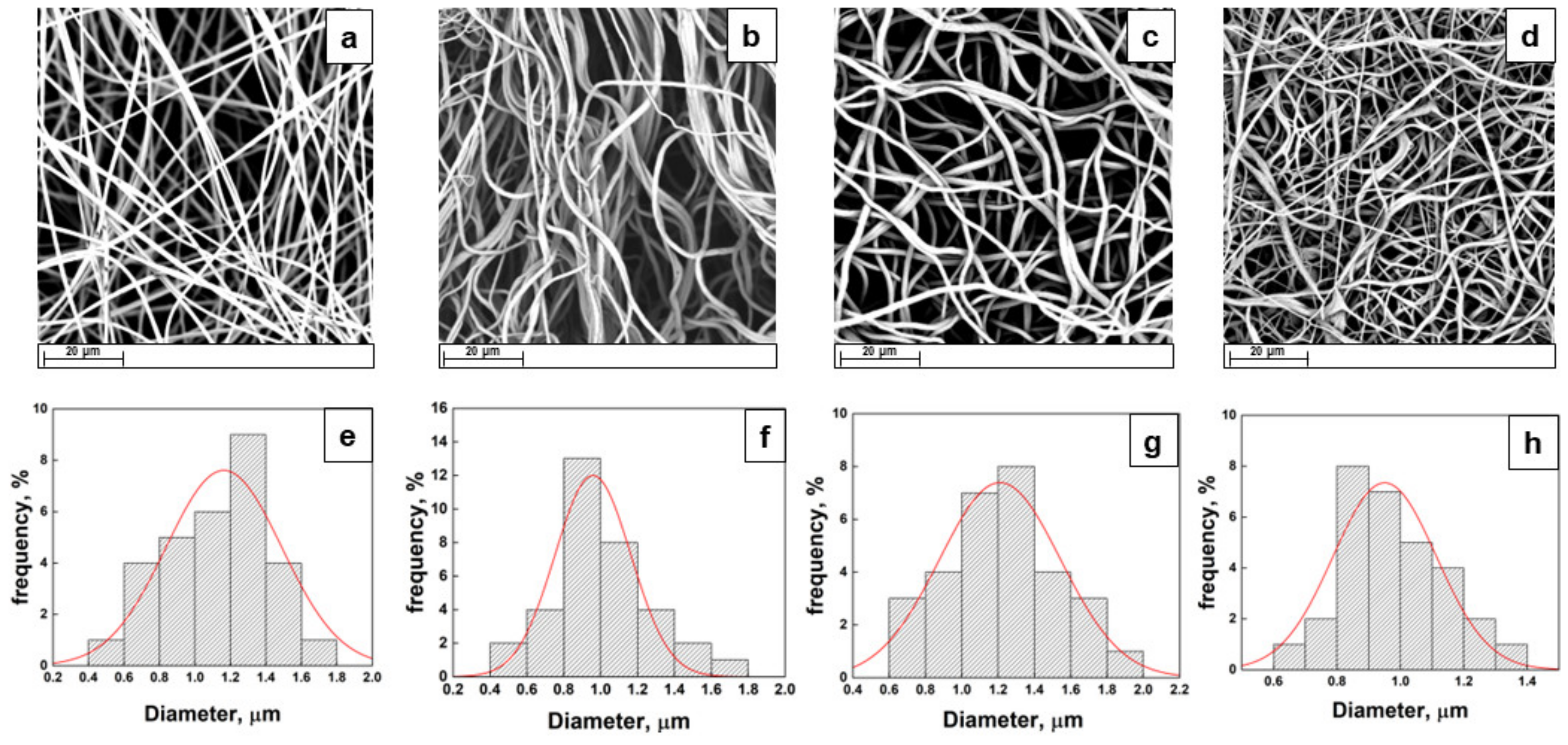
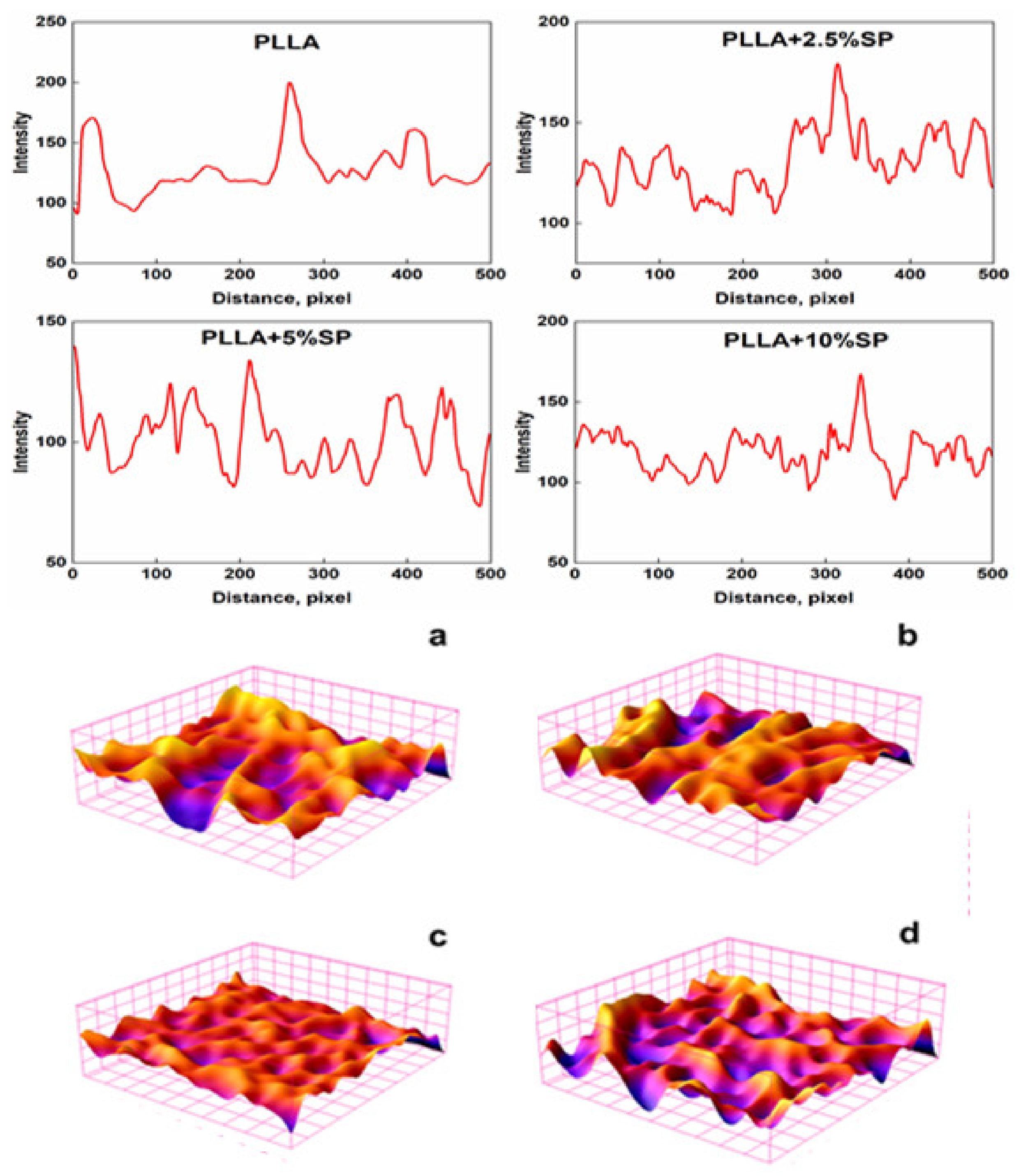
2.2. Morphology and Surface Analysis
2.3. Thermal Properties
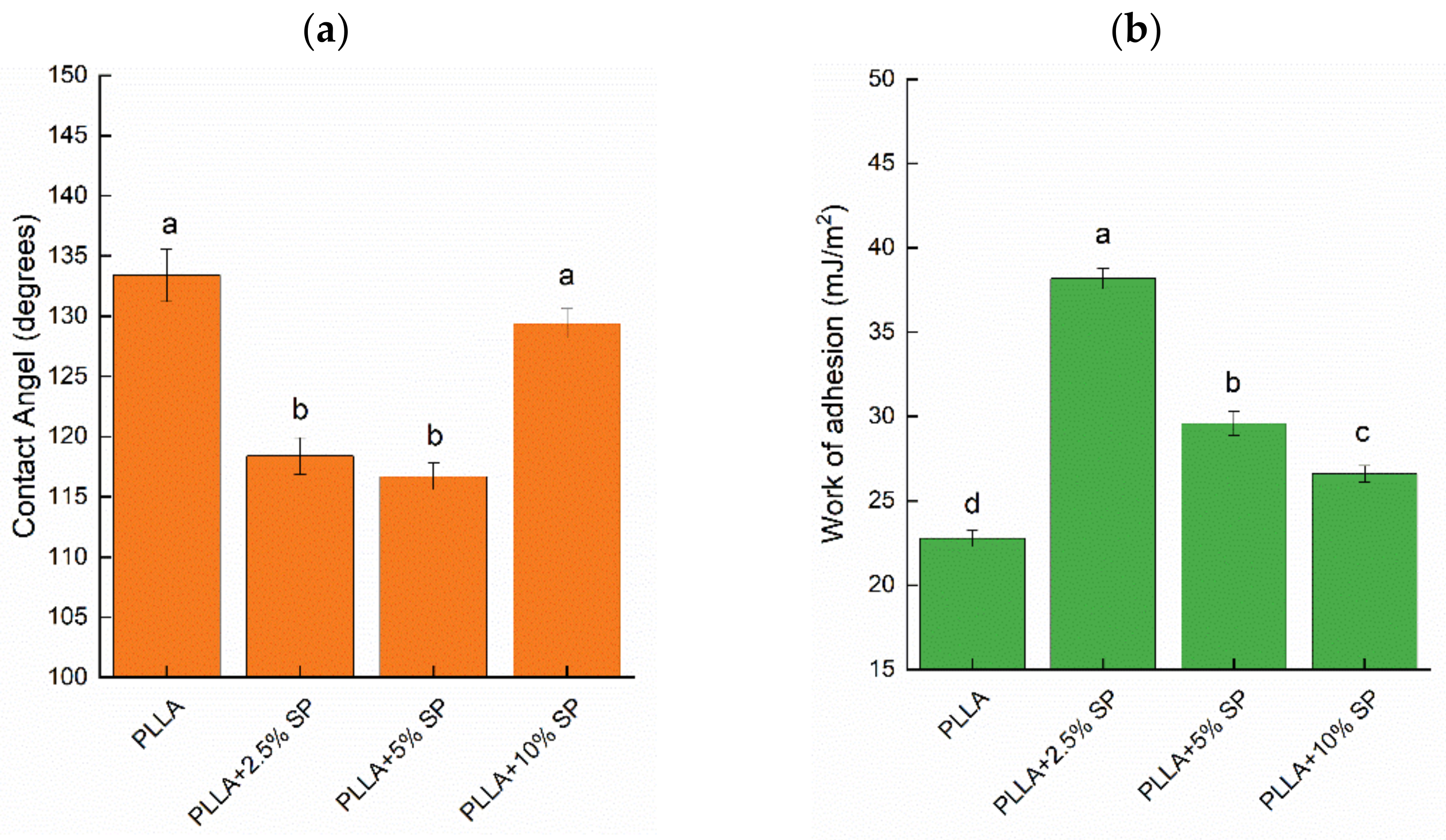
2.4. Wettability and Liquid Retention
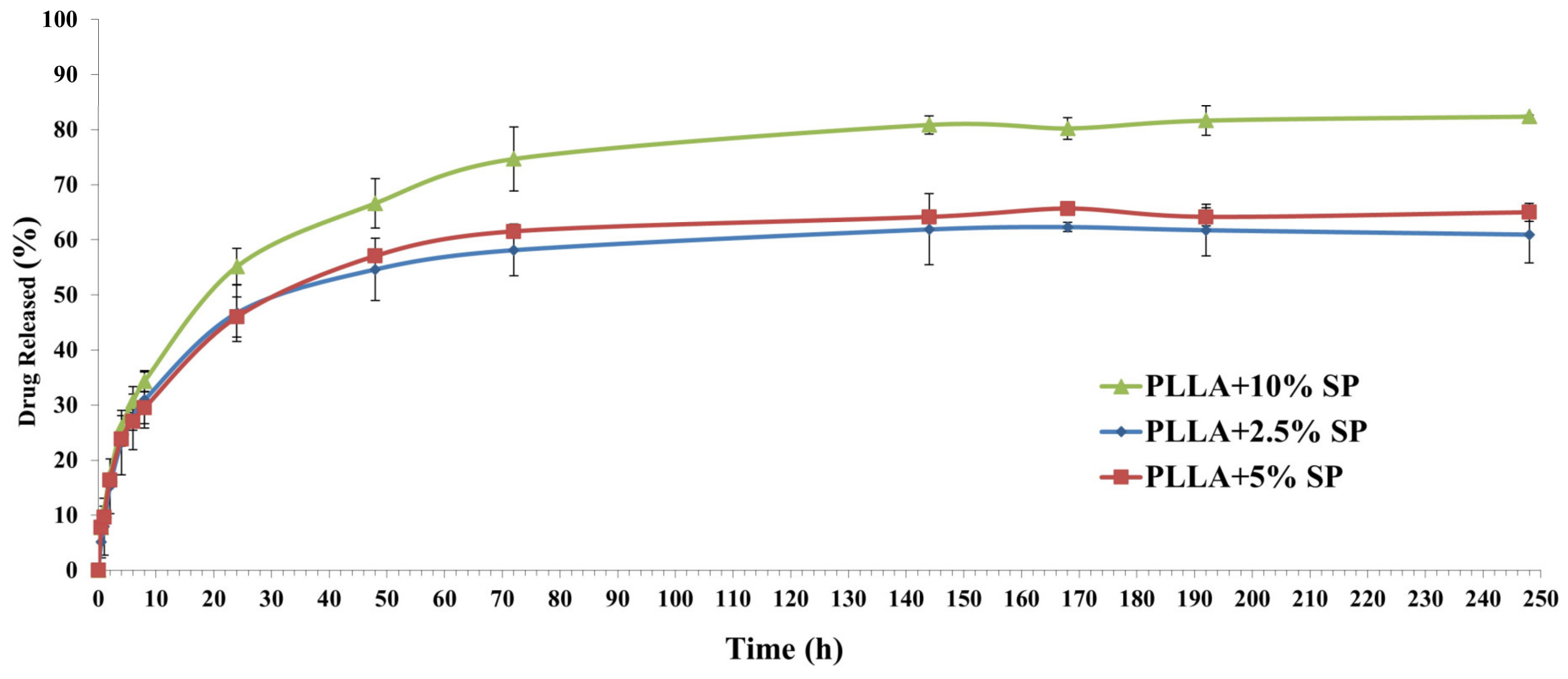
2.5. Drug Release Profile
3. Materials and Methods
3.1. Preparation of Electrospun Membranes
3.2. Physico-Chemical Characterization
3.3. Spironolactone Content
3.4. Spironolactone Release
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Sill, T.J.; Von Recum, H.A.J.B. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.K.; Lee, S.W.; Kim, J.-M.; Oh, J.-E.; Kim, K.-H.; Chung, C.-P.; Choi, S.-C.; Park, W.H.; Min, B.-M. Electrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials 2006, 27, 3934–3944. [Google Scholar] [CrossRef] [PubMed]
- Elsner, J.J.; Zilberman, M. Antibiotic-eluting bioresorbable composite fibers for wound healing applications: Microstructure, drug delivery and mechanical properties. Acta Biomater. 2009, 5, 2872–2883. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.-M.; Park, Y.J.; Hong, S.-D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.-M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef]
