Postmortem-Derived Exosomal MicroRNA 486-5p as Potential Biomarkers for Ischemic Heart Disease Diagnosis
Abstract
1. Introduction
2. Results
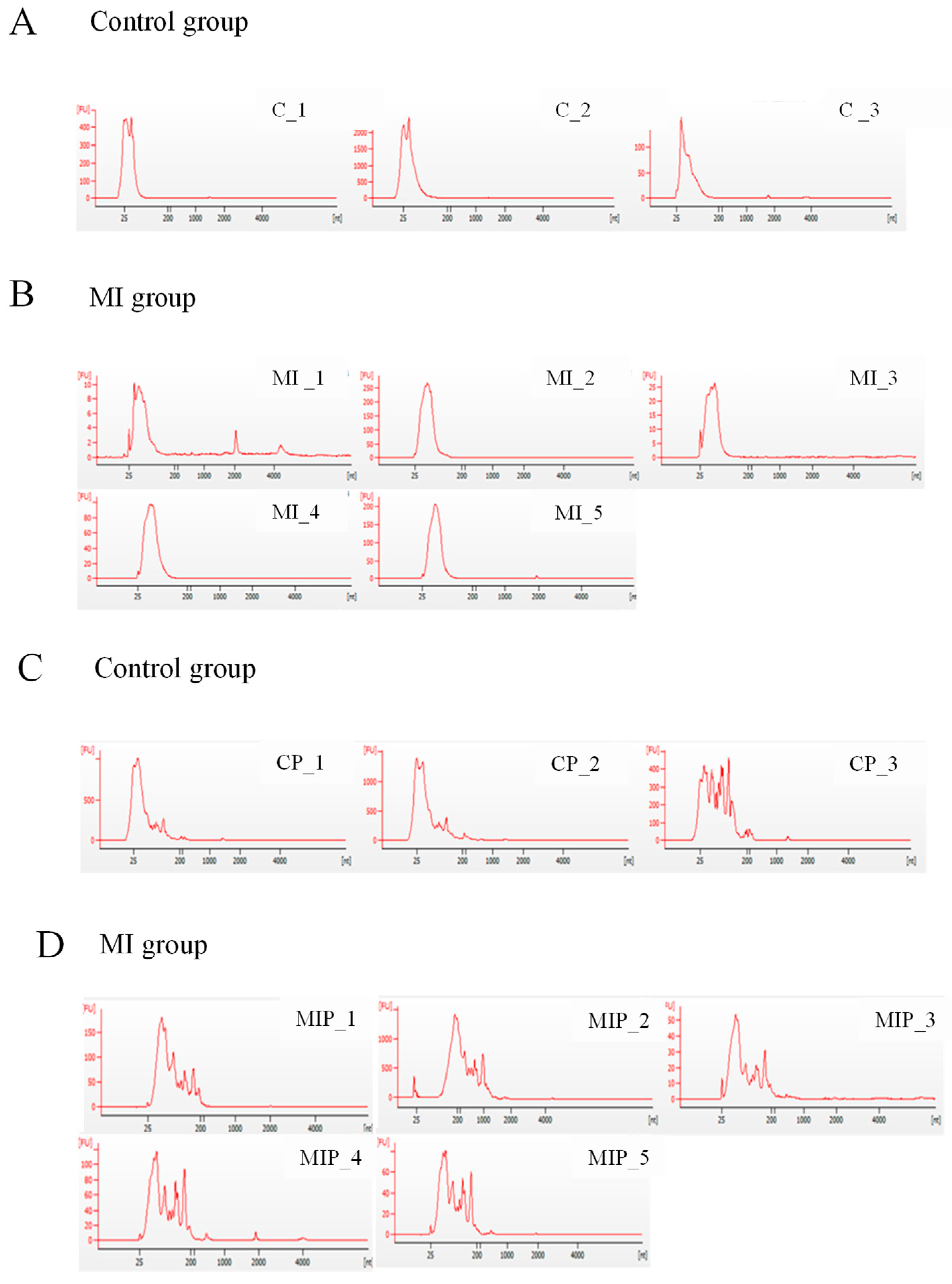
2.1. Quality of Exosomal RNA Isolated from Postmortem Plasma and Pericardial Fluid
2.2. Analysis of Small RNA Profiling of Postmortem Exosomes Using Next-Generation Sequencing
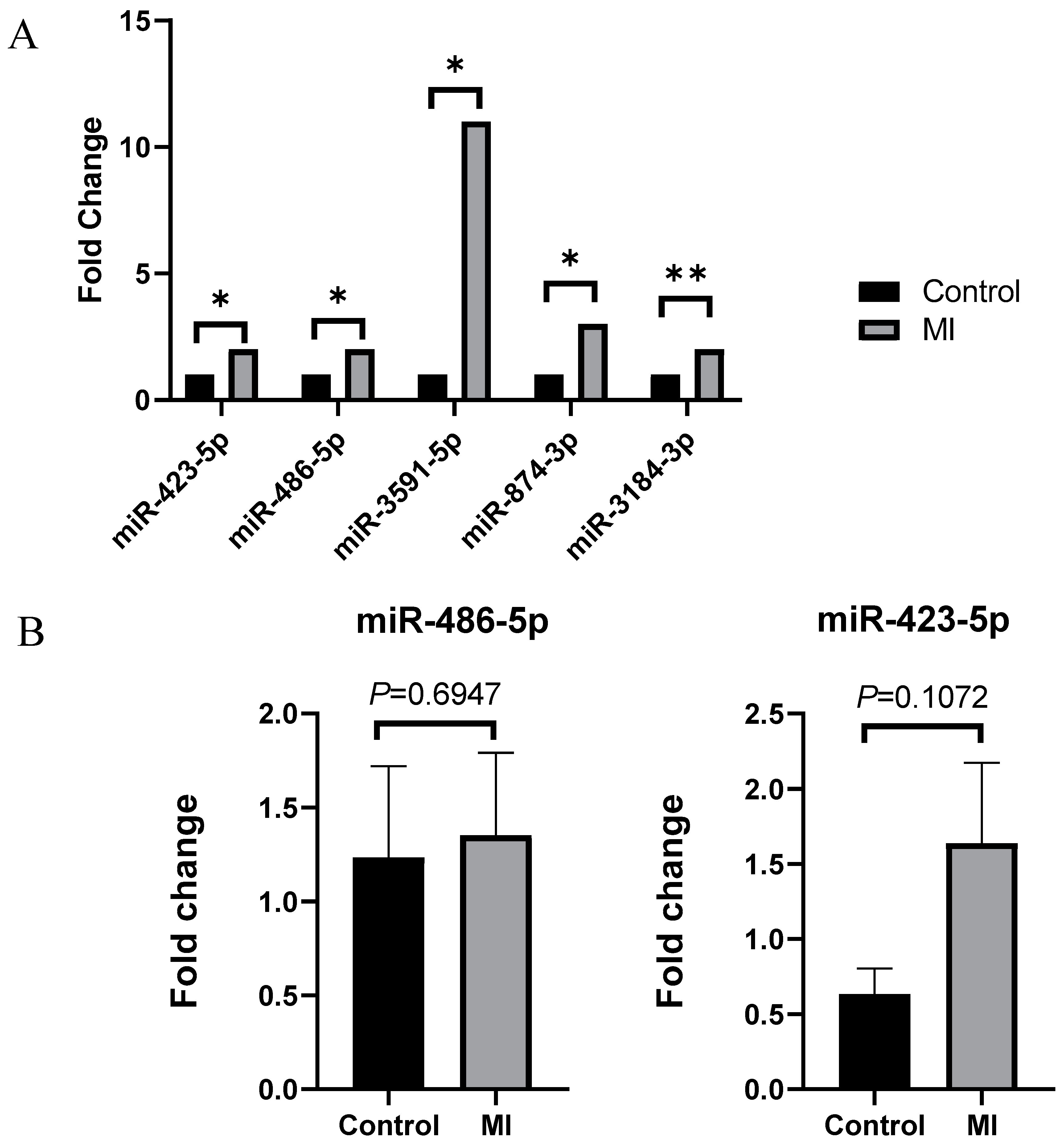
2.3. Validation of Significantly Increased miRNA
2.4. miR-486-5p Expression in Advanced Atherosclerotic Lesions
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Information of Samples Used for Experiments
4.3. Exosomal RNA Isolation and Quality Control Analysis
4.4. Next-Generation Sequencing (NGS) Procedure
4.5. Validation Using Quantitative RT-PCR
4.6. Histopathology for Postmortem Human Coronary Artery
4.7. Statistical Analysis
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Morrow, D.A. Acute myocardial infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Na, J.-Y.; Lee, B.W.; Yang, K.-m.; Choi, Y.S. A statistical analysis on forensic autopsies performed in Korea in 2017. Korean J. Leg. Med. 2018, 42, 111–125. [Google Scholar] [CrossRef]
- Batalis, N.I.; Marcus, B.J.; Papadea, C.N.; Collins, K.A. The role of postmortem cardiac markers in the diagnosis of acute myocardial infarction. J. Forensic Sci. 2010, 55, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Michaud, K.; Basso, C.; d’Amati, G.; Giordano, C.; Kholová, I.; Preston, S.D.; Rizzo, S.; Sabatasso, S.; Sheppard, M.N.; Vink, A. Diagnosis of myocardial infarction at autopsy: AECVP reappraisal in the light of the current clinical classification. Virchows Arch. 2020, 476, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Hiyamizu, S.; Ishida, Y.; Yasuda, H.; Kuninaka, Y.; Nosaka, M.; Ishigami, A.; Shimada, E.; Kimura, A.; Yamamoto, H.; Osako, M.; et al. Forensic significance of intracardiac expressions of Nrf2 in acute myocardial ischemia. Sci. Rep. 2024, 14, 4046. [Google Scholar] [CrossRef]
- Kuninaka, Y.; Ishida, Y.; Nosaka, M.; Ishigami, A.; Taruya, A.; Shimada, E.; Kimura, A.; Yamamoto, H.; Ozaki, M.; Furukawa, F.; et al. Forensic significance of intracardiac heme oxygenase-1 expression in acute myocardial ischemia. Sci. Rep. 2021, 11, 21828. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Bang, C.; Thum, T. Exosomes: New players in cell–cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef]
