Synthesis and Biological Evaluation of Novel Furopyridone Derivatives as Potent Cytotoxic Agents against Esophageal Cancer
Abstract
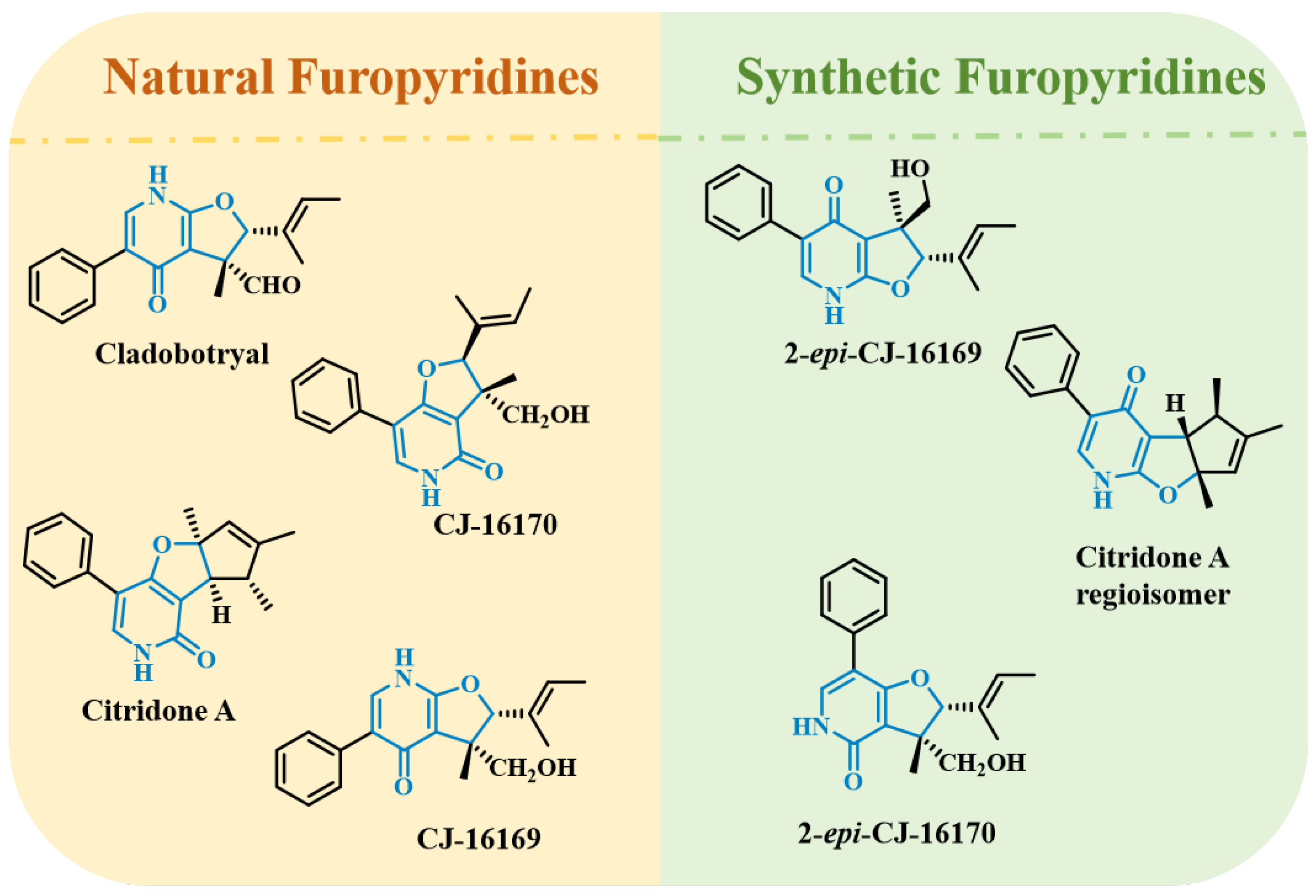
:1. Introduction
2. Results and Discussion
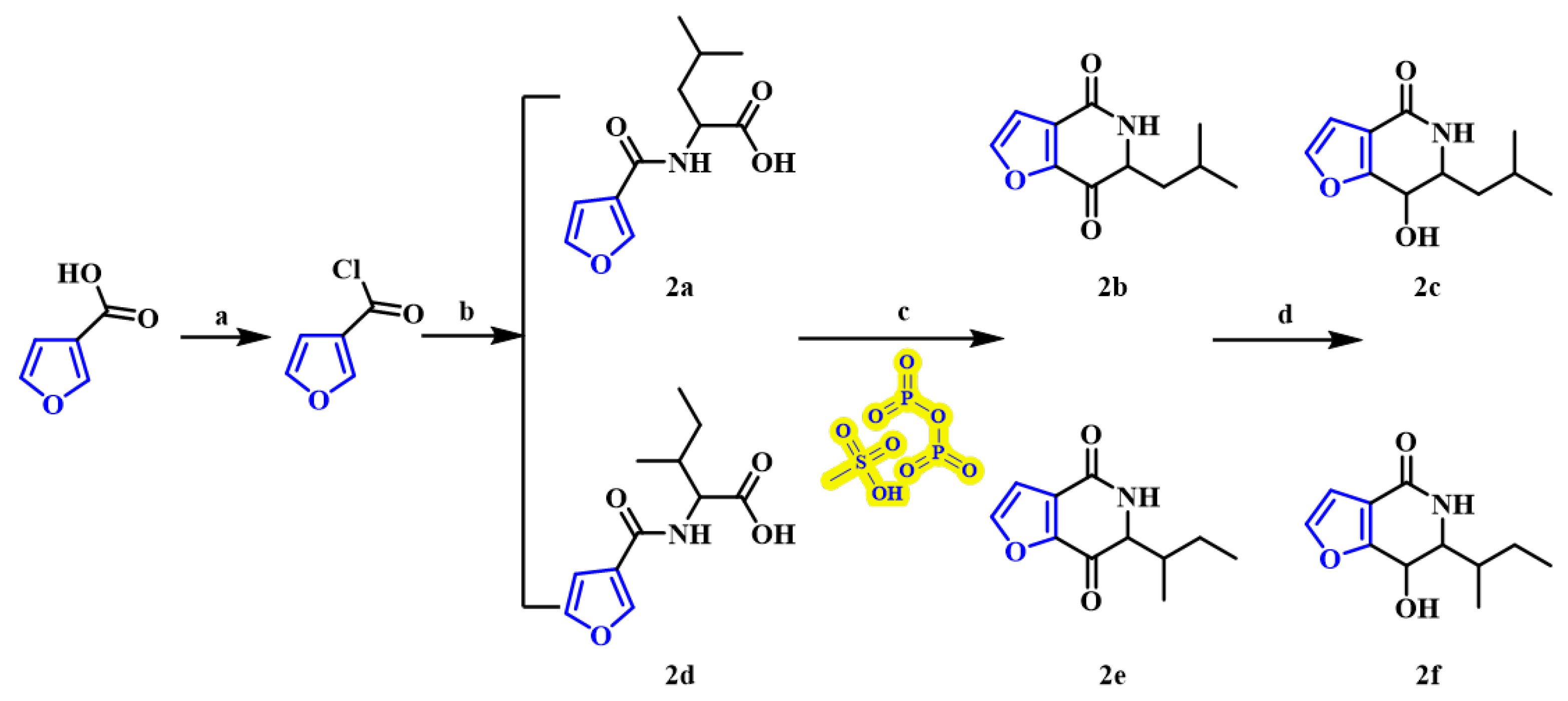
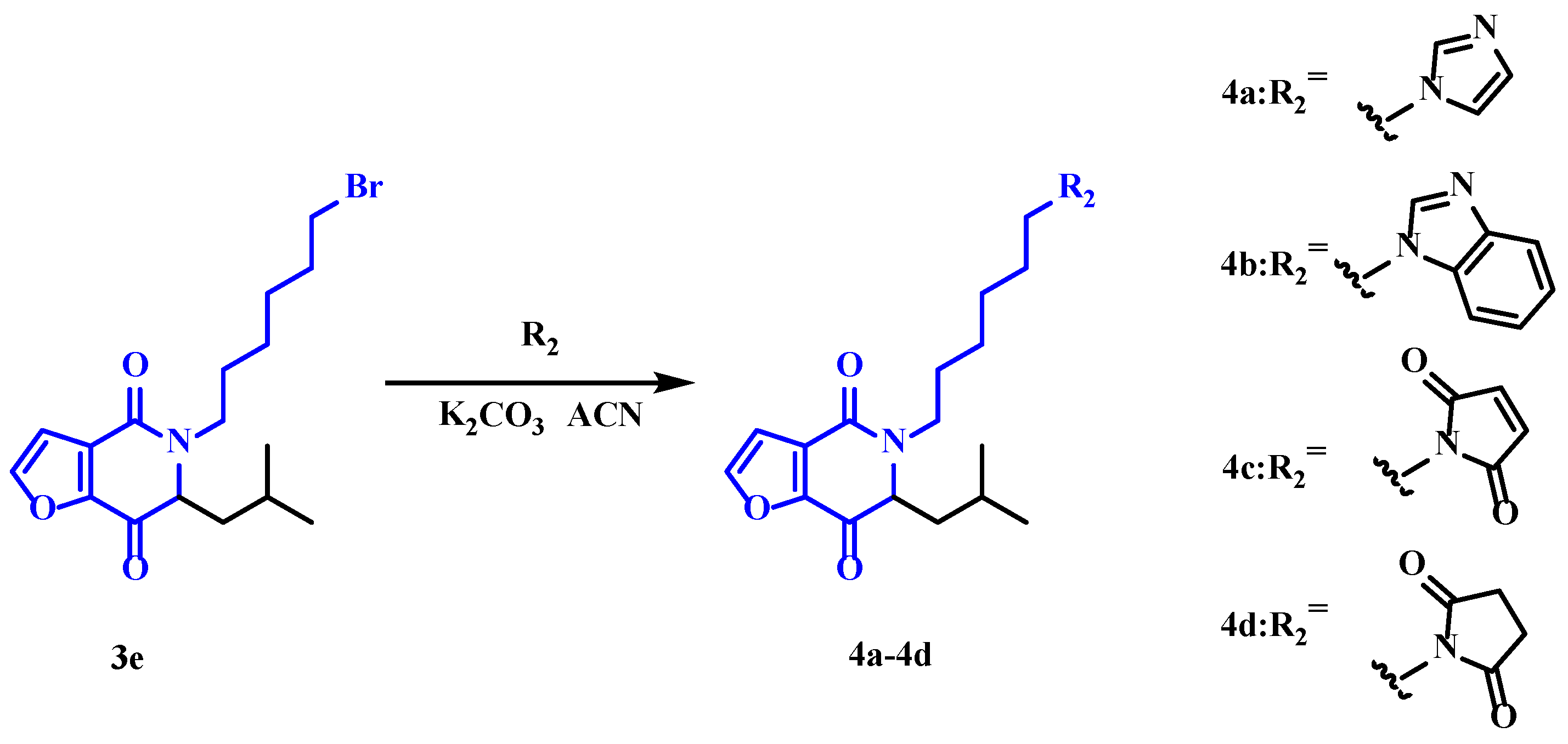
2.1. Chemistry
2.2. Biological Activity
Cytotoxicity Activity
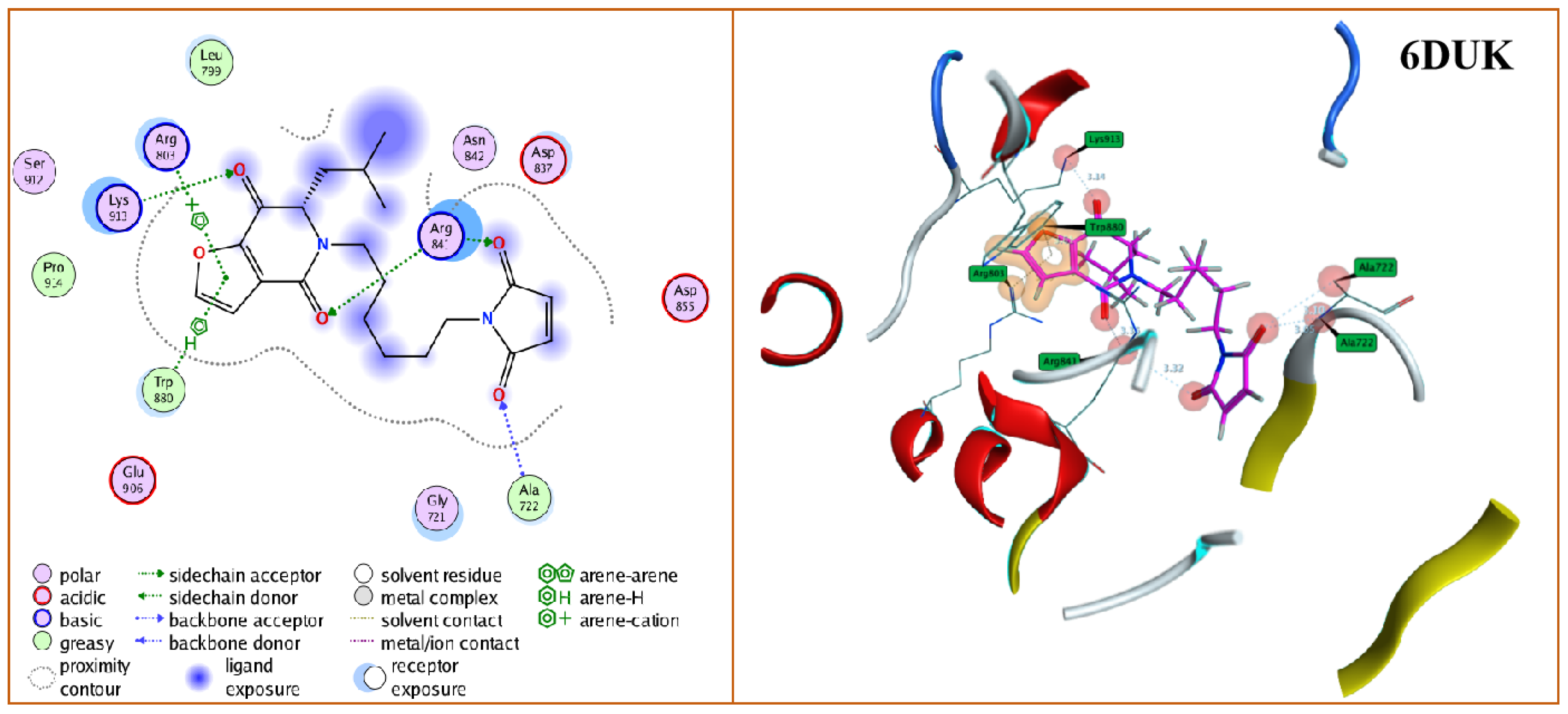
2.3. Molecular Docking Assessment
2.4. Spectral Data 2a–f, 3a–k and 4a–b
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. N-(3-Furanoyl)-L-leucine (2a)
3.2.2. 6-Isobutyl-furan [3,2-c] pyridin-4,7-dione (2b)
3.2.3. 6-Isobutyl-furan [3,2-c] pyridin-4 (5H)-ketone (2c)
3.2.4. 5-(2-Bromoethyl)-6-isobutyl-furano[3,2-c]pyridine-4,7-dione (3a)
3.2.5. 5-(6-(1H-Imidazol-1-yl)hexyl)-6-isobutyl-5,6-dihydrofuro[3,2-c]pyridine-4,7-dione (4a)
3.3. Cancer Cell Viability Assay
3.4. Molecular Docking Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Varmus, H. The new era in cancer research. Science 2006, 312, 1162. [Google Scholar] [CrossRef] [PubMed]
- Fayed, E.A.-O.; Gohar, N.A.; Farrag, A.M.; Ammar, Y.A. Upregulation of BAX and caspase-3, as well as downregulation of Bcl-2 during treatment with indeno[1,2-b]quinoxalin derivatives, mediated apoptosis in human cancer cells. Arch. Pharm. 2022, 355, e2100454. [Google Scholar] [CrossRef]
- Jassem, A.M.; Dhumad, A.M.; Salim, J.K.; Jabir, H.A. An alternative technique for cyclization synthesis, in vitro anti-esophageal cancer evaluation, and molecular docking of novel thiazolidin-4-one derivatives. J. Mol. Struct. 2023, 1280, 135079. [Google Scholar] [CrossRef]