- Grillo, A.; Rusconi, Y.; D’Alterio, M.C.; De Rosa, C.; Talarico, G.; Poater, A. Ring Opening Polymerization of Six-and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials. Int. J. Mol. Sci. 2024, 25, 1647. [Google Scholar] [CrossRef]
- Giram, P.S.; Garnaik, B. Evaluation of biocompatibility of synthesized low molecular weight PLGA copolymers using zinc L-proline through green route for biomedical application. Polym. Adv. Technol. 2021, 32, 4502–4515. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Nandkumar, A.M.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA—Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Tripathi, Y.B.; Tripathi, P.; Arjmandi, B.H. Nutraceuticals and cancer management. Front. Biosci. -Landmark 2005, 10, 1607–1618. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Abate, M.; Pepe, G.; Randino, R.; Pisanti, S.; Basilicata, M.G.; Covelli, V.; Bifulco, M.; Cabri, W.; D’Ursi, A.M.; Campiglia, P. Ganoderma lucidum ethanol extracts enhance re-epithelialization and prevent keratinocytes from free-radical injury. Pharmaceuticals 2020, 13, 224. [Google Scholar] [CrossRef]
- Cheng, P.-G.; Phan, C.-W.; Sabaratnam, V.; Abdullah, N.; Abdulla, M.A.; Kuppusamy, U.R. Polysaccharides-rich extract of Ganoderma lucidum (MA Curtis: Fr.) P. Karst accelerates wound healing in streptozotocin-induced diabetic rats. Evid. Based Complement. Altern. Med. 2013, 2013, 671252. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S. Preparation, characterisation, and biocompatibility of Ganoderma lucidum fibre-based composites with polylactic acid. Compos. Sci. Technol. 2014, 102, 1–9. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun Nanofibres Containing Antimicrobial Plant Extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-F.; Chen, X.; Ahmad, Z.; Li, J.-S.; Chang, M.-W. Engineering of Ganoderma lucidum polysaccharide loaded polyvinyl alcohol nanofibers for biopharmaceutical delivery. J. Drug Deliv. Sci. Technol. 2019, 50, 208–216. [Google Scholar] [CrossRef]
- Dong, Y.; Chaudhary, D.; Haroosh, H.; Bickford, T. Development and characterisation of novel electrospun polylactic acid/tubular clay nanocomposites. J. Mater. Sci. 2011, 46, 6148–6153. [Google Scholar] [CrossRef]
- Balogh, A.; Farkas, B.; Domokos, A.; Farkas, A.; Démuth, B.; Borbás, E.; Nagy, B.; Marosi, G.; Nagy, Z.K. Controlled-release solid dispersions of Eudragit® FS 100 and poorly soluble spironolactone prepared by electrospinning and melt extrusion. Eur. Polym. J. 2017, 95, 406–417. [Google Scholar] [CrossRef]
- Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2368. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Li, C.; Jiang, G.; Wang, F.; Wang, L. Regulating surface roughness of electrospun poly (ε-caprolactone)/β-tricalcium phosphate fibers for enhancing bone tissue regeneration. Eur. Polym. J. 2021, 143, 110201. [Google Scholar] [CrossRef]
- Rietveld, I.B.; Barrio, M.; Lloveras, P.; Céolin, R.; Tamarit, J.-L. Polymorphism of spironolactone: An unprecedented case of monotropy turning to enantiotropy with a huge difference in the melting temperatures. Int. J. Pharm. 2018, 552, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.W.L.; Doriguetto, A.C.; de Araújo, M.B.; Bonfilio, R. Solid-state characterization of spironolactone 1/3 Hydrate. J. Pharm. Sci. 2019, 108, 2458–2464. [Google Scholar] [CrossRef]
- Maleki, H.; Gharehaghaji, A.A.; Moroni, L.; Dijkstra, P.J. Influence of the solvent type on the morphology and mechanical properties of electrospun PLLA yarns. Biofabrication 2013, 5, 035014. [Google Scholar] [CrossRef]
- Tsuji, H.; Nakano, M.; Hashimoto, M.; Takashima, K.; Katsura, S.; Mizuno, A. Electrospinning of poly (lactic acid) stereocomplex nanofibers. Biomacromolecules 2006, 7, 3316–3320. [Google Scholar] [CrossRef]
- Inai, R.; Kotaki, M.; Ramakrishna, S. Structure and properties of electrospun PLLA single nanofibres. Nanotechnology 2005, 16, 208. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly (L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Szabó, E.; Démuth, B.; Nagy, B.; Molnár, K.; Farkas, A.; Szabó, B.; Balogh, A.; Hirsch, E.; Marosi, G.; Nagy, Z.K. Scaled-up preparation of drug-loaded electrospun polymer fibres and investigation of their continuous processing to tablet form. Express Polym. Lett. 2018, 12, 436–451. [Google Scholar] [CrossRef]
- Szabó, E.; Záhonyi, P.; Brecska, D.N.; Galata, D.N.L.; Mészáros, L.A.; Madarász, L.; Csorba, K.F.; Vass, P.; Hirsch, E.; Szafraniec-Szczęsny, J. Comparison of amorphous solid dispersions of spironolactone prepared by spray drying and electrospinning: The influence of the preparation method on the dissolution properties. Mol. Pharm. 2020, 18, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Tiwari, A.P.; Pant, H.R.; Shrestha, B.K.; Kim, H.J.; Park, C.H.; Kim, C.S. In situ generation of cellulose nanocrystals in polycaprolactone nanofibers: Effects on crystallinity, mechanical strength, biocompatibility, and biomimetic mineralization. ACS Appl. Mater. Interfaces 2015, 7, 19672–19683. [Google Scholar] [CrossRef]
- Gomaa, S.F.; Madkour, T.M.; Moghannem, S.; El-Sherbiny, I.M. New polylactic acid/cellulose acetate-based antimicrobial interactive single dose nanofibrous wound dressing mats. Int. J. Biol. Macromol. 2017, 105, 1148–1160. [Google Scholar] [CrossRef]