- Zhu, Q.; Heon, M.; Zhao, Z.; He, M. Microfluidic engineering of exosomes: Editing cellular messages for precision therapeutics. Lab. A Chip 2018, 18, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Beltrami, C.; Besnier, M.; Shantikumar, S.; Shearn, A.I.; Rajakaruna, C.; Laftah, A.; Sessa, F.; Spinetti, G.; Petretto, E.; Angelini, G.D. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol. Ther. 2017, 25, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Bellin, G.; Gardin, C.; Ferroni, L.; Chachques, J.C.; Rogante, M.; Mitrečić, D.; Ferrari, R.; Zavan, B. Exosome in cardiovascular diseases: A complex world full of hope. Cells 2019, 8, 166. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Jang, S.; Lee, S.; Park, J.-T.; Lee, S.-J.; Kim, H.-S. Characterization of Exosomes and Exosomal RNAs Isolated from Post-Mortem Body Fluids for Molecular Forensic Diagnosis. Diagnostics 2022, 12, 2153. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Hutvagner, G.; Zamore, P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Turchinovich, A.; Samatov, T.R.; Tonevitsky, A.G.; Burwinkel, B. Circulating miRNAs: Cell–cell communication function? Front. Genet. 2013, 4, 119. [Google Scholar] [CrossRef]
- Xie, Y.; Dang, W.; Zhang, S.; Yue, W.; Yang, L.; Zhai, X.; Yan, Q.; Lu, J. The role of exosomal noncoding RNAs in cancer. Mol. Cancer 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L. Circulating exosomal miRNA as diagnostic biomarkers of neurodegenerative diseases. Front. Mol. Neurosci. 2020, 13, 53. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, H.; Pan, X.; Liao, M.; Hou, Y. A model for data analysis of microRNA expression in forensic body fluid identification. Forensic Sci. Int. Genet. 2012, 6, 419–423. [Google Scholar] [CrossRef]