- Afonso, L.A.; Cavalcanti, S.M.B.M.N.F.; Cavalcanti, S.M. Human papillomavirus detection and p16 methylation pattern in a case of esophageal papilloma. Braz. J. Med. Biol. Res. 2010, 43, 694. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2018, 15, 1. [Google Scholar] [CrossRef]
- Behranvand, N.; Nasri, F.; Emameh, R.A.-O.Z.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R.A.-O. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Wen, H.; Zhu, J.; Deng, H.; Jiang, X.; Ye, X.-Y.; Wang, L.; Xie, T.; Bai, R. Discovery of plant-derived anti-tumor natural products: Potential leads for anti-tumor drug discovery. Bioorg. Chem. 2024, 142, 106957. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, H.M.; Abdalla, A.N.; Obaidullah, A.J.; Alanazi, M.M.; Almehizia, A.A.; Alanazi, M.G.; Ahmed, A.Y.; Alwassil, O.I.; Darwish, H.W.; Abdel-Aziz, A.A.; et al. Synthesis, cytotoxic evaluation, and molecular docking studies of novel quinazoline derivatives with benzenesulfonamide and anilide tails: Dual inhibitors of EGFR/HER2. Bioorg. Chem. 2020, 95, 103461. [Google Scholar] [CrossRef]
- Mercogliano, M.; Iesce, M.A.-O.; Alfieri, M.L.; Buommino, E.; DellaGreca, M.A.-O.X. Hands-on synthesis of furanamides and evaluation of their antimicrobial activity. Nat. Prod. Res. 2023, 37, 3484. [Google Scholar] [CrossRef]
