Activated THP-1 Macrophage-Derived Factors Increase the Cytokine, Fractalkine, and EGF Secretions, the Invasion-Related MMP Production, and Antioxidant Activity of HEC-1A Endometrium Cells
Abstract
1. Introduction
2. Results
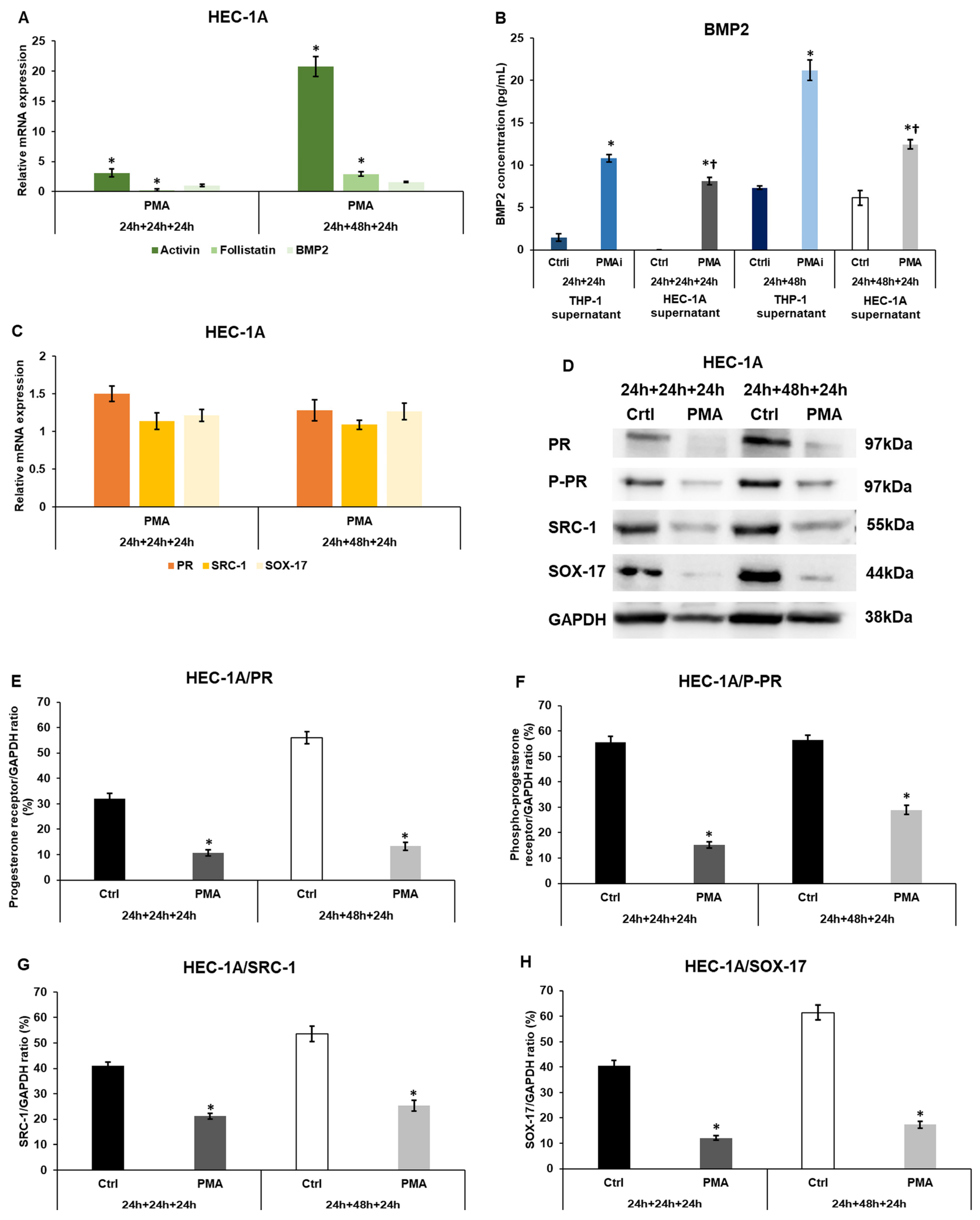
2.1. Macrophage-Derived Factors Regulate the Expression of Receptivity-Related Genes of HEC-1A Cells
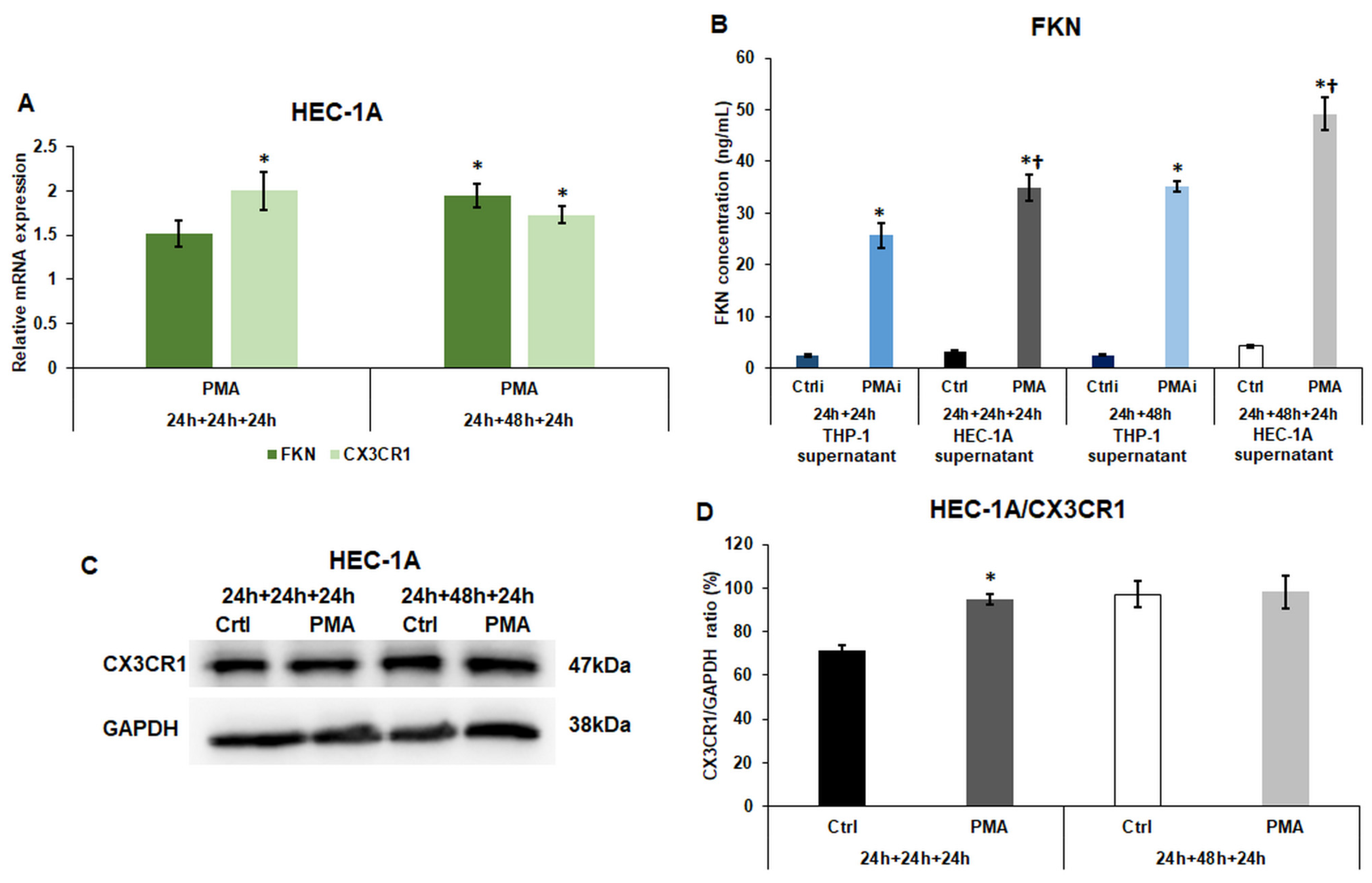
2.2. Macrophage-Derived Factors Modify the Expression of Fractalkine and Its Receptor CX3CR1 of HEC-1A Cells
2.3. The Effect of Macrophage-Derived Factors on the Cytokine Production of the Endometrium Cells
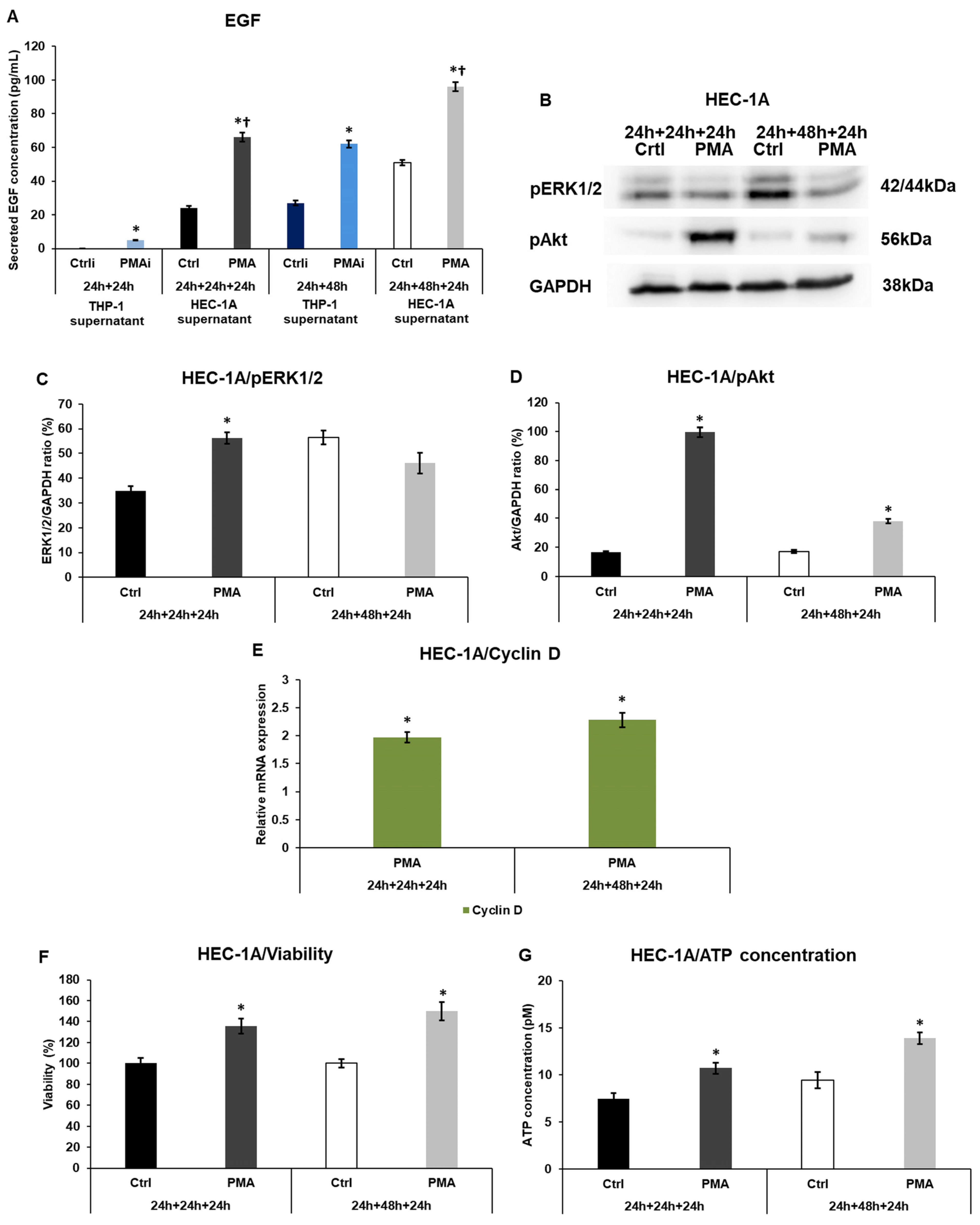
2.4. Macrophage-Related Factors Alter the EGF Secretion, Akt, and ERK1/2 Activation, Regulating the Growth and Proliferation of the Endometrium Cells
2.5. Macrophage-Related Factors Alter the MMP2, and MMP9 Secretion and TIMP1 and 2 Involved in Invasion
2.6. Macrophages Trigger the Antioxidant Protection of the Endometrium Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Treatments
4.2. Real-Time PCR
4.3. Western Blot
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) Measurements
4.5. Cell Viability Measurement
4.6. ATP Concentration Determination
4.7. Total Antioxidant Capacity (TAC) Measurement
4.8. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in Immunoregulation and Therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Jiang, L.; Zhang, C.; Liu, M.; Luo, Y.; Hu, Z.; Mou, X.; Zhu, Y. Biologic Mechanisms of Macrophage Phenotypes Responding to Infection and the Novel Therapies to Moderate Inflammation. Int. J. Mol. Sci. 2023, 24, 8358. [Google Scholar] [CrossRef]
- Woottum, M.; Yan, S.; Sayettat, S.; Grinberg, S.; Cathelin, D.; Bekaddour, N.; Herbeuval, J.-P.; Benichou, S. Macrophages: Key Cellular Players in HIV Infection and Pathogenesis. Viruses 2024, 16, 288. [Google Scholar] [CrossRef]
- Belgiovine, C.; D’Incalci, M.; Allavena, P.; Frapolli, R. Tumor-Associated Macrophages and Anti-Tumor Therapies: Complex Links. Cell. Mol. Life Sci. 2016, 73, 2411–2424. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Thiruchelvam, U.; Dransfield, I.; Saunders, P.T.K.; Critchley, H.O.D. The Importance of the Macrophage within the Human Endometrium. J. Leukoc. Biol. 2013, 93, 217–225. [Google Scholar] [CrossRef]