- Silva, S.S.; Lopes, C.; Teixeira, A.; De Sousa, M.C.; Medeiros, R. Forensic miRNA: Potential biomarker for body fluids? Forensic Sci. Int. Genet. 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Manabe, S.; Morimoto, C.; Ozeki, M.; Hamano, Y.; Hirai, E.; Kotani, H.; Tamaki, K. Distinct spectrum of microRNA expression in forensically relevant body fluids and probabilistic discriminant approach. Sci. Rep. 2019, 9, 14332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Luo, H.; Ye, Y.; Yan, J.; Hou, Y. Screening and confirmation of microRNA markers for forensic body fluid identification. Forensic Sci. Int. Genet. 2013, 7, 116–123. [Google Scholar] [CrossRef]
- Harbison, S.; Fleming, R. Forensic body fluid identification: State of the art. Res. Rep. Forensic Med. Sci. 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Kanno, S.; Sakamoto, T.; Fukuta, M.; Kato, H.; Aoki, Y. Stability of exosomes in the postmortem serum and preliminary study on exosomal miRNA expression profiling in serum from myocardial infarction cadavers. Int. J. Leg. Med. 2023, 137, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sarkar, S.; Choudhury, C.; Singh, L.; Singh, H.; Chakraborti, A. Alpha-1-antitrypsin in serum exosomes and pericardial fluid exosomes is associated with severity of rheumatic heart disease. Mol. Cell Biochem. 2023, 478, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, G.; Giulietti, M.; Principato, G.; Piva, F. The choice of endogenous controls in exosomal microRNA assessments from biofluids. Tumor Biol. 2016, 37, 11657–11665. [Google Scholar] [CrossRef]
- Wei, T.; Folkersen, L.; Ehrenborg, E.; Gabrielsen, A. MicroRNA 486-3P as a stability marker in acute coronary syndrome. Biosci. Rep. 2016, 36, e00351. [Google Scholar] [CrossRef]
- Niculescu, L.S.; Simionescu, N.; Sanda, G.M.; Carnuta, M.G.; Stancu, C.S.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; Simionescu, M. MiR-486 and miR-92a identified in circulating HDL discriminate between stable and vulnerable coronary artery disease patients. PLoS ONE 2015, 10, e0140958. [Google Scholar] [CrossRef]
- Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Rivas, M.; Oliva, P.; Villafani, J.; Navarro, A.; Blanco-López, M.C.; Cernuda-Morollón, E. Characterization of plasma-derived extracellular vesicles isolated by different methods: A comparison study. Bioengineering 2019, 6, 8. [Google Scholar] [CrossRef]
- Stary, H.C. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1177–1178. [Google Scholar] [CrossRef]