- Ramazani, A.; Karimi, M.; Hosseinzadeh, Z.; Rezayati, S.; Hanifehpour, Y.; Joo, S. Syntheses and Antitumor Properties of Furoxan Derivatives. Curr. Org. Chem. 2021, 25, 757. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abbas, E.M.H.; Farghaly, T.A. Antimicrobial Activity of Novel Tetra- and Penta-azaheterocyclic Ring Systems. J. Heterocycl. Chem. 2017, 54, 610. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Samadi, A.; Joukar, F.; Fakheri, H.T.; Hassanipour, S.; Ashoobi, M.T.; Soltanipour, S.; Alizadeh, A.; Rezamand, G.; Fathalipour, M. Efficacy and tolerability of fourteen-day sequential quadruple regimen: Pantoprazole, bismuth, amoxicillin, metronidazole and or furazolidone as first-line therapy for eradication of Helicobacter pylori: A randomized, double-blind clinical trial. EXCLI J. 2019, 18, 644. [Google Scholar]
- Calcaterra, N.E.; Barrow, J.C. Classics in chemical neuroscience: Diazepam (valium). ACS Chem. Neurosci. 2014, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, O.V.; Garifullin, B.F.; Zarubaev, V.V.; Slita, A.V.; Yesaulkova, I.L.; Saifina, L.F.; Shulaeva, M.M.; Belenok, M.G.; Semenov, V.A.-O.; Kataev, V.E. Synthesis of 1,2,3-triazolyl nucleoside analogues and their antiviral activity. Mol. Divers. 2021, 25, 473. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S.; Bérubé, G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2013, 8, 1029. [Google Scholar] [CrossRef]
- Kareem, A.F.A.; Al-Hujaj, H.H.; Jassem, A.M.; Al-Masoudi, N.A. A Click Synthesis, Molecular Docking, Cytotoxicity on Breast Cancer (MDA-MB 231) and Anti-HIV Activities of New 1,4-Disubstituted-1,2,3-Triazole Thymine Derivatives. Russ. J. Bioorg. Chem. 2020, 46, 360. [Google Scholar]