- Thakur, R.A.; Florek, C.A.; Kohn, J.; Michniak, B.B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int. J. Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef] [PubMed]
- D’Alterio, M.C.; D’Auria, I.; Gaeta, L.; Tedesco, C.; Brenna, S.; Pellecchia, C. Are Well Performing Catalysts for the Ring Opening Polymerization of l-Lactide under Mild Laboratory Conditions Suitable for the Industrial Process? The Case of New Highly Active Zn (II) Catalysts. Macromolecules 2022, 55, 5115–5122. [Google Scholar] [CrossRef]
- Kozbial, A.; Li, Z.; Conaway, C.; McGinley, R.; Dhingra, S.; Vahdat, V.; Zhou, F.; D’Urso, B.; Liu, H.; Li, L. Study on the surface energy of graphene by contact angle measurements. Langmuir 2014, 30, 8598–8606. [Google Scholar] [CrossRef]
- Hao, W.; Yao, X.; Ke, Y.; Ma, Y.; Li, F. Experimental characterization of contact angle and surface energy on aramid fibers. J. Adhes. Sci. Technol. 2013, 27, 1012–1022. [Google Scholar] [CrossRef]
- Ebnesajjad, S.; Landrock, A.H. Adhesives Technology Handbook; William Andrew: Norwich, NY, USA, 2014. [Google Scholar]
- Avataneo, V.; De Nicolò, A.; Rabbia, F.; Sciandra, M.; Tosello, F.; Cusato, J.; Perlo, E.; Fatiguso, G.; Allegra, S.; Favata, F. A simple UHPLC-PDA method with a fast dilute-and-shot sample preparation for the quantification of canrenone and its prodrug spironolactone in human urine samples. J. Pharmacol. Toxicol. Methods 2018, 94, 29–35. [Google Scholar] [CrossRef]