- Bhanvadia, V.M.; Desai, N.J.; Agarwal, N.M. Study of coronary atherosclerosis by modified american heart association classification of atherosclerosis-an autopsy study. J. Clin. Diagn. Res. JCDR 2013, 7, 2494. [Google Scholar] [CrossRef]
- Pelisek, J.; Hegenloh, R.; Bauer, S.; Metschl, S.; Pauli, J.; Glukha, N.; Busch, A.; Reutersberg, B.; Kallmayer, M.; Trenner, M. Biobanking: Objectives, requirements, and future challenges—Experiences from the Munich Vascular Biobank. J. Clin. Med. 2019, 8, 251. [Google Scholar] [CrossRef]


| miRNA ID | Control (log2) | Myocardial Infarction (log2) | Fold Change (H/CH) | p-Value | |
|---|---|---|---|---|---|
| Up | hsa-miR-3591-5p | 2.374 | 5.950 | 11.929 | 0.027 |
| hsa-miR-874-3p | 3.845 | 5.575 | 3.319 | 0.024 | |
| hsa-miR-423-5p | 10.870 | 12.405 | 2.897 | 0.012 | |
| hsa-miR-486-5p | 13.363 | 14.698 | 2.522 | 0.045 | |
| hsa-miR-3184-3p | 10.918 | 12.249 | 2.516 | 0.009 | |
| Down | hsa-miR-4755-5p | 5.183 | 0.000 | 0.028 | 0.017 |
| hsa-miR-554 | 4.762 | 0.000 | 0.037 | 0.018 | |
| hsa-miR-4632-3p | 5.916 | 2.006 | 0.067 | 0.030 | |
| hsa-miR-6729-3p | 6.337 | 2.540 | 0.072 | 0.033 | |
| hsa-miR-1296-5p | 4.762 | 1.363 | 0.095 | 0.026 | |
| hsa-miR-7107-5p | 5.586 | 2.483 | 0.116 | 0.005 | |
| hsa-miR-3687 | 6.120 | 3.129 | 0.126 | 0.045 | |
| hsa-miR-411-3p | 4.969 | 2.051 | 0.132 | 0.008 | |
| hsa-miR-1237-3p | 4.762 | 2.006 | 0.148 | 0.043 | |
| hsa-miR-6754-3p | 4.969 | 2.967 | 0.250 | 0.050 | |
| hsa-miR-28-3p | 9.886 | 9.068 | 0.568 | 0.048 |
| miRNA ID | Control (log2) | Myocardial Infarction (log2) | Fold Change (H/CH) | p-Value | |
|---|---|---|---|---|---|
| Up | hsa-miR-4443 | 8.389 | 10.849 | 5.503 | 0.009 |
| hsa-miR-625-3p | 3.916 | 6.253 | 5.053 | 0.005 | |
| Down | hsa-miR-1295a | 6.007 | 0.000 | 0.016 | 0.035 |
| hsa-miR-4494 | 6.007 | 0.000 | 0.016 | 0.035 | |
| hsa-miR-212-5p | 5.593 | 0.000 | 0.021 | 0.046 | |
| hsa-miR-4781-3p | 7.629 | 2.830 | 0.036 | 0.026 | |
| hsa-miR-379-5p | 7.035 | 2.354 | 0.039 | 0.029 | |
| hsa-miR-5585-5p | 6.658 | 2.354 | 0.051 | 0.029 | |
| hsa-miR-214-3p | 6.084 | 2.150 | 0.065 | 0.024 | |
| hsa-miR-1287-5p | 7.808 | 3.899 | 0.067 | 0.039 | |