- Brown, E.; Martínez-Aguilar, R.; Maybin, J.A.; Gibson, D.A. Endometrial Macrophages in Health and Disease. Int. Rev. Cell Mol. Biol. 2022, 367, 183–208. [Google Scholar]
- Chambers, M.; Rees, A.; Cronin, J.G.; Nair, M.; Jones, N.; Thornton, C.A. Macrophage Plasticity in Reproduction and Environmental Influences on Their Function. Front. Immunol. 2021, 11, 607328. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cai, S.; Yang, L.; Wang, L.; Ding, J.; Li, L.; Li, H.; Huang, C.; Diao, L. How Do Pre-Pregnancy Endometrial Macrophages Contribute to Pregnancy? J. Reprod. Immunol. 2022, 154, 103736. [Google Scholar] [CrossRef]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and Functions. ChemTexts 2022, 8, 12. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Robertson, S.A.; Jasper, M.J.; Bromfield, J.J.; Care, A.S.; Nakamura, H.; Ingman, W.V. The Role of Macrophages in Implantation and Early Pregnancy Success. Biol. Reprod. 2008, 78, 274–275. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.J.; Donners, M.M.P.C. Anti-Inflammatory M2, but Not pro-Inflammatory M1 Macrophages Promote Angiogenesis in Vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Pitman, H.; Morgan, H.L.; Innes, B.A.; Agwu, C.N.; Bulmer, J.N. Decidual Macrophages: Key Regulators of Vascular Remodeling in Human Pregnancy. J. Leukoc. Biol. 2016, 100, 315–325. [Google Scholar] [CrossRef]
- Pinhal-Enfield, G.; Leibovich, J.; Vas, N. The Role of Macrophages in the Placenta. In Embryology—Updates and Highlights on Classic Topics; InTech: Philadelphia, PA, USA, 2012. [Google Scholar]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 Macrophages and Their Overlaps—Myth or Reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Krop, J.; Tian, X.; van der Hoorn, M.-L.; Eikmans, M. The Mac Is Back: The Role of Macrophages in Human Healthy and Complicated Pregnancies. Int. J. Mol. Sci. 2023, 24, 5300. [Google Scholar] [CrossRef]