- Al-Salmi, F.A.; Alrohaimi, A.H.; Behery, M.E.; Megahed, W.; Ali, O.A.A.; Elsaid, F.G.; Fayad, E.; Mohammed, F.Z.; Keshta, A.T. Anticancer Studies of Newly Synthesized Thiazole Derivatives: Synthesis, Characterization, Biological Activity, and Molecular Docking. Crystals 2023, 13, 1546. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Pingaew, R.; Worachartcheewan, A.; Sinthupoom, N.; Prachayasittikul, V.; Ruchirawat, S.; Prachayasittikul, V. Roles of Pyridine and Pyrimidine Derivatives as Privileged Scaffolds in Anticancer Agents. Mini Rev. Med. Chem. 2017, 17, 869. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.N.; Poornachandra, Y.; Nagender, P.; Mallareddy, G.; Kumar, N.R.; Ranjithreddy, P.; Kumar, C.G.; Narsaiah, B. Synthesis of novel trifluoromethyl substituted furo [2,3-b] pyridine and pyrido [3′,2′: 4,5] furo [3,2-d] pyrimidine derivatives as potential anticancer agents. Eur. J. Med. Chem. 2016, 108, 68–78. [Google Scholar] [CrossRef]
- Bielenica, A.; Stefańska, J.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Sanna, G.; Madeddu, S.; Boi, S.; Giliberti, G.; Wrzosek, M.; et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl) phenyl moiety. Eur. J. Med. Chem. 2015, 101, 111–125. [Google Scholar] [CrossRef]
- Zuo, Y.; Pu, J.; Chen, G.; Shen, W.; Wang, B. Study on the activity and mechanism of skimmianine against human non-small cell lung cancer. Nat. Prod. Res. 2019, 33, 759. [Google Scholar] [CrossRef]