- Baranowska, I.; Wilczek, A.; Baranowski, J. Rapid UHPLC method for simultaneous determination of vancomycin, terbinafine, spironolactone, furosemide and their metabolites: Application to human plasma and urine. Anal. Sci. 2010, 26, 755–759. [Google Scholar] [CrossRef]

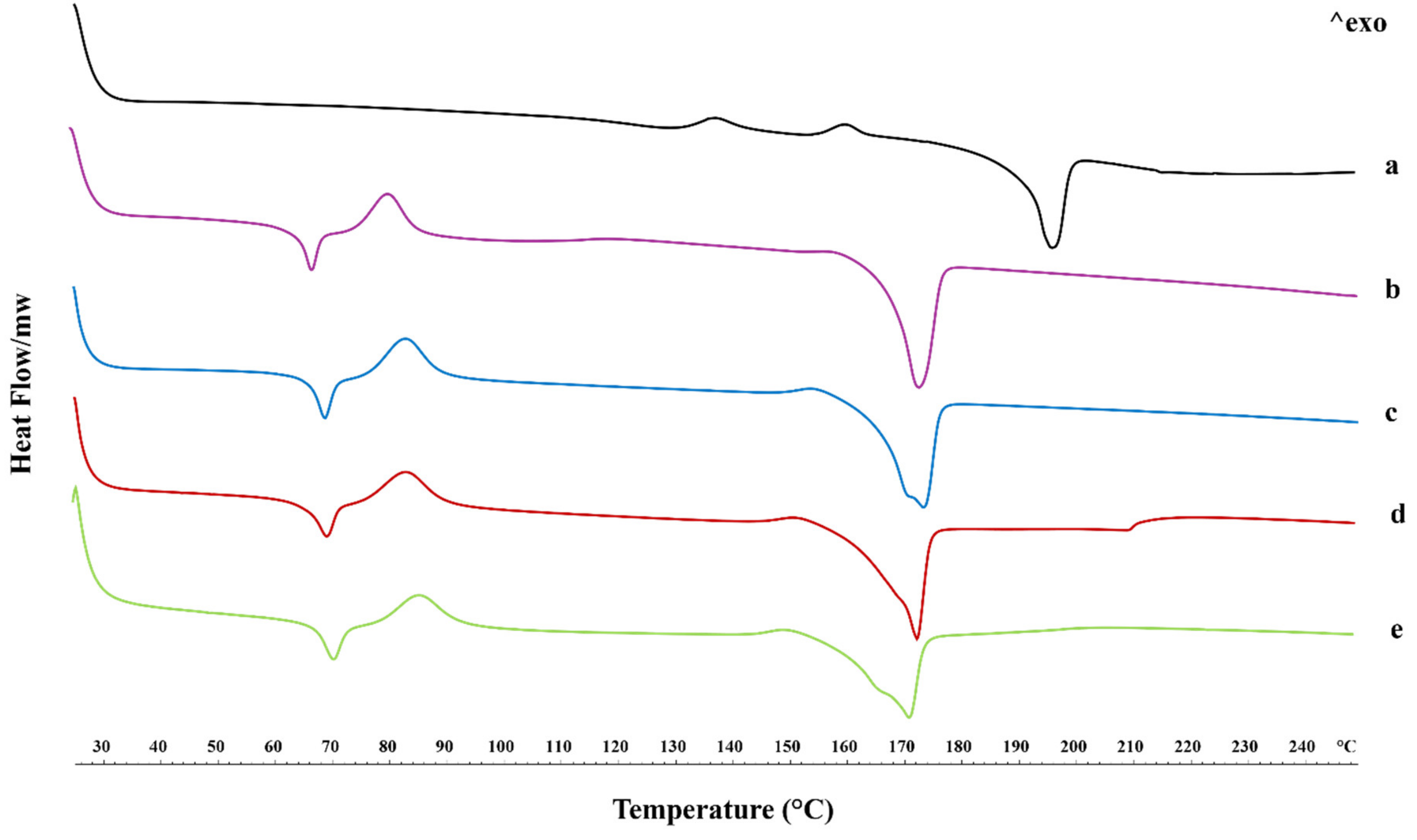

| Tg (°C) | Tcc (°C) | ΔTcc (°C) | Tm (°C) | ΔTm (°C) | |
|---|---|---|---|---|---|
| PLLA | 67.05 | 80.26 | 15.31 | 172.55 | 22.43 |
| PLLA + 2.5% SP | 68.71 | 82.77 | 19.90 | 173.05 | 26.67 |
| PLLA + 5% SP | 69.04 | 82.92 | 21.30 | 172.20 | 27.50 |
| PLLA + 10% SP | 70.57 | 85.60 | 22.34 | 171.14 | 36.32 |
| β (m4/mJ2) | γS (mJ/m2) | |
|---|---|---|
| PLLA | 0.0031 | 49.65 |
| PLLA + 2.5% SP | 0.0061 | 21.92 |
| PLLA + 5% SP | 0.0049 | 54.34 |
| PLLA + 10% SP | 0.0048 | 56.06 |
| Title 1 | γL (mJ/m2) | γdL (mJ/m2) | γpL (mJ/m2) |
|---|---|---|---|
| Water | 72.8 | 21.8 | 51 |
| Glycerol | 63.4 | 33.4 | 30 |
| Ethylene Glycol | 48 | 29 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, G.; Viscusi, G.; D’Alterio, M.C.; Covelli, V.; Gorrasi, G.; Pellecchia, C.; Rizzo, P.; D’Ursi, A.M.; Pepe, G.; Amante, C.; et al. A Spironolactone-Based Prototype of an Innovative Biomedical Patch for Wound Dressing Applications. Int. J. Mol. Sci. 2024, 25, 9608. https://doi.org/10.3390/ijms25179608