- Bajpai, K.; Acharya, N.; Prasad, R.; Wanjari, M.B. Endometrial Receptivity During the Preimplantation Period: A Narrative Review. Cureus 2023, 15, e37753. [Google Scholar] [CrossRef]
- Cope, D.; Monsivais, D. Progesterone Receptor Signaling in the Uterus Is Essential for Pregnancy Success. Cells 2022, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, S.; Salamonsen, L.A.; Francois, M.; Harley, V.; Evans, J. Uterine SOX17: A Key Player in Human Endometrial Receptivity and Embryo Implantation. Sci. Rep. 2019, 9, 15495. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Salamonsen, L.A.; Findlay, J.K. Potential Roles for Endometrial Inhibins, Activins and Follistatin during Human Embryo Implantation and Early Pregnancy. Trends Endocrinol. Metab. 2002, 13, 144–150. [Google Scholar] [CrossRef]
- Coutanceau, B.; Dos Santos, E.; Swierkowski Blanchard, N.; Sanchez Louboutin, A.; Boitrelle, F.; Margueritte, F.; Vialard, F.; Serazin, V.; Fathallah, K. Should the Treatment of Patients with Repeated Embryo Implantation Failure Be Adapted as a Function of the Endometrial Cytokine Profile? A Single-Center Experience. Biomedicines 2023, 11, 817. [Google Scholar] [CrossRef]
- Robertson, S.A.; Moldenhauer, L.M.; Green, E.S.; Care, A.S.; Hull, M.L. Immune Determinants of Endometrial Receptivity: A Biological Perspective. Fertil. Steril. 2022, 117, 1107–1120. [Google Scholar] [CrossRef]
- Pandur, E.; Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L. Fractalkine Enhances Endometrial Receptivity and Activates Iron Transport towards Trophoblast Cells in an in Vitro Co-Culture System of HEC-1A and JEG-3 Cells. Exp. Cell Res. 2021, 403, 112583. [Google Scholar] [CrossRef]
- Pantos, K.; Grigoriadis, S.; Maziotis, E.; Pistola, K.; Xystra, P.; Pantou, A.; Kokkali, G.; Pappas, A.; Lambropoulou, M.; Sfakianoudis, K.; et al. The Role of Interleukins in Recurrent Implantation Failure: A Comprehensive Review of the Literature. Int. J. Mol. Sci. 2022, 23, 2198. [Google Scholar] [CrossRef]
- Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L.; Pandur, E. Fractalkine Regulates HEC-1A/JEG-3 Interaction by Influencing the Expression of Implantation-Related Genes in an In Vitro Co-Culture Model. Int. J. Mol. Sci. 2020, 21, 3175. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, Y.; Murata, H.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; Tanaka, S.; Okada, H. Matrix Metalloproteinases in Human Decidualized Endometrial Stromal Cells. Curr. Issues Mol. Biol. 2021, 43, 2111–2123. [Google Scholar] [CrossRef]
- Didziokaite, G.; Biliute, G.; Gudaite, J.; Kvedariene, V. Oxidative Stress as a Potential Underlying Cause of Minimal and Mild Endometriosis-Related Infertility. Int. J. Mol. Sci. 2023, 24, 3809. [Google Scholar] [CrossRef] [PubMed]
- Artimovič, P.; Badovská, Z.; Toporcerová, S.; Špaková, I.; Smolko, L.; Sabolová, G.; Kriváková, E.; Rabajdová, M. Oxidative Stress and the Nrf2/PPARγ Axis in the Endometrium: Insights into Female Fertility. Cells 2024, 13, 1081. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A Novel and Compact Review on the Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The Choice of Phorbol 12-Myristate 13-Acetate Differentiation Protocol Influences the Response of THP-1 Macrophages to a pro-Inflammatory Stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef]
- Sehring, J.; Beltsos, A.; Jeelani, R. Human Implantation: The Complex Interplay between Endometrial Receptivity, Inflammation, and the Microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Racicot, K.; Mor, G. The Role of Inflammation for a Successful Implantation. Am. J. Reprod. Immunol. 2014, 72, 141–147. [Google Scholar] [CrossRef]
- Gnainsky, Y.; Granot, I.; Aldo, P.B.; Barash, A.; Or, Y.; Schechtman, E.; Mor, G.; Dekel, N. Local Injury of the Endometrium Induces an Inflammatory Response That Promotes Successful Implantation. Fertil. Steril. 2010, 94, 2030–2036. [Google Scholar] [CrossRef]
- Hickman, E.; Smyth, T.; Cobos-Uribe, C.; Immormino, R.; Rebuli, M.E.; Moran, T.; Alexis, N.E.; Jaspers, I. Expanded Characterization of in Vitro Polarized M0, M1, and M2 Human Monocyte-Derived Macrophages: Bioenergetic and Secreted Mediator Profiles. PLoS ONE 2023, 18, e0279037. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Chen, R. An M0 Macrophage-Related Prognostic Model for Hepatocellular Carcinoma. BMC Cancer 2022, 22, 791. [Google Scholar] [CrossRef]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 Macrophage Polarity in Normal and Complicated Pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef]