- Jassem, A.M.; Dhumad, A.M. Synthesis, Antimicrobial Activity, Anti-HIV Activity, and Molecular Docking of Novel 5-, 6- and 7-Membered Ring (1H-Pyrrol-2-yl)aminolactams. ChemistrySelect 2021, 6, 2641. [Google Scholar] [CrossRef]
- Węglarz-Tomczak, E.; Burda-Grabowska, M.; Giurg, M.; Mucha, A. Identification of methionine aminopeptidase 2 as a molecular target of the organoselenium drug ebselen and its derivatives/analogues: Synthesis, inhibitory activity and molecular modeling study. Bioorg. Med. Chem. Lett. 2016, 26, 5254. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, J.L.; Martín-Renedo, J.; García-Palomo, A.; Tuñón, M.J.; González-Gallego, J. Methionine aminopeptidases as potential targets for treatment of gastrointestinal cancers and other tumours. Curr. Drug Targets 2010, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sheppard, G.S.; Lou, P.; Kawai, M.; Park, C.; Egan, D.A.; Schneider, A.; Bouska, J.; Lesniewski, R.; Henkin, J. Physiologically relevant metal cofactor for methionine aminopeptidase-2 is manganese. Biochemistry 2003, 42, 5035. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kawai, H.; Kamishohara, M.; Odagawa, A.; Suzuki, A.; Uchida, T.; Kawasaki, T.; Tsuruo, T.; Otake, N. Structure-antitumor activity relationship of semi-synthetic Spicamycin derivatives. J. Antibiot. 1995, 48, 1467. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235. [Google Scholar] [CrossRef]


| Cells | Compd. | 24 h | 48 h | ||