| hsa-miR-5095 | 7.676 | 5.002 | 0.157 | 0.050 | |
| hsa-miR-4497 | 11.467 | 8.905 | 0.169 | 0.041 | |
| hsa-miR-1285-3p | 7.775 | 5.717 | 0.240 | 0.043 | |
| hsa-miR-107 | 6.973 | 5.387 | 0.333 | 0.023 |
| Control Group (n = 13) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age | Sex | BMI (kg/m) | PMI | Cause of Death | Heart Weight (g) | Risk Factors | Luminal Stenosis (%) | Lesion Type | ||
| DM | HTN | Smoking | |||||||||
| 1 | 52 | F | 22.2 | 26 h 10 m | Hanging | 300 | A + | A | A | 0 | I |
| 2 | 21 | F | 21.5 | 15 h 50 m | Sudden manhood death syndrome | 264 | A | A | A | 0 | I |
| 3 | 47 | F | 19.4 | 75 h 30 m | Drug intoxication | 276 | A | A | A | 56 | III |
| 4 | 70 | F | 23.4 | 42 h 10 m | Manual strangulation | 336 | A | A | A | 0 | I |
| 5 | 65 | M | 26.7 | 65 h 40 m | Drug intoxication | 460 | A | A | A | 83 | VII |
| 6 | 64 | M | 22.5 | 35 h 30 m | Drug intoxication | 372 | A | A | A | 83 | VI |
| 7 | 56 | F | 21.4 | 39 h 30 m | Hanging | 320 | A | A | A | 84 | VI |
| 8 | 58 | M | 16.5 | 53 h 50 m | Asphyxia | 304 | A | A | A | 66 | V |
| 9 | 50 | M | 28.1 | 87 h 00 m | Subarachnoid hemorrhage | 452 | A | A | A | 73 | IV |
| 10 | 74 | F | 19.9 | 65 h 30 m | Non-traumatic cerebral hemorrhage | 402 | A | A | A | 62 | IV |
| 11 | 54 | M | 16.2 | 45 h 30 m | Drug intoxication | 406 | A | A | A | 87 | VII |
| 12 | 25 | M | 27.4 | 86 h 00 m | Drug intoxication | 344 | A | A | A | 0 | I |
| 13 | 59 | F | 22.1 | 25 h 10 m | Drug intoxication | 372 | A | A | A | 0 | Ⅰ |
| MI Group (n = 24) | |||||||||||
| No. | Age | Sex | BMI (kg/m) | PMI | Cause of Death | Heart Weight (g) | Risk Factors | Luminal Stenosis (%) | Lesion Type | ||
| DM | HTN | Smoking | |||||||||
| 1 | 66 | M | 29.3 | 31 h 50 m | Ischemic heart disease including acute myocardial infarction | 580 | A | P | A | 78 | VII |
| 2 | 52 | M | 31.7 | 39 h 20 m | 680 | A | A | A | 88 | V | |
| 3 | 54 | M | 26.2 | 21 h 40 m | 412 | A | A | A | 100 | VI * | |
| 4 | 55 | M | 26.7 | 24 h 20 m | 426 | A | A | A | 88 | V | |
| 5 | 49 | M | 24.8 | 48 h 55 m | 388 | A | A | A | 86 | V | |
| 6 | 59 | M | 22.4 | 37 h 50 m | 378 | A | A | A | 95 | V | |
| 7 | 35 | M | 21.4 | 48 h 15 m | 370 | A | A | A | 76 | VI | |