- DeMayo, F.J.; Lydon, J.P. 90 YEARS OF PROGESTERONE: New Insights into Progesterone Receptor Signaling in the Endometrium Required for Embryo Implantation. J. Mol. Endocrinol. 2020, 65, T1–T14. [Google Scholar] [CrossRef]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef]
- Ryu, B.J.; Han, J.W.; Kim, R.H.; Yun, S.; Kim, T.H.; Hur, S.E.; Kim, C.J.; Lee, S.K. Activation of NOD-1/JNK/IL-8 signal axis in decidual stromal cells facilitates trophoblast invasion. Am. J. Reprod. Immunol. 2017, 78, e12672. [Google Scholar] [CrossRef] [PubMed]
- ARICI, A. Local Cytokines in Endometrial Tissue: The Role of Interleukin-8 in the Pathogenesis of Endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 101–109. [Google Scholar] [CrossRef]
- Nishimoto-Kakiuchi, A.; Sato, I.; Nakano, K.; Ohmori, H.; Kayukawa, Y.; Tanimura, H.; Yamamoto, S.; Sakamoto, Y.; Nakamura, G.; Maeda, A.; et al. A Long-Acting Anti–IL-8 Antibody Improves Inflammation and Fibrosis in Endometriosis. Sci. Transl. Med. 2023, 15, eabq5858. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The Chemokines, CX3CL1, CCL14, and CCL4, Promote Human Trophoblast Migration at the Feto-Maternal Interface1. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Beamer, M.; Ahmed, S. Fractalkine/CX3CL1: A Potential New Target for Inflammatory Diseases. Mol. Interv. 2010, 10, 263. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Haining, R.E.B.; Cameron, I.T.; van Papendorp, C.; Davenport, A.P.; Prentice, A.; Thomas, E.J.; Smith, S.K. Epidermal Growth Factor in Human Endometrium: Proliferative Effects in Culture and Immunocytochemical Localization in Normal and Endometriotic Tissues. Hum. Reprod. 1991, 6, 1200–1205. [Google Scholar] [CrossRef]
- Large, M.J.; Wetendorf, M.; Lanz, R.B.; Hartig, S.M.; Creighton, C.J.; Mancini, M.A.; Kovanci, E.; Lee, K.-F.; Threadgill, D.W.; Lydon, J.P.; et al. The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy. PLoS Genet. 2014, 10, e1004451. [Google Scholar] [CrossRef]