|---|---|---|---|---|---|
| 40.00 (μg/mL) | 20.00 (μg/mL) | 40.00 (μg/mL) | 20.00 (μg/mL) | ||
| KYSE70 | 2a | 4.71 ± 6.10 | 7.02 ± 2.20 | 0.00 | 0.00 |
| 2b | 11.52 ± 2.89 | 8.86 ± 12.18 | 1.42 ± 5.43 | 0.00 | |
| 2c | 15.69 ± 4.52 | 15.38 ± 15.13 | 4.49 ± 5.80 | 0.00 | |
| 2d | 14.43 ± 9.53 | 0.00 | 7.18 ± 0.35 | 1.29 ± 1.09 | |
| 2e | 21.39 ± 4.16 | 3.96 ± 12.27 | 8.72 ± 1.65 | 1.49 ± 3.97 | |
| 2f | 11.48 ± 11.57 | 0.00 | 0.00 | 0.00 | |
| 3a | 26.54 ± 5.93 | 6.44 ± 16.39 | 9.00 ± 18.75 | 3.01 ± 4.53 | |
| 3b | 57.65 ± 3.52 | 38.81 ± 6.40 | 65.13 ± 6.00 | 16.26 ± 12.79 | |
| 3c | 10.06 ± 0.52 | 3.94 ± 6.90 | 6.77 ± 8.02 | 5.14 ± 10.60 | |
| 3d | 23.10 ± 10.92 | 19.98 ± 10.98 | 30.54 ± 9.94 | 1.83 ± 3.95 | |
| 3e | 67.77 ± 3.38 | 38.69 ± 0.72 | 70.16 ± 3.07 | 48.58 ± 5.08 | |
| 3f | 49.37 ± 3.60 | 40.81 ± 3.82 | 53.16 ± 3.77 | 28.58 ± 5.08 | |
| 3g | 35.81 ± 8.99 | 29.22 ± 4.89 | 20.29 ± 7.75 | 9.17 ± 3.32 | |
| 3h | 36.73 ± 8.12 | 20.69 ± 6.59 | 37.00 ± 7.64 | 0.00 | |
| 3i | 45.79 ± 0.62 | 22.15 ± 5.36 | 51.06 ± 2.60 | 16.92 ± 3.84 | |
| 3j | 2.15 ± 0.62 | 0.67 ± 8.49 | 0.00 | 0.00 | |
| 3k | 38.19 ± 4.49 | 2.86 ± 14.53 | 18.91 ± 2.26 | 3.29 ± 5.23 | |
| 4a | 16.41 ± 10.23 | 0.00 | 9.88 ± 4.54 | 7.07 ± 15.37 | |
| 4b | 12.95 ± 7.09 | 6.25 ± 5.28 | 13.15 ± 7.93 | 14.39 ± 4.11 | |
| 4c | 99.97 ± 0.25 | 99.18 ± 0.11 | 100 | 99.90 ± 0.12 | |
| 4d | 20.10 ± 11.43 | 0.00 | 23.62 ± 7.55 | 27.94 ± 14.01 | |
| Cells | Compd. | 24 h | 48 h | ||
|---|---|---|---|---|---|
| 40.00 (μg/mL) | 20.00 (μg/mL) | 40.00 (μg/mL) | 20.00 (μg/mL) | ||