| 8 | 49 | M | 28.2 | 56 h 20 m | 434 | A | A | A | 95 | V | |
| 9 | 32 | M | 24.3 | 26 h 30 m | 422 | A | A | A | 96 | V | |
| 10 | 63 | M | 21 | 27 h 00 m | 444 | A | P | P | 75 | VII | |
| 11 | 55 | M | 24.8 | 42 h 05 m | 460 | A | A | A | 88 | VI | |
| 12 | 46 | M | 26.6 | 25 h 50 m | 404 | A | A | P | 84 | V | |
| 13 | 39 | M | 29.2 | 42 h 10 m | 530 | A | A | A | 94 | VI | |
| 14 | 76 | M | 15.3 | 39 h 00 m | 362 | A | A | A | 100 | V * | |
| 15 | 63 | M | 25.4 | 48 h 25 m | 520 | A | A | A | 85 | VI | |
| 16 | 55 | M | 25.1 | 97 h 05 m | 408 | A | A | A | 82 | VI | |
| 17 | 62 | M | 19.4 | 58 h 50 m | 400 | A | A | A | 87 | V | |
| 18 | 55 | M | 22.0 | 64 h 40 m | 422 | A | A | A | 89 | VI | |
| 19 | 52 | M | 27.4 | 26 h 30 m | 764 | A | A | A | 80 | V | |
| 20 | 57 | M | 25.5 | 67 h 50 m | 420 | A | A | A | 75 | VII | |
| 21 | 66 | M | 23.3 | 26 h 00 m | 610 | P | A | A | 85 | VII | |
| 22 | 59 | F | 18.9 | 97 h 45 m | 446 | A | A | A | 91 | VII | |
| 23 | 40 | M | 29.5 | 35 h 00 m | 462 | A | A | A | 92 | VII | |
| 24 | 44 | M | 22.5 | 29 h 00 m | 452 | A | A | A | 75 | VII | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Lee, S.; Park, J.-T.; Lee, S.-J.; Kim, H.-S. Postmortem-Derived Exosomal MicroRNA 486-5p as Potential Biomarkers for Ischemic Heart Disease Diagnosis. Int. J. Mol. Sci. 2024, 25, 9619. https://doi.org/10.3390/ijms25179619
Kim S-Y, Lee S, Park J-T, Lee S-J, Kim H-S. Postmortem-Derived Exosomal MicroRNA 486-5p as Potential Biomarkers for Ischemic Heart Disease Diagnosis. International Journal of Molecular Sciences. 2024; 25(17):9619. https://doi.org/10.3390/ijms25179619
Chicago/Turabian StyleKim, So-Yeon, Sookyoung Lee, Jong-Tae Park, Su-Jin Lee, and Hyung-Seok Kim. 2024. "Postmortem-Derived Exosomal MicroRNA 486-5p as Potential Biomarkers for Ischemic Heart Disease Diagnosis" International Journal of Molecular Sciences 25, no. 17: 9619. https://doi.org/10.3390/ijms25179619
APA StyleKim, S.-Y., Lee, S., Park, J.-T., Lee, S.-J., & Kim, H.-S. (2024). Postmortem-Derived Exosomal MicroRNA 486-5p as Potential Biomarkers for Ischemic Heart Disease Diagnosis. International Journal of Molecular Sciences, 25(17), 9619. https://doi.org/10.3390/ijms25179619