Aquino G, Viscusi G, D’Alterio MC, Covelli V, Gorrasi G, Pellecchia C, Rizzo P, D’Ursi AM, Pepe G, Amante C, et al. A Spironolactone-Based Prototype of an Innovative Biomedical Patch for Wound Dressing Applications. International Journal of Molecular Sciences. 2024; 25(17):9608. https://doi.org/10.3390/ijms25179608
Chicago/Turabian StyleAquino, Giovanna, Gianluca Viscusi, Massimo Christian D’Alterio, Verdiana Covelli, Giuliana Gorrasi, Claudio Pellecchia, Paola Rizzo, Anna Maria D’Ursi, Giacomo Pepe, Chiara Amante, and et al. 2024. "A Spironolactone-Based Prototype of an Innovative Biomedical Patch for Wound Dressing Applications" International Journal of Molecular Sciences 25, no. 17: 9608. https://doi.org/10.3390/ijms25179608
APA StyleAquino, G., Viscusi, G., D’Alterio, M. C., Covelli, V., Gorrasi, G., Pellecchia, C., Rizzo, P., D’Ursi, A. M., Pepe, G., Amante, C., Del Gaudio, P., & Rodriquez, M. (2024). A Spironolactone-Based Prototype of an Innovative Biomedical Patch for Wound Dressing Applications. International Journal of Molecular Sciences, 25(17), 9608. https://doi.org/10.3390/ijms25179608