| KYSE150 | 2a | 0.13 ± 3.62 | 3.62 ± 4.43 | 1.71 ± 0.62 | 0.68 ± 13.88 |
| 2b | 2.22 ± 1.82 | 4.72 ± 4.18 | 0.00 | 2.12 ± 7.69 | |
| 2c | 4.87 ± 3.21 | 3.56 ± 3.55 | 0.00 | 0.00 | |
| 2d | 19.03 ± 1.47 | 0.00 | 0.00 | 0.13 ± 11.98 | |
| 2e | 12.47 ± 1.85 | 0.13 ± 0.16 | 0.56 ± 7.22 | 0.00 | |
| 2f | 12.68 ± 6.89 | 0.00 | 3.93 ± 4.44 | 0.00 | |
| 3a | 13.09 ± 3.96 | 0.00 | 6.43 ± 1.92 | 0.00 | |
| 3b | 20.37 ± 10.73 | 19.12 ± 9.67 | 41.25 ± 7.33 | 27.47 ± 7.33 | |
| 3c | 10.06 ± 0.52 | 3.94 ± 6.90 | 6.77 ± 8.02 | 5.14 ± 10.60 | |
| 3d | 23.10 ± 10.92 | 19.98 ± 10.98 | 30.54 ± 9.94 | 1.83 ± 3.95 | |
| 3e | 61.47 ± 4.58 | 40.96 ± 13.22 | 51.62 ± 8.18 | 28.21 ± 13.17 | |
| 3f | 15.41 ± 7.27 | 4.50 ± 14.14 | 28.51 ± 8.46 | 13.81 ± 13.28 | |
| 3g | 11.5 ± 13.92 | 0.00 | 18.71 ± 9.04 | 18.71 ± 9.04 | |
| 3h | 13.14 ± 10.98 | 9.47 ± 10.32 | 39.76 ± 4.69 | 39.76 ± 4.69 | |
| 3i | 35.82 ± 8.24 | 26.56 ± 4.11 | 30.37 ± 3.49 | 6.25 ± 11.25 | |
| 3j | 3.35 ± 25.41 | 11.19 ± 6.11 | 8.14 ± 4.83 | 17.15 ± 12.21 | |
| 3k | 34.43 ± 7.57 | 11.05 ± 23.83 | 25.83 ± 5.21 | 2.85 ± 5.24 | |
| 4a | 26.34 ± 25.72 | 0.00 | 47.63 ± 5.85 | 25.61 ± 8.92 | |
| 4b | 33.30 ± 42.72 | 12.91 ± 2.28 | 0.05 ± 3.96 | 7.39 ± 4.71 | |
| 4c | 99.45 ± 0.49 | 99.24 ± 0.21 | 100.00 | 99.88 ± 0.14 | |
| 4d | 37.55 ± 16.59 | 11.62 ± 36.06 | 30.59 ± 2.99 | 28.7 ± 13.12 | |
| Time | Cells | Compd. | IC50 (μg/mL) | 95% Confidence Interval | Regression Equation | R2 |
|---|---|---|---|---|---|---|