- Zou, J.; Lei, T.; Guo, P.; Yu, J.; Xu, Q.; Luo, Y.; Ke, R.; Huang, D. Mechanisms Shaping the Role of ERK1/2 in Cellular Sene Scence (Review). Mol. Med. Rep. 2018, 19, 759–770. [Google Scholar] [CrossRef]
- Xu, N.; Lao, Y.; Zhang, Y.; Gillespie, D.A. Akt: A Double-Edged Sword in Cell Proliferation and Genome Stability. J. Oncol. 2012, 2012, 951724. [Google Scholar] [CrossRef]
- Caudron-Herger, M.; Diederichs, S. Insights from the Degradation Mechanism of Cyclin D into Targeted Therapy of the Cancer Cell Cycle. Signal Transduct. Target. Ther. 2021, 6, 311. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Ou, J.; Zhang, S.; Ming, Y. Crosstalk between the CX3CL1/CX3CR1 Axis and Inflammatory Signaling Pathways in Tissue Injury. Curr. Protein Pept. Sci. 2019, 20, 844–854. [Google Scholar] [CrossRef]
- Loh, S.X.; Ekinci, Y.; Spray, L.; Jeyalan, V.; Olin, T.; Richardson, G.; Austin, D.; Alkhalil, M.; Spyridopoulos, I. Fractalkine Signalling (CX3CL1/CX3CR1 Axis) as an Emerging Target in Coronary Artery Disease. J. Clin. Med. 2023, 12, 4821. [Google Scholar] [CrossRef] [PubMed]
- Siarkou, C.M.; Prapas, Y.; Petousis, S.; Milias, S.; Ravanos, K.; Kalogiannidis, I.; Mavromatidis, G.; Haitoglou, C.; Prapas, N.; Rousso, D. LIF and LIF-R Expression in the Endometrium of Fertile and Infertile Women: A Prospective Observational Case-Control Study. Mol. Med. Rep. 2016, 13, 4721–4728. [Google Scholar] [CrossRef] [PubMed]
- Perrier d’Hauterive, S.; Charlet-Renard, C.; Dubois, M.; Berndt, S.; Goffin, F.; Foidart, J.-M.; Geenen, V. Human Endometrial Leukemia Inhibitory Factor and Interleukin-6: Control of Secretion by Transforming Growth Factor-β-Related Members. Neuroimmunomodulation 2005, 12, 157–163. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Noss, E.H.; Mizoguchi, F.; Huppertz, C.; Wei, K.S.; Watts, G.F.M.; Brenner, M.B. Autocrine Loop Involving IL-6 Family Member LIF, LIF Receptor, and STAT4 Drives Sustained Fibroblast Production of Inflammatory Mediators. Immunity 2017, 46, 220–232. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef]