| 24 h | KYSE70 | 4c | 1.463 | 1.300–1.600 | Y = −0.34 + 2.09 × X | 0.989 |
| 48 h | KYSE70 | 4c | 1.329 | 1.216–1.443 | Y = −0.3 + 2.65 × X | 0.968 |
| 24 h | KYSE150 | 4c | 0.888 | 0.801–0.973 | Y = −0.15 + 2.69 × X | 0.978 |
| 48 h | KYSE150 | 4c | 0.655 | 0.569–0.739 | Y = −0.39 + 2.15 × X | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Zhang, J.; Dai, A.; Sun, P.; Zhang, Y.; Jin, L.; Pan, L. Synthesis and Biological Evaluation of Novel Furopyridone Derivatives as Potent Cytotoxic Agents against Esophageal Cancer. Int. J. Mol. Sci. 2024, 25, 9634. https://doi.org/10.3390/ijms25179634
Ren X, Zhang J, Dai A, Sun P, Zhang Y, Jin L, Pan L. Synthesis and Biological Evaluation of Novel Furopyridone Derivatives as Potent Cytotoxic Agents against Esophageal Cancer. International Journal of Molecular Sciences. 2024; 25(17):9634. https://doi.org/10.3390/ijms25179634
Chicago/Turabian StyleRen, Xingyu, Jiaojiao Zhang, Anying Dai, Pengzhi Sun, Yibo Zhang, Lu Jin, and Le Pan. 2024. "Synthesis and Biological Evaluation of Novel Furopyridone Derivatives as Potent Cytotoxic Agents against Esophageal Cancer" International Journal of Molecular Sciences 25, no. 17: 9634. https://doi.org/10.3390/ijms25179634
APA StyleRen, X., Zhang, J., Dai, A., Sun, P., Zhang, Y., Jin, L., & Pan, L. (2024). Synthesis and Biological Evaluation of Novel Furopyridone Derivatives as Potent Cytotoxic Agents against Esophageal Cancer. International Journal of Molecular Sciences, 25(17), 9634. https://doi.org/10.3390/ijms25179634