- Tapia, A.; Salamonsen, L.A.; Manuelpillai, U.; Dimitriadis, E. Leukemia Inhibitory Factor Promotes Human First Trimester Extravillous Trophoblast Adhesion to Extracellular Matrix and Secretion of Tissue Inhibitor of Metalloproteinases-1 and -2. Hum. Reprod. 2008, 23, 1724–1732. [Google Scholar] [CrossRef]
- Kothari, P.; Pestana, R.; Mesraoua, R.; Elchaki, R.; Khan, K.M.F.; Dannenberg, A.J.; Falcone, D.J. IL-6–Mediated Induction of Matrix Metalloproteinase-9 Is Modulated by JAK-Dependent IL-10 Expression in Macrophages. J. Immunol. 2014, 192, 349–357. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S. Understanding the Molecular Mechanisms of Macrophage Polarization and Metabolic Reprogramming in Endometriosis: A Narrative Review. Reprod. Med. Biol. 2022, 21, e12488. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for Study of Human Embryo Implantation: Choice of Cell Lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Riera-Escamilla, A.; Gou, G.; Piñol-Felis, C.; Motilva, M.-J. Design, Optimization and Validation of Genes Commonly Used in Expression Studies on DMH/AOM Rat Colon Carcinogenesis Model. PeerJ 2019, 7, e6372. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Pap, R.; Jánosa, G.; Horváth, A.; Sipos, K. Fractalkine Improves the Expression of Endometrium Receptivity-Related Genes and Proteins at Desferrioxamine-Induced Iron Deficiency in HEC-1A Cells. Int. J. Mol. Sci. 2023, 24, 7924. [Google Scholar] [CrossRef]
- Ansar Ahmed, S.; Gogal, R.M.; Walsh, J.E. A New Rapid and Simple Non-Radioactive Assay to Monitor and Determine the Proliferation of Lymphocytes: An Alternative to [3H]Thymidine Incorporation Assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.D.; Sutherland, L.A.; Hunter, E.M.; Cree, I.A. Comparison of MTT and ATP-based Assays for the Measurement of Viable Cell Number. J. Biolumin. Chemilumin. 1995, 10, 29–34. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Fritz, F.R.; Nagy, T.; Agócs, A.; Deli, J. Protective Effects of 3′-Epilutein and 3′-Oxolutein against Glutamate-Induced Neuronal Damage. Int. J. Mol. Sci. 2023, 24, 12008. [Google Scholar] [CrossRef]




| Primer | Sequence 5′ → 3′ |
|---|---|
| Activin forward | TGTTCCAATATGATTCCACCC |
| Activin reverse | CCACTTGATTTTGGAGGGAT |
| BMP2 forward | TAAGTTCTATCCCCACGGAG |
| BMP2 reverse | AGCATCTTGCATCTGTTCTC |
| CX3CR1 forward | ATTTGTTGGTAGTGTTTGCC |
| CX3CR1 reverse | CAGGTTCAGGAGGTAAATGT |
| Cyclin D forward | CTGGTGAACAAGCTCAAGTG |
| Cyclin D reverse | GCGGATGATCTGTTTGTTCT |
| Follistatin forward | CAAAGCAAAGTCCTGTGAAG |
| Follistatin reverse | CCTCTCCCAACCTTGAAATC |
| Fractalkine forward | TACCTGTAGCTTTGCTCATC |
| Fractalkine reverse | GTCTCGTCTCCAAGATGATT |
| GAPDH forward | TGTTCCAATATGATTCCACCC |
| GAPDH reverse | CCACTTGATTTTGGAGGGAT |
| Progesterone receptor forward | CCAAAGGCCGCAAATTCT |
| Progesterone receptor reverse | TGAGGTCAGAAAGGTCATCG |
| SOX-17 forward | CAGTATCTGCACTTCGTGTG |
| SOX-17 reverse | AGTAATATACCGCGGAGCTG |
| SRC-1 forward | AGACCCAACCTTTATTCCCA |
| SRC-1 reverse | GGTGTTACTTGAACAGGCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandur, E.; Pap, R.; Sipos, K. Activated THP-1 Macrophage-Derived Factors Increase the Cytokine, Fractalkine, and EGF Secretions, the Invasion-Related MMP Production, and Antioxidant Activity of HEC-1A Endometrium Cells. Int. J. Mol. Sci. 2024, 25, 9624. https://doi.org/10.3390/ijms25179624
Pandur E, Pap R, Sipos K. Activated THP-1 Macrophage-Derived Factors Increase the Cytokine, Fractalkine, and EGF Secretions, the Invasion-Related MMP Production, and Antioxidant Activity of HEC-1A Endometrium Cells. International Journal of Molecular Sciences. 2024; 25(17):9624. https://doi.org/10.3390/ijms25179624
Chicago/Turabian StylePandur, Edina, Ramóna Pap, and Katalin Sipos. 2024. "Activated THP-1 Macrophage-Derived Factors Increase the Cytokine, Fractalkine, and EGF Secretions, the Invasion-Related MMP Production, and Antioxidant Activity of HEC-1A Endometrium Cells" International Journal of Molecular Sciences 25, no. 17: 9624. https://doi.org/10.3390/ijms25179624
APA StylePandur, E., Pap, R., & Sipos, K. (2024). Activated THP-1 Macrophage-Derived Factors Increase the Cytokine, Fractalkine, and EGF Secretions, the Invasion-Related MMP Production, and Antioxidant Activity of HEC-1A Endometrium Cells. International Journal of Molecular Sciences, 25(17), 9624. https://doi.org/10.3390/ijms25179624






